Abstract
Background:
Any residual pigment left in patients of universal vitiligo is managed with topical treatments, cryotherapy, and lasers.
Aim:
The study aims to assess the efficacy and safety of Q-switched Nd: YAG laser in treating the residual pigmentation in patients with universal vitiligo.
Materials and Methods:
Fifteen patients of universal vitiligo with residual pigmentation on the face, hands, or feet, resistant to topical treatments, were treated with single or multiple sessions of Q-switched Nd: YAG laser treatment. Topical treatments were continued in between the laser sessions and the depigmentation achieved was monitored by clinical examination and repeat digital photographs. Response to the treatment was labelled as excellent if the residual pigment could be reduced by at least 90% while 50–90% resolution of pigmentation was labelled as a partial response. Adverse effects to the treatment offered were also monitored.
Results:
Thirteen of the 15 patients enrolled for the study showed an excellent response to the treatment offered. Two other patients showed a poor response with less than 50% resolution of pigmentation. The number of laser sessions needed at a particular site ranged from 1 to 3 and no patient was offered more than three sessions of laser treatment at any site. No significant adverse events were reported by any patient.
Conclusions:
Residual pigmentation in patients with universal vitiligo that does not respond to topical treatment options alone can be managed quite effectively with Q-switched Nd: YAG laser without any significant adverse effects.
Keywords: Depigmentation, lasers, Q-switched Nd: YAG laser, treatment, vitiligo
INTRODUCTION
Vitiligo can progress in some patients with or without medical treatment to involve a major part of the body surface area. Depigmentation is the only treatment option possible in such patients who develop vitiligo on more than 90% body surface area, for which the term ‘universal vitiligo’ is commonly used.[1,2] The residual pigmentation, seen in these patients, causes a lot of disfigurement as the sites most commonly involved are the face and other exposed parts of the body.[3] Moreover, this residual pigment tends to come again and again with the approach of summer months even after successful depigmentation therapy.[3,4]
The first-line treatment options used for depigmentation treatment in universal vitiligo include topical treatment with monobenzyl ether of hydroquinone (MBEH),[5–7] phenol peels,[8,9] cryotherapy,[10,11] and lasers.[12–14] Topical treatments used for this indication are associated with certain limitations like local irritation of the skin, incomplete depigmentation and most importantly, a really long time to achieve a clinical response.[3,5,6,15,16] In usual cases, it takes about 1 year for the topical treatment to act in this condition.[3,5] Lasers overcome many of these limitations as they are much more rapidly acting, relatively safer and usually do not require months of treatment. In addition, they have also been shown to help those patients who do not respond to topical treatments alone.[12,14] Lasers are claimed to be more effective in those vitiligo patients who show a positive Koebner’s phenomenon. For a patient who requires treatment of a cosmetically important area like the face urgently, lasers probably provide the only possible alternative.
Lasers that have been used for depigmentation therapy in vitiligo till date include the Q-switched Ruby laser and Q-switched Alexandrite laser.[12–14] Q-switched Ruby laser uses a wavelength of 694 nm at nanosecond pulses to destroy the melanocytes present in the pigmented areas. A larger area can be treated in a single session and the treatment can be combined with topical MBEH treatment to accelerate the depigmenting process.[13] Q-switched Alexandrite laser, which uses a wavelength of 755 nm, has also been reported to be useful in treating the residual pigmentation present in vitiligo.[14]
For treating pigmented lesions, Q-switched Nd: YAG laser is the one that is most commonly used in India.[15–17] The popularity of this laser system in India is because of its ‘safer’ wavelength at 1064 nm as it is less likely to cause a postinflammatory pigmentation in dark skin. Q-switched Nd: YAG laser can deliver energy at two different wavelengths of 1064 nm and 532 nm in nanosecond pulses to cause both a photothermolytic and photoacoustic damage to melanosomes and even melanocytes.[18,19] While the former wavelength is useful for its deeper penetration and is thus used to treat dermal pigmentation, the 532-nm wavelength is commonly used to target the epidermal pigment.[20–22]
MATERIALS AND METHODS
Fifteen patients of vitiligo universalis, in whom the residual pigmentation had not responded satisfactorily to topical application of MBEH for at least 3 months, were enrolled for this prospective open-label study. All the patients had more than 80% body surface area involved with vitiligo and had residual pigmentation on cosmetically important areas like the face, hands, forearms, and feet. Pregnant females, patients with keloidal tendency, and children less than 15 years of age were excluded from the study.
The patients enrolled for the study were photographed at the initial visit and an informed consent was obtained from each one of them. The areas with residual pigment were noted down and a management plan was chalked out depending on the patient’s preferences. In all enrolled patients only one area of the body was treated in a single session. The area that was the most troublesome for the patient was treated first (in all cases the face) followed by the other areas involved. Before each laser session, topical treatment with MBEH was discontinued for about 5–7 days on the area to be treated.
Treatment was carried out with a frequency-doubled Q-switched Nd: YAG laser at 532-nm wavelength. The fluence used was in the range of 1–2 J/cm2 with a spot size of 2–3 mm. No topical anaesthesia was used in any patient and after the laser session the patients were advised to use a mid-potent topical steroid with an antibacterial cream for 2–3 days and to limit their sun exposure. A broad-spectrum sunscreen lotion was also mandatorily used in every patient. In patient requiring further laser sessions at an already treated site a minimum gap of 6 weeks was kept in between the sessions. Topical treatment with MBEH was continued at bedtime along with the laser sessions on all the treated areas. Patients were called for follow-up at 1st and 2nd weeks after each laser session and the response to treatment was noted down. At this follow-up visit, any other untreated area of the body was taken up for laser treatment if required.
Any change in the residual pigment was assessed clinically as well as with repeat photography at the follow-up visits after 1st, 2nd and 6 weeks. Any need for further laser sessions was assessed at the 6-week follow-up visit. Results to treatment offered were termed as excellent if there was more than 90% resolution of pigment. A 50–90% resolution of pigment was termed as a partial response and anything less than 50% pigment resolution was termed as a poor response. Adverse events noted down were pain and burning at the time of laser treatment and any post-treatment sequalae. All the patients were also followed for a period of 3 months to look for any relapse of pigmentation on the treated areas.
RESULTS
The age of our patients ranged from 15 to 42 years with a mean of 27 years. The youngest patient in our study was a 15-year-old male student while the eldest one was a female aged 42 years. There were 11 females and four males in the study group.
The duration of vitiligo ranged from 2 to 25 years with a mean of 10.6 years.
Face was the commonest area involved with the residual pigmentation and was thus the commonest site treated. In fact, face was treated in all the 15 enrolled patients [Table 1]. However, the type and degree of involvement of face varied among the enrolled patients, being patchy and limited to malar area and forehead [Figure 1a and b] in majority (11 patients) and residual pigmentation on hands in four patients [Figure 2a and b].
Table 1.
Various sites treated with depigmentation therapy

Figure 1.
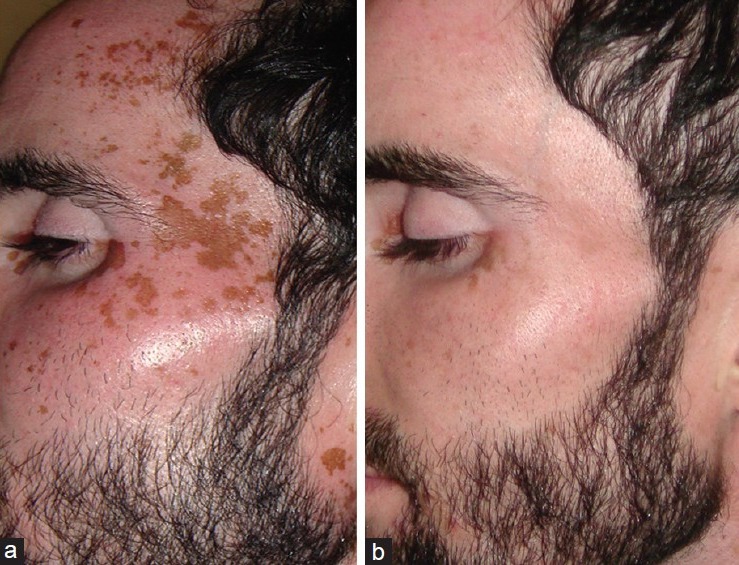
(a) Patchy residual pigmentation on face, (b) after treatment with Q-switched Nd:YAG laser
Figure 2.
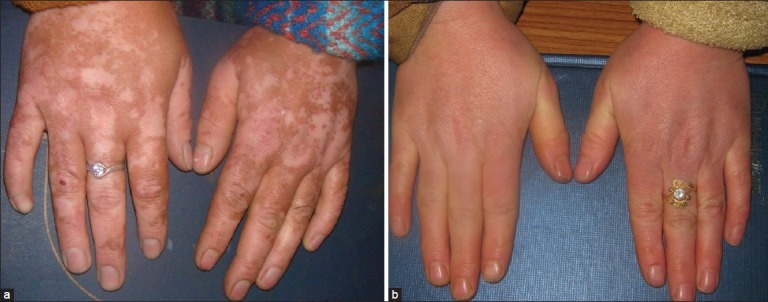
(a) Residual pigmentation on hands and (b) after 2 sessions of Q-switched Nd:YAG laser
The next commonest site treated was the hands (six patients) followed by the forearms (three patients) and the feet (two patients). Thus, a total of 26 sites were treated in the 15 patients enrolled [Table 1].
Most of the patients responded well to treatment and excellent results with more than 90% resolution of pigment were seen in 13 out of the 15 patients enrolled. Only two patients had a poor response with less than 50% resolution of pigment. Both of these partially responding patients had a diffuse involvement of the face and these patients refused treatment at other body sites involved.
All the body sites responded almost equally to the treatment offered and there was no site-specific difference in the results achieved. Majority of the sites treated responded with just one or two laser sessions but in three cases, the treatment had to be repeated thrice on the face to achieve a satisfactory response. However, no patient was given more than three sessions of laser treatment at any site.
No significant adverse effects were noted in any patient and the procedure was termed as ‘tolerable’ by all patients enrolled. All patients experienced a mild burning sensation during the laser sessions. Similarly, there were no serious post-treatment sequalae in any patient.
On follow-up no patient was seen to experience a relapse over 3 months. However, the follow-up period was too short to comment on the long-term efficacy of the treatment offered.
DISCUSSION
Topical depigmenting treatments in vitiligo have two main inherent limitations; first, they are too slow to act and second, they too often cause an irritant reaction on the skin.[5,6,15,16] Patients of universal vitiligo who have residual pigment on the cosmetically important areas demand a treatment that is quick to act and is devoid of side effects. Lasers are of help in such situations as their therapeutic effect is visible in days and the treatment is devoid of any serious adverse effects in usual circumstances.
Lasers act by two different mechanisms in causing depigmentation in universal vitiligo. First, they target the melanosomes in the residual melanocytes by means of a selective photothermolysis and photoacoustic effect. In addition they are also believed to stimulate Koebnerisation of the vitiligo process and this adds to the depigmenting effect.[12,14,17] Among the different lasers used to cause further depigmentation in vitiligo, Q-switched Ruby laser has been the most commonly used and reported till date. This laser has been used in patients after tanning to cause a permanent depigmenting effect.[12] Tanning in such cases is thought to activate melanocytes and these activated melanocytes can then be more easily targeted by laser light.[12] In addition to Q-switched Ruby laser the other laser that has been reported to be useful in causing depigmentation in vitiligo patients is the Q-switched Alexandrite laser.[14] This laser system has been used in combination with MBEH to cause a long-lasting depigmenting effect in patient who did not respond to Q-switched Ruby laser. The investigators used 10 sessions of laser treatment and within 22 months, they were able to achieve a permanent depigmenting effect in their patient.[14]
In the present study, we used the 532-nm wavelength of Q-switched Nd: YAG laser as the target was the epidermal pigment only and we wanted some inflammation and thus a Koebnerisation of the vitiligo process to take place. We were able to achieve a total or near-total depigmentation with just one or two sessions in combination with the use of a topical depigmenting agent MBEH in majority of our patients. We did not advise our patient to get tanned but we did stop the topical depigmenting agent for a few days before each laser session to have some active melanocytes to work on. The main advantage that we found with this combination was that the therapeutic effect was very rapid in onset and the patients could notice some pigment loss within just days after the laser treatment. It is noteworthy that all these patients had already been using topical depigmenting creams with no or minimal effect on the residual pigment. We did follow-up with our patients for 3 months and we did not encounter any relapses over the follow-up period. However, the follow-up of 3 months is too short to really comment upon the stability of the therapeutic effect achieved with the laser treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo: Meta-analysis of the literature. Arch Dermatol. 1998;134:1532–40. doi: 10.1001/archderm.134.12.1532. [DOI] [PubMed] [Google Scholar]
- 2.James WD, Berger TG, Elston DM, editors. Andrew’s Disease of The Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier; 2006. pp. 860–2. [Google Scholar]
- 3.Alghamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25:749–57. doi: 10.1111/j.1468-3083.2010.03876.x. [DOI] [PubMed] [Google Scholar]
- 4.Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159:1051–76. doi: 10.1111/j.1365-2133.2008.08881.x. [DOI] [PubMed] [Google Scholar]
- 5.Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone: A retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol. 1977;97:669–81. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolognia JL, Lapia BK, Somma S. Depigmentation therapy. Dermatol Ther. 2001;14:29–34. [Google Scholar]
- 7.Oakley A. Rapid repigmentation after depigmentation therapy: Vitiligo treated with monobenzyl ether of hydroquinone. Australas J Dermatol. 1996;37:96–8. doi: 10.1111/j.1440-0960.1996.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 8.Van doon Boorn JG, Melief CJ, Luiten RM. Monobenzone induced depigmentation: From enzymatic blockade to autoimmunity. Pigment Cell Melanoma Res. 2011;24:673–9. doi: 10.1111/j.1755-148X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 9.Di Nuzzo S, Masotti A. Depigmentation therapy in vitiligo universalis with cryotherapy and 4-hydroxyanisole. Clin Exp Dermatol. 2010;35:215–6. doi: 10.1111/j.1365-2230.2009.03412.x. [DOI] [PubMed] [Google Scholar]
- 10.Zanini M. Depigmentation therapy for generalized vitiligo with topical 88% phenol solution. An Bras Dermatol. 2005;80:415–6. [Google Scholar]
- 11.Kavuossi H. Induction of depigmentation in a universal vitiligo patient with combination of cryotherapy and phenol. J Pak Assoc Dermatol. 2009;19:112–4. [Google Scholar]
- 12.Kim YJ, Chung BS, Choi KC. Depigmentation therapy with Q-switched ruby laser after tanning in vitiligo universalis. Dermatol Surg. 2001;27:969–70. doi: 10.1046/j.1524-4725.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 13.Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol. 2000;42:760–9. doi: 10.1067/mjd.2000.103813. [DOI] [PubMed] [Google Scholar]
- 14.Rao J, Fitzpatrick RE. Use of the Q-switched 755 nm alexandrite laser to treat recalcitrant pigment after depigmentation therapy for vitiligo. Dermatol Surg. 2004;30:1043–5. doi: 10.1111/j.1524-4725.2004.30313.x. [DOI] [PubMed] [Google Scholar]
- 15.Nordlund JJ, Forget B, Kirkwood J, Lerner AB. Dermatitis produced by applications of monobenzone in patients in patients with active vitiligo. Arch Dermatol. 1985;121:1141–4. [PubMed] [Google Scholar]
- 16.Lyon CC, Beck MH. Contact hypersensitivity to monobenzyl ether of hydroquinone used to treat vitiligo. Contact Dermatitis. 1998;39:132–56. doi: 10.1111/j.1600-0536.1998.tb05863.x. [DOI] [PubMed] [Google Scholar]
- 17.Thissen M, Westerhof W. Laser treatment for further depigmentation in vitiligo. Int J Dermatol. 1997;36:386–8. doi: 10.1046/j.1365-4362.1997.00144.x. [DOI] [PubMed] [Google Scholar]
- 18.Goel A. Clinical applications of Q-switched NdYAG laser. Indian J Dermatol Venereol Leprol. 2008;74:682–6. doi: 10.4103/0378-6323.45135. [DOI] [PubMed] [Google Scholar]
- 19.Aurangabadkar S. QYAG% Q-switched Nd: YAG laser treatment of nevus of ota: An Indian study of 50 patients. J Cutan Aesthet Surg. 2008;1:80–4. doi: 10.4103/0974-2077.44164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aurangabadkar S, Mysore V. Standard guidelines of care: Lasers for tattoos and pigmented lesions. Indian J Dermatol Venereol Leprol. 2009;75:111–26. [Google Scholar]
- 21.Kilmer SL, Garden JM. Laser treatment of pigmented lesions and tattoos. Semin Cutan Med Surg. 2000;19:232–44. doi: 10.1053/sder.2000.18363. [DOI] [PubMed] [Google Scholar]
- 22.Kar HK, Gupta L. 1064 nm Q switched Nd: YAG laser treatment of nevus of Ota: An Indian open label prospective study of 50 patients. Indian J Dermatol Venereol Leprol. 2011;77:565–70. doi: 10.4103/0378-6323.84057. [DOI] [PubMed] [Google Scholar]


