Abstract
Background:
Invasive aspergillosis (IA) is a leading cause of mortality in acute leukemia and hematopoietic stem cell transplantation (HSCT).
Aims:
To determine the yield of galactomannan (GM) assay for the diagnosis of probable IA, its temporal relationship with the computed tomography (CT) scans and correlation with mortality in AL and HSCT.
Patients and Methods:
Consecutive neutropenic episodes (n=150) among inpatients aged ≥15 years with AL or recipients of HSCT were prospectively evaluated over 1½ years. All patients underwent weekly serum GM assay and optical density index >0.5 for ≥2 samples was defined as positive. IA was diagnosed according to EORTC 2008 guidelines.
Results:
Of the 150 episodes enrolled, 43 (28.7%) were diagnosed with IA: possible 25 (16.7%), probable 17 (11.3%) and proven 1 (0.7%). The yield of GM assay in diagnosing probable IA was 17/42 (40.5%). In 88.2% of probable IA episodes, GM was positive before high-resolution CT at a median of 10 days (range 1-16). In the episodes with ≥2 samples tested, fatality was higher in those ≥2 values positive for GM, compared to the rest (31% vs. 13.2%, odd ratio 2.96, 95% CI 1.09-8.00; P=0.04).
Conclusions:
In AL and HSCT, GM assay could identify patients with probable IA earlier than CT chest and also predicted a higher risk of death.
Keywords: Acute leukemia, computed tomography, galactomannan assay, hematopoietic stem cell transplantation, invasive Aspergillosis
INTRODUCTION
Invasive Aspergillosis (IA) currently constitutes the most common cause of infectious pneumonic mortality in patients undergoing hematopoietic stem-cell transplant (HSCT) and is an important cause of opportunistic respiratory and disseminated infection in other immune-compromised patients. Conventional diagnosis of IA is dependent on culture and histopathologic examination of the tissues involved.[1] Microscopy and culture of sputum and bronchoalveolar lavage (BAL) samples are insufficiently sensitive for diagnostic approaches, and BAL and biopsies are not always feasible in patients with a severe underlying condition and thrombocytopenia.[2] Thus, in most cases, diagnosis depends on a combination of clinical scenario (i.e., setting of high-risk neutropenia, persistent fever despite broad-spectrum antibiotics, suggestive clinical features) along with radiologic abnormalities and clinical experience.
Currently several non-invasive tests are available to facilitate the diagnosis of IA, like enzyme-linked immunosorbent assay (ELISA) for circulating serum galactomannan (GM), assay for circulating 1-3-β-D-glucan (BDG) and polymerase chain reaction (PCR) for fungal DNA in the blood. The PCR assays are not routinely available, and are not considered standard for diagnosis, as of now.[3] The BDG assay is in turn, not specific for Aspergillosis and has numerous confounding factors, which may give rise to false-positive and false-negative results.[3]
The ELISA for GM detects the circulating GM, which is a major constituent of Aspergillus cell walls. The sensitivity and specificity of this test may vary considerably according to the patient population and cut off level used.[4] In addition, false-positive results with antibiotics, such as amoxicillin-clavulanate and piperacillin-tazobactam, are a cause of concern. Although the test kit is readily available in India and some centers are using it, there is no published data from India except a small series as an abstract.[5] We planned this study to prospectively evaluate the utility of weekly serum GM assay as an adjunct to computed tomography (CT) scans in neutropenic patients of acute leukemia (AL) or HSCT recipients, at high-risk for IA. The primary objective was to estimate the yield of GM assay in detecting probable IA in this high-risk population. Secondary objectives were to assess its temporal relationship with high-resolution computed tomographic (HRCT) scans and correlation with mortality.
PATIENTS AND METHODS
This was a prospective, single institutional, cohort study conducted during the period October 2008 to February 2010. A convenient sample of 150 consecutive episodes of high-risk neutropenia was chosen. We included patients aged ≥15 years, with a diagnosis of AL (AL induction, high-dose cytarabine consolidation, relapsed/refractory AL on re-induction therapy) or recipients of auto- and allo-HSCT. Episodes in patients diagnosed with possible or probable IA were also eligible for inclusion. Patients who were planned for discharge soon after completion of chemotherapy and those not willing to participate were excluded. The study was approved by the institutional ethics committee and informed consent was taken from all enrolled subjects.
Clinical management
Enrolled patients were hospitalized from the start of chemotherapy until neutrophil recovery. Antifungal prophylaxis was with either itraconazole 200 mg/day orally, or amphotericin B (AmB) 0.5-0.7 mg/kg IV every alternate day. Febrile neutropenia was managed by intravenous broad-spectrum antibiotics as per existing guidelines.[6] First-line IV antibiotic therapy consisted of cefoperazone-sulbactam plus amikacin. Levofloxacin use was allowed in lieu of amikacin, at the discretion of the treating physician (renal impairment, elderly patient, etc.,). Empiric AmB (1-1.5 mg/kg/day) was added in the following circumstances-on day 4 or 5 in patients with persisting fever, fever relapsing after 48 h of defervescence, progressive pneumonia, organ dysfunction or signs of sepsis unresponsive to broad-spectrum antibiotics.
GM assay
Samples for GM assay were collected at the time of enrolment and then continued weekly until recovery of absolute neutrophil count (ANC) and discharge from hospital or death, whichever was later. Samples were collected and transported in sealed containers. Serum was separated immediately after collection and stored at −70°C until analysis. A double-sandwich ELISA GM assay (Platelia Aspergillus, ®Bio-Rad laboratories) capable of detecting GM at concentrations as low as 0.5 ng/mL was used. The assay was carried out at the Medical Oncology Laboratory of the hospital as per manufacturer's guidelines. A cut-off of optical density index (ODI) >0.5 was taken as positive.
CT scans
A HRCT chest was carried out in episodes with any clinical features of chest infection or after 4-6 days of persistent fever. HRCT paranasal sinus was carried out in cases with the clinical or radiologic suspicion of sinusitis.
Diagnosis of IA
Each episode was categorized as no IA, possible IA, probable IA, or proven IA according to European Organization for Research and Treatment of Cancer (EORTC) 2008 criteria.[7] BAL was attempted in cases with clinico-radiologic diagnosis of pneumonia. All cases fitting into possible, probable, and proven IA were treated with therapeutic doses of AmB (1-1.5 mg/kg/day) or voriconazole (300 mg for first two doses followed by 200 mg twice daily) until recovery. Oral voriconazole was continued after discharge for as long as the patient was immunosuppressed and at least for a total of 6 weeks of therapy. Study end-points were either recovery from neutropenia and clinical resolution of IA (irrespective of persistence of radiologic abnormalities) or death.
Statistical analysis
The yield of GM assay was calculated as a percentage of possible cases reclassified as probable on the basis of GM results. Positive GM results in episodes classified as no IA were taken as false-positives. Association between GM-positive results and CT scan findings, frequency of therapeutic antifungal usage or survival at the end of the episode was analyzed by Chi-squared test or Fisher's exact test as applicable. A P<0.05 was considered statistically significant. All statistical analysis was done by SPSS version 11.5.0.
RESULTS
Baseline characteristics
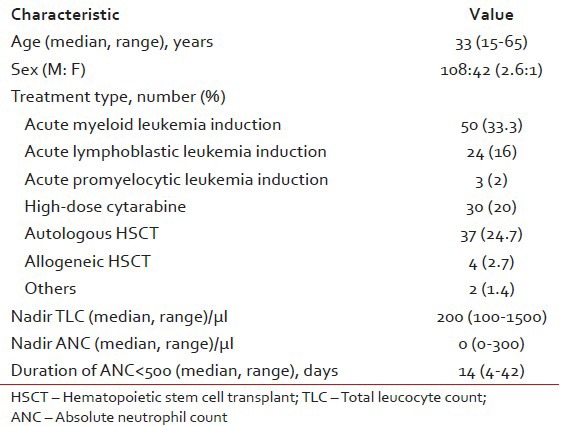
During the study period, a total of 150 episodes (in 126 unique patients) of neutropenia were included in the study. The baseline characteristics of the episodes are summarized in Table 1. Median age of the patients in the episodes was 33 years (range 15-65 years). Male sex predominated with M:F ratio of 2.6:1. Acute myeloid leukemia induction constituted 50 episodes (33.3%), consolidation with high-dose cytarabine 30 episodes (20%) and recipients of autologous hematopoietic stem cell transplantation (auto-HSCT) 37 episodes (24.7%), respectively. Six episodes had a prior history of IA (three possible and three probable).
Table 1.
Baseline characteristics of the episodes
Based on clinico-radiologic and microbiologic criteria, diagnosis of IA was made in 43 episodes (28.7%). Of these, 25 were possible and 17 were probable. The lone episode of proven IA was diagnosed on lung autopsy in a fatal case of acute lymphoblastic leukemia undergoing induction chemotherapy. We could do BAL in only seven episodes, none showed evidence of Aspergillosis. The probable cases were diagnosed on the basis of two or more serum samples positive for GM. The nadir total leucocyte count, nadir ANC, and duration of ANC<500/μl were comparable in all groups.
GM samples
A total of 368 samples for GM were collected during the study. Median number of samples was 2 (range 1-8) per episode for the whole cohort. Median number of samples for GM was higher for the probable IA group as compared to possible IA group (3 vs. 2, P=0.03). The first sample for GM had an ODI of ≥0.5 in 48 episodes, of which 19 were ≥1.0 and 6 were ≥2.0. Among the episodes not having a diagnosis of IA, 88.8% had none or only one sample positive for GM (ODI>0.5) while 39.5% of the episodes with a diagnosis of possible, probable or proven IA had two samples of GM with ODI>0.5 [Table 2]. Out of the two samples sent for the patient with proven IA, one was positive; the patient died before further samples could be collected.
Table 2.
Number of positive galactomannan samples in each group. Only 11.2% episodes with an eventual diagnosis of no Aspergillosis had two or more samples positive for galactomannan whereas 43% had atleast one sample positive
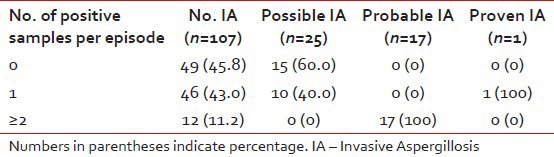
Of the 42 episodes with possible and probable IA, 17 were classified as probable on the basis of GM assay. Thus, the yield of GM assay (considering two ODI values>0.5 as positive) to detect probable IA was 40.5%. The false positive rate of a single GM result with ODI>0.5 was very high (54.2%), but that of at least two values>0.5 was much lower (11.2%). When the cut-off was raised to 1.0 (single value), false-positive rate was 23.1% with the yield of probable IA being 14/42 (33.3%). False positive rate was only 7.5% if a cut-off of 2.0 or more was used, but yield of probable IA was reduced to 10/42 (23.8%).
Correlation with CT scan
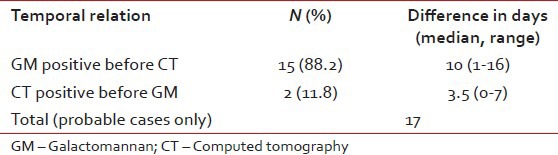
A CT scan of chest and/or paranasal sinuses were carried out in 78 episodes (46.4%). CT chest was carried out in all but two cases with probable or proven IA and showed signs of IA in all. The median interval between enrolment (not from the onset of fever) and the first CT scan was 14 days for the entire cohort; 12.5 days (range 1-24) in the no IA group, and 15 (range 2-30) in the IA group. Of the 32 episodes in the no IA group, who required empiric AmB or voriconazole, CT was carried out in 21 episodes, 7 of which were abnormal but not consistent with IA. If only the probable IA were considered, GM was positive before CT in 88.2% episodes while CT was positive before GM in the remaining 11.8% episodes [Table 3].
Table 3.
Temporal relation between CT scans and galactomannan positivity in cases with a diagnosis of probable Aspergillosis. Galactomannan was positive before CT in 88% episodes at a median of 10 days earlier
Effect of antibiotics
In those samples collected during the amoxicillin-clavulanate therapy, 27 (62.7%) had an ODI >0.5 while in 12 episodes (31.7%) it was >1.0. In the samples collected during the piperacillin-tazobactam therapy, 11 (35.4%) had ODI>0.5 and 5 (16.1%) had ODI>1.0. In the 294 samples collected while the patient was not on any of these antibiotics, 88 (29.9%) and 37 (12.5%) had a value >0.5 and >1.0, respectively. Among the no IA group (n=107), overall 30% samples were false-positive. In this group, 23 samples were collected while the patient was on amoxicillin-clavulanate, out of these 17 were positive (73.9%). For the samples while on piperacillin-tazobactam, 5/14 were positive (35.7%). A cut-off of >1.0 could eliminate 3/5 false positives for piperacillin-tazobactam and 10/17 for amoxycillin-clavulanate.
Correlation with antifungal usage
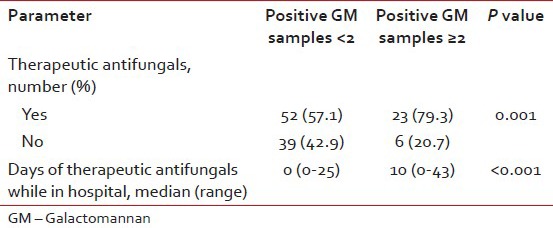
Therapeutic anti-mould agent usage (AmB or voriconazole) was assessed in the episodes where at least two GM samples were collected [Table 4]. Of the 91 episodes with <2 samples having ODI >0.5, 42.9% received therapeutic AmB or voriconazole, corresponding figure for those with ≥2 samples with ODI >0.5 was 79.3% (P=0.001). The median duration of in-hospital antifungal usage was also significantly high in the 29 episodes with ≥2 GM samples positive (10 vs. 0 days, P<0.001). Of these, the 17 probable cases received antifungals for a median of 14 days (17 days in those who survived versus 12 in the fatal cases). This excludes oral voriconazole taken after discharge. Among the 52 episodes with positive CT criteria, 18 were GM negative (all values <0.5) and out of these eight completed >2 weeks antifungal therapy. Of the remaining 10 episodes, five had a fatal outcome and antifungals were stopped earlier than 2 weeks in the rest.
Table 4.
Therapeutic antifungal usage while in hospital. In episodes with two or more galactomannan positive, 79.3% received antifungals as compared to 57.1% with none or one positive sample
Correlation with mortality
There were total 23 deaths (15.3%). Six of 107 died in the no IA group (5.6%) as compared to 9/25 (36%) in possible IA group and 7/17 (41.1%) in the probable IA group. The lone proven case was fatal. The odds ratio (OR) for death for those with any category of IA versus no IA was 11.06 (95% CI 3.94-30.69, P<0.001).
Considering only the 120 episodes with at least two samples analyzed, in the episodes with <2 GM samples positive (ODI > 0.5), 13.2% had a fatal outcome while in those with at least two values positive, 31.0% died [Figure 1]. The OR for death for episodes with at least 2 values positive was 2.96 (95% CI 1.09-8.00; P=0.04).
Figure 1.
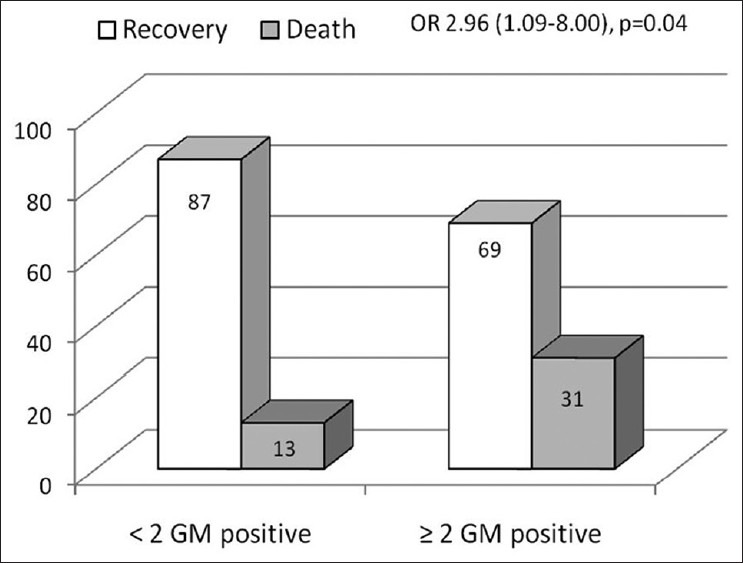
Bar diagram showing higher mortality in episodes with two or more samples positive (optical density index >0.5) for galactomannan
DISCUSSION
This is the first large study from India on the role of GM assay in IA in patients of hematological malignancies and recipients of HSCT. On evaluating 150 episodes of neutropenia among patients with above-mentioned diagnoses, we found that the incidence of IA according to EORTC guidelines was quite high (28.7%). Assessing the sensitivity of GM assay was problematic because we could diagnose only one proven case. This was partly because of difficulty in getting BAL done, which required the patients to be shifted to another building and hence, not possible for critically ill patients. In cases where BAL was carried out, none was positive for Aspergillosis. In the lone proven case, one of the two GM samples was strongly positive. Of the 42 episodes who would be otherwise diagnosed with possible IA, 17 (40.5%) could be assigned a diagnosis of probable IA, based upon two or more serum GM samples having an ODI>0.5. False positivity of a single value >0.5 was high (54.2%), but if positivity for at least two samples was taken, it decreased to 11.2%. The reason for lower specificity of a single value is unclear, but it may be due to confounding antibiotics such s amoxycillin-clavulanate, or translocation of GM from contaminated food through chemotherapy-induced breached gut mucosal barrier. It is clear that in most cases these may be eliminated by repeated sampling. When we used a cut-off of 1.0, the specificity of a single value improved markedly to 76.6%, but still lower to that in the Cochrane meta-analysis (91%).[4]
The false-positivity rate in case of samples collected during therapy with amoxycillin-clavulanate was 73.9% that with piperacillin-tazobactam 35.7% and for those samples where no such antibiotics were used, it was 24.6%. These results are in accordance with published reports.[8,9,10,11,12,13,14,15,16] It has been shown earlier that, for piperacillin-tazobactam, raising the cut-off to >0.7 can eliminate the false-positives. In our study also, raising the cut-off to >1.0 could eliminate more than half of the false-positives for piperacillin-tazobactam and amoxycillin-clavulanate, but with a resultant decrease in sensitivity. Collecting the sample just before a dose of antibiotic has been administered could reduce false-positives.[14] However, we did not collect samples according to a pre-specified time-frame with respect to administration of antibiotics in our study.
In contrast to one report of high false-positivity rate in multiple myeloma,[17] in our study only one out of 20 myeloma patients (undergoing auto-HSCT) had two false-positive values. However, though this patient did not have clinical signs of IA and chest radiograph was normal on more than one occasion, he did have fever >96 h on antibiotics and received AmB for 9 days. Interestingly, he also had both GM values >1.0. Therefore, it is unclear whether this case was truly a ‘false-positive’.
Earlier reports on the relationship between GM levels and CT scans have shown conflicting results.[18,19] We found that serum GM had an earlier time to positivity as compared to CT scans of the chest or paranasal sinuses. Logistically, this is important as CT cannot be repeated frequently in one patient, although, serum GM assay can be repeated even twice weekly. Earlier detection can lead to earlier initiation of therapy and has the potential to improve survival.
The high case-fatality rate of IA is well-known and mortality can be taken as a surrogate for IA. In one literature review, serum GM assay had an excellent correlation with survival and autopsy findings in proven and probable IA.[20] GM assay outcome has also been proposed as a surrogate end-point for IA trials.[21] In our study, those episodes with two or more samples positive for GM received AmB or voriconazole more frequently and had a higher probability of death. This could not have been due to longer duration of neutropenia and more samples in the fatal episodes as only episodes with at least two samples were considered for this analysis. The mortality of all episodes with IA (proven + probable + possible) was 39.5%, which compares favorably with other studies, but obviously not all in this group were true IA.
From this study, we could infer that GM assay (≥2 samples with ODI values >0.5) can identify patients having a higher probability of harboring IA and have a higher risk of mortality. It can also identify these patients earlier than CT scan and is much easier to perform frequently. Thus, it may be helpful to identify patients who should receive therapeutic doses of mould-active antifungals (AmB or voriconazole) as early as possible. This approach can also reduce the use of antifungals in episodes with two consecutive negative values of GM. In fact, it has been observed in another study that the response rate of empirical antifungal therapy was higher in patients with the positive results of serum GM tests and/or chest CT scan than that in others.[22] We could identify one study, which studied this pre-emptive approach in a randomized fashion. In this small study of 52 episodes, the pre-emptive approach saved up to 14% of patients from empirical antifungals.[23] However, further randomized studies are needed to test this hypothesis, where GM results are used in one arm in a protocol-defined manner as compared to the control arm where GM assay is either not done or the treating physician is blinded to the results.
Footnotes
Source of Support: The study was entirely funded by the Department of Medical Oncology, Dr. B.R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
Conflict of Interest: None declared.
REFERENCES
- 1.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 3.Thornton CR. Detection of invasive aspergillosis. Adv Appl Microbiol. 2010;70:187–216. doi: 10.1016/S0065-2164(10)70006-X. [DOI] [PubMed] [Google Scholar]
- 4.Leeflang MM, Debets-Ossenkopp YJ, Visser CE, Scholten RJ, Hooft L, Bijlmer HA, et al. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst Rev. 2008;4:CD007394. doi: 10.1002/14651858.CD007394. [DOI] [PubMed] [Google Scholar]
- 5.Anjan M, Oberoi J, Yadav SP, Sachdeva A. Diagnosis of invasive aspergillosis using Galactomannan assay in pediatric patients with cancer. J Clin Oncol. 2008;26(Suppl 15S):20522. [Google Scholar]
- 6.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 7.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam O, Aupérin A, Wilquin F, Bourhis JH, Gachot B, Chachaty E. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin Infect Dis. 2004;38:917–20. doi: 10.1086/383148. [DOI] [PubMed] [Google Scholar]
- 9.Aubry A, Porcher R, Bottero J, Touratier S, Leblanc T, Brethon B, et al. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J Clin Microbiol. 2006;44:389–94. doi: 10.1128/JCM.44.2.389-394.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattei D, Rapezzi D, Mordini N, Cuda F, Lo Nigro C, Musso M, et al. False-positive Aspergillus galactomannan enzyme-linked immunosorbent assay results in vivo during amoxicillin-clavulanic acid treatment. J Clin Microbiol. 2004;42:5362–3. doi: 10.1128/JCM.42.11.5362-5363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh TJ, Shoham S, Petraitiene R, Sein T, Schaufele R, Kelaher A, et al. Detection of galactomannan antigenemia in patients receiving piperacillin-tazobactam and correlations between in vitro, in vivo, and clinical properties of the drug-antigen interaction. J Clin Microbiol. 2004;42:4744–8. doi: 10.1128/JCM.42.10.4744-4748.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscoli C, Machetti M, Cappellano P, Bucci B, Bruzzi P, Van Lint MT, et al. False-positive galactomannan platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin Infect Dis. 2004;38:913–6. doi: 10.1086/382224. [DOI] [PubMed] [Google Scholar]
- 13.Bart-Delabesse E, Basile M, Al Jijakli A, Souville D, Gay F, Philippe B, et al. Detection of Aspergillus galactomannan antigenemia to determine biological and clinical implications of beta-lactam treatments. J Clin Microbiol. 2005;43:5214–20. doi: 10.1128/JCM.43.10.5214-5220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlopp K, von Lilienfeld-Toal M, Marklein G, Reiffert SM, Welter A, Hahn-Ast C, et al. False positivity of the Aspergillus galactomannan Platelia ELISA because of piperacillin/tazobactam treatment: Does it represent a clinical problem? J Antimicrob Chemother. 2008;62:1109–12. doi: 10.1093/jac/dkn308. [DOI] [PubMed] [Google Scholar]
- 15.Boonsarngsuk V, Niyompattama A, Teosirimongkol C, Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scand J Infect Dis. 2010;42:461–8. doi: 10.3109/00365541003602064. [DOI] [PubMed] [Google Scholar]
- 16.Zandijk E, Mewis A, Magerman K, Cartuyvels R. False-positive results by the platelia Aspergillus galactomannan antigen test for patients treated with amoxicillin-clavulanate. Clin Vaccine Immunol. 2008;15:1132–3. doi: 10.1128/CVI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori Y, Nagasaki Y, Kamezaki K, Takenaka K, Iwasaki H, Harada N, et al. High incidence of false-positive Aspergillus galactomannan test in multiple myeloma. Am J Hematol. 2010;85:449–51. doi: 10.1002/ajh.21697. [DOI] [PubMed] [Google Scholar]
- 18.Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: A prospective feasibility study. Clin Infect Dis. 2005;41:1242–50. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]
- 19.Weisser M, Rausch C, Droll A, Simcock M, Sendi P, Steffen I, et al. Galactomannan does not precede major signs on a pulmonary computerized tomographic scan suggestive of invasive aspergillosis in patients with hematological malignancies. Clin Infect Dis. 2005;41:1143–9. doi: 10.1086/444462. [DOI] [PubMed] [Google Scholar]
- 20.Miceli MH, Grazziutti ML, Woods G, Zhao W, Kocoglu MH, Barlogie B, et al. Strong correlation between serum aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: Clinical and research implications. Clin Infect Dis. 2008;46:1412–22. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 21.Anaissie EJ. Trial design for mold-active agents: Time to break the mold – Aspergillosis in neutropenic adults. Clin Infect Dis. 2007;44:1298–306. doi: 10.1086/514352. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Xu LP, Liu DH, Chen YH, Han W, Zhang XH, et al. Positive results of serum galactomannan assays and pulmonary computed tomography predict the higher response rate of empirical antifungal therapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:759–64. doi: 10.1016/j.bbmt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Tan BH, Low JG, Chlebicka NL, Kurup A, Cheah FK, Lin RT, et al. Galactomannan-guided preemptive vs. empirical antifungals in the persistently febrile neutropenic patient: A prospective randomized study. Int J Infect Dis. 2011;15:e350–6. doi: 10.1016/j.ijid.2011.01.011. [DOI] [PubMed] [Google Scholar]


