Abstract
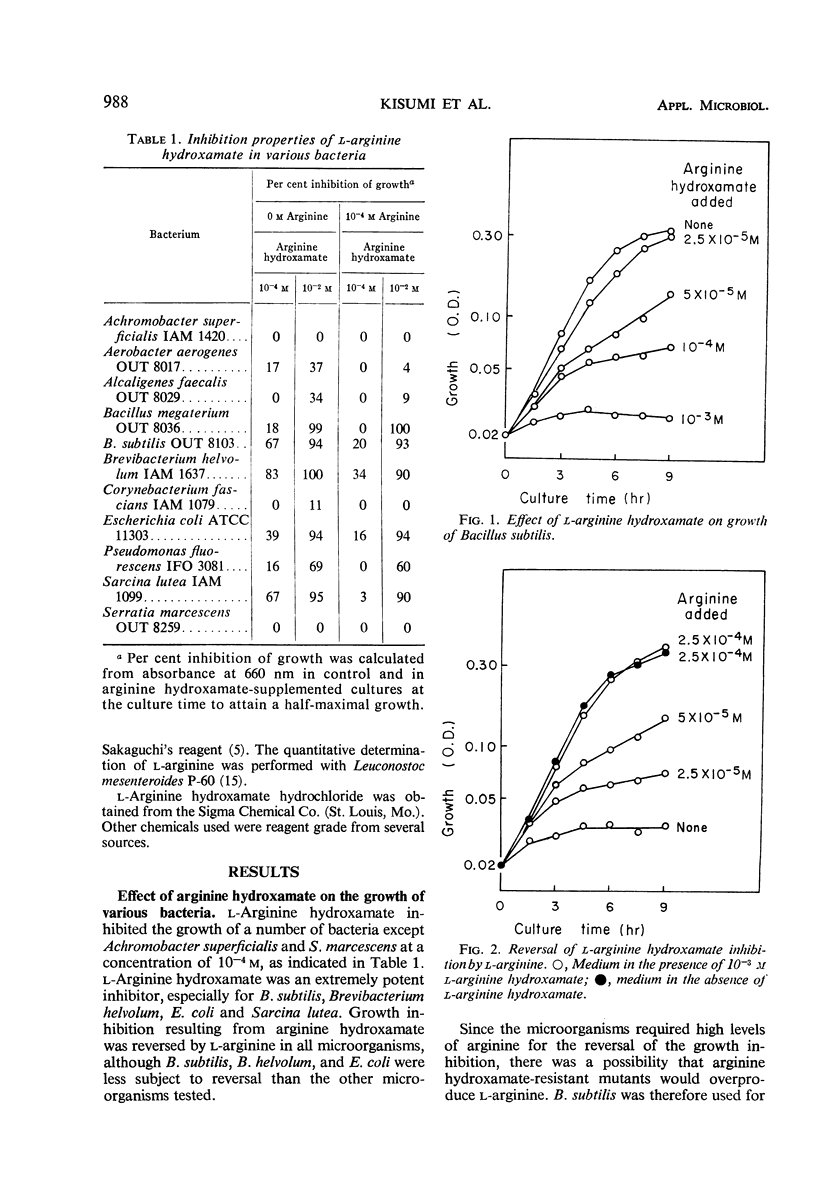
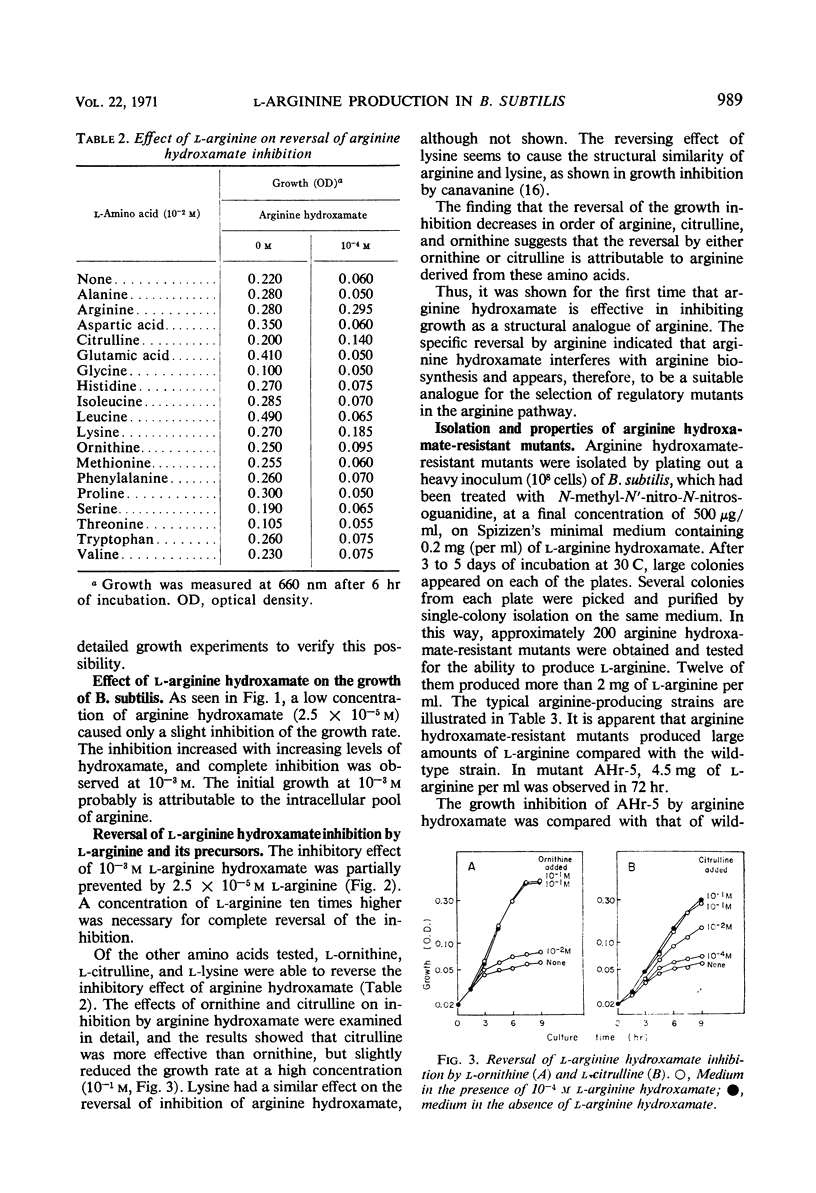
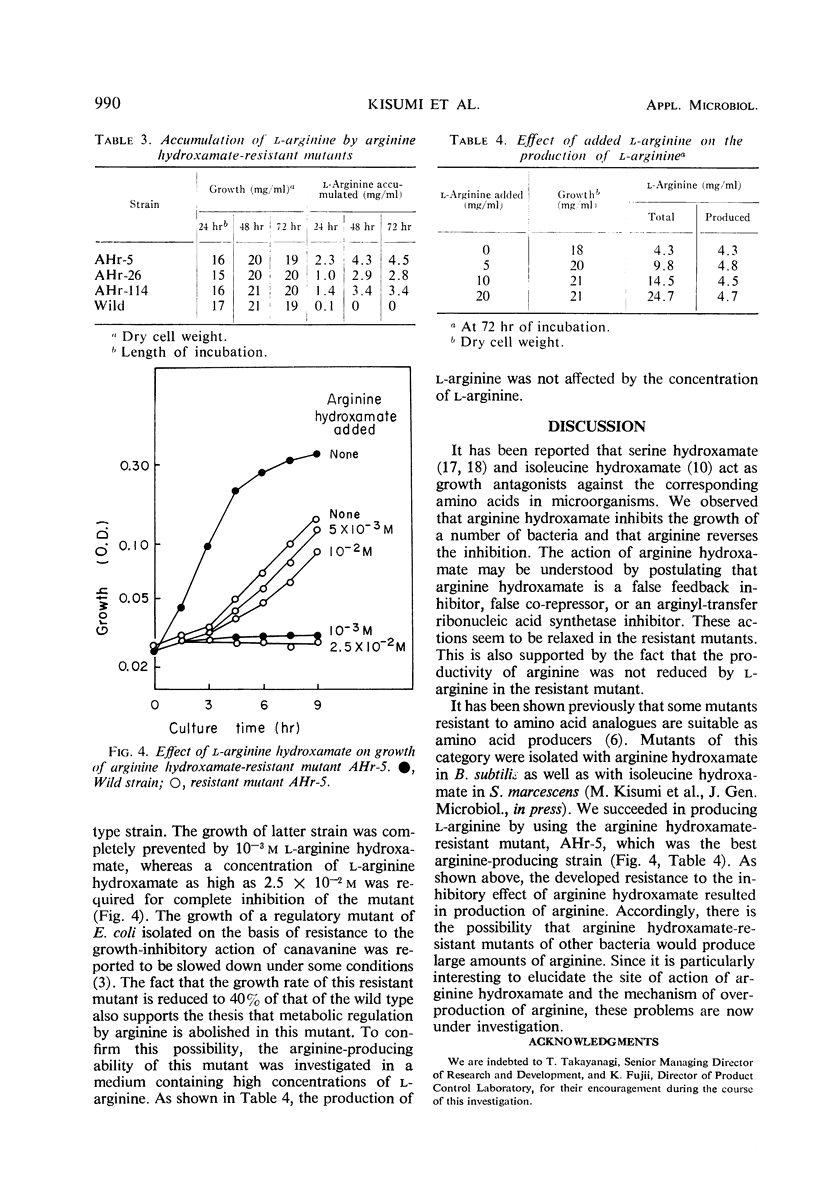
l-Arginine hydroxamate inhibited the growth of various bacteria, and the inhibition was readily reversed by arginine. l-Arginine hydroxamate (10−3m) completely inhibited the growth of Bacillus subtilis. This inhibitory effect was prevented by 2.5 × 10−4ml-arginine, which was the most effective of all the natural amino acids in reversing the inhibition. l-Arginine hydroxamate-resistant mutants of Bacillus subtilis were isolated and found to excrete l-arginine in relatively high yields. One of the mutants, strain AHr-5, produced 4.5 mg of l-arginine per ml in shaken culture in 3 days.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A. Selection of bacterial mutants which excrete antagonists of antimetabolites. J Bacteriol. 1958 Sep;76(3):326–326. doi: 10.1128/jb.76.3.326-326.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Horn P. C., Hopwood D. A., Maas W. K., DeDeken R. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. 3. Repression of enzymes of arginine biosynthesis in arginyl-tRNA synthetase mutants. J Mol Biol. 1968 Jul 14;35(1):83–93. doi: 10.1016/s0022-2836(68)80038-5. [DOI] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Kato J., Komatsubara S., Chibata I. Increase in isoleucine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. Appl Microbiol. 1971 Apr;21(4):569–574. doi: 10.1128/am.21.4.569-574.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Valine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. J Bacteriol. 1971 May;106(2):493–499. doi: 10.1128/jb.106.2.493-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- Peyru G. M., Maas W. K. Inhibition of Escherichia coli B by homoarginine. J Bacteriol. 1967 Sep;94(3):712–718. doi: 10.1128/jb.94.3.712-718.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAS H. J. Effect of canavanine on mutants of Neurospora and Bacillus subtilis. J Biol Chem. 1951 May;190(1):369–375. [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol. 1971 Jun;106(3):966–971. doi: 10.1128/jb.106.3.966-971.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka S. Pathway-specific pattern of control of arginine biosynthesis in bacteria. J Bacteriol. 1966 Feb;91(2):617–621. doi: 10.1128/jb.91.2.617-621.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J. Aspects of repression in the regulation of enzyme synthesis: pathway-wide control and enzyme-specific response. Cold Spring Harb Symp Quant Biol. 1961;26:163–172. doi: 10.1101/sqb.1961.026.01.021. [DOI] [PubMed] [Google Scholar]
- VYAS S., MAAS W. K. Feedback inhibition of acetylglutamate synthetase by arginine in Escherichia coli. Arch Biochem Biophys. 1963 Mar;100:542–546. doi: 10.1016/0003-9861(63)90124-3. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Canavanine and homoarginine as antimetabolites of arginine and lysine in yeast and algae. J Biol Chem. 1955 Jan;212(1):207–215. [PubMed] [Google Scholar]
- WALKER J. B. Homoarginine inhibition of Escherichia coli B. J Biol Chem. 1955 Feb;212(2):617–622. [PubMed] [Google Scholar]



