Abstract
Plasmablastic lymphoma (PL) is a type of non-Hodgkin's lymphoma (NHL) having a strong association with immunosuppression, especially, human immunodeficiency virus (HIV) infection. It generally has a poor prognosis with most patients dying within 2 years from initial presentation, and long-term survivors are very few. We report the case of a 10-years-old child, presenting in 2003 with swelling on the right side of the face and fever of 2 months. Evaluation revealed a mass in the right palatal and upper alveolar region with extensive spread and bone destruction, regional adenopathy, mass lesion in the liver and hepatosplenomegaly without bone marrow involvement. Histopathology was suggestive of the PL and patient tested positive for HIV. He was started on high grade NHL chemotherapy protocol along with highly-active anti-retroviral therapy HAART. He responded well and is in complete remission since 8 years of completion of treatment and is on HAART.
Keywords: Human immunodeficiency virus-associated non-Hodgkin's lymphoma, long-term survivor, plasmablastic lymphoma
INTRODUCTION
Plasmablastic lymphoma (PL) of the oral cavity, a form of non-Hodgkin's lymphoma (NHL) was first, reported by Delecluse et al.[1] It has a clear association with immunosuppression and is one of the most frequent oral malignancies in human immunodeficiency virus (HIV) infected patients. In the pre- highly-active anti-retroviral therapy (HAART) era, the incidence of NHL was as high as 60-200 times greater in HIV-infected patients than in non-HIV-infected patients.[2] However, since the introduction of HAART in the mid-1990s, the incidence of NHL appears to have been declining.[3]
The average survival time reported by Delecluse et al. was a few months, and half of the original 16 patients died within 1 year; however, more recent reports have reported improved survival when treatment with both HAART and appropriate chemotherapy are used, similar to outcomes of HIV-infected patients with other NHLs.[4,5,6]
Common primary sites of presentation include the oral cavity, gastrointestinal tract and lymph nodes. Case reports in HIV negative patients and HIV positive patients with extra oral sites have been described with cases presenting as paravertebral mass, cervical adenopathy, skin manifestation, anal canal, lung, and breast involvement.[7]
CASE REPORT
A 10-year-old male child presented to our center 7 years ago with a history of swelling of the right side of the face and intermittent fever of 2 months duration. On examination, he had pallor, palatal and upper alveolar mass, bilateral cervical adenopathy and hepatosplenomegaly. Baseline investigation showed Hb–7.3g/dL, TLC − 5.6 × 109/L, platelet count of 181 × 109/L. There was reversal of A: G ratio. Biopsy from oral mass was suggestive of PL. Bone marrow was not involved. ELISA for HIV-1 was reactive. Computed tomography, scan of the face, neck, chest, and abdomen showed a large oral cavity mass lesion with an extensive local spread and bony destruction, retroperitoneal adenopathy, multiple hypo-dense masses in both lobes of liver, with the hepatosplenomegaly. Serum protein electrophoresis showed no M band. Biopsy from oral mass showed sheets of small round cells with eccentric nuclei, vesicular nuclei and prominent nucleoli with plasmacytoid appearance. These cells were immunopositive for leucocyte common antigen (LCA), CD 79a, kappa light chain, epithelial membrane antigen (EMA) and CD138 and immunonegative for CD 20, CD3, MPO, and CD56 [Figures 1 and 2].
Figure 1.
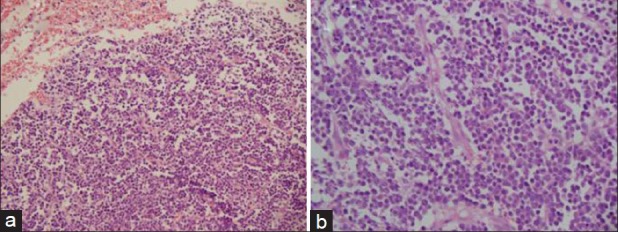
(a) Sheets of small round cells with eccentric nuclei, vesicular nuclei and prominent nucleoli (H and E, ×100); (b) Higher magnification of the same showing plasmacytoid appearance of cells (H and E,×400)
Figure 2.
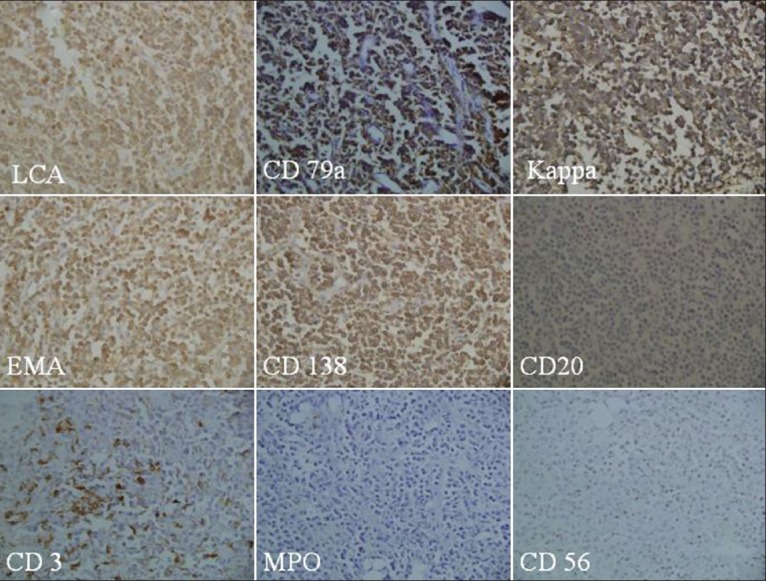
Immunopositivity for LCA, CD79a, kappa lightchain, EMA and CD138 but tumour cells are immunonegative for CD20, CD3, MPO, and 56 (×200 each)
Final diagnosis of HIV associated high-grade lymphoma, plasmablastic type Stage IV was made.
He was treated with the high grade NHL protocol (MCP 842 protocol) that included – cyclophosphamide, vincristine, adriamycin, high-dose ara C, ifosfamide, etoposide along-with the central nervous system (CNS) prophylaxis.[8] He was simultaneously started on HAART with lamivudine, stavudine and nevirapine. He completed the chemotherapy in 2003 and is in remission until last follow-up about 3 months ago. He is continuing HAART with same medicines and his latest CD4 cell count is 280 cells/mm3.
DISCUSSION AND REVIEW OF LITERATURE
PLof oral cavity was first described in 1997 as a separate entity.[1] It is a highly malignant, B cell disorder, morphologically similar to diffuse large B-cell lymphoma (DLBCL). It probably arises from a post-germinal center B cell, related to Epstein-Barr virus (EBV) but not to human herpes virus 8, as opposed to primary effusion lymphoma. The defining feature of PL is positive staining for VS38c, CD38, or CD138 and negativity for the common B-cell markers CD20 and CD79a; however, patient described here was CD79a positive, which can be seen in a small number of patients. It is described in both HIV positive and HIV negative patients. In HIV-infected patients, NHLs have been observed around a median age of 40 years.[9] There is a notable male predominance in PL. The reason for the over-representation of male patients with PL is not clear; however, may correlate to the over-representation of HIV infection in men compared with women in developed countries.[10]
There is no standard chemotherapy protocol for treatment of this lymphoma. Anthracycline based protocols (Cyclophosphamide, Adriamycin, Vincristine, Prednisolone (CHOP) or CHOP like) have been the back-bone. Of late there are some reports of use of rituximab based therapy. Two years OS reported in a series of 35 patients was 42%.[11] Despite, an initial good response to therapy, the prognosis remains poor even after polychemotherapy with or without radiotherapy. In a recent review of 131 HIV positive patients with PL,[12] the median age at presentation was 39 years with a male predominance of 7:1. Afflicted patients had a median CD4+ count of 173 cells/mm3. PL presented on average 6 years after the initial diagnosis of HIV while many (59%) patients were already on HAART. Most (95%) cases presented with Stage I or IV. There was variable expression of B-cell markers (CD20 9%; CD79a 25%). Plasma cell markers were expressed in all cases. The average proliferation index was 83%. EBV was detected in 75% and human herpes virus-8 in 16% of cases. Complete remission (CR) was obtained in 93% of the cases; the majority of these responses were seen after CHOP. Median survival was 14 months from diagnosis. In the original report by Delecluse et al.,[1] half of 16 patients with available follow-up died, within 12 months of diagnosis. In one series of 13 patients, all died within 34 months with a median survival of 7 months.[13] Of the 90 cases in the literature that mention survival data, almost half (47%) had died within 1 year of diagnosis. Others have also reported very short survival,[4,14] with the exception of one outlier alive 3.5 years after diagnosis.
In summary, PL has been recognized as a new aggressive NHL in immunocompetent and immunocompromized patients. Anthracycline based aggressive chemotherapy protocols along with HAART are the back bone of therapeutic interventions. Prognosis usually is poor, but occasional patients may do well. We have described a HIV positive child who was treated with aggressive protocol and is disease free 8 years after the initial diagnosis.
Long-term survival in our patient may be due to the following reason:
Pediatric tumors are more chemo responsive
A probable inherent difference in tumor biology of pediatric and adult PL.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, et al. Plasmablastic lymphomas of the oral cavity: A new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–20. [PubMed] [Google Scholar]
- 2.Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: Changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14–9. doi: 10.1097/01.qco.0000200295.30285.13. [DOI] [PubMed] [Google Scholar]
- 3.Clarke CA, Glaser SL. Epidemiologic trends in HIV-associated lymphomas. Curr Opin Oncol. 2001;13:354–9. doi: 10.1097/00001622-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Nasta SD, Carrum GM, Shahab I, Hanania NA, Udden MM. Regression of a plasmablastic lymphoma in a patient with HIV on highly active antiretroviral therapy. Leuk Lymphoma. 2002;43:423–6. doi: 10.1080/10428190290006260. [DOI] [PubMed] [Google Scholar]
- 5.Lester R, Li C, Phillips P, Shenkier TN, Gascoyne RD, Galbraith PF, et al. Improved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of highly active antiretroviral therapy: A report of two cases. Leuk Lymphoma. 2004;45:1881–5. doi: 10.1080/10428190410001697395. [DOI] [PubMed] [Google Scholar]
- 6.Panos G, Karveli EA, Nikolatou O, Falagas ME. Prolonged survival of an HIV-infected patient with plasmablastic lymphoma of the oral cavity. Am J Hematol. 2007;82:761–5. doi: 10.1002/ajh.20807. [DOI] [PubMed] [Google Scholar]
- 7.Nicol I, Boye T, Carsuzaa F, Feier L, Collet Villette AM, Xerri L, et al. Post-transplant plasmablastic lymphoma of the skin. Br J Dermatol. 2003;149:889–91. doi: 10.1046/j.1365-2133.2003.05544.x. [DOI] [PubMed] [Google Scholar]
- 8.Advani S, Pai S, Adde M, Vaidya S, Vats T, Naresh K, et al. Preliminary report of an intensified, short duration chemotherapy protocol for the treatment of pediatric non-Hodgkin's lymphoma in India. Ann Oncol. 1997;8:893–7. doi: 10.1023/a:1008228529397. [DOI] [PubMed] [Google Scholar]
- 9.Matthews GV, Bower M, Mandalia S, Powles T, Nelson MR, Gazzard BG. Changes in acquired immunodeficiency syndrome-related lymphoma since the introduction of highly active antiretroviral therapy. Blood. 2000;96:2730–4. [PubMed] [Google Scholar]
- 10.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004;23:6524–34. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 11.Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, Maestre L, Sanchez-Verde L, Roncador G, et al. Aggressive large B-cell lymphoma with plasma cell differentiation: Immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010;95:1342–9. doi: 10.3324/haematol.2009.016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo J, Dezube BJ, Pantanowitz L. Plasmablastic lymphoma in HIV. J Clin Oncol. 2008;26:19519. [Google Scholar]
- 13.Dong HY, Scadden DT, deLeval L, Tang Z, Isaacson PG, Harris NL. Plasmablastic lymphoma in HIV-positive patients: An aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol. 2005;29:1633–41. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 14.Flaitz CM, Nichols CM, Walling DM, Hicks MJ. Plasmablastic lymphoma: An HIV-associated entity with primary oral manifestations. Oral Oncol. 2002;38:96–102. doi: 10.1016/s1368-8375(01)00018-5. [DOI] [PubMed] [Google Scholar]


