Background: TRPM5 channel is a monovalent cation channel activated by intracellular Ca2+.
Results: TRPM5 was inhibited by extracellular Zn2+. The inhibition involves His-896, Glu-926, and Glu-939.
Conclusion: TRPM5 is inhibited by physiological concentrations of Zn2+ through interaction with the pore-loop domain.
Significance: Zn2+ is a TRPM5 inhibitor and the inhibition might be related to its physiological functions.
Keywords: Calcium, Ion Channels, Patch Clamp Electrophysiology, TRP Channels, Zinc
Abstract
The transient receptor potential melastatin 5 (TRPM5) channel is a monovalent cation channel activated by intracellular Ca2+. Expression of this channel is restricted to taste cells, the pancreas and brainstem, and is thought to be involved in controlling membrane potentials. Its endogenous ligands are not well characterized. Here, we show that extracellular application of Zn2+ inhibits TRPM5 activity. In whole-cell patch-clamp recordings, extracellular application of ZnCl2 inhibited step-pulse-induced TRPM5 currents with 500 nm free intracellular Ca2+ in a dose-dependent manner (IC50 = 4.3 μm at −80 mV). ZnSO4 also inhibited TRPM5 activity. Extracellular application of ZnCl2 inhibited TRPM5 activation at several temperatures. Furthermore, inhibition by 30 μm ZnCl2 was impaired in TRPM5 mutants in which His at 896, and Glu at 926 and/or Glu at 939 in the outer pore loop were replaced with Gln. From these results, we conclude that extracellular Zn2+ inhibits TRPM5 channels, and the residues in the outer pore loop of TRPM5 are critically involved in the inhibition.
Introduction
Transient receptor potential melastatin 5 (TRPM5)3 is a cation-permeable channel. Whereas monovalent cations are permeable, divalent cations are not. TRPM5 is thought to function as a tetramer of each subunit containing intracellular N and C termini, 6 transmembrane (TM) domains, and a pore-forming loop between TM5 and TM6 like other TRP channels (1, 2). This channel is restrictedly expressed in taste cells, the pancreas, and the brainstem, and is thought to be involved in controlling membrane potentials (2). Some reports have shown its physiological functions. TRPM5 activity in pancreatic β-cells controls insulin secretion by modulating the oscillation frequency in the membrane potentials (3). TRPM5 activation in taste cells potentiates sweet taste signals by depolarizing the cells downstream of taste receptor activation (4). These functions are thought to result from activation of TRPM5 mediated by increases in intracellular Ca2+ concentrations ([Ca2+]i). Although most TRP channels have endogenous ligands, there are few reports about endogenous stimuli or inhibitors of TRPM5 except for intracellular Ca2+, PIP2, and increased temperature (stimuli), and extracellular acidification (an inhibitor) (5–8).
Zinc is one of the most abundant metals in organisms. Zinc is an important nutrient and the recommended dietary allowance is 11 mg/day for men and 8 mg/day for women in the United States (9). Zinc deficiency affects about two billion people in the developing world and is associated with many diseases. In children, it causes growth retardation, delayed sexual maturation, susceptibility to infection, and diarrhea, which contributes to the death of about 800,000 children per year worldwide. Zinc is also the only metal used in all enzyme classes including DNA polymerase and alcohol dehydrogenase (10, 11). In addition, many reports have shown that zinc modulates a variety of ion channels such as the NMDA receptor, voltage-gated Ca2+ channels, P2X, voltage-gated K+ channels, two-pore K+ channels, ASIC channel, and epithelial Na+ channels (12–17). These findings suggest that zinc could have more functions than previously thought. Some of the TRP channels were reported to be modulated by Zn2+ or to be involved in zinc-related physiological functions. For example, TRPA1 is activated by about 20 μm ZnCl2, and TRPM1 is inhibited by high concentrations (mm) of ZnCl2 (18, 19). TRPM2 is inactivated by more than 30 μm ZnCl2, and Zn2+ entering cells through TRPM7 channel is involved in Zn2+-mediated neuronal injury (20, 21). In this study, we found extracellular Zn2+ to be a potential blocker of TRPM5 activity. Furthermore, we determined the amino acid residues required for the inhibition.
MATERIALS AND METHODS
Cell Culture
Human embryonic kidney-derived 293 (HEK293) cells were maintained in DMEM (WAKO Pure Chemical Industries, Ltd., Osaka, Japan) containing 10% FBS (Biowest SAS, Caille, France), 100 units/ml penicillin (Invitrogen), 100 mg/ml streptomycin (Invitrogen), and 2 mm l-glutamine (GlutaMAX, Invitrogen) at 37 °C in 5% CO2. For patch-clamp recordings, 1 μg of plasmid DNA containing mouse TRPM5 (TRPM5, a gift from Assoc. Prof. Misaka, Tokyo University, Japan) in pME18S and 0.1 μg pGreen Lantern 1 cDNA in OPTI-MEM medium (Invitrogen) were transfected to HEK293 cells using Lipofectamine Plus Reagent (Invitrogen). Mock-transfected cells were prepared by using pME18S (empty plasmid vector). After incubating for 3 to 4 h, cells were reseeded on coverslips and further incubated at 37 °C in 5% CO2. Whole-cell patch-clamp recordings were performed 1 day after transfection.
Construction of TRPM5 Mutants
The mutants of mTRPM5 were made using a modified QuickChange Site-directed Mutagenesis method (Stratagene). In detail, PCR was performed using mTRPM5 expression vectors as templates, two synthetic oligonucleotide primers containing specific mutations (supplemental Table S1), and primeSTAR HS DNA polymerase (Takara Bio Inc., Shiga, Japan). The PCR products were digested with Dpn1 at 37 °C for 1 h and transformed into DH5α competent cells. Double mutants other than E923Q/E926Q mutant were made by using single mutants as templates. The entire sequence including the desired substitution in the mutants was confirmed.
Electrophysiology
HEK293 cells on coverslips were mounted in an open chamber (Warner Instruments LLC) and superfused with a standard bath solution containing 150 mm NaCl, 5 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES (pH 7.4 with NaOH). Zn2+-free bath solution was made by adding 10 μm diethylenetriaminepentaacetic acid (Sigma), a membrane impermeable zinc chelator. The pipette solution contained 50 mm NaCl, 100 mm NMDG, 1.55 mm CaCl2, 2 mm EGTA, and 10 mm HEPES (pH 7.2 with HCl). It was adjusted to a free Ca2+ concentration of 500 nm (calculated by CaBuf; www.kuleuven.be/fysio/trp/cabuf). For the experiments in which Na+ concentrations were changed, we used a standard bath solution and a low Na+ bath solution containing 5 mm NaCl, 145 mm NMDG, 5 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES (pH 7.4 with HCl). For these experiments, 150 mm Na+ or 5 mm Na+ pipette solutions were used. The 150 mm Na+ pipette solution contained 150 mm NaCl, 1.55 mm CaCl2, 2 mm EGTA, and 10 mm HEPES (pH 7.4 with NaOH), and the 5 mm Na+ pipette solution contained 5 mm NaCl, 145 mm NMDG, 1.55 mm CaCl2, 2 mm EGTA, and 10 mm HEPES (pH 7.4 with HCl). Data from whole-cell voltage-clamp recordings were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Axon Instruments). The temperature of the solutions perfusing the cell was controlled by an in-line heater (Warner Instruments, LLC). The membrane potential was clamped at +25 mV and voltage step-pulses from −160 to +160 mV (200 ms) were applied every second. Ramp-pulses from −100 to +100 mV for 500 ms were applied every 5 s. All experiments other than the experiment changing temperature were performed at room temperature.
Statistical Analysis
Data are expressed as means ± S.E. When analyzing dose-response profiles, the data were fit into a logistic curve by using Origin 8.6 software, using the formula: y = A2 + (1 − A2)/(1 + (x/x0)^p) Statistical analysis was performed by Student's t test or one-way analysis of variance (ANOVA) followed by a two-tailed multiple t test with Bonferroni correction. p values less than 0.05 were considered significant.
RESULTS
First, we examined whether zinc ion inhibited TRPM5 using whole-cell patch-clamp recordings. Because TRPM5 is an intracellular Ca2+-activated channel, we used a pipette solution containing 500 nm free Ca2+ to check the inhibition by ZnCl2. In HEK293 cells expressing TRPM5 (but not in mock-transfected cells), step-pulses with 500 nm free intracellular Ca2+ activated currents with slowly activated large outward components and rapidly-desensitized inward components (Fig. 1A). Extracellular application of 20 μm ZnCl2 reduced both outward and inward currents, and this inhibition was partially recovered after washout of ZnCl2 (Fig. 1A). The recovery was observed to a lesser extent after application of high concentrations (more than 30 μm) of ZnCl2 (data not shown). Next, we established a dose-response curve of ZnCl2-induced inhibition of TRPM5, using 0.1 to 100 μm of ZnCl2. TRPM5 activity was inhibited in a dose-dependent manner, similar at both positive and negative potentials, and the dose-dependent curves could be fit to a logistic function with IC50 values of 4.3 ± 3.0 μm at −80 mV and 4.3 ± 0.7 μm at +160 mV (Fig. 1B). In addition, we analyzed the peak currents with or without 30 μm ZnCl2 at each membrane potential. As shown in Fig. 1C, significant inhibition of TRPM5 currents by 30 μm ZnCl2 was observed at both positive and negative membrane potentials, indicating that the inhibition by ZnCl2 was independent of membrane potentials. Next we investigated whether Zn2+ acted as a pore blocker. We examined the effect of changing the extracellular and intracellular Na+ concentrations on the inhibition of TRPM5 channel by extracellular ZnCl2 at a concentration of 30 μm. When both extracellular and intracellular Na+ concentrations were 150 mm, we observed TRPM5 currents with an outwardly rectifying current-voltage relationship by 500 nm [Ca2+]i, and the currents were inhibited by 30 μm ZnCl2 without voltage-dependence (Fig. 2A) as in the step-pulse protocol (Fig. 1C). When the intracellular or extracellular Na+ concentration was reduced to 5 mm, similar TRPM5-mediated currents with outward rectification were observed, and the currents were inhibited by 30 μm ZnCl2, similarly without voltage dependence (Fig. 2, B and C). The data indicated that the changes in extracellular and intracellular Na+ concentrations did not affect the inhibition of the TRPM5 channel by 30 μm extracellular ZnCl2. Such voltage-independent inhibition of TRPM5 currents by 30 μm extracellular ZnCl2 was more clearly shown when the extent of inhibition was plotted at each membrane potential (Fig. 2D). Reversal potentials were as follows: (A) −4.0 ± 3.4 mV in 150 mm Na+ intra/150 mm Na+ extra, (B) 26.0 ± 4.0 mV in 5 mm Na+ intra/150 mm Na+ extra, and (C) −33.9 ± 3.4 mV in 150 mm Na+ intra/5 mm Na+ extra, and they were somehow not well shifted as a Na+-selective channel.
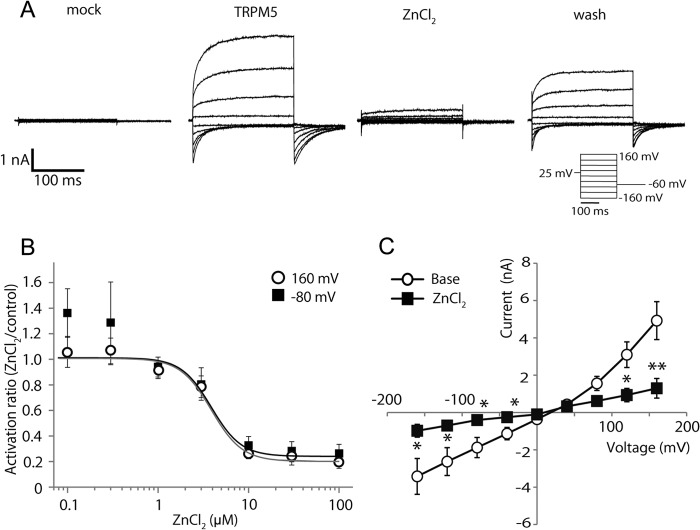
FIGURE 1.
Extracellular application of Zn2+ inhibits TRPM5 activation. A, representative traces of whole-cell currents stimulated by step-pulses with intracellular 500 nm Ca2+ in mock-transfected (mock) and TRPM5-expressing HEK293 cells at 25 °C. 20 μm ZnCl2 reduced the currents in a HEK293 cell expressing TRPM5 (ZnCl2). The inhibition was partially recovered after wash out (wash). The inset shows the step-pulse protocol. B, dose-response profiles of the inhibitory effect of ZnCl2 on TRPM5 activation at +160 mV and −80 mV potentials. Gray and black lines indicate logistic curves fitted for the data at 160 mV and −80 mV, respectively. Each symbol represents the mean ± S.E. from 5 to 8 cells. C, current-voltage relationship of the TRPM5 currents in the presence and absence (Base) of 30 μm ZnCl2. TRPM5 currents evoked by a step-pulse protocol shown in the inset of Fig. 1A. Each symbol represents the mean ± S.E. from 8 cells. *, p < 0.05; **, p < 0.01.
FIGURE 2.
Extracellular and intracellular Na+ concentrations do not alter the inhibition of TRPM5 activity by extracellular ZnCl2. A–C, representative current-voltage curves from the 500 nm free Ca2+-induced currents recorded in HEK293 cells expressing TRPM5 with different intracellular (intra) and extracellular (extra) Na+ concentrations. D, mean fraction of the remaining currents (IZnCl2/Ibase) at different voltages following application of 30 μm ZnCl2. Each symbol represents the mean ± S.E. from 4 or 5 cells.
Next, to determine whether TRPM5 inhibition mediated by ZnCl2 was due to the effects of Zn2+ itself, we examined the effect of another zinc compound, ZnSO4, on TRPM5 activity. As shown in Fig. 3, 30 μm ZnSO4 significantly inhibited TRPM5 activity at both positive and negative membrane potentials as did 30 μm ZnCl2.
FIGURE 3.
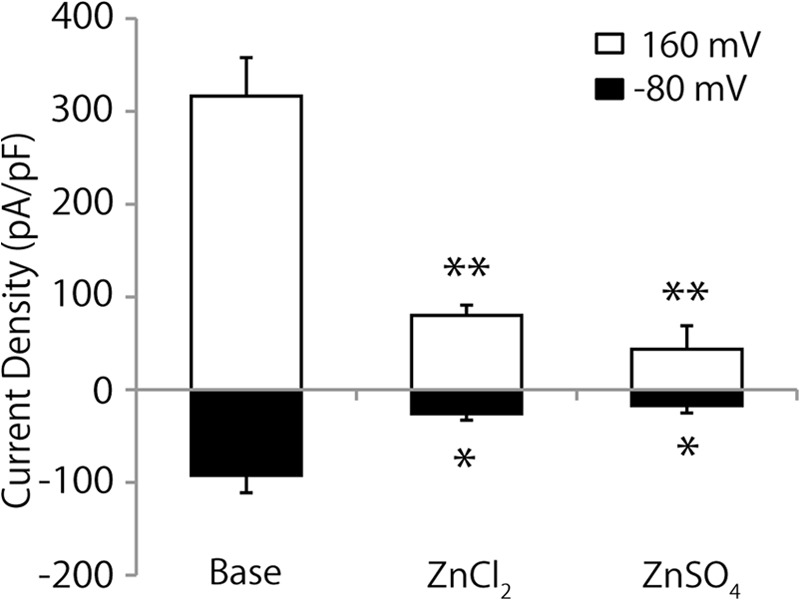
Extracellular Zn2+ ion inhibits TRPM5 activation. TRPM5 activation was significantly inhibited by both 30 μm ZnCl2 and 30 μm ZnSO4 at positive (160 mV) and negative (−80 mV) potentials. Each bar represents the mean ± S.E. from 8 to 16 cells. *, p < 0.05; **, p < 0.01.
It is known that TRPM5 has thermosensitivity and its activity is potentiated by temperature increases (8). Therefore, we checked the effect of Zn2+ on TRPM5 activity at different temperatures. The experimental data obtained at room temperature (about 25 °C) (Figs. 1, 2, and 3) might contain a temperature-dependent component of TRPM5 activity, as shown in previous study (8). Thus, we examined TRPM5 activity at higher (32 °C) and lower (20 °C) temperatures. In this experiment, we also used a pipette solution with 500 nm [Ca2+]i. As shown in Fig. 4, TRPM5 activation by 500 nm [Ca2+]i at both 20 °C and 32 °C was inhibited by 30 μm ZnCl2 in HEK293 cells expressing TRPM5. These results indicated that Zn2+ inhibited the temperature effects on TRPM5.
FIGURE 4.

Extracellular application of ZnCl2 inhibits TRPM5 at different temperature condition. A, representative traces of whole-cell currents at 32 °C in HEK293 cell expressing TRPM5. This activation was inhibited by extracellular application of 30 μm ZnCl2. B, TRPM5-mediated currents at 25 °C and 32 °C were significantly larger than that at 20 °C. Extracellular application of 30 μm ZnCl2 inhibited TRPM5 activation at 20 °C, 25 °C, and 32 °C. Each bar represents the mean ± S.E. for 7 to 9 cells. *, p < 0.05; **, p < 0.01 versus control. ##, p < 0.01 versus 20 °C.
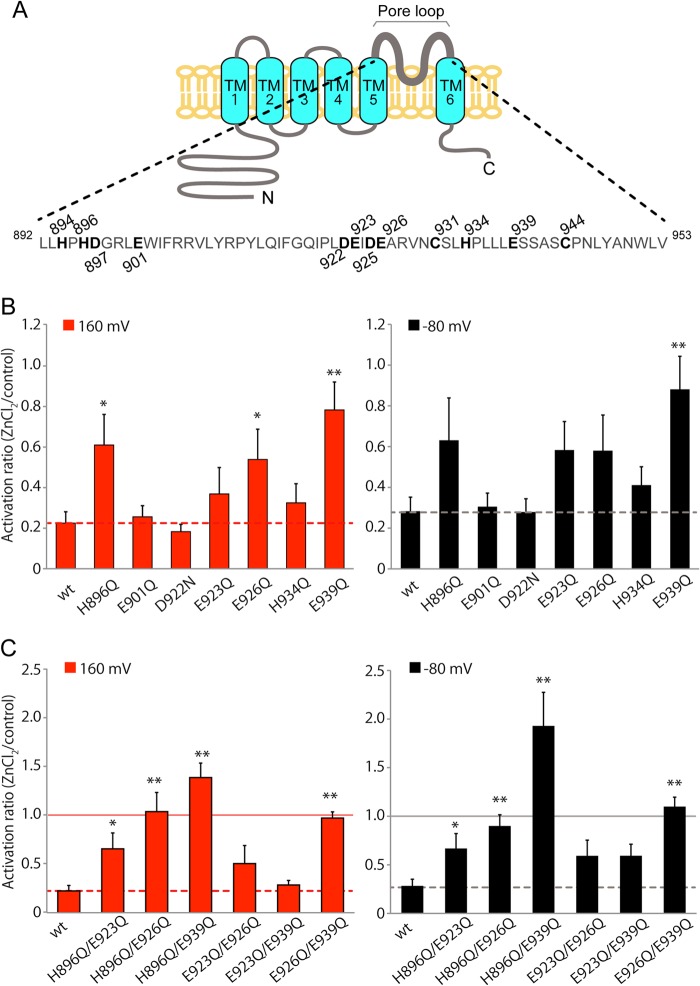
To clarify the amino acids involved in Zn2+-mediated inhibition of TRPM5 activity, we examined the effect of Zn2+ on TRPM5 mutants. We hypothesized that Zn2+ interacted with extracellular domains of TRPM5 because 1) extracellular Zn2+ inhibits TRPM5 activity and 2) divalent cations are not permeable to TRPM5. In addition, many reports have shown amino acid residues such as His, Cys, Lys, Asp, and Glu interact with Zn2+ (11, 16, 22). Based on these facts, we constructed TRPM5 mutants in which His, Cys, Lys, Asp, and Glu in the outer pore loop, the largest extracellular domain, were mutated. As shown in Fig. 5A, there are twelve such amino acids (His, Cys, Asp, and Glu) in the outer pore loop of TRPM5. First, we constructed single mutants in which Gln was substituted for His and Glu (H894Q, H896Q, E901Q, E923Q, E926Q, and E939Q), and Asn was substituted for Asp (D897N, D922N, and D925N), or Ser for Cys (C931S and C944S). Unfortunately, we had to exclude H894Q, D897N, D925N, C931S and C944S from analysis because these mutants showed no current activation by step-pulses with intracellular 500 nm Ca2+ (data not shown). In the single mutants analyzed, inhibition of TRPM5 by 30 μm ZnCl2 was significantly impaired in H896Q, E926Q, and E939Q mutants at +160 mV (Fig. 5B). Some reduction in ZnCl2-induced inhibition was observed in the E923Q mutant although it was not statistically significant (Fig. 5B). Then, we focused on H896, E923, E926, and E939, and constructed double mutants (H896Q/E923Q, H896Q/E926Q, H896Q/E939Q, E923Q/E926Q, E923Q/E939Q, E926Q/E939Q). As shown in Fig. 5C, inhibition of TRPM5 at both +160 mV and −80 mV by 30 μm ZnCl2 was completely lost in H896Q/E926Q and E926Q/E939Q mutants. In the H896Q/E939Q mutant, TRPM5 activity was even increased after application of ZnCl2 especially at −80 mV.
FIGURE 5.
His and Glu residues in the pore region are necessary for Zn2+-mediated inhibition of TRPM5 activation. A, candidate residues for Zn2+ interaction in the outer pore region. Top: schematic diagram. Bottom: amino acids in the region. TM: transmembrane domain. B, summary of the residual currents upon exposure to 30 μm extracellular ZnCl2 in HEK293 cells expressing single mutants of TRPM5, expressed as the ratio of the currents to those before application of ZnCl2, at +160 mV (left) and −80 mV (right) potentials. Dotted lines indicate the ratio of residual currents in wild-type TRPM5 channel (wt). Each bar represents the mean ± S.E. from 6 to 8 cells. *, p < 0.05; **, p < 0.01. C, summary of the residual currents upon exposure to 30 μm extracellular ZnCl2 in HEK293 cells expressing double mutants of TRPM5, expressed as the ratio of the currents to those before application of ZnCl2, at +160 mV (left) and −80 mV (right) potentials. Solid and dotted lines indicate the ratios of 1.0 and residual currents in wild-type TRPM5 channel (wt). Each bar represents the mean ± S.E. for 6 to 8 cells. *, p < 0.05; **, p < 0.01.
DISCUSSION
TRPM5 is a monovalent cation permeable channel and its activation can modulate membrane potentials. A proposed endogenous activator is intracellular Ca2+, and some molecules such as PIP2 enhance TRPM5 activity. However, inhibitors of TRPM5 are not known. In this study, we found an endogenous inhibitor, Zn2+. Zn2+-mediated inhibition was observed in the μm range. Under physiological conditions, serum zinc concentrations are about 14 μm (23). However, the precise concentration of free Zn2+ is not known because Zn2+ binds to many proteins in plasma, such as albumin and transferrin. In addition, many reports have indicated that release of vesicular Zn2+ from pre-synapses might cause a transient increase in Zn2+ concentrations from one to 100 μm in the brain (24). Given these facts, free Zn2+ concentrations in vivo could be high enough to modulate TRPM5 activity under physiological condition.
We identified the Zn2+-interacting sites as His at 896 and Glu at 926 and 939 in the outer pore loop of mouse TRPM5. TRPA1, which is activated by extracellular Zn2+, reportedly interacts with Zn2+ in its intracellular domain (18). In the report, they concluded that Zn2+ entered the cell through TRPA1 channels from the extracellular space and activated TRPA1. Intracellular Zn2+ levels are thought to be controlled by Zn2+ movement through TRPM7 (20). However, TRPM5 is a divalent cation-impermeable channel (25), suggesting that Zn2+ acts on the extracellular domains of TRPM5. One report showed that extracellular acidification inhibits mouse TRPM5 through interaction with the extracellular domain (Glu at 830 in TM3–4 linker and His at 934 in the pore loop) (5), different from those involved in Zn2+ action in our study. The Zn2+-mediated inhibition of TRPM5 was only partially reversed after wash out. A similar phenomenon was observed in the inhibition of TRPM5 by protons (5). Protons irreversibly inhibited TRPM5 activity, and His at 896, which is a critical amino acid residue for TRPM5 inhibition by Zn2+, was also critically involved in the reversibility of TRPM5 inhibition by protons. Although the same His residue was involved in the action of both Zn2+ and protons, the mechanisms of inhibition might be quite different. The fact that the involvement of Glu at 926 is close to the putative selective filter (26, 27) raises the possibility that Zn2+ could act as a pore blocker. Pore blockers generally act in a membrane potential-dependent manner and the inhibition could be modulated by permeating ions. In this study, we found that the inhibition of TRPM5 activity by Zn2+ did not depend on membrane potentials and was not modulated by changes in either extracellular or intracellular Na+ concentrations. These results suggest that Zn2+ is not a channel pore blocker.
TRPM5 is restricted in its distribution to taste cells, the pancreas and the brain stem in mammals. Zn2+ is reportedly released from pre-synapses like a transmitter in neurons and pancreatic β-cells in response to insulin. Zn2+ released by glutamate following pre-synapse activation could modulate synaptic transduction through the modulation of receptors such as the NMDA receptor (12, 24). In the case of the pancreas, Zn2+ released from β-cells might modulate glucagon secretion from pancreatic α-cells after increases in blood glucose levels (28, 29). Therefore, it would be intriguing to speculate that modulation of TRPM5 by Zn2+ in neurons and the pancreas in an autocrine or paracrine manner could have important roles under physiological conditions. Recently, one report showed that TRPM5 might be involved in cholecystokinin secretion stimulated by linoleic acid in an enteroendocrine cell line (30), suggesting the possibility that TRPM5 could have more physiological functions than those reported to date.
In conclusion, we found that Zn2+ is an effective inhibitor of TRPM5. Furthermore, His and Glu residues in the pore-loop domain are critical for the inhibition. Such inhibition by Zn2+ could be important for the biological or physiological functions of TRPM5.
Acknowledgments
We thank N. Fukuta of the National Institute for Physiological Sciences (NIPS) for technical assistance.

This article contains supplemental Table S1.
- TRPM
- transient receptor potential melastatin
- TM
- transmembrane.
REFERENCES
- 1. Enklaar T., Esswein M., Oswald M., Hilbert K., Winterpacht A., Higgins M., Zabel B., Prawitt D. (2000) Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics 67, 179–187 [DOI] [PubMed] [Google Scholar]
- 2. Liman E. R. (2007) TRPM5 and taste transduction. in Handbook of Experimental Pharmacology (Veit Flockerzi B. N. ed), Volume 179, pp. 287–298, Springer; Berlin Heidelberg: [DOI] [PubMed] [Google Scholar]
- 3. Colsoul B., Schraenen A., Lemaire K., Quintens R., Van Lommel L., Segal A., Owsianik G., Talavera K., Voets T., Margolskee R. F., Kokrashvili Z., Gilon P., Nilius B., Schuit F. C., Vennekens R. (2010) Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc. Natl. Acad. Sci. U.S.A. 107, 5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pérez C. A., Huang L., Rong M., Kozak J. A., Preuss A. K., Zhang H., Max M., Margolskee R. F. (2002) A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 5, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 5. Liu D., Zhang Z., Liman E. R. (2005) Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3-S4 and S5-S6 extracellular domains. J. Biol. Chem. 280, 20691–20699 [DOI] [PubMed] [Google Scholar]
- 6. Liu D., Liman E. R. (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. U.S.A. 100, 15160–15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prawitt D., Monteilh-Zoller M. K., Brixel L., Spangenberg C., Zabel B., Fleig A., Penner R. (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc. Natl. Acad. Sci. U.S.A. 100, 15166–15171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talavera K., Yasumatsu K., Voets T., Droogmans G., Shigemura N., Ninomiya Y., Margolskee R. F., Nilius B. (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025 [DOI] [PubMed] [Google Scholar]
- 9. Lindeman R. D. (2009) in Handbook of Clinical Nutrition and Aging (Connie W., Bales C. S. R., ed), pp. 650, 2nd Ed., Springer [Google Scholar]
- 10. Kusakabe T., Richardson C. C. (1996) The role of the zinc motif in sequence recognition by DNA primases. J. Biol. Chem. 271, 19563–19570 [DOI] [PubMed] [Google Scholar]
- 11. Billeter S. R., Webb S. P., Agarwal P. K., Iordanov T., Hammes-Schiffer S. (2001) Hydride transfer in liver alcohol dehydrogenase: quantum dynamics, kinetic isotope effects, and role of enzyme motion. J. Am. Chem. Soc. 123, 11262–11272 [DOI] [PubMed] [Google Scholar]
- 12. Nozaki C., Vergnano A. M., Filliol D., Ouagazzal A. M., Le Goff A., Carvalho S., Reiss D., Gaveriaux-Ruff C., Neyton J., Paoletti P., Kieffer B. L. (2011) Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat. Neurosci. 14, 1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke C. E., Veale E. L., Green P. J., Meadows H. J., Mathie A. (2004) Selective block of the human 2-P domain potassium channel, TASK-3, and the native leak potassium current, IKSO, by zinc. J. Physiol. 560, 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J., Winarski K. L., Myerburg M. M., Pitt B. R., Sheng S. (2012) Probing the structural basis of Zn2+ regulation of the epithelial Na+ channel. J. Biol. Chem. 287, 35589–35598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alvarez-Collazo J., Díaz-Garcia C. M., López-Medina A. I., Vassort G., Alvarez J. L. (2012) Zinc modulation of basal and β-adrenergically stimulated L-type Ca2+ current in rat ventricular cardiomyocytes: consequences in cardiac diseases. Pflugers Arch 464, 459–470 [DOI] [PubMed] [Google Scholar]
- 16. Liu X., Surprenant A., Mao H. J., Roger S., Xia R., Bradley H., Jiang L. H. (2008) Identification of key residues coordinating functional inhibition of P2X7 receptors by zinc and copper. Mol. Pharmacol. 73, 252–259 [DOI] [PubMed] [Google Scholar]
- 17. Teisseyre A., Mozrzymas J. W. (2002) Inhibition of the activity of T lymphocyte Kv1.3 channels by extracellular zinc. Biochem. Pharmacol 64, 595–607 [DOI] [PubMed] [Google Scholar]
- 18. Hu H., Bandell M., Petrus M. J., Zhu M. X., Patapoutian A. (2009) Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 5, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambert S., Drews A., Rizun O., Wagner T. F., Lis A., Mannebach S., Plant S., Portz M., Meissner M., Philipp S. E., Oberwinkler J. (2011) Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J. Biol. Chem. 286, 12221–12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inoue K., Branigan D., Xiong Z. G. (2010) Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J. Biol. Chem. 285, 7430–7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang W., Manna P. T., Zou J., Luo J., Beech D. J., Sivaprasadarao A., Jiang L. H. (2011) Zinc inactivates melastatin transient receptor potential 2 channels via the outer pore. J. Biol. Chem. 286, 23789–23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathie A., Sutton G. L., Clarke C. E., Veale E. L. (2006) Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 111, 567–583 [DOI] [PubMed] [Google Scholar]
- 23. Terrés-Martos C., Navarro-Alarcón M., Martín-Lagos F., López G., de la Serrana H., Pérez-Valero V., López-Martinez M. C. (1998) Serum zinc and copper concentrations and Cu/Zn ratios in patients with hepatopathies or diabetes. J. Trace Elem. Med. Biol. 12, 44–49 [DOI] [PubMed] [Google Scholar]
- 24. Paoletti P., Vergnano A. M., Barbour B., Casado M. (2009) Zinc at glutamatergic synapses. Neuroscience 158, 126–136 [DOI] [PubMed] [Google Scholar]
- 25. Hofmann T., Chubanov V., Gudermann T., Montell C. (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr. Biol. 13, 1153–1158 [DOI] [PubMed] [Google Scholar]
- 26. Tóth B., Csanády L. (2012) Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents. Proc. Natl. Acad. Sci. U.S.A. 109, 13440–13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nilius B., Prenen J., Janssens A., Owsianik G., Wang C., Zhu M. X., Voets T. (2005) The selectivity filter of the cation channel TRPM4. J. Biol. Chem. 280, 22899–22906 [DOI] [PubMed] [Google Scholar]
- 28. Slucca M., Harmon J. S., Oseid E. A., Bryan J., Robertson R. P. (2010) ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 59, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qian W. J., Peters J. L., Dahlgren G. M., Gee K. R., Kennedy R. T. (2004) Simultaneous monitoring of Zn2+ secretion and intracellular Ca2+ from islets and islet cells by fluorescence microscopy. BioTechniques 37, 922–924, 926,, 928–930 [DOI] [PubMed] [Google Scholar]
- 30. Shah B. P., Liu P., Yu T., Hansen D. R., Gilbertson T. A. (2012) TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am. J. Physiol. Cell Physiol. 302, C210–C219 [DOI] [PMC free article] [PubMed] [Google Scholar]