Background: PTP-PEST regulates cell migration as part of many protein complexes.
Results: SKAP-Hom is a substrate of PTP-PEST that is required for proper fibroblasts migration. Enhanced migration was observed when SKAP-Hom-deficient fibroblasts are rescued with its SH3 domain mutant.
Conclusion: As a novel substrate of PTP-PEST, SKAP-Hom is important in cellular migration.
Significance: PTP-PEST regulates migration through a new complex involving SKAP-Hom.
Keywords: Cell Migration, Phosphorylation, Protein-Protein Interactions, SH3 domains, Tyrosine Protein Phosphatase (Tyrosine Phosphatase)
Abstract
PTP-PEST is a cytosolic ubiquitous protein tyrosine phosphatase (PTP) that contains, in addition to its catalytic domain, several protein-protein interaction domains that allow it to interface with several signaling pathways. Among others, PTP-PEST is a key regulator of cellular motility and cytoskeleton dynamics. The complexity of the PTP-PEST interactome underscores the necessity to identify its interacting partners and physiological substrates in order to further understand its role in focal adhesion complex turnover and actin organization. Using a modified yeast substrate trapping two-hybrid system, we identified a cytosolic adaptor protein named Src kinase-associated phosphoprotein 55 homologue (SKAP-Hom) as a novel substrate of PTP-PEST. To confirm PTP-PEST interaction with SKAP-Hom, in vitro pull down assays were performed demonstrating that the PTP catalytic domain and Proline-rich 1 (P1) domain are respectively binding to the SKAP-Hom Y260 and Y297 residues and its SH3 domain. Subsequently, we generated and rescued SKAP-Hom-deficient mouse embryonic fibroblasts (MEFs) with WT SKAP-Hom, SKAP-Hom tyrosine mutants (Y260F, Y260F/Y297F), or SKAP-Hom SH3 domain mutant (W335K). Given the role of PTP-PEST, wound-healing and trans-well migration assays were performed using the generated lines. Indeed, SKAP-Hom-deficient MEFs showed a defect in migration compared with WT-rescued MEFs. Interestingly, the SH3 domain mutant-rescued MEFs showed an enhanced cell migration corresponding potentially with higher tyrosine phosphorylation levels of SKAP-Hom. These findings suggest a novel role of SKAP-Hom and its phosphorylation in the regulation of cellular motility. Moreover, these results open new avenues by which PTP-PEST regulates cellular migration, a hallmark of metastasis.
Introduction
Cytoskeleton rearrangement is crucial in a number of cellular responses to environmental cues. Cellular differentiation, response to osmotic and other cellular stresses, as well as cell migration and invasion, all require a coordinate reorganization of actin fibers. Cell migration in particular is a dynamic and multistep process of leading edge protrusion, turnover of focal adhesions, generation of traction forces, and tail retraction and detachment (1). As the major cellular receptors for extracellular matrix (ECM),5 integrin family of cell adhesion receptors is essential for each of these steps of cell migration. Although they have relatively short cytoplasmic domains, integrins regulate cell migration as well as other cellular functions through their coupling to multiple cytoskeletal and signaling molecules, many of which co-cluster with integrins in focal adhesions in adherent cells (2). The focal adhesion kinase (FAK) is one of the most prominent signaling molecules among these proteins. Through its tyrosine kinase activity FAK influence protein tyrosine phosphorylation during cell migration to initiate various reorganizations of actin fibers and cellular cytoskeleton (3).
PTP-PEST (PTPN12) is a non-receptor classical protein tyrosine phosphatase (PTP) that has a significant role in the regulation of cellular migration. It is ubiquitously expressed in mammalian cells with the murine and human orthologs sharing 97.9% homology (4). In addition to the conserved PTPase domain at the N terminus, murine PTP-PEST is characterized by the presence of four proline rich domains, three of which (Pro1, Pro2, and Pro4) have been identified as SH3 binding motifs. Pro3 domain has not yet been linked to any SH3-containing proteins (5). The C-terminal domain has also been found to possess dimerizing properties as well providing association to PST-PIP, a protein involved in linking integrin signaling to the arp2/3 complex, hence controlling actin reorganizations (6). Moreover, the Tremblay laboratory first identified a non-canonical NPLH interacting motif that is recognized by the PTB domain of p66SHC adapter protein (7). To understand the function of PTP-PEST, we generated a mouse deficient for PTP-PEST. Removal of PTP-PEST causes embryonic lethality between day 9.5p.c. and 10.5p.c., therefore demonstrating the crucial role of this PTP in embryonic development (8). Recently, it was shown that PTP-PEST plays a crucial role in the proper endothelial cells migration and adhesion. Absence of PTP-PEST leads to a defective vascularization, thus explaining the lethality observed during embryogenesis (9).
PTP-PEST−/− mouse embryonic fibroblasts (MEFs) showed a defect in motility and an increased number of focal adhesion contacts indicating a role for PTP-PEST. Indeed, many cytoskeleton-associated proteins such as paxillin (10), Hic-5 (11), leupaxin (12), p130Cas (13), PSTPIP (6), filamin-A (14), p50csk (15), and others were identified as both substrates and interacting partners of PTP-PEST. As a major cell motility modulator, PTP-PEST influences this process partly by regulating the actin cytoskeleton polymerization through the interaction with WASP proteins via PSTPIP, and by the formation of lamellipodia and cell retraction through Rac1 and RhoA via the regulation of upstream effectors such as VAV and RhoGAP (6, 16, 17). Moreover, PTP-PEST is phosphorylated by ERK1/2 in response to Ras activation, leading to its isomerization by PIN1. Subsequently, PTP-PEST interacts and dephosphorylates FAK, thus contributing to cell migration and invasion in a RAS-dependent mechanism (18). Therefore, determining PTP-PEST substrates as well as the physiological interacting partners will allow deciphering the diverse mechanism used by this PTP to control migration. In addition to its role in cellular motility, its interaction with the cytokinesis protein PSTPIP is required to contribute to the proper separation of cleavage furrow (6, 19, 20).
PTP-PEST has been coupled to the epidermal growth factor receptor (EGFR) through its binding to the Src homology 3 (SH3) domain of Grb2 via its first proline-rich domain and to p66SHC via the NPLH domain (7, 21). More recently, PTP-PEST was identified as a potent suppressor of mammary epithelial cell transformation and of breast cancer cells proliferation and metastasis through its interaction and dephosphorylation of the prominent breast cancer proto-oncogenic receptor tyrosine kinases (RTKs), EGFR, and HER2 (22).
As for regulation, PTP-PEST is modulated during the apoptotic response, where it is cleaved by caspase-3, rendering it catalytically more active to further dismantle the cytoskeleton network (23). In addition, PKA and PKC are capable of inhibiting PTP-PEST phosphatase activity by phosphorylating residue S39, while the outcome of phosphorylating S434 is still unclear (24). Recently, PP1α was determined to dephosphorylate PTP-PEST on S39 (25). Other modifications to PTP-PEST include ROS inactivation (26).
Many efforts are directed at the identification of new PTP-PEST substrates in order to better understand its various functions in human diseases such as autoimmune disorders and cancer. Here, we used a modified yeast two-hybrid screening strategy to search for novel substrates of PTP-PEST and identified Src kinase-associated phosphoprotein 55 homologue (SKAP-Hom) as a novel substrate of PTP-PEST. SKAP-Hom is a 55 kDa adaptor protein that contains a coiled-coil domain, a central PH binding domain and an N-terminal SH3 domain and is ubiquitously expressed in mammalian tissues (27). It shares high homology with SKAP55 with the main difference being the absence of the coiled-coil domain in SKAP55 and the exclusive expression of the latter in T lymphocytes (28). Additionally, SKAP-Hom has been implicated in immune-cell regulation where PTP-PEST was also found to negatively regulate receptor-mediated activation by dephosphorylating SHC, CAS, Pyk2, and FAK in B-cells and T-cells (29–32). We report the identification of SKAP-Hom as a novel substrate of PTP-PEST and characterize the interaction in vitro. We also highlight the importance of SKAP-Hom in PTP-PEST-dependent control of cellular migration, therefore adding a new node in the PTP-PEST cell migration interactome.
EXPERIMENTAL PROCEDURES
DNA Constructs
Site-directed mutagenesis was used to insert the S39A, S39D, S434A, and S434D mutations into the previously described pcDNA3.1(+)Zeo-PTP-PEST expression vector (6). Constructs containing deleted proline regions were also constructed similarly. PTP-PEST was inserted into the BamHI and ClaI sites of the pEBG vector (33). A cDNA encoding murine SKAP-Hom (ATCC) was cloned into the SpeI and NotI sites of the pEBG vector for an N-terminal GST-SKAP-Hom. Site-directed mutagenesis was used to create the Y75F, Y93F, Y237F, Y260F, Y297F, and W335K GST-SKAP-Hom mutants. The C terminus myc fusion SKAP-Hom was obtained by subcloning into pCDNA 3.1 expression vector (Invitrogen). Flag-tagged SKAP-Hom Y260F, Y297F, Y260F/Y297F, and W335K were cloned into the EcoRI and XhoI sites of the pMSCV-IRES-GFP vector. The full-length PTP-PEST D199A bait expression construct for two-hybrid screening was generated by cloning the PTP-PEST cDNA into the BamHI site of the pBridge/LexA/v-src plasmid (34).
Antibodies
For immunodetection, the following primary antibodies were used: anti-glutathione S-transferase (GST) (SCBT), anti-Lex A (SCBT), anti-phosphotyrosine (Upstate Biotechnology), anti-p130cas (BD Biosciences), anti-SHC (Upstate), anti-PTP-PEST (in-house), anti-myc (BD Biosciences), anti-paxillin (BD), anti-Fyn (Dr. A. Veillette), anti-v-src (Dr. Steven Martin), anti-SKAP-Hom (ProteinTech Group). Specific binding was detected using the appropriate HRP-conjugated goat anti-rabbit or rabbit anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories).
Cells, Transfection, Pull-down Assays, and Immunoprecipitation
HeLa299, Mouse embryonic fibroblasts and Phoenix Ecotropic cells were cultured in DMEM media supplemented with 10% (v/v) fetal bovine serum (Invitrogen) and 50 μg/ml Gentamycin (Invitrogen). All transfections were done using Lipofectamine 2000 (Invitrogen) reagent. Cells were lysed in ice-cold HNMETG lysis buffer (50 mm HEPES pH 7.5, 150 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 1% (v/v) Triton X-100, 10% (v/v) glycerol) containing 1× Complete Mini-protease inhibitors (Roche). Pull-downs were carried out for 2 h at 4 °C on a rotating platform using 20–40 μl of a 50% slurry of glutathione-conjugated Sepharose beads (GE Healthcare). Following incubation samples were washed three times in lysis buffer prior to elution using 80 μl of 1× SDS sample buffer.
PTP-PEST−/− Mouse embryonic fibroblasts were lysed in ice-cold RIPA lysis buffer (50 mm Tris pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.25% Na deoxycholate) containing 1× Complete Mini-protease inhibitors (Roche). Immunoprecipitations were carried out overnight at 4 °C on a rotating platform using 1 μg of SKAP-Hom IgG (Proteintech) and 20 μl of protein A/G mix with 1 mg of total cell lysates. Following incubation, samples were washed three times in lysis buffer prior to elution with 20 μl of 1× SDS sample buffer.
Modified Yeast Two-hybrid Screening
Yeast-two-hybrid screening was performed using the Saccharomyces cerevisiae strain L40, which harbors the reporter genes HIS 3 and LacZ under the control of an upstream LexA-binding site. pBridgeLexA-PTP-PEST D/A and a mouse 17-day embryo MATCHMAKER cDNA library (Clontech) were transformed in the yeasts cells as previously described (Kawachi, 2001, Fukada 2005). This library has an estimated diversity of 3.5 million independent clones. 400,000 clones were screened in our assay and positives clones were selected on media lacking leucine, tryptophan, histidine and methionine, and verified by a β-galactosidase filter-lift assay ± methionine as described by Fukada et al. (34).
Mouse Embryonic Fibroblasts and Migration Assay
Primary mouse embryonic fibroblasts isolated from SKAP-Hom−/− embryos (Dr. M. Togni) were immortalized according to the 3T3 protocol (35). Phoenix Ecotropic packaging lines (Nolan Lab) were transfected with pMSCV-IRES-GFP retroviral vectors containing either WT SKAP-Hom, Y260F, Y260F/Y297F, or W335K mutants. Viral supernatants were collected 48 h post-transfection, and MEFs were subsequently transduced and sorted by FACSAria for GFP-positive cells. Western blot using anti-SKAP-Hom was performed to verify ectopic protein expression in the sorted population. The trans-well migration assays were performed in CIM-16 plates with 8 μm pore membranes (Roche). Wells of the bottom chamber were filled with 160 μl of 10% serum-containing DMEM media and the top and bottom chambers of the CIM-16 plates were assembled together. 40 μl of serum-free media were added to the top chamber and the assembled CIM-16 plate was allowed to equilibrate for 2 h at 37 °C, 5% CO2. For seeding, cells were rinsed with PBS, trypsinized for 2 min, centrifuged at 1200 rpm for 5 min, and washed with DMEM with 10% serum before washing and resuspension in serum-free DMEM. Cells (4 × 104 cells/well) were seeded onto the top chambers of CIM-16 plates and placed into the xCELLigence system for data collection after 30 min incubation at room temperature to allow cells to settle at the bottom of the top wells. The xCELLigence software was set to collect impendence data (reported as cell index) at 15-min intervals. Rate of cellular migration was calculated from the slope of the cell index.
Confocal Immunofluorescence Microscopy
3 × 104 MEFs were seeded on glass coverslips in 6-well plates and left overnight. 20 h later, Coverslips were washed with PBS and then fixed in 4% paraformaldehyde in PBS for 20 min. Cells were then washed in PBS and permeabilized with 0.2% Triton X-100/PBS and washed with 100 μm glycine/PBS. Cells were then blocked for 30 min with blocking buffer (2% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS). Cells were incubated with Alexa Fluor 488 Phalloidin (Molecular Probes, Life Technologies) diluted in blocking buffer for 1 h at room temperature. Coverslips were washed in IF buffer (0.2% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS) between primary and secondary antibodies. Coverslips were mounted with Immumount (Thermo-Shandon, Pittsburgh, PA). Confocal images were taken using a LSM 510, Axiovert200M (Carl Zeiss Canada Ltd, Toronto, ON, Canada) with a 63×/1.4 Oil DIC objective. Image analysis was carried out using Zen 2011 software, Blue edition (Carl Zeiss Microscopy). Actin staining intensity was assessed using MetaXpress (V5) analysis software (Molecular Devices).
RESULTS
SKAP-Hom Is a Novel Substrate of PTP-PEST
To screen for substrates and/or binding partners of PTP-PEST, we utilized a modified yeast two-hybrid system, which is described in greater detail elsewhere (34, 36). The bait construct constitutively expressed a full-length substrate-trapping mutant (D199A) of PTP-PEST with an N-terminal fusion to the LexA DNA binding domain. In addition, this construct also expressed a nucleus-targeted kinase, v-src, under a methionine-suppressive promoter. Given the very low level of tyrosine phosphorylation in yeast (36, 37), the expression of v-src was used to promote substrate interactions with the PTP-PEST trapping mutant. The full-length PTP-PEST trapping mutant was screened against 17-day-old mouse embryo cDNA library. A stable interaction between PTP-PEST and putative substrates and/or binding partners results in the expression of these reporters. Our assumption is that interactions with the substrate trapping PTP-PEST construct that are strengthened through increased v-src expression indicate putative PTP-PEST substrates. Our first experiments confirmed that v-src expression could be modulated by methionine culture levels (Fig. 1A). In addition, the increased v-src expression led to a dramatic increase in the global phosphorylation level of protein tyrosine residues (Fig. 1B). Of the estimated 3.5 million independent cDNA clones in the MATCHMAKER library, we succeeded in screening ∼400,000 independent clones. Despite this relatively low library representation, we managed to identify the known PTP-PEST binding partners Hic-5 and PST-PIP2, as well as a novel interaction with an N-terminal truncation of SKAP-Hom (amino acids 86–358) (data not shown). This clone was purified and the interaction was reconfirmed in yeast as evidenced by growth on minimal media and induction of β-galactosidase (Fig. 1C). We tested various PTP-PEST bait constructs to ascertain whether the interaction was dependent on PTP domain recognition. We confirmed that the PTP domain D199A region was sufficient for binding to SKAP-Hom.
FIGURE 1.
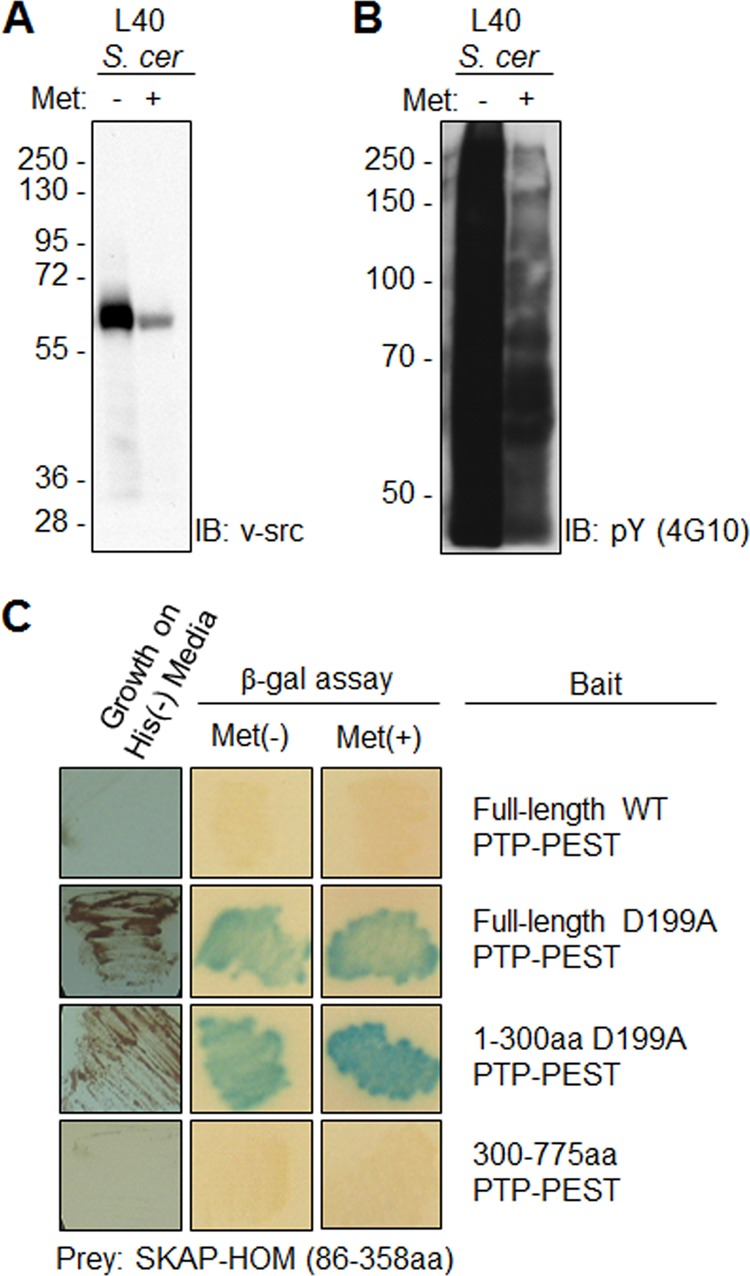
Modified yeast two-hybrid screening identifies SKAP-Hom as a novel PTP-PEST substrate. A, yeast (S. cerevisiae) lysates were immunoblotted for exogenous v-src expression in the absence (−) and presence (+) of methionine. B, immunoblot analysis examining global tyrosine phosphorylation (4G10 antibody) in yeast lysates confirmed that expressed v-src was enzymatically active. C, verification of the interaction in yeast by a β-galactosidase filter-lift assay in the presence or absence of methionine. Substrates should display an enhanced interaction and increased β-galactosidase activity in the presence of v-src. In contrast, binding partners should display the same level of β-galactosidase activity regardless of v-src expression. Positive clones were sent for sequencing for identification.
PTP-PEST: SKAP-Hom Interaction
We sought to determine whether the PTP-PEST D199A:SKAP-Hom interaction in our yeast system was also valid in a mammalian cell system. A constitutively active mutant of the kinase FynT (FynT Y528F) was used to promote the interaction. FynT was used instead of v-src, because some literature described SKAP-Hom as preferentially phosphorylated by Fyn (38, 39). We found that GST-SKAP-Hom could co-precipitate with PTP-PEST D199A, but not with WT PTP-PEST (Fig. 2). Moreover, GST-SKAP-Hom phosphorylation was protected when bound to the trapping mutant of PTP-PEST. In the absence of PTP-PEST D199A, GST-SKAP-Hom phosphorylation levels were considerably lower (Fig. 2A). As a reciprocal experiment, a vector expressing C terminus myc tag fused in-frame to SKAP-Hom was co-transfected with GST-PTP-PEST WT and D199A. It was observed that GST-PTP-PEST D199A but not WT was capable of pulling down SKAP-Hom. Binding and phosphorylation of SKAP-Hom was not detected in the GST-PTP-PEST WT pull-down (Fig. 2B). Moreover, as a substrate of PTP-PEST, SKAP-Hom tyrosine phosphorylation is expected to be higher in the absence of the phosphatase Indeed, endogenous immunoprecipitated SKAP-Hom showed an enhanced level of phosphotyrosine in PTP-PEST−/− MEFs compared with PTP-PEST-WT-rescued MEFs (Fig. 2C). Altogether these results support SKAP-Hom as a bona fide binding partner and a substrate of PTP-PEST.
FIGURE 2.
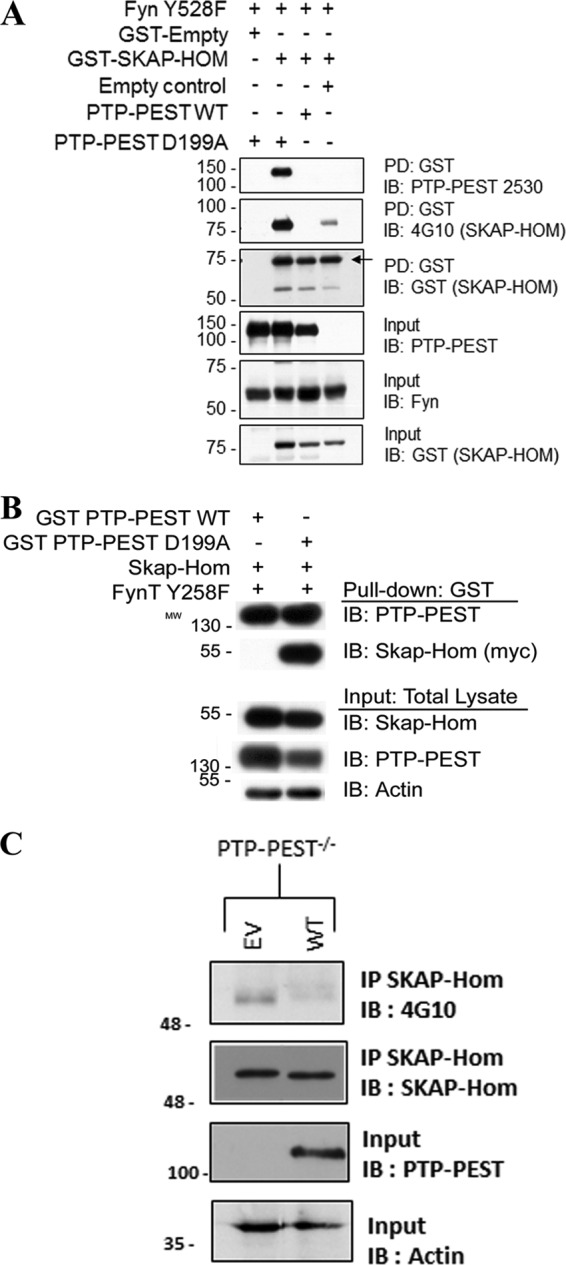
Confirmation of the PTP-PEST:SKAP-Hom interaction in HeLa299 cells. A, ability of PTP-PEST D199A to interact with GST-SKAP-Hom was examined by protein overexpression studies followed by GST-pull-down assays in HeLa299 cells. Cells were transfected with the indicated vectors and incubated at 37 °C overnight prior to lysis in HNMETG buffer. The lysates were cleared and quantitated for GST pull-down at 4 °C for 2 h on a rotator. The eluates obtained from GST pulldowns were immunoblotted using specific antibodies to confirm GST-SKAP-Hom precipitation and phosphorylation status, and to determine if any co-precipitation of PTP-PEST D199A occurred. Immunoblotting was also used to confirm equal protein overexpression of GST-SKAP-Hom, PTP-PEST WT or D199A, and Fyn Y528F in total cell lysates/input material. B, reciprocal GST-pull-down assay of PTP-PEST followed by immunoblotting showing the precipitation of SKAP-Hom with GST-PTP-PEST. C, PTP-PEST−/− MEFs were lysed in RIPA buffer and total protein lysates were subject to SKAP-Hom immunoprecipitation. The eluates obtained were immunoblotted with 4G10 and SKAP-Hom antibodies to assess the phosphotyrosine status and the total SKAP-Hom, respectively. PTP-PEST and actin immunoblots of input material were performed to confirm PTP-PEST ectopic expression.
PTP-PEST Dephosphorylates SKAP-Hom on Y260 and Y297 Residues
PTP-PEST D199A:GST-SKAP-Hom interaction is PTP domain dependent, thus we aim to identify the phosphorylation site(s) in SKAP-Hom required for binding to PTP-PEST. SKAP-Hom phosphorylation has been reported on residue Y260 (29), however, bioinformatics sequence analysis using NetPhos 2.0 predicted that tyrosine residues 75, 93, 197, 237, 260, and 297 could also be candidate phosphorylation sites (38). Since residue Y75 was not present in the truncated version of SKAP-Hom (amino acids 86–358) in the initial yeast two-hybrid screen, this site was not investigated. Instead, tyrosine-to-phenylalanine mutants were generated at each of the remaining sites. As predicted, the GST-SKAP-Hom Y260F mutant displayed significantly reduced tyrosine phosphorylation and reduced binding to PTP-PEST D199A (Fig. 3A). Additionally, the GST-SKAP-Hom Y297F mutant also displayed reduced tyrosine phosphorylation and reduced binding to PTP-PEST D199A (Fig. 3A). It was only when both Y260F and Y297F mutants were used that binding to PTP-PEST D199A was undetected (Fig. 3B). None of the GST-SKAP-Hom tyrosine mutants disrupted binding to FynT kinase (Fig. 3, A and B) even though phosphorylation of Y260 was reported to be essential for this interaction (29). These results suggest that PTP-PEST recognizes two tyrosine sites, Y260 and Y297, as the main sites of phosphorylation on SKAP-Hom.
FIGURE 3.
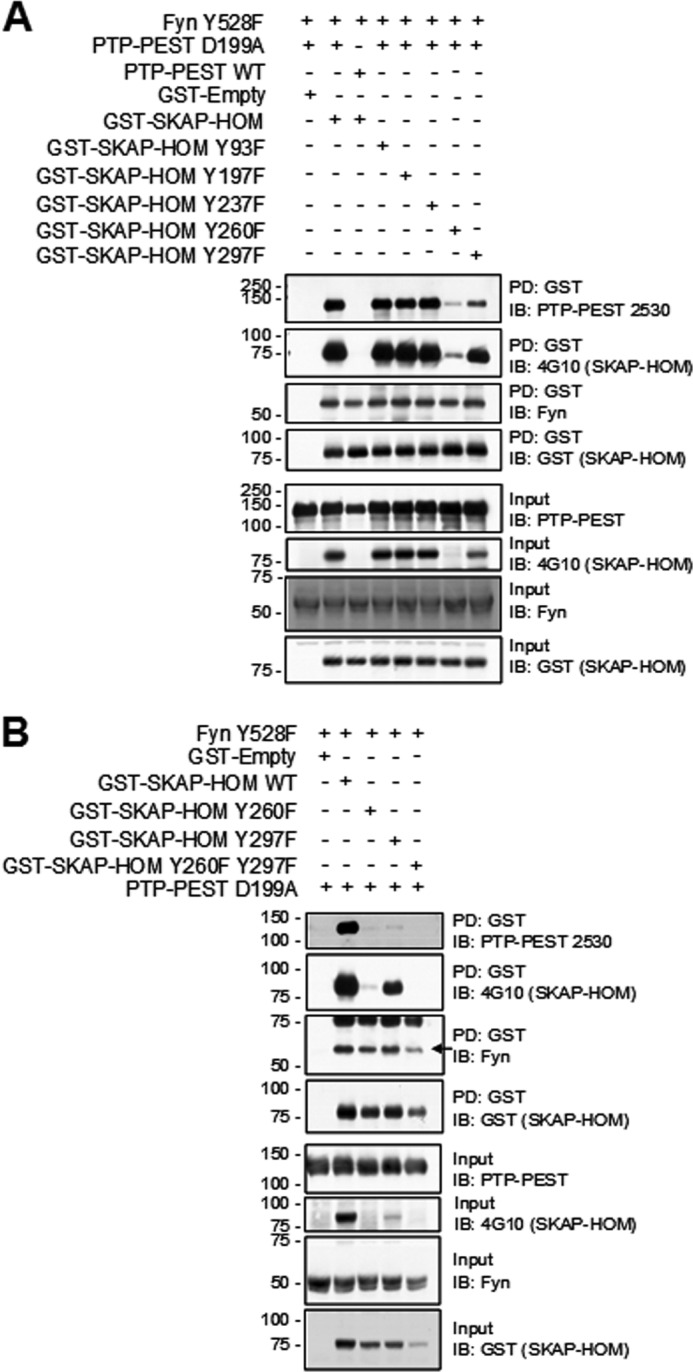
PTP-PEST dephosphorylates SKAP-Hom on Y260 and Y297 residues. A, identification of the phosphotyrosine residues in GST-SKAP-Hom that are trapped by PTP-PEST D199A was done by protein overexpression studies in HeLa299 cells where GST-SKAP-Hom Y-F mutants were used followed by GST pull-down analysis. Eluates obtained from GST pull-downs were immunoblotted for confirming SKAP-Hom precipitation, phosphorylation status, and co-precipitation of PTP-PEST D199A and FynT Y528F. Input lysates were also probed as a control of the presence of each of the indicated proteins. B, Y260 and Y297 were identified to be essential for the interaction. Pull down and Western blot conditions are the same as previously described.
The PTP-PEST: SKAP-Hom Complex Interacts via the PTP, the Pro1, and SH3 Domains
PTP-PEST has five proline rich regions in its C-terminal tail, which mediate interactions with other proteins often contributing a first stage of phosphorylation independent interaction allowing for subsequent tyrosine dephosphorylation by the PTP-PEST catalytic domain (6, 10–12, 14, 15, 17). Our yeast two-hybrid data revealed that the substrate trapping assay with the D-A PTP domain is sufficient for promoting the interaction with SKAP-Hom, yet as with other SH3 domain-containing proteins interacting with PTP-PEST (13), we postulated that the SKAP-HOM SH3 domain may also serve to stabilize this interaction in mammalian cells. To test this hypothesis, we generated proline region deletion mutants of PTP-PEST D199A, and dominant negative SH3 domain mutant of GST-SKAP-Hom (W335K), where the first tryptophan of the characteristic tryptophan doublet in the SH3 domain was mutated to lysine (39, 40). The disruption of SKAP-Hom's SH3 domain or PTP-PEST's ΔPro1 region dramatically reduced the PTP-PEST D199A associated with GST SKAP-Hom in a GST pull-down assay (Fig. 4, A and B). This suggests that the SH3 and ΔPro1 domains of SKAP-Hom and PTP-PEST, respectively, serve to stabilize the interaction.
FIGURE 4.
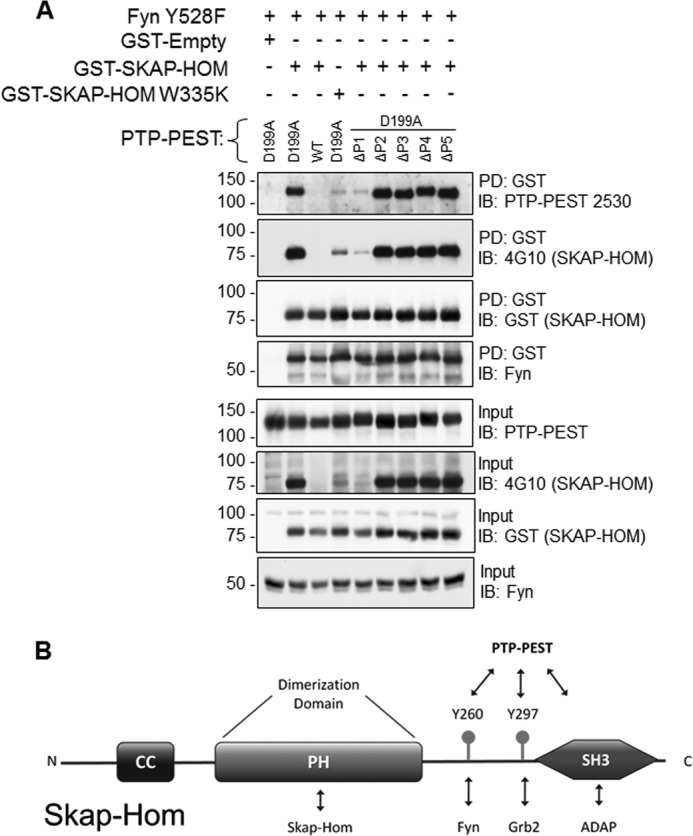
The PTP-PEST:SKAP-Hom interaction is dependent upon a dual mechanism of PTP domain recognition and SH3 domain recognition. A, PTP-PEST D199A domain mutants containing deletions of each of the proline-enriched domains, from P1 to P5 were utilized to determine the specific binding sites for SKAP-Hom. The mutated SH3 domain (W335K) on SKAP-Hom was also used to determine the binding requirement of the interaction. Procedures were performed as previously described. B, schematic representation of SKAP-Hom structural domains featuring the tyrosine residues substrates of PTP-PEST and the SH3 domain required for the interaction with the proline-rich domain 1 (P1) of the phosphatase.
PTP-PEST Residues S39 and S434 Modulate SKAP-Hom Substrate Recognition
It was previously reported that human PTP-PEST is phosphorylated in vitro by both PKA and PKC on residues S39 and S435 (S434 in mouse PTP-PEST) (24). Moreover, it was found that phosphorylation of the S39 site decreased enzymatic activity by lowering PTP-PEST's affinity for substrates, while the S434 site was unclear (24). PKA and PKC signaling pathways could potentially modulate PTP-PEST function in vivo. To test whether these serine residues are important for the PTP-PEST:SKAP-Hom interaction, serine-to-alanine and serine-to-aspartic acid mutants were made at each site in a GST-PTP-PEST D199A construct. The alanine and aspartic mutants were chosen to simulate the non-phosphorylated and charged nature of the phosphorylated state, respectively. Both the S39A and S39D mutants prevented the PTP-PEST D199A trapping mutant from binding GST-SKAP-Hom in a GST pull-down assay (Fig. 5A). Interestingly, these same PTP-PEST mutants displayed normal binding to p130cas, paxillin, and SHC (Fig. 5B), suggesting that this effect is specific to SKAP-Hom and that protein misfolding or mislocalization are not responsible. The same observations were made concerning the S434A mutant but not the S434D mutant (Fig. 5, A and B). The S434D mutant of PTP-PEST D199A was able to interact with GST-SKAP-Hom as well or better than PTP-PEST D199A S434 (Fig. 5A). These data illustrate how differential phosphorylation of PTP-PEST could potentially regulate its ability to interact with SKAP-Hom. We conclude here that phosphorylation at serine 39 could disrupt the SKAP-Hom interaction with PTP-PEST, while phosphorylation at serine 434 could promote the interaction.
FIGURE 5.
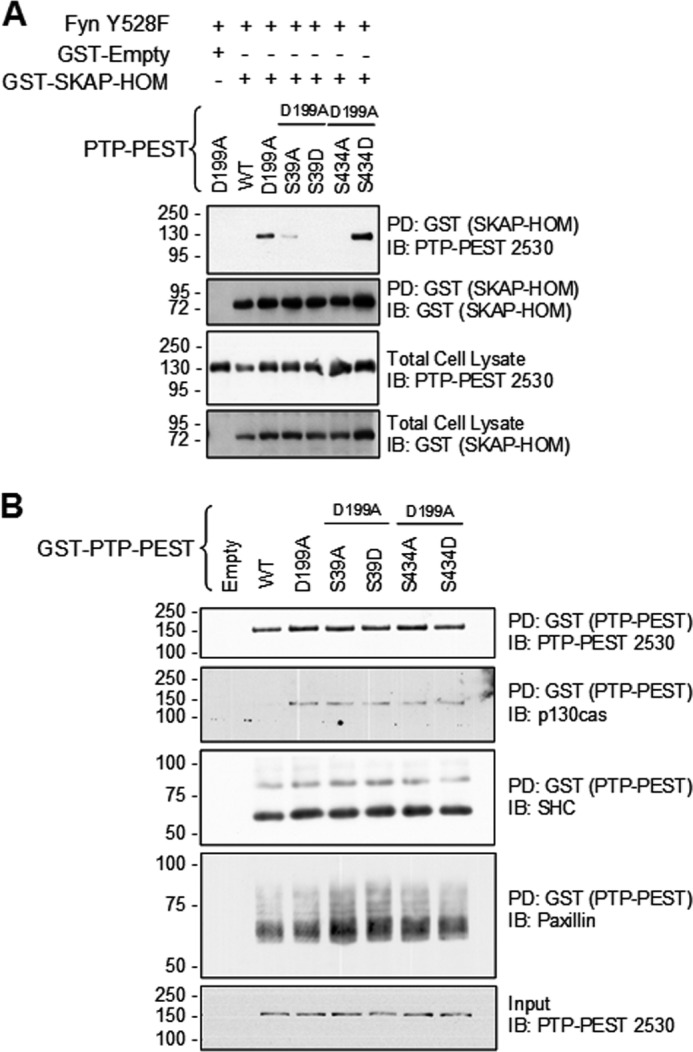
PTP-PEST phosphorylation on residues S39 and S434 modulates SKAP-Hom substrate recognition. A, mutational studies of PTP-PEST regulatory serine phosphorylation sites S39 and S434 were used to determine the effect on GST-SKAP-Hom binding. S39A, S39D, and S434A disrupted the binding of SKAP-Hom while S434D slightly enhanced the binding. B, effect of serine modulation of PTP-PEST D199A was also tested in the ability to co-precipitate some common binding partners, of p130Cas, Shc, and paxillin.
FynT Kinase Contributes to the PTP-PEST:SKAP-Hom Interaction
It was previously reported that phosphorylation of Y260 residue of murine SKAP-Hom is required for binding to Fyn kinase (29). GST-SKAP-Hom mutants co-precipitated FynT (Figs. 3, A and B, 4 and 6) and endogenous Fyn found in HeLa 299 cells (Fig. 6). This led to the investigation whether in addition to phosphorylation, FynT kinase could contribute to the PTP-PEST: SKAP-Hom interaction in other ways. A GST-SKAP-Hom pull-down in the presence of the catalytically dead FynT Y528F K296M mutant resulted in less PTP-PEST D199A co-precipitation compared with that obtained when using the constitutively active FynT Y528F mutant. This was expected, however, the amount of PTP-PEST D199A co-precipitated in the presence of catalytically dead FynT was still greater than that obtained in the absence of FynT (Fig. 6). This suggests that FynT promotes the PTP-PEST D199A:GST-SKAP-Hom interaction through some other mechanism. This hypothesis is further supported by the fact that tyrosine phosphorylation of GST-SKAP-Hom is higher in the presence of catalytically dead FynT compared with the condition where none is over-expressed. By helping to recruit PTP-PEST D199A, phosphorylated GST-SKAP-Hom residues are protected by the trapping ability of PTP-PEST D199A (Fig. 6).
FIGURE 6.
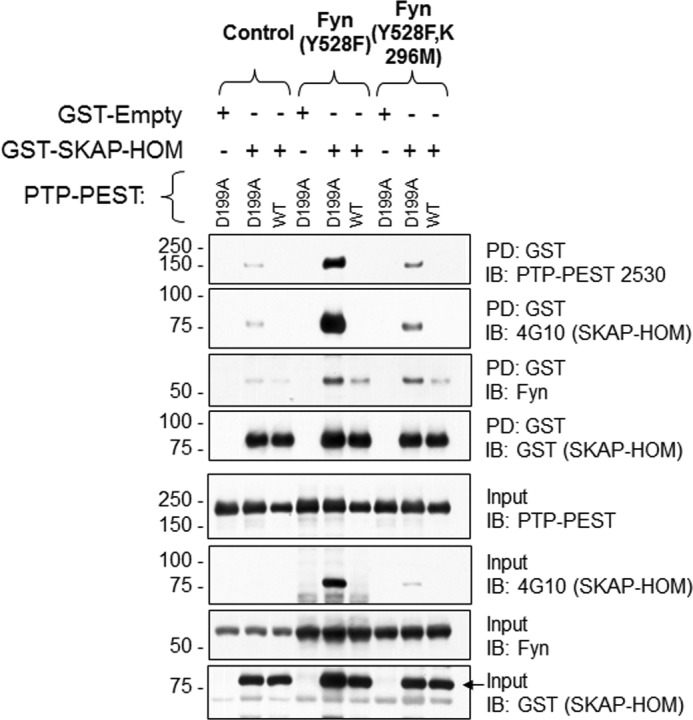
Fyn Kinase contributes to PTP-PEST:SKAP-Hom interaction. The amount of SKAP-Hom successfully pull-down in the absence and presence of Fyn was determined using the PTP-PEST WT and D199A mutant. The constitutively active Fyn kinase mutant Y528F, and the catalytically dead Fyn kinase mutant Y528F, K296M were used to determine whether the kinase activity is required for the SKAP-Hom:PTP-PEST interaction. The phosphorylation of GST SKAP-Hom is blotted with 4G10 and GST antibodies and the amount of PTP-PEST is determined.
SKAP-Hom:PTP-PEST Interaction Is Essential in the Regulation of Cellular Migration
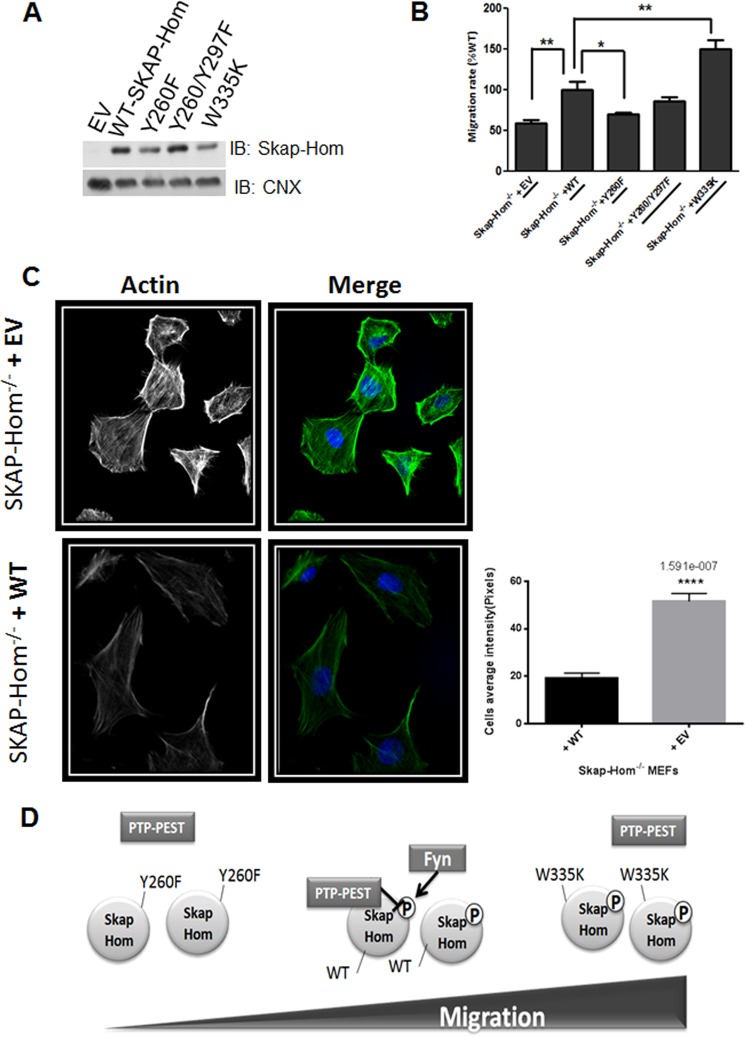
PTP-PEST interaction with numerous cytoskeleton-associated proteins and its regulation of cellular mobility has been well documented. Interestingly, SKAP-Hom is implicated in integrin signaling. BCR-induced adhesion to integrin ligands, such as fibronectin and ICAM-1, is strongly decreased in SKAP-Hom−/− B cells (31). Therefore, it is intriguing to investigate whether SKAP-Hom is a novel module through which PTP-PEST contributes to proper cell migration. Consequently, we carried trans-well migration assays using immortalized SKAP-Hom−/− fibroblasts rescued with WT SKAP-Hom, SKAP-Hom tyrosine mutants (Y260F, Y260F/Y297F), or SH3 domain mutant (W335K) (Fig. 7A). Indeed, SKAP-Hom−/− MEFs showed a defect in migration rate compared with WT-rescued MEFs (Fig. 7B). Similarly, SKAP-Hom Y260F-rescued cells showed a decrease in migration rate, indicating the importance of phosphorylation of this residue in the control of cellular migration. Interestingly, fibroblasts expressing the mutated SH3 domain of SKAP-Hom, the essential domain for the interaction with the PTP-PEST, showed an enhanced trans-well migration rate (Fig. 7B). This domain is believed to stabilize the interaction with PTP-PEST as shown previously (Fig. 4A), allowing dephosphorylation. Supporting the defect observed in SKAP-Hom−/− migration, actin staining in those cells showed an increase in stress fibers formation compared with WT-rescued fibroblasts (Fig. 7C), as higher stress fiber content is often associated with slower migration (41).
FIGURE 7.
SKAP-Hom is essential in the regulation of cellular migration. A, immortalized SKAP-Hom−/− MEFs were transduced with plasmids expressing either WT SKAP-Hom or Y260F, Y26F/Y297F or the SH3 domain mutant (W335K). Positive clones were analyzed by Western blotting using SKAP-Hom antibody to verify the ectopic expression of SKAP-Hom variants. Calnexin was used as a protein loading control. B, Transwell migration assay (Xcelligence system, Roche) was performed to assess the importance of SKAP-Hom and its interaction with PTP-PEST in the regulation of cellular migration. 10% FBS was used as a chemoattractant and the cells were allowed to migrate for 6 h at 37 °C. Migrating cells signals were monitored in real-time. The rate of cellular migration was normalized against the control (WT rescued SKAP-Hom−/− MEFs). The results represent three independent experiments. One-way ANOVA followed by Dunnett's Multiple Comparison Test was used to assess statistical significance. Error bars represent the S.E., **, p < 0.01, *, p < 0.05. C, SKAP-Hom−/− and WT-rescued MEFs were seeded on coverslips, fixed and stained with Phalloidin (F-actin in green) and DAPI (nuclei in blue). F-actin staining intensity was measured as cells average intensity using MetaXpress Analysis software (Molecular Devices). The graph is a representative of three independent experiments. Two-tailed non-parametric Student's t test followed by Mann-Whitney test was used to assess statistical significance. Error bars represent the S.E., ****, p < 0.0001. D, proposed model for SKAP-Hom regulation of cellular migration. SKAP-Hom phosphorylation on Y260 is required for proper cellular migration. When mutated, Y260 reside can no longer be phosphorylated by Fyn kinase and the cells show a defect in migration. On the other hand, when SKAP-Hom SH3 domain is mutated, it loses the ability to interact and to be dephosphorylated by PTP-PEST, hence retaining a higher phosphorylation state compared with WT protein, conferring a better migratory capacity.
Hence, when SKAP-Hom SH3 domain is mutated, the protein is expected to maintain phosphorylated Y260 and Y297 residues (Fig. 7D). Wound-healing assay coupled to time-lapse imaging was also performed with SKAP-Hom−/− -rescued MEFs and resulted in similar migration trend as the trans-well migration assay (data not shown).
DISCUSSION
Using a modified yeast two-hybrid substrate-trapping assay, we identified SKAP-Hom as a novel substrate of PTP-PEST. We mapped the binding domains from both proteins, including the key phosphorylation sites of PTP-PEST and SKAP-Hom that are required for optimal interaction. The Pro 1 domain of PTP-PEST region was determined as the required binding site for SKAP-Hom, as abrogation of this region significantly reduced the interaction with SKAP-Hom. Likewise, a mutation in the WW motif of the SH3 domain of SKAP-Hom disrupted the binding to PTP-PEST D199A. The wild-type PTP-PEST failed to co-precipitate SKAP-Hom under these same conditions. We have observed that the phosphorylation status of PTP-PEST may play a role in modulating SKAP-Hom binding. We also document the binding of Fyn kinase that further stabilizes this interaction.
Importantly, we showed that SKAP-Hom interaction with PTP-PEST and its dephosphorylation impacts cellular migration as SKAP-Hom phosphorylation levels correlates with the observed migration trend. We also showed that in the absence of SKAP-Hom the cells display more stress fibers. Our results demonstrate for the first time a role for SKAP-Hom in the regulation of fibroblasts migration.
While SKAP-Hom Y260F/Y297F showed no significant effect, cells expressing the Y260F, mimicking a non-phosphorylated residue, showed a defect in migration comparable to the complete absence of SKAP-Hom. By dephosphorylating this residue, PTP-PEST could be interrupting a docking site for SH2 domain-containing proteins such as Fyn (27). Consequently, SKAP-Hom adaptor protein function that is likely needed to coordinates cytoskeleton and focal adhesion protein complexes is disturbed. It has been reported that Y260 is phosphorylated by Fyn kinase and that a mutation to phenylalanine was shown to disrupt the binding to Fyn kinase (29), an observation that was not detected in our assays. Furthermore, we believe that Y297 provides an additional regulatory site and may serve as a binding site for other proteins, which could compensate for the defect of migration seen in the single Y260F mutant and not with the double mutant Y260/297F.
In a recently published screen for substrate-specific motifs of the lymphoid-specific tyrosine phosphatase (Lyp), a closely related phosphatase to PTP-PEST, Y75 of SKAP-Hom was identified as a putative target. However, the physiological relevance of this interaction is yet to be determined (42). Altogether, these data reflect on the specificity of closely related tyrosine phosphatases. Although it is likely that each PTP will dephosphorylate several substrates that may act in the same signaling pathways, many examples have been reported that position specific PTPs as regulators of completely independent signaling activities (i.e. PTP1B in insulin receptor compared with JAK-STAT pathway regulation) (43, 44).
SKAP-Hom is functionally well characterized in immune cells, specifically in macrophages, dendritic cells, B- and T-lymphocytes. Upon macrophage M-CSF-stimulation, SKAP-Hom is tyrosine phosphorylated and recruited to actin complexes where it gets dephosphorylated (45), suggesting that PTP-PEST is the likely implicated PTP since it is also recruited to actin networks in the WAVE complex (46). Moreover, SKAP-Hom is tyrosine phosphorylaled and localizes to membrane periphery of macrophages in response to adhesion to fibronectin (47). Likewise, we have reported similar PTP-PEST localization to membrane periphery in response to the same integrin ligand in COS cells (48). Subsequently, we confirmed the co-localization of SKAP-Hom and PTP-PEST at the cell membrane upon COS1 cells adhesion to fibronectin (data not shown). Moreover, SKAP-Hom was recently identified as an essential adaptor protein, part of a protein complex, that functions downstream of integrins in order to modulate macrophage migration and actin reorganization (49). Identifying SKAP-Hom as a substrate reemphasize the importance of PTP-PEST in integrin signaling complexes where it was previously found to regulate cellular adhesion and motility.
SKAP-Hom−/− MEFs showed a decreased mobility and more stress fibers similarly to PTP-PEST−/− MEFs (48). The increased number of stress fibers could be a consequence of altered functions of members of Rho family of small GTPases, such as RhoA, which is indirectly activated by PTP-PEST (16). Paradoxically, overexpression of PTP-PEST in MEFs shows a similar phenotype. Hence, either enhanced or impaired PTP-PEST activity inhibits cell motility (48, 50, 51). We believe that SKAP-Hom phosphorylation is an example of a key substrate that is tyrosine phosphorylated to promote motility, providing a possible explanation of the defective migration of cells with enhanced PTP-PEST activity.
In addition to its role in immune cells, a genetic screening of various pancreatic ductal adenocarcinoma samples revealed that SKAP-Hom is frequently amplified (in 63% of the cases) (52). Interestingly, another study determined that PTP-PEST is associated with ArgBP2/WAVE signaling which regulates pancreatic cancer cell mobility and which, when deregulated, could lead to metastasis (46).
Our findings attributed a new function to SKAP-Hom as a regulator of cellular migration through its phosphorylation regulation via Fyn kinase and PTP-PEST. This provides an additional mechanism through which PTP-PEST controls cellular adhesion and migration that are likely to contribute to metastatic pathways in cancer cells.
Acknowledgments
We thank Jean-Francois Theberge and to Drs. S. Martin, M. Togni, and A. Veillette for the kind gift of reagents. Microscopy image processing and analysis for this manuscript were performed in the McGill University Life Sciences Complex Imaging Facility.
This work was funded by the CRS/Rob Lutterman Pancreatic Cancer Research Grant and the Samuel Champlain foundation (to M. L. T. and A. B.).
- ECM
- extracellular matrix
- PTP
- protein tyrosine phosphatase
- SKAP-Hom
- Src kinase-associated phosphoprotein 55 homologue
- MEF
- mouse embryonic fibroblast
- FAK
- focal adhesion kinase.
REFERENCES
- 1. Petrie R. J., Doyle A. D., Yamada K. M. (2009) Random versus directionally persistent cell migration. Nature Rev. Mol. Cell Biol. 10, 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Streuli C. H., Akhtar N. (2009) Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 [DOI] [PubMed] [Google Scholar]
- 3. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase: in command and control of cell motility. Nature Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 4. Charest A., Wagner J., Shen S. H., Tremblay M. L. (1995) Murine protein tyrosine phosphatase-PEST, a stable cytosolic protein tyrosine phosphatase. Biochem. J. 308, 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charest A., Wagner J., Muise E. S., Heng H. H., Tremblay M. L. (1995) Structure of the murine MPTP-PEST gene: genomic organization and chromosomal mapping. Genomics 28, 501–507 [DOI] [PubMed] [Google Scholar]
- 6. Côté J. F., Chung P. L., Théberge J. F., Hallé M., Spencer S., Lasky L. A., Tremblay M. L. (2002) PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J. Biol. Chem. 277, 2973–2986 [DOI] [PubMed] [Google Scholar]
- 7. Charest A., Wagner J., Jacob S., McGlade C. J., Tremblay M. L. (1996) Phosphotyrosine-independent binding of SHC to the NPLH sequence of murine protein-tyrosine phosphatase-PEST. Evidence for extended phosphotyrosine binding/phosphotyrosine interaction domain recognition specificity. J. Biol. Chem. 271, 8424–8429 [DOI] [PubMed] [Google Scholar]
- 8. Sirois J., Côté J. F., Charest A., Uetani N., Bourdeau A., Duncan S. A., Daniels E., Tremblay M. L. (2006) Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech. Dev. 123, 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Souza C. M., Davidson D., Rhee I., Gratton J. P., Davis E. C., Veillette A. (2012) The phosphatase PTP-PEST/PTPN12 regulates endothelial cell migration and adhesion, but not permeability, and controls vascular development and embryonic viability. J. Biol. Chem. 287, 43180–43190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Côté J. F., Turner C. E., Tremblay M. L. (1999) Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J. Biol. Chem. 274, 20550–20560 [DOI] [PubMed] [Google Scholar]
- 11. Nishiya N., Iwabuchi Y., Shibanuma M., Côté J. F., Tremblay M. L., Nose K. (1999) Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3 domain. J. Biol. Chem. 274, 9847–9853 [DOI] [PubMed] [Google Scholar]
- 12. Sahu S. N., Nunez S., Bai G., Gupta A. (2007) Interaction of Pyk2 and PTP-PEST with leupaxin in prostate cancer cells. Am. J. Physiol. Cell Physiol. 292, C2288–C2296 [DOI] [PubMed] [Google Scholar]
- 13. Garton A. J., Burnham M. R., Bouton A. H., Tonks N. K. (1997) Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene 15, 877–885 [DOI] [PubMed] [Google Scholar]
- 14. Playford M. P., Lyons P. D., Sastry S. K., Schaller M. D. (2006) Identification of a filamin docking site on PTP-PEST. J. Biol. Chem. 281, 34104–34112 [DOI] [PubMed] [Google Scholar]
- 15. Davidson D., Cloutier J. F., Gregorieff A., Veillette A. (1997) Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J. Biol. Chem. 272, 23455–23462 [DOI] [PubMed] [Google Scholar]
- 16. Sastry S. K., Rajfur Z., Liu B. P., Cote J. F., Tremblay M. L., Burridge K. (2006) PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J. Biol. Chem. 281, 11627–11636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen Y., Schneider G., Cloutier J. F., Veillette A., Schaller M. D. (1998) Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 273, 6474–6481 [DOI] [PubMed] [Google Scholar]
- 18. Zheng Y., Yang W., Xia Y., Hawke D., Liu D. X., Lu Z. (2011) Ras-induced and extracellular signal-regulated kinase 1 and 2 phosphorylation-dependent isomerization of protein tyrosine phosphatase (PTP)-PEST by PIN1 promotes FAK dephosphorylation by PTP-PEST. Mol. Cell. Biol. 31, 4258–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spencer S., Dowbenko D., Cheng J., Li W., Brush J., Utzig S., Simanis V., Lasky L. A. (1997) PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J. Cell Biol. 138, 845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y., Dowbenko D., Lasky L. A. (1998) PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J. Biol. Chem. 273, 30487–30496 [DOI] [PubMed] [Google Scholar]
- 21. Charest A., Wagner J., Kwan M., Tremblay M. L. (1997) Coupling of the murine protein tyrosine phosphatase PEST to the epidermal growth factor (EGF) receptor through a Src homology 3 (SH3) domain-mediated association with Grb2. Oncogene 14, 1643–1651 [DOI] [PubMed] [Google Scholar]
- 22. Sun T., Aceto N., Meerbrey K. L., Kessler J. D., Zhou C., Migliaccio I., Nguyen D. X., Pavlova N. N., Botero M., Huang J., Bernardi R. J., Schmitt E., Hu G., Li M. Z., Dephoure N., Gygi S. P., Rao M., Creighton C. J., Hilsenbeck S. G., Shaw C. A., Muzny D., Gibbs R. A., Wheeler D. A., Osborne C. K., Schiff R., Bentires-Alj M., Elledge S. J., Westbrook T. F. (2011) Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 144, 703–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hallé M., Liu Y. C., Hardy S., Théberge J. F., Blanchetot C., Bourdeau A., Meng T. C., Tremblay M. L. (2007) Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol. Cell. Biol. 27, 1172–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garton A. J., Tonks N. K. (1994) PTP-PEST: a protein tyrosine phosphatase regulated by serine phosphorylation. EMBO J. 13, 3763–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura K., Palmer H. E., Ozawa T., Mashima K. (2010) Protein phosphatase 1alpha associates with protein tyrosine phosphatase-PEST inducing dephosphorylation of phospho-serine 39. J. Biochem. 147, 493–500 [DOI] [PubMed] [Google Scholar]
- 26. Wu W. S., Wu J. R., Hu C. T. (2008) Signal cross talks for sustained MAPK activation and cell migration: the potential role of reactive oxygen species. Cancer Metastasis Rev. 27, 303–314 [DOI] [PubMed] [Google Scholar]
- 27. Marie-Cardine A., Verhagen A. M., Eckerskorn C., Schraven B. (1998) SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 435, 55–60 [DOI] [PubMed] [Google Scholar]
- 28. Marie-Cardine A., Bruyns E., Eckerskorn C., Kirchgessner H., Meuer S. C., Schraven B. (1997) Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 272, 16077–16080 [DOI] [PubMed] [Google Scholar]
- 29. Curtis D. J., Jane S. M., Hilton D. J., Dougherty L., Bodine D. M., Begley C. G. (2000) Adaptor protein SKAP55R is associated with myeloid differentiation and growth arrest. Exp. Hematol. 28, 1250–1259 [DOI] [PubMed] [Google Scholar]
- 30. Davidson D., Veillette A. (2001) PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 20, 3414–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Togni M., Swanson K. D., Reimann S., Kliche S., Pearce A. C., Simeoni L., Reinhold D., Wienands J., Neel B. G., Schraven B., Gerber A. (2005) Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 25, 8052–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H., Rudd C. E. (2008) SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 18, 486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen P., Hutter D., Liu P., Liu Y. (2002) A mammalian expression system for rapid production and purification of active MAP kinase phosphatases. Protein Expr. Purif. 24, 481–488 [DOI] [PubMed] [Google Scholar]
- 34. Fukada M., Kawachi H., Fujikawa A., Noda M. (2005) Yeast substrate-trapping system for isolating substrates of protein tyrosine phosphatases: Isolation of substrates for protein tyrosine phosphatase receptor type z. Methods 35, 54–63 [DOI] [PubMed] [Google Scholar]
- 35. Todaro G. J., Green H. (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawachi H., Fujikawa A., Maeda N., Noda M. (2001) Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase ζ/β by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. U.S.A. 98, 6593–6598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castellanos R. M., Mazón M. J. (1985) Identification of phosphotyrosine in yeast proteins and of a protein tyrosine kinase associated with the plasma membrane. J. Biol. Chem. 260, 8240–8242 [PubMed] [Google Scholar]
- 38. Blom N., Gammeltoft S., Brunak S. (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 [DOI] [PubMed] [Google Scholar]
- 39. Gupta R. W., Mayer B. J. (1998) Dominant-negative mutants of the SH2/SH3 adapters Nck and Grb2 inhibit MAP kinase activation and mesoderm-specific gene induction by eFGF in Xenopus. Oncogene 17, 2155–2165 [DOI] [PubMed] [Google Scholar]
- 40. Tanaka M., Gupta R., Mayer B. J. (1995) Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell. Biol. 15, 6829–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tojkander S., Gateva G., Lappalainen P. (2012) Actin stress fibers–assembly, dynamics and biological roles. J. Cell Science 125, 1855–1864 [DOI] [PubMed] [Google Scholar]
- 42. Yu X., Chen M., Zhang S., Yu Z. H., Sun J. P., Wang L., Liu S., Imasaki T., Takagi Y., Zhang Z. Y. (2011) Substrate specificity of lymphoid-specific tyrosine phosphatase (Lyp) and identification of Src kinase-associated protein of 55 kDa homolog (SKAP-HOM) as a Lyp substrate. J. Biol. Chem. 286, 30526–30534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dubé N., Tremblay M. L. (2005) Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: from diabetes, obesity to cell cycle, and cancer. Biochim. Biophys. Acta 1754, 108–117 [DOI] [PubMed] [Google Scholar]
- 44. St-Pierre J., Tremblay M. L. (2012) Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 15, 292–297 [DOI] [PubMed] [Google Scholar]
- 45. Bourette R. P., Thérier J., Mouchiroud G. (2005) Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell. Signal. 17, 941–949 [DOI] [PubMed] [Google Scholar]
- 46. Taieb D., Roignot J., André F., Garcia S., Masson B., Pierres A., Iovanna J. L., Soubeyran P. (2008) ArgBP2-dependent signaling regulates pancreatic cell migration, adhesion, and tumorigenicity. Cancer Res. 68, 4588–4596 [DOI] [PubMed] [Google Scholar]
- 47. Black D. S., Marie-Cardine A., Schraven B., Bliska J. B. (2000) The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2, 401–414 [DOI] [PubMed] [Google Scholar]
- 48. Angers-Loustau A., Côté J. F., Charest A., Dowbenko D., Spencer S., Lasky L. A., Tremblay M. L. (1999) Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144, 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alenghat F. J., Baca Q. J., Rubin N. T., Pao L. I., Matozaki T., Lowell C. A., Golan D. E., Neel B. G., Swanson K. D. (2012) Macrophages require Skap2 and Sirpalpha for integrin-stimulated cytoskeletal rearrangement. J. Cell Science 125, 5535–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garton A. J., Flint A. J., Tonks N. K. (1996) Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol. Cell. Biol. 16, 6408–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sastry S. K., Lyons P. D., Schaller M. D., Burridge K. (2002) PTP-PEST controls motility through regulation of Rac1. J. Cell Science 115, 4305–4316 [DOI] [PubMed] [Google Scholar]
- 52. Harada T., Chelala C., Crnogorac-Jurcevic T., Lemoine N. R. (2009) Genome-wide analysis of pancreatic cancer using microarray-based techniques. Pancreatology 9, 13–24 [DOI] [PubMed] [Google Scholar]