Abstract
Background
As an alternative to transurethral resection of the prostate (TURP), photoselective vaporization of the prostate (PVP) provides a bloodless, relatively painless relief of lower urinary tract symptoms for men with benign prostatic hyperplasia. Following a review of the evidence in 2006, the Ontario Health Technology Advisory Committee recommended that a study be conducted to evaluate PVP in Ontario.
Objectives
To compare the clinical effectiveness, safety, cost-effectiveness, and budget impact of PVP compared to conventional TURP for the treatment of benign prostatic hyperplasia in Ontario.
Methods
A prospective, nonrandomized trial was conducted in 3 Ontario centres. Consenting subjects were assessed at baseline and 1, 3, and 6 months following surgery. Outcome measures included International Prostate Symptom Score (IPSS), peak urinary flow rate (Qmax), post-void residual (PVR) volume, prostate-specific antigen (PSA), health-related quality of life (HRQOL) using the EuroQol 5 Domain questionnaire, and the Sexual Health Inventory for Men (SHIM) score. Adverse events, resource utilization, and productivity losses were also assessed. Cost-effectiveness and budget impact analyses were completed using data from the study.
Results
Between February 2008 and August 2010, 164 subjects were enrolled in the study (n = 140 for PVP and n = 24 for TURP). Treatment outcomes were similar between the 2 groups at 6 months, with the IPSS decreasing similarly over time (P = 0.718). For other treatment outcomes (Qmax, PSA, HRQOL, SHIM) both treatments provided similar benefit over time; only changes in PVR volume favoured PVP (P = 0.018). The majority of PVP patients were managed on an outpatient basis, with only 7.1% requiring admission (all TURP subjects were inpatients). At 6 months, PVP was less costly than TURP ($3,891 versus $4,863; P = 0.001), with similar quality-adjusted life-years (0.448 versus 0.441; P = 0.658). PVP remained the most cost-effective treatment across all decision-making thresholds, with the technology costing less and providing similar clinical outcomes. Extrapolating the results to a provincial level indicated (based on an estimated case volume of 12,335 TURPs) that there is an opportunity to reallocate just over $14 million (Cdn), primarily related to the reduced need for hospital admission.
Limitations
This study was nonrandomized, and the results should be interpreted with some caution, despite generally similar baseline characteristics between the 2 groups. Recruiting individuals to the TURP arm was a challenge, resulting in a size imbalance between treatment arms.
Conclusions
Based on this analysis, PVP appears to be a cost-effective alternative to TURP, providing similar clinical benefit at a lower cost to the health system.
Plain Language Summary
For men with lower urinary tract symptoms due to an enlarged prostate, a laser treatment called photoselective vaporization of the prostate (or PVP) is just as effective as surgery. PVP does not require an overnight stay in the hospital for most men, and it costs almost $1,000 less. This report describes the results of a study that collected information about treatment outcomes, quality of life, and health care use related to PVP and surgery in Ontario.
Background
Study Objectives
The objective of this study was to evaluate the clinical effectiveness, safety, cost-effectiveness, and budget impact of photoselective vaporization of the prostate (PVP) using a 120 W potassium titanyl phosphate (KTP) laser (Greenlight™ HPS-120 Laser Therapy) compared to conventional transurethral resection of the prostate (TURP) for the treatment of benign prostatic hyperplasia (BPH) in Ontario.
The primary objective was to compare the effectiveness of 120 W PVP versus TURP in the treatment of BPH, as measured by the change from baseline in International Prostate Symptom Score (IPSS) (1) at 6 months following surgical intervention. Secondary objectives were to examine differences between PVP and TURP with respect to standard clinical efficacy outcomes (e.g., peak urinary flow rate [Qmax], post-void residual (PVR) volume, quality of life, sexual function); intra- and postoperative complication rates; durability at 12 and 24 months following the procedure; resource utilization and costs (i.e., intra- and postoperative costs for patient care and evaluation); and cost-effectiveness.
Clinical Need and Target Population
Description of Condition
BPH is a noncancerous enlargement of the prostate gland. As the prostate increases in size, lower urinary tract symptoms (LUTS) can gradually develop as a result of irritation or obstruction of the urethra and typically include nocturia, urinary frequency, urinary urgency, incomplete emptying, urinary hesitancy, weak stream, and acute urinary retention. (2) The severity of bothersome symptoms can have a negative effect on a patient’s quality of life by interfering with normal daily activities.
Burden
The prevalence of BPH and LUTS increases with age; in men over 60 years of age, the prevalence of BPH is 50%, and in men over 85, the prevalence is 90%. (3) Left untreated, complications of BPH can include upper urinary tract dilatation and hydronephrosis, chronic renal failure, bladder wall hypertrophy, bladder stones, bladder diverticula, and urinary infection. (3)
Treatment
Conventional treatment for BPH includes watchful waiting, pharmacotherapy, and surgery. (4)
Watchful waiting, in which the patient receives no active treatment but is monitored by his physician, is an appropriate treatment strategy for patients with mild symptoms of BPH.
In recent years, medical therapies have been considered for first-line treatment of BPH; the most commonly prescribed pharmacotherapy is alpha-adrenergic blockers, which work by inhibiting the alpha-adrenergic-mediated contraction of smooth muscle in the prostate and bladder, allowing urine to flow more easily. (5) However, although alpha-adrenergic blockers can provide symptom relief, they do not reduce prostate volume or prevent progression of the disease. Another class of medications used is 5-alpha-reductase inhibitors; they work by decreasing dihydrotestosterone levels, leading to a reduction in prostate size. Treatment with 5-alpha-reductase inhibitors can improve LUTS and urinary flow, but symptom relief can take 6 to 12 months to occur. (5)
Surgical intervention may be recommended for patients with moderate to severe LUTS or other BPH-related complications. Conventional surgical options include TURP, transurethral incision of the prostate, and open prostatectomy, but the gold standard for the surgical treatment of LUTS secondary to BPH is TURP. (6;7) With TURP, the prostate is accessed with a resectoscope via the urethra, and the inner portion of the prostate is resected and cauterized using an electrified loop. Recent technological improvements to the TURP procedure have led to a reduction in perioperative complications, transurethral resection syndrome, clot retention, and urinary tract infection. (7) The incidence of transurethral resection syndrome (a serious complication that occurs when irrigation solution is absorbed into the bloodstream) has decreased from > 2% to < 1%, but other complications (such as sexual dysfunction, irritative voiding symptoms, bladder neck contracture, blood transfusions, urinary tract infections, and hematuria) have been reported in > 5% of patients. (4;7)
Technology
Recent innovations in energy-based interventions have provided alternative treatment options for patients with BPH. These techniques, which use laser energy, electrovaporization (transurethral electrovaporization of the prostate), microwaves (transurethral microwave thermotherapy), radiofrequency waves (transurethral needle ablation of the prostate), or ultrasound (high-intensity focused ultrasound), may have clinical and economic benefits compared to conventional surgical treatment with TURP. (8)
Laser energy can be used to produce coagulation necrosis, vaporization of tissue, or resection of the prostate tissue. (4) For the treatment of BPH, 4 types of laser energy have been studied: Nd:YAG, diode, holmium:YAG, and KTP. The KTP laser is produced by halving the wavelength and doubling the frequency of the Nd:YAG laser; this lower wavelength is in the visible green area of the electromagnetic spectrum. Unlike the Nd:YAG laser, which disperses laser energy over a large volume of tissue and penetrates up to 10 mm into tissue, the KTP laser penetrates to a depth of only 0.8 mm. (9) KTP laser energy is strongly absorbed by blood vessels and hemoglobin, and the heat is confined to a small volume of tissue, resulting in rapid vaporization.
PVP
The use of the KTP laser for vaporization of prostatic tissue has been called PVP. This system produces a light beam at a wavelength of 532 nm, which is fully transmitted through water and is selectively absorbed by hemoglobin. The laser light emitted by this system vaporizes the prostate tissue and allows for tissue coagulation to a depth of 1 to 2 mm. This rapidly evolving technology offers the efficient debulking of prostate tissue seen in TURP, but with the clinical benefits of laser vaporization techniques.
PVP can provide nearly bloodless tissue removal and can be used in patients with large prostates, patients with comorbidities, or patients taking oral anticoagulants. (9) It has advantages over the gold standard TURP in terms of improved perioperative safety, shorter catheterization time, shorter hospitalization, faster symptomatic improvement, and lower morbidity. (10) As a result, PVP patients may return to work and normal daily activities more quickly than TURP patients. In terms of sexual function, treatment with PVP may result in less erectile dysfunction and a lower incidence of retrograde ejaculation than with TURP. (11) PVP also has the potential to be cost-effective compared to TURP, due to the cost savings associated with shorter hospital stay (PVP can be performed in an outpatient setting) and lower incidence of postoperative complications. (12)
Ontario Context
At the onset of this evaluation, initial clinical experiences with PVP for the treatment of BPH were with lower-powered 60 W and 80 W KTP lasers. (13) Although the 60 W KTP laser was shown to be as safe and effective as conventional TURP, the lower energy density resulted in long procedure times. (9) This led to the development of the higher-power 80 W KTP laser to increase the speed of tissue ablation. The systematic review of energy-based interventions for the treatment of BPH performed by the Medical Advisory Secretariat (now known as Evidence Development and Standards, Health Quality Ontario) in 2006 (8) identified only 1 randomized controlled trial comparing the use of low-power 60 W PVP with TURP (8;14) and only 1 prospective cohort study using 80 W PVP. (10) Both studies reported similar outcomes for PVP compared to TURP. However, the 80 W KTP was rapidly replaced by 120 W technology, which provided more efficient vaporization of tissue and shorter procedure times. In addition, PVP using the 120 W laser may result in fewer postoperative voiding difficulties, such as burning when passing urine and bladder irritability. With the increased power density, treatment of larger prostates has also become feasible. (9;15)
Following a review of the evidence initially presented by the Medical Advisory Secretariat, (8) the Ontario Health Technology Advisory Committee, recommended that “a registry study be conducted to establish longer term effectiveness and complication rates for PVP given the likelihood of increasing diffusion of this technology.” (16)
The replacement of the older 80 W KTP laser occurred during the development of this clinical trial protocol, and as of April 30, 2007, the Medical Devices Bureau of the Therapeutic Products Directorate, Health Canada, licensed a 120 W KTP laser system (Greenlight HPS) for sale in Canada. (17) At the time of licensing, no clinical trials comparing the 120 W KTP laser to TURP were available in the medical literature.
Clinical Analysis
Methods
Study Design
This study was a prospective, nonrandomized, multicentre (3 centres), controlled trial to evaluate the safety, effectiveness, and cost-effectiveness of PVP compared to TURP for the treatment of BPH. All subjects enrolled in the study were treated once and followed for 6 months via clinical visits and then for 24 months by telephone.
The study was completed in 2 phases. Phase I consisted of the surgical intervention and subsequent short-term clinical follow-up. During this phase, the efficacy, safety, and cost-effectiveness of PVP compared to TURP were examined prospectively; subjects were assessed for clinical and economic outcomes during the postoperative period (Days 1 to 10) and at 1, 3, and 6 months after the initial treatment. These evaluations were conducted in the urologist’s office.
In Phase II, participants were contacted by telephone at 12 and 24 months following surgery and asked about the long-term durability of the procedures, health care resource use, and overall health-related quality of life using structured telephone interviews and questionnaires.
The study protocol was reviewed and approved by the Research Ethics Boards at all of the participating sites, and the study was registered at www.ClinicalTrials.gov: NCT00527371. A schematic summarizing the study design can be found in Appendix 1.
Study Population
Patients who were already booked for surgical treatment of BPH using either PVP or TURP at 1 of the study hospitals were approached to participate in the study. Consenting individuals who met the inclusion criteria were enrolled in the study. Eligible subjects were males who:
were > 40 years of age
had been diagnosed with symptomatic/obstructive symptoms secondary to BPH requiring surgical intervention as determined by a urologist
had LUTS > 3 months in duration
had a baseline IPSS > 12
had a Qmax ≤ 20 mL/sec on minimum of 125 mL voided volume
had a prostate size < 100 cm3 as measured by transrectal ultrasound
had an American Society of Anesthesiology classification of physical status class 1-3
were able to read, understand, and sign the informed consent
were willing and able to comply with all follow-up requirements, including multiple follow-up visits
Patients were excluded from the study if any 1 of the following were present:
transvesically measured PVR volume of > 400 mL
currently in urinary retention or with chronic urinary retention
receiving medication that impaired bladder contractibility
uncorrectable bleeding disorders or long-term anticoagulation that could not be stopped
a recent myocardial infarction or coronary artery stent placement
any of the following diseases, which appeared to involve the bladder: myasthenia gravis, diabetes neuropathy, multiple sclerosis, spinal cord injury, or Parkinson’s disease
idiopathic atonic bladder
previous major pelvic fractures that involved damage to the external urinary sphincter
recently completed definitive radiation therapy for prostate cancer
active localized or systemic infections, including active urinary tract infection, active cystolithiasis, urethral strictures, or bladder neck contracture
acute prostatitis affecting bladder function
prostate-specific antigen (PSA) value greater than the age-adjusted normal value (patient needed to have a negative biopsy before participating in the study)
confirmed malignancy of the prostate
bladder cancer treated with transurethral resection of bladder tumour within 12 months
treated with Bacillius Calmette-Guerin, bilateral hydronephrosis on renal ultrasound, urethral strictures or a residual volume > 400 mL, immunocompromised, or a previous TURP procedure
Study Interventions
PVP
PVP procedures were performed using the GreenLight HPS laser system (American Medical Systems, Minnetonka, Minnesota), a high-power (120 W) KTP laser. The GreenLight HPS laser system was provided on loan from American Medical Systems to each of the centres participating in the study with no conditions or obligation to acquire the technology following the completion of the study. All PVP procedures were performed by investigators familiar with laser surgery of the urinary tract and trained to use the GreenLight HPS laser system.
PVP was carried out using standard technique by drawing the fibre back and forth with a slow sweeping motion across the surface of the prostatic tissue. The type of anesthesia was determined by discussion between the patient and anesthesiologist and was based on the health of the patient. A 3-way catheter with continuous irrigation was inserted following the procedure. PVP was completed as an outpatient procedure, and the catheter was removed the next postoperative day.
TURP
TURP was performed with a continuous flow resectoscope and unipolar cautary using standard technique. As with PVP, the type of anesthesia was determined by discussion between the patient and anesthesiologist and was based on the health of the patient. Also similar to PVP, a 3-way catheter with continuous irrigation was inserted following the procedure. As per standard care, the subject was admitted to hospital overnight and the catheter removed as clinically indicated.
Baseline Assessments
Baseline information was obtained from consenting subjects at the preoperative visit, including demographic data, medical and surgical history, and concomitant medications.
An initial assessment of BPH symptoms was completed using the IPSS, (18) which was developed and validated by the American Urological Association. (1) This widely used symptom index includes 7 questions related to incomplete emptying, frequency, intermittence, urgency, weak urine stream hesitancy, and nocturia, which are scored from 0 to 5. The total score is used to categorize the severity of symptoms as mild, moderate, or severe (a higher score indicates more severe symptoms). IPSS has been extensively validated and is recommended not only for the objective assessment of symptoms at initial diagnosis but also to quantify symptomatic improvement in response to treatment.
An initial baseline physical examination was also conducted, and the following urological measures were completed: Qmax, PVR volume, PSA, and transrectal ultrasound.
Quality of life assessment was conducted using the EuroQol 5 Domain (EQ-5D) questionnaire. (19;20) The EQ-5D is a self-administered questionnaire that requires 1 minute to complete. The EQ-5D measures health status in terms of 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. It provides a simple descriptive health profile and generates a single utility value for health status; full health is assigned a value of 1 and death a value of 0. Quality of life was also assessed using the bothersomeness question, which is included in the IPSS (Bother score). This single BPH-specific question was developed to assess the effect of LUTS on quality of life and measures the extent to which symptoms were bothersome.
Sexual function assessment was conducted using the Sexual Health Inventory for Men (SHIM). (21) The SHIM questionnaire is self-administered and is applicable to adult male subjects. This 5-item questionnaire was developed to diagnose the presence and severity of erectile dysfunction. The items focus on erectile function and intercourse satisfaction and are scored on a scale of 0 to 5. A total score obtained by summing all individual scores indicates the severity level. Erectile dysfunction was classified according to 5 severity levels, ranging from none (22-25) through severe. (5-7) Because there is no validated questionnaire to evaluate retrograde ejaculation, additional specific questions were developed.
Follow-up Assessments: Phase I
Clinical and economic data for the period during and immediately following the intervention were collected, including complication rates and resource utilization.
Data collected for postoperative Day 1 to Day 10 included duration of catheterization and resource utilization (e.g., operating room time). As per usual patient work-up, postprocedure laboratory samples were collected, including complete blood count and serum electrolytes to evaluate changes in hemoglobin and serum creatinine values. The need for blood transfusions was also recorded. For individuals receiving medical therapy for LUTS prior to surgery (either alpha-adrenergic blockers or 5-alpha-reductase inhibitors), therapy was discontinued following the surgical intervention.
During Phase I, regular scheduled follow-up visits occurred at 1, 3, and 6 months after the date of the intervention. At each regularly scheduled follow-up, information was collected on IPSS, quality of life, sexual function, rate of reoperation, adverse events, resource utilization, and productivity losses. Rates of re-bleed requiring hospitalization were recorded at 1 and 3 months postintervention. At 3 and 6 months after surgery, the rate of urethral stricture or bladder neck contracture requiring reoperation was also recorded. Tests for Qmax and PVR volume were also done at the 1-, 3-, and 6-month follow-up visits. Although not part of standard care at 3 months after surgery, these noninvasive tests were done in the urologist’s clinic/office and incurred no additional costs or risk to the patient.
Follow-up Assessments: Phase II
The durability of PVP and TURP was assessed at 12 and 24 months by evaluating reinitiation of BPH-related medication and rates of reoperation or rehospitalization using scripted telephone interview methods.
Quality of life was also evaluated via telephone interview at 12 and 24 months.
Resource Utilization and Productivity Losses
Resource utilization and productivity losses incurred by both PVP and TURP patients were captured throughout the study. Information on the 2 procedures was documented, including length of stay in hospital, operating room time, recovery time, number of fibres used for PVP, anesthesia time, and surgery-related medications. These health care resource utilization events were measured to assess the clinical efficacy and safety of the interventions, as well as to capture associated downstream health care costs for each treatment arm, to be used in the economic analysis.
For Phase I, BPH-related resource utilization—such as visits to health care professionals (including nurses’ visits at home), tests and procedures, medications, emergency department visits, rehospitalization, BPH-related medications, or any nonscheduled visits—was captured using a questionnaire at the 3- and 6-month follow-up visits. Hospital medical records and physician questionnaires were used to document resource utilization that the patient would not know about, such as operating room time or other characteristics of the procedure itself (e.g., number of fibres for PVP). For patients who were employed at the time of surgery, information on productivity losses (defined as the number of days out of work following the procedure) was also collected at regular intervals.
For Phase II, patients were asked to identify any resource utilization directly related to BPH (e.g., rehospitalization, BPH-related medication, visits to urologists) during the 12- and 24-month follow-up interviews (the recall period was 6 and 12 months for the 12- and at 24-month interviews, respectively). While this may introduce some bias compared to a recall period of 3 months, the economic evaluation used techniques to deal with uncertainty.
Statistical Analyses
Because of the nonrandomized nature of the study, there may have been selection bias in patients receiving 1 treatment versus the other. To test for differences in baseline comorbidities, t-statistics, chi-squared, and Fischer exact tests were employed for continuous, count, or rare data, respectively. If differences in baseline comorbidities existed between patients receiving TURP or PVP, adjustments for the potential bias in the outcomes were addressed.
For other secondary outcomes, the durability of PVP versus TURP was assessed at 12 and 24 months by reporting rates of readmission or initiation of BPH-related medications. Resource utilization was assessed with either count data (for example, readmission rates), or by using methods for normal data (for example, length of hospitalization stay).
The primary statistical analysis was conducted at 6 months (IPSS). The secondary analysis to evaluate long-term durability was conducted at 24 months.
Results
Participant Recruitment
Recruitment occurred between February 2008 and August 2010, and 164 subjects were enrolled in the study. Because this was an observational study and treatment options were based on patient choice in consultation with a physician, 140 were assigned to PVP and 24 were assigned to TURP.
Baseline Characteristics
At baseline, the PVP and TURP groups were similar with respect to prostate size, prostate volume, average flow rate, voiding, and PSA, but PVP subjects had a higher Qmax at baseline and were significantly younger. Baseline characteristics are provided in Table 1.
Table 1: Baseline Characteristics.
| PVP a n = 140 | TURP a n = 24 | P value | |
|---|---|---|---|
| Age, years | 67.4 (7.6) (48, 85) | 70.8 (7.6) (55, 82) | 0.045 |
| Prostate size, cc | 48.0 (18.8) (20, 102) | 54.5 (20.5) (19, 90) | 0.123 |
| Prostate volume, cc | 53.8 (26.2) (14, 152) | 54.5 (22.4) (19, 99) | 0.900 |
| Qmax, mL/sec | 11.1 (4.2) (2.0, 29.8) | 8.8 (4.1) (2.9, 15.4) | 0.017 |
| Average flow rate, mL/sec | 5.6 (2.2) (1.4, 13.2) | 4.7 (2.3) (1.7, 9.4) | 0.066 |
| Total voiding duration, sec | 51.7 (30.4) (15.0, 219.0) | 58.9 (52.5) (9.0, 263.0) | 0.345 |
| PVR volume, mL | 106.9 (108.5) (0, 395) | 68.8 (69.1) (0, 233) | 0.114 |
| PSA, ng/dL | 2.9 (2.5) (0.2, 13.4) | 3.0 (2.1) (0.4, 9.1) | 0.782 |
Abbreviations: PSA, prostate-specific antigen; PVP, photoselective vaporization of the prostate; PVR, post-void residual; Qmax, peak urinary flow rate; TURP, transurethral resection of the prostate.
Mean (SD) (min, max).
There was no statistically significant difference in employment status at baseline: 51 PVP patients (36%) and 5 TURP patients (21%) were employed on a full- or part-time basis (P = 0.137).
There were no significant differences in use of medications for the management of LUTS (Table 2).
Table 2: Medication Use at Baseline for the Management of LUTS.
| Medication Use | PVP, n (%) n = 140 | TURP, n (%) n = 24 | P value |
|---|---|---|---|
| Alpha-adrenergic blocker | 101 (72%) | 20 (83%) | 0.320 |
| 5-alpha reductase inhibitor | 50 (36%) | 11 (46%) | 0.367 |
| Alpha-adrenergic blocker and 5-alpha reductase inhibitor | 35 (25%) | 8 (33%) | 0.452 |
Abbreviations: LUTS, lower urinary tract symptoms; PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
Phase I
Procedural Details
All procedures were completed between March 2008 and February 2011. The operating time for PVP was significantly longer than for TURP (63 minutes versus 40.9 minutes, P = 0.001). Less postoperative bleeding occurred in PVP-treated patients than in TURP patients (based on data provided for 60% of patients in the study). Postoperative mean (SD) hemoglobin was 140.3 (12.1) g/L for the PVP group and 119.5 (16.3) g/L for the TURP group (P < 0.001). Similarly, mean (SD) postoperative hematocrit was 0.41 (0.03) for the PVP group and 0.35 (0.05) for the TURP group (P < 0.001). Catheter reinsertion was required within 48 hours of the procedure in 4% of PVP patients and 14% of TURP patients (P = 0.075).
PVP procedures were conducted primarily in an outpatient setting, with only 10 of 140 PVP patients (7.1%) requiring hospital admission after the procedure; all TURP patients were admitted after the procedure. For those admitted to hospital, the PVP group had a significantly shorter length of stay (LOS): the mean (SD) LOS for PVP patients (n = 10) was 2.0 (0.5) days (generally overnight stays), compared to 2.5 (0.5) days for the TURP group (P = 0.021). The prescribing of postprocedural analgesia was similar between the 2 groups, with 10 PVP subjects (7%) and 3 TURP subjects (13%) receiving prescriptions (P = 0.369).
Serious adverse events reported postoperatively (e.g., hematuria, urinary retention, or bleeding) occurred in 6% of PVP patients (no deaths) and in no TURP patients (P = 0.253). The overall rate of serious or non-serious adverse events was the same between groups, with no significant differences (P = 0.253). Recatheterization was required in 15 individuals: 12 PVP (9%) and 3 TURP (17%) (P = 0.537).
Outcome Measures
The change in IPSS scores was similar over time (Figure 1), with both groups experiencing similar reductions in LUTS at 6 months (Table 3). For other clinical measures, such as Qmax, urinary frequency, and erectile function (SHIM score), no statistically significant differences were observed. Differences in PVR volume and PSA were found (Table 3).
Figure 1: IPSS From Baseline to 6 Months Following Procedure.

Abbreviations: IPSS, International Prostate Symptom Score; PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
Table 3: Changes From Baseline in Clinical Outcomes.
| Treatment Group | Baseline, mean (SD) | 6-Month, mean (SD) | % Change a | P value | |
|---|---|---|---|---|---|
| IPSS | PVP | 21.4 (6.4) | 8.2 (6.1) | -62% | 0.718 |
| TURP | 24.4 (4.4) | 10.5 (8.3) | -57% | ||
| Urinary frequency (IPSS 2 hours) | PVP | 3.7 (1.2) | 1.7 (1.4) | -53% | 0.544 |
| TURP | 3.7 (1.2) | 2.0 (1.5) | -46% | ||
| Qmax, mL/sec | PVP | 11.1 (4.2) | 17.2 (10.1) | +55% | 0.705 |
| TURP | 8.8 (4.1) | 15.8 (8.7) | +79% | ||
| PVR volume, mL | PVP | 106.9 (108.7) | 30.6 (50.2) | -71% | 0.018 |
| TURP | 68.8 (69.1) | 43.4 (69.1) | -31% | ||
| PSA, ng/dL | PVP | 2.9 (2.5) | 2.8 (2.9) | -2% | 0.050 |
| TURP | 3.0 (2.1) | 2.2 (1.7) | -29% | ||
| PSA before 5-alpha reductase inhibitor use, ng/dL | PVP | 2.4 (2.1) | 3.2 (3.8) | +36% | 0.581 |
| TURP | 2.7 (2.0) | 1.9 (2.0) | -28% | ||
| EQ-5D score | PVP | 0.87 (0.1) | 0.91 (0.1) | +5% | 0.134 |
| TURP | 0.89 (0.1) | 0.88 (0.1) | -1% | ||
| Erectile function (SHIM score) | PVP | 12.4 (7.8) | 11.4 (8.7) | -7% | 0.569 |
| TURP | 9.4 (8.8) | 9.3 (9.3) | -2% |
Abbreviations: EQ-5D, EuroQol 5 Domain; IPSS, International Prostate Symptom Score; PSA, prostate-specific antigen; PVP, photoselective vaporization of the prostate; PVR, post-void residual; Qmax, maximum flow rate; SD, standard deviation; SHIM, Sexual Health Inventory for Men; TURP, transurethral resection of the prostate.
Calculations may appear inexact due to rounding.
Health Care Resource Utilization
Health care resource utilization over the 6-month follow-up did not differ between the 2 groups. The need for repeat or crossover procedures was required in 1 patient (4.1%) in the TURP group and 5 patients (3.6%) in the PVP group (P = 0.886). Similarly, there was no statistically significant difference in emergency department visits (6% versus 11%, P = 0.318), admissions (0% versus 2%, P = 0.469), or additional physician visits (39% versus 49%, P = 0.068) for TURP compared to PVP, respectively. The only difference between groups was related to the use of diagnostic tests and procedures: 11% of TURP patients and 36% of PVP patients (P = 0.020) required additional testing.
Phase II
Long-term follow-up at 24 months was available for 116/140 (83%) of the PVP group and 16/24 (67%) of the TURP group, for an overall 24-month follow-up rate of 80%. In those with follow-up data, the rate of repeat procedures was not significantly different between the 2 groups after 24 months, with 1 patient in the TURP group (6%) requiring a repeat TURP, and 5 patients in the PVP group (4%) with repeat procedures (2 TURPs and 3 PVPs).
There was no difference in IPSS (P = 0.182) or quality of life (P = 0.118) between the 2 groups. In the PVP group, 11/116 (9.5%) individuals were receiving medication to manage their LUTS, whereas none of the patients originally treated with TURP restarted pharmacotherapy.
Economic Analysis
Cost-Effectiveness Analysis
Methods
An economic evaluation was conducted to assess the cost-effectiveness of PVP compared to TURP. The economic analysis compared the patient-level costs of both procedures in terms of incremental cost per quality-adjusted life-year (QALY) gained. The analysis was conducted from both the Ministry of Health and Long-Term Care and societal perspectives at 6 months following surgery. Direct and indirect costs associated with PVP and TURP, as well as resource utilization data collected during follow-up visits/interviews, were valued using public and private sources (e.g., hospital medical records, Ontario Schedule of Benefits for Physician Services (22), American Medical Systems [manufacturer of the laser] for the cost of PVP/fibres).
The costs of the device per procedure and the procedures themselves are outlined in Tables 4 and 5. To calculate QALYs, utilities derived from the EQ-5D questionnaire were weighted by time spent in health states. QALYs were adjusted for potential differences in baseline utilities and patient characteristics between the 2 groups. The health care resources consumed over 6 months were multiplied by the appropriate cost and added to the procedural costs to determine the total cost of care over the period. The expected costs and QALYs for PVP and TURP were also calculated. In the absence of dominance (e.g., PVP being less costly and more effective than TURP), results were expressed in terms of incremental cost-utility ratios to compare PVP and TURP. To deal with sample variability, bootstrap techniques were used to generate confidence intervals around the incremental cost-utility ratios. Uncertainty was summarized using cost-effectiveness acceptability curves.
Table 4: Estimated Costa of PVP Device Per Procedure.
| Input Variables | Amount |
|---|---|
| Device cost | $130,000.00 |
| Amortization of device—annuity factor (5 years at an interest rate of 5%) | 4.3295% |
| Equivalent annual cost | $30,026.72 |
| Annual operating cost | $10,000.00 |
| Total cost per year of device | $40,026.72 |
| Total annual PVP case volume | 165 cases |
| Total cost per procedure of device | $242.59 |
Abbreviation: PVP, photoselective vaporization of the prostate.
All costs are in Canadian dollars.
Table 5: Cost of PVP and TURPa.
| Item | PVP | TURP | ||
|---|---|---|---|---|
| Description | Estimate | Description | Estimate | |
| Physician | TURP (code S655) | $450.60 | TURP (code S655) | $450.60 |
| Anesthesiologist | 7 basic units + 6 time unitsb | $190.45 | 7 basic units + 4 time unitsb | $161.15 |
| Hospital | Day surgery (OCCI procedure code 1QT59BAAG) | $1,550.94 | Acute inpatient (OCCI procedure code 1QT87BA) | $3,849.90 |
| Consumables | PVP fibre (estimated 1 per procedure) | $850.00 | Resecting loop | $100.00 |
| Device, capital | Cost per procedure, PVP device | $242.59 | NA | — |
| Total Cost per Case | $3,284.58 | $4,561.65 | ||
Abbreviations: NA, not applicable; OCCI, Ontario Case Costing Initiative; PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
All costs are in Canadian dollars.
Unit fee = $14.65.
Results
Health Care Resource Utilization Costing
The mean total cost of care after 6 months for the TURP arm (including the cost of the primary procedure) was $4,863 (Cdn) (SD $971), and for the PVP arm was $3,891 (Cdn) (SD $1,315), for a statistically significant difference in cost of $971 (Cdn) between interventions (P = 0.001). The majority of this cost difference was attributable to differences in cost between initial procedures.
From Phase II, the 24-month overall estimated average health care costs associated with the 2 groups were $4,946 (Cdn) for the TURP arm and $4,116 (Cdn) for the PVP arm, with PVP having a lower average cost of care of $830 (Cdn) after 24 months.
Cost Utility Analysis
The results of the 1,000 bootstrap replicates are presented as a scatter plot in the cost-effectiveness plane (Figure 2). PVP was less costly and provided generally improved effectiveness; the QALYs associated with PVP and TURP were calculated to be 0.448 (SD 0.048) and 0.441 (SD 0.071), respectively (P = 0.658), out of a maximum of 0.5 QALYs over 6 months (utility of 1 * 0.5 year = 0.5 QALY). As a result, PVP was the preferred strategy in the base case. The results of the bootstrap analysis showed that the majority of the simulations lay in the southeast quadrant, indicating improved QALYs and lower costs for the majority of simulations.
Figure 2: Cost-Effectiveness Planea.

Abbreviations: QALY, quality-adjusted life-year.
AII costs are in Canadian dollars.
The probabilistic analysis of uncertainty is presented as a cost-effectiveness acceptability curve in Figure 3, which indicates that PVP was the most cost-effective treatment across all decision-making thresholds.
Figure 3: Cost-Effectiveness Acceptability Curvea.

Abbreviations: QALY, quality-adjusted life-year.
All costs are in Canadian dollars.
Budget Impact Analysis
Methods
Two budget impact analyses were conducted by applying the cost of TURP and PVP to estimated procedural volumes for the province of Ontario and for an average Ontario urology department.
The average annual volume for TURP was determined for fiscal years 2008/2009 to 2012/2013 using data from the Canadian Institute for Health Information (Discharge Abstract Database for inpatient procedures and National Ambulatory Care Reporting System for outpatient procedures). Records with an International Statistical Classification of Diseases and Related Health Problems (10th revision, Canadian version) diagnosis code for hyperplasia of prostate (N40) and procedure codes for TURP (excision, partial prostate—endoscopic per orifice approach [transurethral] [1.QT.87.BA, 1.QT.87.BA-GX, 1.QT.87.BA.AK]) were extracted. (23-25) The substitution of PVP for TURP (using the same calculated average annual volume) was then evaluated using different rates: 100%, 75%, 50%, and 25%.
The economic consequences of substituting PVP for TURP were evaluated using cost (as calculated for the economic evaluation) and number of patient days. Admission rates from the clinical study were used to determine the number of patients who would require admission for both PVP and TURP. If a patient was admitted, the cost of the procedure and hospital stay was assumed to be similar to that of TURP; however, this may overestimate the cost of a PVP admission, since the average length of stay for PVP patients was 2.0 days, compared to 2.5 days for the TURP patients, as found in the study.
Results
The average annual volume for TURP in the 5-year period from fiscal year 2008/2009 to fiscal year 2012/2013 was 12,335 procedures. The estimated potential cost averted by substituting PVP for 100% of TURP was $14,195,193.11 (Cdn) (Table 6). This averted cost was associated primarily with the ability to conduct PVP on an outpatient basis and avoid hospital admission in approximately 93% of cases. Providing PVP as an outpatient procedure could avert up to 28,213 days of inpatient care. When substitution rates were lower, costs averted and number of inpatient days averted were also lower.
Table 6: Provincial Budget Impact and Inpatient Length of Stay Analysis, Substituting PVP for TURPa.
| Cost of TURP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number of TURPs performed for BPH in Ontario per yearb | 12,335 | 12,335 | 12,335 | 12,335 | ||||
| Proportion of patients admitted following procedure | 97% | 97% | 97% | 97% | ||||
| Number of inpatient TURP procedures performedb | 11,965 | 11,965 | 11,965 | 11,965 | ||||
| Average cost of TURP (Table 5) | $4,561.65 | $4,561.65 | $4,561.65 | $4,561.65 | ||||
| Total estimated cost of TURP | $54,579,914 | $54,579,914 | $54,579,914 | $54,579,914 | ||||
| Substitution of PVP for TURP | ||||||||
| Substitution rate (PVP for TURP) | 100% | 75% | 50% | 25% | ||||
| Average number of inpatient TURPs performed after substitution | 0 | 2,991 | 5,982 | 8,974 | ||||
| Cost of PVP | ||||||||
| Number of PVP procedures performed per year | 11,965 | 8,974 | 5,982 | 2,991 | ||||
| Proportion of patients admitted following PVP | 7.1% | 7.1% | 7.1% | 7.1% | ||||
| Number of PVPs performed as an outpatient procedure | 11,115 | 8,337 | 5,558 | 2,779 | ||||
| Average cost of PVP outpatient procedure (Table 5) | $3,284.58 | $3,284.58 | $3,284.58 | $3,284.58 | ||||
| Total estimated cost of PVP outpatient procedures | $36,509,547.15 | $27,382,160.36 | $18,254,773.58 | $9,127,386.79 | ||||
| Number of PVPs performed with inpatient admission | 850 | 637 | 425 | 212 | ||||
| Estimated cost of PVP inpatient procedure (assumed to be same as TURP) | $4,561.65 | $4,561.65 | $4,561.65 | $4,561.65 | ||||
| Total estimated cost of PVP inpatient procedures | $3,875,173.91 | $2,906,380.43 | $1,937,586.95 | $968,793.48 | ||||
| Total estimated cost of all PVP procedures | $40,384,721.06 | $30,288,540.79 | $20,192,360.53 | $10,096,180.26 | ||||
| Total estimated cost of remaining TURP procedures | $0 | $13,644,978.54 | $27,289,957.08 | $40,934,935.63 | ||||
| Total cost of care for surgical intervention for BPH (PVP and TURP) | $40,384,721.06 | $43,933,519.34 | $47,482,317.61 | $51,031,115.89 | ||||
| Cost difference of substituting PVP for TURP | $14,195,193.11 | $10,646,394.83 | $7,097,596.55 | $3,548,798.28 | ||||
| Inpatient Length of Stay Analysis | ||||||||
| Average length of stay per TURP | 2.5 days | 2.5 days | 2.5 days | 2.5 days | ||||
| Inpatient days with TURP | 29,912 | 22,434 | 14,956 | 7,478 | ||||
| Average length of stay for PVP (if admitted) | 2.0 days | 2.0 days | 2.0 days | 2.0 days | ||||
| Inpatient days with PVP | 1,699 | 1,274 | 850 | 425 | ||||
| Inpatient days averted | 28,213 | 21,160 | 14,107 | 7,053 | ||||
| Differences in Consumable Device Costs Associated With Procedures | ||||||||
| TURP consumables, per resecting loop | $100 | $100 | $100 | $100 | ||||
| PVP consumable, per fibre | $850 | $850 | $850 | $850 | ||||
| Total cost of resecting loops for all TURPs | $1,233,500 | $897,371 | $598,248 | $299,124 | ||||
| Total cost of fibres for all PVPs | $10,170,208 | $7,627,656 | $5,085,104 | $2,542,552 | ||||
| Incremental cost of consumables associated with PVP | $8,936,708 | $6,730,284 | $4,486,856 | $2,243,428 | ||||
Abbreviations: BPH, benign prostatic hyperplasia; PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
All costs are in Canadian dollars. Calculations may appear inexact due to rounding.
Average for fiscal years 2008/2009 to 2012/2013.
Applying the same calculations to a hospital with a TURP case volume of 165 procedures on an annual basis (or approximately 3 procedures per week), the budget impact for a representative hospital in Ontario was also determined (using a substitution rate of 100% only). The estimated potential cost averted by substituting PVP for TURP for a representative hospital was $195,755.67 (Cdn) (Table 7). The availability of this outpatient procedure could avert 389 days of inpatient care for a hospital.
Table 7: Hospital Budget Impact and Inpatient Length of Stay Analysis, Substituting PVP for TURPa.
| Cost of TURP | |
|---|---|
| Number of TURPs performed in a representative hospital per year | 165 |
| Proportion of patients admitted following procedure | 100% |
| Average cost of TURP (Table 5) | $4,561.65 |
| Total estimated cost of TURP procedures | $752,672.25 |
| Cost of PVP | |
| Percentage substitution of PVP with TURP | 100% |
| Number of PVPs performed in a representative hospital per year | 165 |
| Proportion of patients admitted following PVP | 7.1% |
| Number of PVPs performed as an outpatient procedure | 153 |
| Average cost of PVP outpatient procedure (Table 5) | $3,284.58 |
| Total estimated cost of PVP outpatient procedures | $503,476.85 |
| Number of PVPs performed with inpatient admission | 12 |
| Estimated cost of PVP inpatient procedure (assumed to be same as TURP) | $4,561.65 |
| Total estimated cost of PVP inpatient procedures | $53,439.73 |
| Total estimated cost of all PVP procedures | $556,916.58 |
| Cost difference of substituting TURP for PVP | $-195,755.67 |
| Inpatient Length of Stay Analysis | |
| Average length of stay per TURP | 2.5 days |
| Inpatient days with TURP | 412.5 days |
| Average length of stay for PVP (if admitted) | 2.0 days |
| Inpatient days with PVP | 23.4 days |
| Inpatient days averted | 389 days |
| Differences in Consumable Device Costs Associated With Procedures | |
| TURP consumable: resecting loop | $100 per resecting loop |
| PVP consumable: fibre | $850 per fibre |
| Total cost of resecting loops for all TURPs | $16,500 |
| Total cost of fibres for all PVPs | $140,250 |
| Incremental cost of consumables associated with PVP | $123,750 |
Abbreviations: PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
All costs are in Canadian dollars. Calculations may appear inexact due to rounding.
Discussion
In the surgical treatment of BPH, a number of energy-based interventions have been developed as an alternative to the standard TURP. Recent randomized controlled trials, along with this observational study, have demonstrated that the efficacy of PVP is not different from TURP with respect to IPSS at 6 months or other outcome measures, such as flow rates, quality of life, or sexual function. (11;26;27) The ability to perform PVP on an outpatient basis meant that only a few individuals required hospital admission after the procedure, compared to TURP, which is done on an inpatient basis. This resulted in a lower cost for PVP, for the both index procedure and 6-month follow-up. The durability of PVP relative to TURP and lower overall costs were also apparent 24 months following the index procedures.
Limitations
This study was nonrandomized, and the results should be interpreted with some caution, despite generally similar baseline characteristics between the 2 groups. In addition, the ability to recruit individuals to the TURP arm was a challenge, as has been found by other investigators conducting studies with this technology. (28;29) In this study, men who were offered a choice of procedure chose PVP more frequently.
Conclusions
Based on this analysis, PVP appears to be a cost-effective alternative to TURP, providing similar clinical benefit at a lower cost to the health system. This trial-based cost-effectiveness analysis is unique; it is the first time the cost-effectiveness of PVP has been examined within a study. The opportunity to avert inpatient stays and redirect funds to other areas by using PVP over TURP could free up over 28,000 inpatient days and just over $14 million (Cdn) for other uses. The provision of funding for the PVP devices and consumable laser fibres used would have to be considered.
Acknowledgements
The authors would like to thank the Evidence Development and Standards group (formerly the Medical Advisory Secretariat) at Health Quality Ontario and the Ontario Health Technology Advisory Committee for their support of the study. We would also like to thank Erica Nunes, Craig MacDougald, Simone Sutherland, and the surgeons and research coordinators at each of the sites for their work on the study. Drs. O’Reilly, Tarride, and Xie are recipients of Career Scientist Awards from the Ontario Ministry of Health and Long-Term Care.
Ontario Ministry of Health and Long-Term Care (MOHLTC) funding was acquired through an independent Health Technology Assessment and Economic Evaluation Program research grant awarded to Prof. Ron Goeree (Grant 06129) through the Programs for Assessment of Technology in Health (PATH Research Institute at St. Joseph’s Healthcare Hamilton) and to Dr. Jean-Eric Tarride for the specific funding of this study.
Appendices
Appendix 1: Study Schematic
Figure A1: Study Schematic.
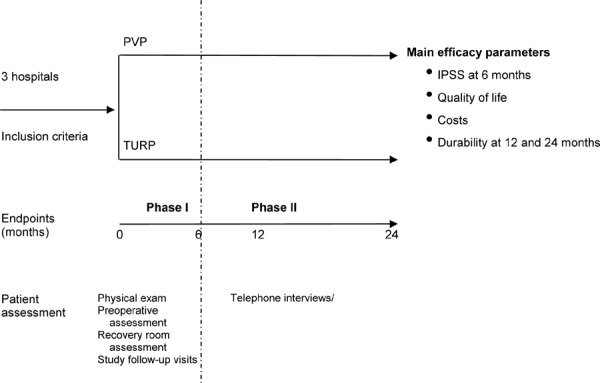
Abbreviations: IPSS, International Prostate Symptom Score; PVP, photoselective vaporization of the prostate; TURP, transurethral resection of the prostate.
Suggested Citation
This report should be cited as follows:
Bowen JM, Whelan JP, Hopkins RB, Burke N, Woods EA, McIsaac GP, et al. Photoselective vaporization for the treatment of benign prostatic hyperplasia. Ont Health Technol Assess Ser [Internet]. 2013 August;13(2):1-34. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-PVP.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/EMBASE, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398
ISBN 978-1-4606-1469-3
© Queen’s Printer for Ontario, 2013
About Health Quality Ontario
Health Quality Ontario is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians, and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. The Evidence Development and Standards branch works with expert advisory panels, clinical experts, scientific collaborators, and field evaluation partners to conduct evidence-based reviews that evaluate the effectiveness and cost-effectiveness of health interventions in Ontario.
Based on the evidence provided by Evidence Development and Standards and its partners, the Ontario Health Technology Advisory Committee—a standing advisory subcommittee of the Health Quality Ontario Board—makes recommendations about the uptake, diffusion, distribution, or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders, and policy-makers.
Health Quality Ontario’s research is published as part of the Ontario Health Technology Assessment Series, which is indexed in MEDLINE/PubMed, Excerpta Medica/Embase, and the Centre for Reviews and Dissemination database. Corresponding Ontario Health Technology Advisory Committee recommendations and other associated reports are also published on the Health Quality Ontario website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, Evidence Development and Standards and its research partners review the available scientific literature, making every effort to consider all relevant national and international research; collaborate with partners across relevant government branches; consult with expert advisory panels, clinical and other external experts, and developers of health technologies; and solicit any necessary supplemental information.
In addition, Evidence Development and Standards collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review.
The Ontario Health Technology Advisory Committee uses a unique decision determinants framework when making recommendations to the Health Quality Ontario Board. The framework takes into account clinical benefits, value for money, societal and ethical considerations, and the economic feasibility of the health care intervention in Ontario. Draft Ontario Health Technology Advisory Committee recommendations and evidence-based reviews are posted for 21 days on the Health Quality Ontario website, giving individuals and organizations an opportunity to provide comments prior to publication. For more information, please visit: http://www.hqontario.ca/evidence/evidence-process/evidence-review-process/professional-and-public-engagement-and-consultation.
Disclaimer
This report was prepared by Health Quality Ontario or one of its research partners for the Ontario Health Technology Advisory Committee and was developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to Health Quality Ontario. It is possible that relevant scientific findings may have been reported since the completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the Health Quality Ontario website for a list of all publications: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations.
List of Tables
| Table 1: Baseline Characteristics |
| Table 2: Medication Use at Baseline for the Management of LUTS |
| Table 3: Changes From Baseline in Clinical Outcomes |
| Table 4: Estimated Cost of PVP Device Per Procedure |
| Table 5: Cost of PVP and TURP |
| Table 6: Provincial Budget Impact and Inpatient Length of Stay Analysis, Substituting PVP for TURP |
| Table 7: Hospital Budget Impact and Inpatient Length of Stay Analysis, Substituting PVP for TURP |
List of Figures
List of Abbreviations
- AMS
American Medical Systems
- BPH
Benign prostatic hyperplasia
- EQ-5D
EuroQol 5 Domain
- IPSS
International Prostate Symptom Score
- KTP
Potassium titanyl phosphate
- LUTS
Lower urinary tract symptoms
- NA
Not applicable
- OCCI
Ontario Case Costing Initiative
- PSA
Prostate-specific antigen
- PVR
Post-void residual
- PVP
Photoselective vaporization of the prostate
- QALY
Quality-adjusted life-year
- Qmax
Peak urinary flow rate
- SD
Standard deviation
- SHIM
Sexual Health Inventory for Men
- TURP
Transurethral resection of the prostate
Footnotes
Presented to the Ontario Health Technology Advisory Committee on November 11, 2011, and April 26, 2013.
Final report submitted to Health Quality Ontario June 2013.
References
- 1.Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992 Nov;148(5):1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 2.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005 Apr;173(4):1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 3.Barry M, Roehrborn C. Management of benign prostatic hyperplasia. Annu Rev Med. 1997;48:177–89. doi: 10.1146/annurev.med.48.1.177. [DOI] [PubMed] [Google Scholar]
- 4.American Urological Association. American Urological Association guideline: management of benign prostatic hyperplasia (BPH) [Internet]. Linthicum (MD): American Urological Association Education and Research; 2010. [[cited 2012 Oct 9]. 62 p.]. Available from: http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm?sub=bph .
- 5.Naslund MJ, Chiao E, Black L, Eaddy MT. The hidden condition: status, challenges, and opportunities in the management of enlarged prostate for managed care. Am J Manag Care. 2006 Mar;12(4 Suppl):S76–82. [PubMed] [Google Scholar]
- 6.Nickel JC, Herschorn S, Corcos J, Donnelly B, Drover D, Elhilali M. et al. Canadian guidelines for the management of benign prostatic hyperplasia. Can J Urol. 2005 Jun;12(3):2677–83. [PubMed] [Google Scholar]
- 7.Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol. 2006 Nov;50(5):969–79. doi: 10.1016/j.eururo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Medical Advisory Secretariat Energy delivery systems for treatment of benign prostatic hyperplasia: an evidence-based analysis. [[cited 3013 Jul 22].];Ont Health Technol Assess Ser. 2006 Aug;6(17):1–121. [Internet] Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/energy-delivery-systems-for-treatment-of-benign-pr . [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann A, Ruszat R. The KTP-(greenlight-) laser--principles and experiences. Minim Invasive Ther Allied Technol. 2007;16(1):5–10. doi: 10.1080/13645700601157885. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann A, Schurch L, Ruszat R, Wyler SF, Seifert HH, Muller A. et al. Photoselective vaporization (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol. 2005 Dec;48(6):965–71. doi: 10.1016/j.eururo.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Capitan C, Blazquez C, Martin MD, Hernandez V. de la Pena E, Llorente C. GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: a randomized clinical trial with 2-year follow-up. Eur Urol. 2011;60(4):734–9. doi: 10.1016/j.eururo.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Bouchier-Hayes DM, Van AS, Bugeja P, Crowe H, Challacombe B, Costello AJ. A randomized trial of photoselective vaporization of the prostate using the 80-W potassium-titanyl-phosphate laser vs transurethral prostatectomy, with a 1-year follow-up. BJU Int. 2010 Apr;105(7):964–9. doi: 10.1111/j.1464-410X.2009.08961.x. [DOI] [PubMed] [Google Scholar]
- 13.Malek RS, Kuntzman RS, Barrett DM. High power potassium-titanyl-phosphate laser vaporization prostatectomy. J Urol. 2000 Jun;163(6):1730–3. [PubMed] [Google Scholar]
- 14.Shingleton WB, Farabaugh P, May W. Three-year follow-up of laser prostatectomy versus transurethral resection of the prostate in men with benign prostatic hyperplasia. Urology. 2002 Aug;60(2):305–8. doi: 10.1016/s0090-4295(02)01697-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee R, Gonzalez RR, Te AE. The evolution of photoselective vaporization prostatectomy (PVP): advancing the surgical treatment of benign prostatic hyperplasia. World J Urol. 2006 Sep;24(4):405–9. doi: 10.1007/s00345-006-0094-y. [DOI] [PubMed] [Google Scholar]
- 16.Ontario Health Technology Advisory Committee (OHTAC). OHTAC recommendation: energy delivery systems for treatment of benign prostatic hyperplasia [Internet]. Toronto (ON): Queen’s Printer for Ontario; 2006 Aug. [[cited 2013 Jul 22]. 5 p.]. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/energy-delivery-systems-for-treatment-of-benign-pr .
- 17.Health Canada. Medical Devices Active Licence Listing (MDALL) [Internet]. Ottawa (ON): Health Canada; 2007 Mar 22. [[cited 2007 Jun 4].]. Available from: http://www.hc-sc.gc.ca/dhp-mps/md-im/licen/mdlic_e.html .
- 18.Barry MJ, O’Leary MP. Advances in benign prostatic hyperplasia. The developmental and clinical utility of symptom scores. Urol Clin North Am. 1995 May;22(2):299–307. [PubMed] [Google Scholar]
- 19.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990 Dec;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 20.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997 Nov;35(11):1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999 Dec;11(6):319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 22.Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act (April 1, 2013) [Internet]. [[cited 2013 Jul 22].]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html .
- 23.International statistical classification of diseases and related health problems (ICD-10-CA). 10th rev. Ottawa (ON): Canadian Institute for Health Information; 2009. Diseases of male genital organs (N40-N51).
- 24.Canadian classification of health interventions (CCI). Volume three - tabular list. Ottawa (ON): Canadian Institute for Health Information; 2009. 1.QT.87.∧∧: excision partial, prostate.
- 25.Canadian classification of health interventions (CCI). Volume four - alphabetical index. Ottawa (ON): Canadian Institute for Health Information; 2012. 1.QT.87.∧∧: excision partial, prostate.
- 26.Lukacs B, Loeffler J, Bruyere F, Blanchet P, Gelet A, Coloby P. et al. Photoselective vaporization of the prostate with GreenLight 120-W laser compared with monopolar transurethral resection of the prostate: a multicenter randomized controlled trial. Eur Urol. 2012;61(6):1165–73. doi: 10.1016/j.eururo.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ansari A, Younes N, Sampige VP, Al-Rumaihi K, Ghafouri A, Gul T. et al. GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a randomized clinical trial with midterm follow-up. Eur Urol. 3. 2010;58:349–55. doi: 10.1016/j.eururo.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Pereira-Correia JA, de Moraes Sousa KD, Santos JB, de Morais PD, Lopes-da-Silva LF, Krambeck RL. et al. GreenLight HPS 120-W laser vaporization vs transurethral resection of the prostate (<60 mL): a 2-year randomized double-blind prospective urodynamic investigation. BJU Int. 2012 Oct;110(8):1184–9. doi: 10.1111/j.1464-410X.2011.10878.x. [DOI] [PubMed] [Google Scholar]
- 29.Madersbacher S. After three randomised controlled trials comparing 120-W high-performance-system potassium-titanyl-phosphate laser vaporisation to transurethral resection of the prostate (TURP), is this procedure finally first-line, outdated, or still not surpassing TURP? Eur Urol. 2012;61(6):1174–5. doi: 10.1016/j.eururo.2012.02.046. [DOI] [PubMed] [Google Scholar]


