Background: Heparan sulfate proteoglycan (HSPG) co-receptors modulate VEGFA signaling.
Results: MicroRNA-24 targets NDST1 to reduce HS sulfation and HS affinity for VEGFA, suppressing VEGFA signaling and endothelial cell migration.
Conclusion: MicroRNAs targeting HS biosynthesis can regulate VEGFA-induced chemotaxis, essential during angiogenesis.
Significance: HSPG-dependent signaling of pathophysiological importance can be targeted via microRNA interference with HS biosynthesis.
Keywords: Angiogenesis, Biosynthesis, Heparan Sulfate, MicroRNA, Vascular Endothelial Growth Factor (VEGF), NDST1, miR-24
Abstract
Heparan sulfate (HS) proteoglycans, present at the plasma membrane of vascular endothelial cells, bind to the angiogenic growth factor VEGFA to modulate its signaling through VEGFR2. The interactions between VEGFA and proteoglycan co-receptors require sulfated domains in the HS chains. To date, it is essentially unknown how the formation of sulfated protein-binding domains in HS can be regulated by microRNAs. In the present study, we show that microRNA-24 (miR-24) targets NDST1 to reduce HS sulfation and thereby the binding affinity of HS for VEGFA. Elevated levels of miR-24 also resulted in reduced levels of VEGFR2 and blunted VEGFA signaling. Similarly, suppression of NDST1 using siRNA led to a reduction in VEGFR2 expression. Consequently, not only VEGFA binding, but also VEGFR2 protein expression is dependent on NDST1 function. Furthermore, overexpression of miR-24, or siRNA-mediated reduction of NDST1, reduced endothelial cell chemotaxis in response to VEGFA. These findings establish NDST1 as a target of miR-24 and demonstrate how such NDST1 suppression in endothelial cells results in reduced responsiveness to VEGFA.
Introduction
It is well established that proteoglycans (PGs)2 carrying heparan sulfate (HS) polysaccharide chains are required for angiogenesis, the process whereby new blood vessels are formed from pre-existing vasculature (1–4). HSPGs act as co-receptors for a number of secreted proangiogenic signaling proteins. Sulfated motifs present in the HS chains bind mainly via electrostatic interactions to positively charged amino acid residues at the surface of protein ligands. HSPGs have been shown to bind vascular endothelial growth factor (VEGFA) to modulate signaling through VEGF-receptor 2 (VEGFR2) (1, 5, 6).
Biosynthesis of HS involves stepwise addition of alternating glucuronic acid (GlcUA) and N-acetylglucosamine (GlcNAc) units to acceptor linkage region oligosaccharides covalently attached to PG core proteins (7). During polymerization, the HS chains are modified by the action of several biosynthetic enzymes residing in the Golgi compartment. A key step in HS biosynthesis is the early N-deacetylation and N-sulfation of GlcNAc residues in the HS chain, catalyzed by the bifunctional N-deacetylase/N-sulfotransferases (NDSTs). Four NDSTs (NDST1-4) have been identified, and between these, NDST1 and NDST2 display the broadest tissue distribution (8). The actions of the NDSTs are generally incomplete, resulting in HS chains having a highly variable pattern of N-sulfated regions separated by N-acetylated regions. The N-sulfation step is followed by C5-epimerization of GlcUA to iduronic acid (IdoUA) and O-sulfation at various positions. Because of the substrate specificities of the biosynthetic enzymes involved, epimerization and O-sulfation of the HS chain occur predominantly in regions rich in N-sulfation. The highly modified N-sulfated regions mediate most interactions with protein ligands (9).
MicroRNAs (miRNAs) are small, endogenous, noncoding RNAs involved in posttranscriptional regulation of gene expression (10). miRNAs may mediate either degradation of target mRNAs or repression of mRNA translation (11, 12). Several recent studies have demonstrated important roles for miRNAs in endothelial cells and vascular development (13–16). However, little is known about how miRNAs may regulate the structure of HS chains and the functions of HSPG co-receptors, which are required for proper signaling of many growth factors in endothelial cells (17–19).
Here, we identified miR-24 as a potential suppressor of NDST1 expression, a key enzyme in HS biosynthesis vital for the formation of sulfated protein-binding HS domains. We confirm that miR-24 does suppress NDST1 and decreases the level of HS sulfation. This resulted in reduced affinity of HS for VEGFA, which was accompanied by reduced levels of VEGFR2 and blunted VEGFA signaling. Endothelial cell chemotaxis in response to VEGFA gradients was suppressed both by miR-24 and by siRNA-mediated suppression of NDST1, underlining the key role of NDST1 in HS biosynthesis and angiogenic growth factor signaling.
EXPERIMENTAL PROCEDURES
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were cultured in EBM-2 medium supplemented with EGM-2-MV bullet kit (Lonza Group Ltd.). VEGFA-165 (PeproTech) was used to stimulate cells. Cells were used at passages 2–6.
Cell Transfection with Pre-miRNAs, Anti-miRNAs, and siRNA
2 μg of synthetic mature miR-24 double-stranded RNA (dsRNA; pre-miR-24; Applied Biosystems) or 2 μg of negative control dsRNA (pre-miR negative control; Applied Biosystems) was used to transfect ∼700,000 HUVECs by electroporation using either the Nucleofector system (Amaxa Inc.) and the basic endothelial cell kit (Lonza) or the Ingenio electroporation solution (Mirus Bio LLC). Similarly, cells were transfected with 3 μg of pre-miR-23a or 3 μg of negative control dsRNA, 3 μg of anti-miR-24 or 3 μg of anti-miR negative control, or 4 μg of anti-miR-23a or 4 μg of anti-miR negative control, all acquired from Applied Biosystems. siRNA targeting human NDST1 (catalog number 4390824) was obtained from Invitrogen.
Real-time qPCR
miRNeasy mini spin columns (Qiagen) were used for RNA isolation. 300 ng of total RNA was used for cDNA synthesis (miScript reverse transcription kit, Qiagen), and miRNA expression was determined using miScript primer assays (Qiagen); qRT-PCR reactions were carried out using SSO Fast EvaGreen supermix (Bio-Rad) on a 7900HT real-time PCR system (Bio-Rad). mRNA levels were determined 48 h after transfection using the following primers: Ndst1 (forward 5′-ctcgaagctgcgtgccctc-3′ and reverse 5′-atgtccatcactttggcaggttctg-3′) and GAPDH (forward 5′-atgggtgtgaaccatgagaagta-3′ and reverse 5′-ggcagtgatggcatggac-3′). hsa-miR-24 (5′-tggctcagttcagcaggaacag-3′), hsa-miR-9 (5′-tctttggttatctagctgtatga-3′), and hsa-miR-23a (5′-atcacattgccagggatttcc-3′) were used as primers in the reverse transcription step to generate cDNA from mRNA isolated from HUVECs followed by qPCR analysis using the above described Ndst1 primers.
Luciferase Reporter Assays
Reporter assays were performed as described (20) with minor modifications. Briefly, HUVECs were co-transfected with 10 ng of miR-24 pMIR-REPORT vectors containing either the predicted miR-24 Ndst1 3′-UTR target site 1 or target site 2, along with 25 ng of pCMV β-gal reference plasmid and pre-miR-control or pre-miR-24 (100 pmol, respectively; Applied Biosystems). The single miR-23a-binding site was cloned into a pMIR-REPORT vector, and HEK293 cells were co-transfected with 10 ng of the miR-23a pMIR-REPORT vector along with 25 ng of pCMV β-gal reference plasmid and either negative control dsRNA or pre-miR-23a (10 pmol, respectively; Applied Biosystems). Cells were harvested after 48 h, and extracts were assayed for luciferase and β-galactosidase activities in a microplate luminometer/photometer reader (Wallac VICTOR 1420 multilabel counter; PerkinElmer). The luciferase signals were normalized to β-galactosidase activity and compared with control groups.
Bioinformatical Analysis
TargetScan (21) was used to identify miRNAs that have target sites in the Ndst1 3′-UTR.
N-Deacetylase Assay
The assay for measuring NDST N-deacetylase activity was carried out as described previously (22).
Isolation of HS and CS and Generation of HS and CS Disaccharide Species for Structural Analysis
Disaccharides were generated as described previously (23) from HS and chondroitin sulfate (CS) produced by HUVECs transfected with pre-miR-24 or control dsRNA. Per condition, disaccharides from two confluent 175-cm2 cell culture flasks were analyzed by RPIP-HPLC as described previously (24).
Metabolic Labeling of Endothelial HS
Metabolic labeling of HS with [3H]glucosamine (100 μCi/ml, specific activity 37 Ci/mmol, PerkinElmer) and analysis of HS chain length were carried out on a Superose 6 column (GE Healthcare) essentially as described previously (25).
Nitrocellulose Filter Binding Assay
The interaction between radiolabeled HS and VEGFA-165 (PeproTech) was probed by using a filter binding assay, previously described in detail by Kreuger et al. (26). The KD values were calculated using GraphPad Prism (La Jolla, CA) using the following parameters: nonlinear regression, binding saturation, and one-site specific binding.
Antibodies for Western Blotting
The primary antibodies used were: goat anti-VEGFR2 (R&D Systems; AF357), rabbit anti-phospho (Tyr-1175)-VEGFR2 (Cell Signaling; 2478), rabbit anti-phospho-AKT (Ser-473) (Cell Signaling; 9271), mouse anti-total-AKT (Cell Signaling; 2966), rabbit anti-phospho-ERK (Cell Signaling; 9101), and mouse anti-total-ERK (Cell Signaling; 9107). The secondary antibodies used were Alexa Fluor 680 (Invitrogen) and IR-dye 800 (Rockland). All membranes were scanned using the Odyssey Imaging System (LI-COR).
Endothelial Cell Chemotaxis in Response to VEGFA Gradients
The microfluidic cell migration assay was carried out as described (27, 28). HUVECs were transfected with either pre-miR-24 or pre-miR-control, and after 24 h, transferred to a 3-cm cell culture dish coated with type A gelatin (Sigma). Briefly, the cells were starved in serum-free cell medium containing 0.2% BSA for 12 h, and thereafter, the microfluidic device was assembled, and the cells were exposed to a stable hill-shaped gradient of VEGFA-165 (0–50 ng/ml over a distance of 400 μm) generated by diffusion. Cell migration was analyzed for 4 h by time-lapse microscopy using an Axiovert 200 microscope followed by cell tracking (Zeiss).
Statistical Analysis
Statistical significance was evaluated using the two-tailed Student's t test as the means of the parameters measured are assumed to be normally distributed and the variances are assumed to be equal.
RESULTS
A bioinformatical survey using TargetScan (21) was initially performed to identify miRNAs that may regulate NDST1 as well as other genes involved in HS biosynthesis. 14 different miRNAs were predicted to bind to the 3′-UTR of Ndst1 mRNA (Table 1). Among these miRNAs, miR-24 and miR-23a/b have been reported to be selectively expressed in endothelial cells of microvessels (20, 29) and were therefore selected for further analysis. miR-24 and miR-23a/b are located together with miR-27a in two separate clusters in the human genome on chromosome 9 and chromosome 19 and may thus be co-transcribed (30, 31). miR-24 was predicted to have two binding sites in the Ndst1 3′-UTR, whereas miR-23a/b had one. Based on these target predictions, we hypothesized that miR-24 and miR23a/b should have the capacity to reduce NDST1 function and thus decrease sulfation of HSPG co-receptors in endothelial cells.
TABLE 1.
MicroRNAs predicted to target NDST1
A summary of microRNAs predicted to target NDST1 is shown. Predictions and PCT values reflecting the probability of conserved targeting were achieved using TargetScan.
| MicroRNA | Number of conserved Ndst13′-UTR binding sites | Ranking based on PCT value |
|---|---|---|
| miR-34a | 3 | 0.98 |
| miR-24 | 2 | 0.94 |
| miR-29a | 1 | 0.92 |
| miR-124 | 1 | 0.92 |
| miR-137 | 1 | 0.89 |
| miR-128 | 2 | 0.85 |
| miR-200b | 1 | 0.75 |
| miR-23a/b | 1 | 0.72 |
| miR-140–5p | 1 | 0.64 |
| miR-1 | 1 | 0.5 |
| miR-96 | 1 | 0.47 |
| miR-183 | 1 | 0.36 |
| miR-191 | 1 | 0.35 |
| miR-7 | 1 | 0.3 |
miR-24 Targets Ndst1 mRNA
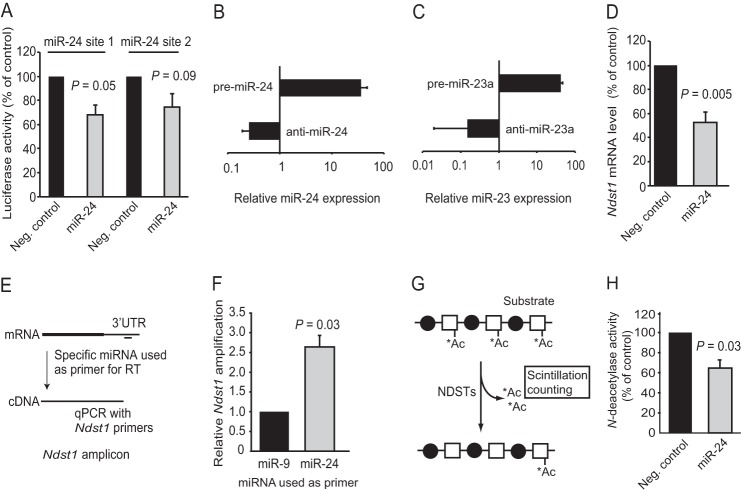
Two luciferase reporter vectors were constructed to initially investigate whether miR-24 can target NDST1. The vectors consisted of a CMV-luciferase reporter followed by a portion of the Ndst1 3′-UTR containing either of the two predicted binding sites for miR-24. Overexpression was achieved by transfection of HEK293 cells with synthetic mature pre-miR-24 dsRNA that was processed by the cells into functional miRNA (10), and miR-24 was shown to suppress luciferase activity (Fig. 1A).
FIGURE 1.
Regulation of NDST1 by miR-24. A, ∼60-bp regions containing either of the two conserved miR-24-binding sites present in the Ndst1 3′-UTR were individually cloned into pMIR-REPORT vectors. Luciferase activity was assayed after 48 h in cell lysates from HEK293 cells co-transfected with pMIR-REPORT vectors and either negative control (Neg. control) dsRNA or pre-miR-24. The luciferase signals were normalized to control groups. B, qPCR quantification of miR-24 levels in HUVECs after transfection with pre-miR-24 or anti-miR-24, respectively (n = 4). Values are presented as mean relative expression (log scale) compared with untransfected cells, and the expression levels were normalized against GAPDH mRNA. C, qPCR quantification of miR-23 levels in HUVECs after transfection with pre-miR-23a (n = 3) or anti-miR-23a (n = 2). D, HUVECs were transfected with pre-miR-24, and the level of Ndst1 mRNA was determined by qRT-PCR (n = 6). E, schematic overview of the PCR approach used in panel F to investigate the binding of miR-24 to the Ndst1 3′-UTR. miR-9 (not predicted to target NDST1) was included for comparison. G, outline of the assay used to measure NDST activity; N-deacetylase activity was detected by measuring the release of 3H-labeled acetyl (*Ac) groups using biphasic scintillation counting. H, effects on N-deacetylase activity in lysates from HUVECs transfected with pre-miR-24. Error bars represent S.E. (n = 3 unless indicated otherwise).
Primary endothelial cells (HUVECs) were next used to analyze the effects of miR-24 on Ndst1 mRNA levels. miR-24 overexpression (36-fold, Fig. 1B) reduced the Ndst1 mRNA levels by ∼50% (Fig. 1D) as compared with control. Transfection with anti-miR-24 in loss-of-function experiments to reduce the endogenous miRNA levels resulted in a tendency for increased cellular Ndst1 mRNA; however, this increase was not statistically significant (data not shown).
As a third approach to verify that Ndst1 mRNA is a target for miR-24, qRT-PCR was performed using miR-24 as primer in a reverse transcriptase reaction (Fig. 1E) followed by cDNA amplification using specific Ndst1 primers (17). The results showed that miR-24 binds more efficiently to the Ndst1 3′-UTR as compared with miR-9, predicted not to target Ndst1 (Fig. 1F).
Overexpression of miR-24 Reduces NDST Activity
NDST1 protein can only be detected by Western blotting in cells overexpressing NDST1 as the endogenous NDST1 levels are too low for reliable detection (32, 33). A sensitive N-deacetylase assay (22), previously demonstrated to faithfully reflect the levels of NDST1 protein, was therefore employed to determine the effects of miR-24 on the levels of functional NDST1 protein (Fig. 1G). N-[3H]Acetyl-labeled K5 capsular polysaccharide was used as a substrate to probe the N-deacetylase activity in HUVEC lysates by measuring the release of [3H]acetate using biphasic scintillation counting. Overexpression of miR-24 decreased N-deacetylase activity by 36% (Fig. 1H). Transfection with anti-miR-24 to suppress endogenous miR-24 did not significantly increase N-deacetylase activity, suggesting that the endogenous levels of miR-24 do not limit NDST1 expression in cultured HUVECs (data not shown).
We next evaluated whether miR-23a/b may also target Ndst1 mRNA to suppress NDST1 activity. As the sequences of miR-23a and miR-23b differ only at the position of a single nonseed nucleotide, and thus can be expected to have similar effects, we concentrated our efforts on miR-23a. miR-23a was shown to bind to its predicted target site in the Ndst1 3′-UTR (Fig. 2A), but miR-23a overexpression (42-fold, Fig. 1C) had no significant effect on the Ndst1 mRNA levels nor N-deacetylase activity under the conditions tested (Fig. 2, B and D). Also, transfection with anti-miR-23a had no effect on the Ndst1 mRNA levels (data not shown). miR-23a was therefore not investigated further.
FIGURE 2.
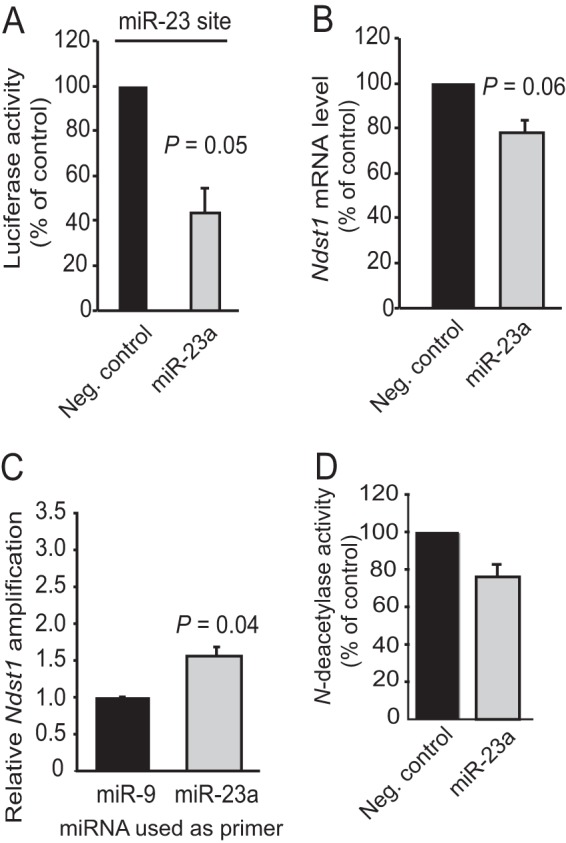
Regulation of NDST1 by miR-23a. A, the single miR-23a-binding site present in the Ndst1 3′-UTR was cloned into a pMIR-REPORT vector. Luciferase activity was assayed after 48 h in cell lysates from HEK293 cells co-transfected with the miR-23a pMIR-REPORT vector and either negative control (Neg. control) dsRNA or pre-miR-23a. The luciferase signals were normalized to control groups. B, HUVECs were transfected with pre-miR-23a, and Ndst1 mRNA levels were determined by qRT-PCR. C, a PCR approach using miR-23a as a primer for the reverse transcription step (see Fig. 1E for comparison) was used to investigate the binding of miR-23a to the Ndst1 3′-UTR. miR-9 was included for comparison. D, effects of pre-miR-23a on N-deacetylase activity in HUVEC lysates. Error bars represent S.E. (n = 3).
Reduced HS Sulfation in Endothelial Cells Overexpressing miR-24
Next, we investigated how miR-24 may affect the sulfation of HS. The related polysaccharide CS was also included in the analysis as the biosynthesis of HS and CS to some extent is interconnected (7, 25, 34). HS and CS were isolated from HUVECs transfected with pre-miR-24 or pre-miR-control and thereafter subjected to HS compositional analysis using RPIP-HPLC (24). Overexpression of miR-24 was shown to reduce N-sulfation of the HS-derived disaccharides (Fig. 3A), in line with the effects of miR-24 on Ndst1 mRNA levels and enzyme activity. The reduction of glucosamine N-sulfation by 5% as compared with the control was mirrored by an increase in N-acetylation of the same residues. The miR-24-mediated reduction in N-sulfation was accompanied by decreased 2-O-sulfation, explained by the fact that the 2-O-sulfotransferase requires N-sulfated HS substrates for enzymatic activity. Altogether an overall reduction of HS sulfation of 10% was detected (Fig. 3A). Interestingly, although miR-24 overexpression did not change the degree of total CS sulfation, an increase in CS 6-O-sulfation together with a decrease in 4-O-sulfation was detected (Fig. 3B). The effects on CS sulfation caused by suppression of HS biosynthesis are most likely indirect as recently suggested (25, 34).
FIGURE 3.
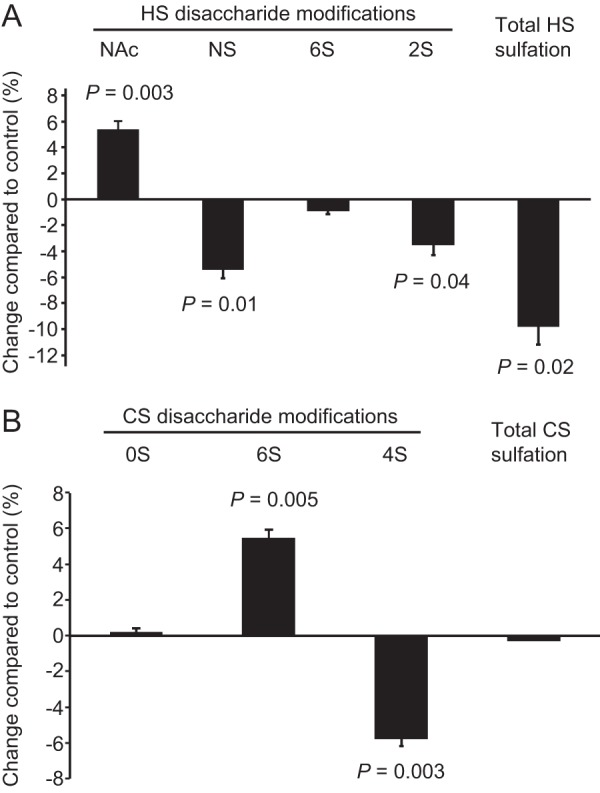
Reduced HS sulfation in endothelial cells in response to miR-24. A and B, HS (A) and CS (B) were isolated from HUVECs transfected with pre-miR-24 or negative control dsRNA and analyzed with regard to disaccharide composition using RPIP-HPLC. A reduction of HS N-sulfation (NS) (accompanied by an increased occurrence of N-acetyl groups) and 2-O-sulfation was recorded in response to miR-24. B, the overall sulfation degree of CS was unaffected, although the CS sulfation pattern was altered due to increased 6-O-sulfation and reduced 4-O-sulfation. Error bars represent S.E. (n = 3).
HS from miR-24-overexpressing Endothelial Cells Has Reduced Affinity for VEGFA
To examine whether the reduced degree of HS sulfation had an effect on the interaction with the angiogenic growth factor VEGFA, HS from HUVECs overexpressing miR-24 was isolated. A nitrocellulose filter binding assay was used to generate saturation binding curves, and it could be shown that miR-24-mediated reduction of HS sulfation reduced the affinity of full-length HS chains for VEGFA by ∼50% (Fig. 4A). This reduction in affinity was not due to altered HS chain length as the analysis of intact HS chains by size exclusion chromatography showed no difference between HS chains from miR-24 and control cells (Fig. 4B). Thus, the reduction in VEGFA affinity could be attributed to reduced HS sulfation.
FIGURE 4.
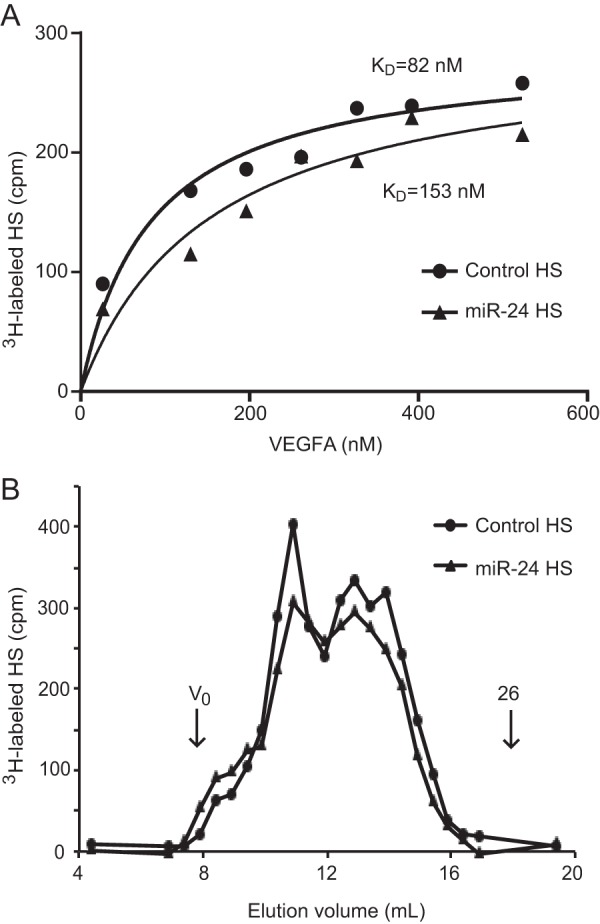
HS isolated from miR-24-overexpressing endothelial cells has reduced affinity for VEGFA. A, 3H-labeled HS was isolated from HUVECs transfected with pre-miR-24 or pre-miR-control, and binding to VEGFA was analyzed using a nitrocellulose filter binding assay. Increasing amounts of VEGFA were added to generate saturation binding curves. Analysis of the experimental data using GraphPad Prism yielded the indicated KD values. B, increased levels of miR-24 did not alter HS chain length. 3H-labeled HS samples were analyzed on Superose 6. The void volume (Vo) and the elution position of a heparin 26-mer oligosaccharide (26) are indicated by arrows.
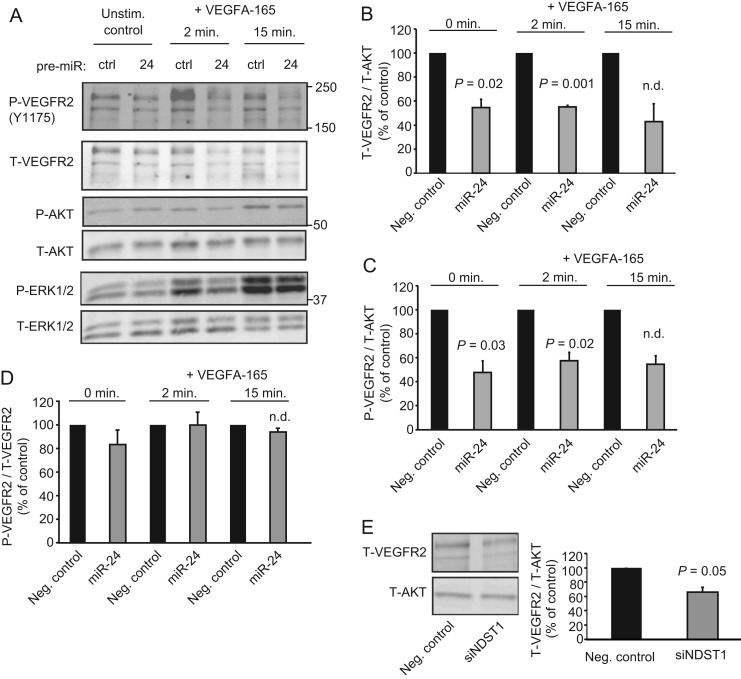
Increased miR-24 Activity and Suppression of NDST1 Leads to Reduced Levels of VEGFR2
Next, the effects of elevated miR-24 levels on VEGFA signaling in HUVECs were analyzed. miR-24 overexpression reduced the total levels of VEGFR2 (Fig. 5, A and B), and as a consequence, the levels of phosphorylated VEGFR2 (Fig. 5, A and C). Notably, the degree of phosphorylation per VEGFR2 molecule was comparable in miR-24-overexpressing cells and control cells (Fig. 5D). Phosphorylation of the signaling effectors ERK1/2 acting downstream of VEGFR2 was also reduced in cells with elevated levels of miR-24 as compared with the control (Fig. 5A). Strikingly, siRNA-mediated suppression of NDST1 by 75% as judged by qPCR also resulted in reduced VEGFR2 levels in HUVECs (Fig. 5E).
FIGURE 5.
Overexpression of miR-24 or suppression of NDST1 leads to reduced levels of VEGFR2. A, Western blot analysis showed reduced total levels of VEGFR2 and reduced phosphorylation of the downstream targets ERK1/2 in HUVECs in response to increased miR-24. Total (T) as well as phosphorylated (P) VEGFR2, AKT, and ERK1/2 were detected using dual labeling with fluorescent secondary antibodies. ctrl, control. B and C, quantifications of total VEGFR2 (B) and phosphorylated VEGFR2 (C) normalized to total AKT. Neg. control, negative control. D, overexpression of miR-24 did not reduce the level of phosphorylation per VEGFR2 in response to VEGFA-165. E, Western blot analysis showed reduced levels of total VEGFR2 in response to siRNA-mediated suppression of NDST1 as compared with control. Error bars represent S.E. (n = 3 except where indicated n.d., not determined; n = 2).
NDST1 Is Required for VEGFA-induced Chemotaxis
A microfluidic chemotaxis assay was employed to assess the effects of miR-24 and the dependence on NDST1 in a functional assay (20, 27). The microfluidic assay makes it possible, via time-lapse microscopy, to follow endothelial cell migration in response to stable gradients of VEGFA (Fig. 6A). In this assay, VEGFA has previously been shown to induce chemotaxis of human endothelial cells, including HUVECs (27, 28). Cells transfected with either pre-miR-24 or siRNA against NDST1 were analyzed. In both cases, the ability of the cells to chemotax in response to VEGFA was impaired (Fig. 6, B and D). Overexpression of miR-24 also led to a reduction of total (nondirectional) migration distances (Fig. 6C), whereas siRNA against NDST1 was shown not to reduce the total migration distances per cell (Fig. 6E).
FIGURE 6.
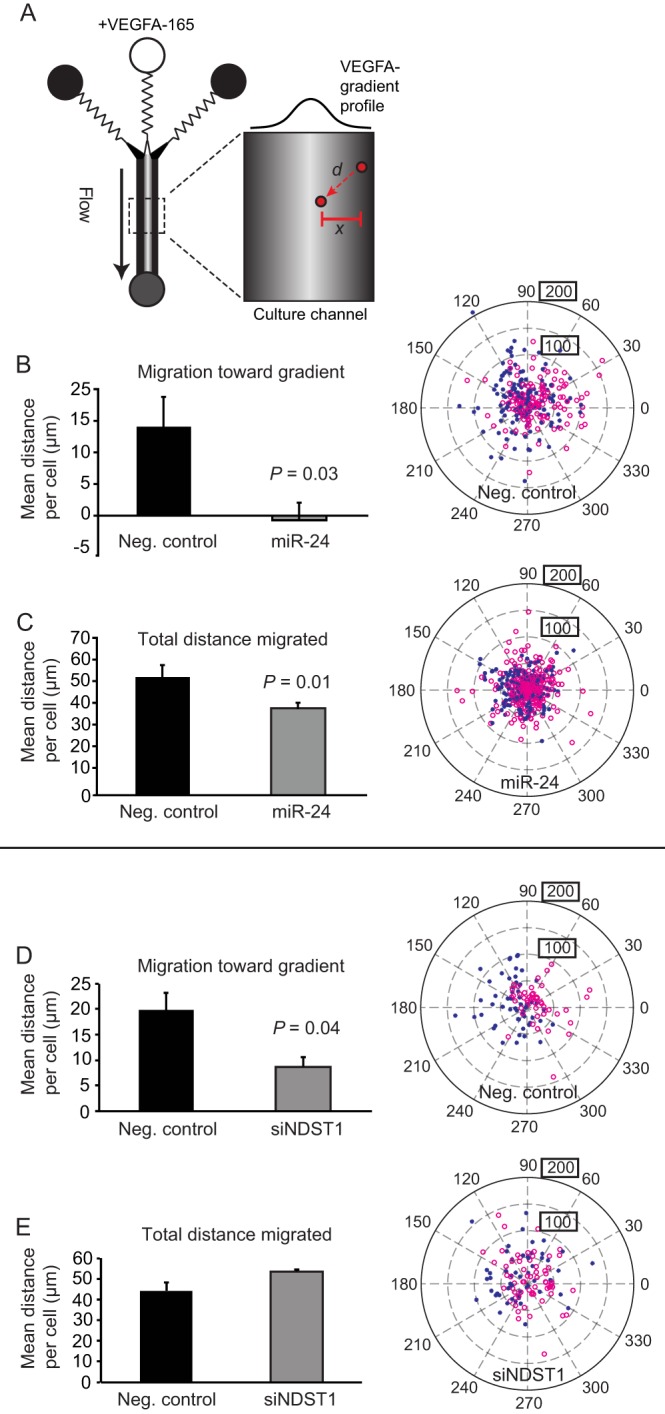
Elevated levels of miR-24 or suppression of NDST1 inhibit VEGFA-induced chemotaxis. A, overview of the microfluidic chemotaxis chamber used to study HUVEC cell migration in response to stable, diffusion-limited gradients of VEGFA-165 generated in the device (27). Cell chemotaxis (x, the distance migrated perpendicular toward the gradient) as well as total cell migration (d, distance migrated irrespective of directionality) was analyzed. B and C, the ability of cells transfected with pre-miR-24 to chemotax toward VEGFA was completely lost (B), whereas the total average migration distances per cell were reduced by ∼20% (C) (n = 4). Neg. control, negative control. D and E, endothelial cells treated with siRNA against NDST1 exhibited an ∼60% decrease of chemotaxis in response to VEGFA (D), but exhibited no reduction in total cell migration (E) (n = 3). Error bars represent S.E. The tracked migration distances and the directionality of migration of all endothelial cells are shown in polar plots to the right for each condition. Here, the starting positions of the cells are assigned to the middle of the plot, and the relative positions of the individual cells at the end of the chemotaxis experiment are shown. Red open circles indicate cells migrating on the left side of the chamber, and blue stars indicate cells migrating on the right side; a separation of stars from circles indicates that the cells respond to the hill-shaped gradient. Boxed values indicate net migration distances (μm).
DISCUSSION
In this study, we have shown that NDST1 is a target of miR-24. The major findings include the following: (a) miR-24-mediated suppression of NDST1 reduced HS N- and 2-O-sulfation, resulting in decreased affinity of HS for VEGFA; (b) miR-24 can suppress the levels of VEGFR2 and thereby VEGFA signaling; and (c) endothelial cell chemotaxis in response to VEGFA is dependent on NDST1 and can be suppressed by miR-24. These findings provide proof for the principle that miRNA-mediated regulation of HS biosynthesis represents an additional mechanism whereby VEGFA signaling can be modulated.
It has been shown that several miRNAs are selectively expressed in endothelial cells (13, 14, 20, 29, 36). We have recently demonstrated that miR-24 and miR-23a/b are expressed in microvessels and that both miR-24 and miR-23a/b are up-regulated 6–10-fold in vascular fragments isolated from mature mouse kidneys as compared with vessels isolated from developing kidneys (embryonic day 14) (20). This suggests that blood vessel maturation, characterized by reduced proangiogenic growth factor signaling, could involve increased expression of miR-24 and miR-23a/b. This concept is supported by the present study as we show that increased miR-24 expression reduced the affinity of HS for VEGFA and blunted signaling via the VEGFA-VEGFR2 axis.
With regard to the involvement of HS in VEGFA interactions with VEGFR2, Xu et al. (5) recently employed a sensitive proximity ligation assay to show that HS can form complexes directly with VEGFR2 in endothelial cells. Furthermore, it has been suggested that VEGFA-VEGFR2 complexes contain HS, for example analogous to the ternary complexes observed for HS, FGF1, and FGFR2 (37). Here, the finding that reduced NDST1 activity results in lowered VEGFR2 expression levels is intriguing (Fig. 5). We have previously shown that HSPGs, present on perivascular cells, affect the activation and turnover of VEGFRs present in trans on adjacent NDST1/2−/− endothelial cells (1). Thus, HSPGs are indeed critical regulators of VEGFR2 signaling, affecting not only receptor activation by VEGFA, but also the dynamics of receptor turnover (38). The results presented in the current study extend these findings by providing evidence that miR-24-mediated suppression of NDST1 leads to reduced VEGFR2 expression and signaling. Notably, miR-24 suppressed HUVEC chemotaxis to a greater extent than direct suppression of NDST1 by siRNA (Fig. 6), although direct NDST1 suppression led to lower levels of Ndst1 mRNA (∼75% suppression, as compared with ∼50% suppression in response to miR-24). miR-24 also significantly reduced the average total distance migrated by the cells, whereas siRNA against NDST1 did not. These differences may be due to the fact that miR-24, like most miRNAs, has more than one target. In addition, miRNA binding to its target mRNA may not immediately result in transcript degradation, but may, however, impair translation (11, 12). Nonetheless, the data presented here demonstrate that NDST1 is a target for miR-24 and that a reduction of NDST1 activity leads to reduced VEGFR2 expression and signaling, resulting in impaired chemotaxis of endothelial cells in response to VEGFA.
It is further to be expected that miRNA-mediated regulation of HS biosynthesis, affecting the expression of sulfated protein-binding domains, will affect other angiogenic signaling proteins whose activities are modulated by HSPGs co-receptors. For example, there is ample evidence to suggest that signaling events in response to FGF2, PDGFB, and TGF-β are modulated by HSPGs (4, 39).
The finding that elevated levels of miR-24 also altered the sulfation profile of CS warrants further discussion (Fig. 3). The effects on CS biosynthesis are most likely indirect. It has previously been shown that cells deficient in the exostosin (EXT) polymerases that generate the HS backbone (shown not to synthesize HS) instead produce increased amounts of CS (34, 40, 41). In addition, overexpression of NDST1 in HEK293 cells resulted in decreased CS sulfation (42). The precise mechanism underlying the communication between HS and CS biosynthesis is unclear, but it is considered likely that the inability to produce HS leads to larger pools of available sulfate precursors (3′-phosphoadenosine-5′-phosphosulfate, PAPS) as well as more available UDP-sugar precursors that also may be used for CS biosynthesis (25). Also, interactions between some of the enzymes involved in HS and CS biosynthesis, and their localization in the Golgi apparatus, may be such that a reduction of a particular enzyme may affect the activities of several other biosynthetic enzymes by yet unknown mechanisms (7).
Several studies suggest that miR-24 is important for blood vessel formation and function of the cardiovascular system. For example, overexpression of miR-24 in zebrafish embryos results in aberrant blood vessel formation. miR-24 has also been reported to be up-regulated after cardiac ischemia (36) as well as during cardiac hypertrophy (43). The levels of miR-24 may also be altered in different types of cancer. In some cases, the levels of miR-24 in tumor cells are elevated (44, 45), but there are also examples of reduced miR-24 expression in cancer cells (46). Further, microRNA expression data from The Cancer Genome Atlas (TCGA) show that the miR-24 levels vary up to 34-fold between individual invasive breast carcinomas (47) and that the miR-24 levels vary up to 61-fold between different ovarian serous carcinomas (48).
In light of the results presented in this study, it will be of great future interest to see whether altered miR-24 expression in cancer cells or tumor-associated vessels correlates with altered HS biosynthesis and aberrant signaling of HSPG-dependent growth factors. Of note, a reduction of NDST1 activity in endothelial cells has in an experimental model been shown to result in reduced microvascular density and vessel branching, accompanied by decreased tumor growth (49).
Efforts are currently ongoing to see whether inhibition of HS biosynthesis can be used for therapeutic purposes (35). Interfering with HS biosynthesis via miRNAs or miRNA inhibitors could possibly represent a new way to target HSPG-dependent growth factor signaling of pathophysiological importance.
This work was supported by grants from the Swedish Research Council (to J. K., L. K., and P. G.), the Swedish Cancer Society (to J. K., L. K., and P. G.) the Swedish Childhood Cancer Foundation (to J. K. and P. G), the Swedish Foundation for Strategic Research (to J. K.), the Foundation for Proteoglycan Research at Uppsala University (to J. K. and L. K.) and Uppsala University (to J. K., L. K., and P. G.).
- PG
- proteoglycan
- HS
- heparan sulfate
- HSPG
- heparan sulfate proteoglycan
- CS
- chondroitin sulfate
- miRNA
- microRNA
- miR
- microRNA
- NDST
- N-deacetylase/N-sulfotransferase
- HUVEC
- human umbilical vein endothelial cell
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative RT-PCR
- VEGFR
- VEGF receptor
- RPIP-HPLC
- reversed-phase ion-pairing HPLC.
REFERENCES
- 1. Jakobsson L., Kreuger J., Holmborn K., Lundin L., Eriksson I., Kjellén L., Claesson-Welsh L. (2006) Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell 10, 625–634 [DOI] [PubMed] [Google Scholar]
- 2. Abramsson A., Kurup S., Busse M., Yamada S., Lindblom P., Schallmeiner E., Stenzel D., Sauvaget D., Ledin J., Ringvall M., Landegren U., Kjellén L., Bondjers G., Li J. P., Lindahl U., Spillmann D., Betsholtz C., Gerhardt H. (2007) Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev. 21, 316–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikari N., Basi D. L., Townsend D., Rusch M., Mariash A., Mullegama S., Watson A., Larson J., Tan S., Lerman B., Esko J. D., Selleck S. B., Hall J. L. (2010) Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. J. Mol. Cell. Cardiol. 49, 287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iozzo R. V., San Antonio J. D. (2001) Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest. 108, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu D., Fuster M. M., Lawrence R., Esko J. D. (2011) Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J. Biol. Chem. 286, 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson C. J., Mulloy B., Gallagher J. T., Stringer S. E. (2006) VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J. Biol. Chem. 281, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 7. Kreuger J., Kjellén L. (2012) Heparan sulfate biosynthesis: regulation and variability. J. Histochem. Cytochem. 60, 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grobe K., Esko J. D. (2002) Regulated translation of heparan sulfate N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes by structured 5′-untranslated regions and internal ribosome entry sites. J. Biol. Chem. 277, 30699–30706 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher J. T. (2006) Multiprotein signalling complexes: regional assembly on heparan sulphate. Biochem. Soc. Trans. 34, 438–441 [DOI] [PubMed] [Google Scholar]
- 10. Bushati N., Cohen S. M. (2008) MicroRNAs in neurodegeneration. Curr. Opin. Neurobiol. 18, 292–296 [DOI] [PubMed] [Google Scholar]
- 11. Eulalio A., Huntzinger E., Izaurralde E. (2008) Getting to the root of miRNA-mediated gene silencing. Cell 132, 9–14 [DOI] [PubMed] [Google Scholar]
- 12. Filipowicz W., Bhattacharyya S. N., Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 [DOI] [PubMed] [Google Scholar]
- 13. Suárez Y., Fernández-Hernando C., Yu J., Gerber S. A., Harrison K. D., Pober J. S., Iruela-Arispe M. L., Merkenschlager M., Sessa W. C. (2008) Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 105, 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuehbacher A., Urbich C., Zeiher A. M., Dimmeler S. (2007) Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 101, 59–68 [DOI] [PubMed] [Google Scholar]
- 15. Harris T. A., Yamakuchi M., Ferlito M., Mendell J. T., Lowenstein C. J. (2008) MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. U.S.A. 105, 1516–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang S., Aurora A. B., Johnson B. A., Qi X., McAnally J., Hill J. A., Richardson J. A., Bassel-Duby R., Olson E. N. (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 15, 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X., Hu G., Zhou J. (2010) Repression of versican expression by microRNA-143. J. Biol. Chem. 285, 23241–23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Small E. M., Sutherland L. B., Rajagopalan K. N., Wang S., Olson E. N. (2010) MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ. Res. 107, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi X., Su S., Long J., Mei B., Chen Y. (2011) MicroRNA-191 targets N-deacetylase/N-sulfotransferase 1 and promotes cell growth in human gastric carcinoma cell line MGC803. Acta Biochim. Biophys. Sin. 43, 849–856 [DOI] [PubMed] [Google Scholar]
- 20. Larsson E., Fredlund Fuchs P., Heldin J., Barkefors I., Bondjers C., Genové G., Arrondel C., Gerwins P., Kurschat C., Schermer B., Benzing T., Harvey S. J., Kreuger J., Lindahl P. (2009) Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 1, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. (2003) Prediction of mammalian microRNA targets. Cell 115, 787–798 [DOI] [PubMed] [Google Scholar]
- 22. Pettersson I., Kusche M., Unger E., Wlad H., Nylund L., Lindahl U., Kjellén L. (1991) Biosynthesis of heparin: purification of a 110-kDa mouse mastocytoma protein required for both glucosaminyl N-deacetylation and N-sulfation. J. Biol. Chem. 266, 8044–8049 [PubMed] [Google Scholar]
- 23. Dagälv A., Holmborn K., Kjellén L., Abrink M. (2011) Lowered expression of heparan sulfate/heparin biosynthesis enzyme N-deacetylase/N-sulfotransferase 1 results in increased sulfation of mast cell heparin. J. Biol. Chem. 286, 44433–44440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ledin J., Staatz W., Li J. P., Götte M., Selleck S., Kjellén L., Spillmann D. (2004) Heparan sulfate structure in mice with genetically modified heparan sulfate production. J. Biol. Chem. 279, 42732–42741 [DOI] [PubMed] [Google Scholar]
- 25. Holmborn K., Habicher J., Kasza Z., Eriksson A. S., Filipek-Gorniok B., Gopal S., Couchman J. R., Ahlberg P. E., Wiweger M., Spillmann D., Kreuger J., Ledin J. (2012) On the roles and regulation of chondroitin sulfate and heparan sulfate in zebrafish pharyngeal cartilage morphogenesis. J. Biol. Chem. 287, 33905–33916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreuger J., Lindahl U., Jemth P. (2003) Nitrocellulose filter binding to assess binding of glycosaminoglycans to proteins. Methods Enzymol. 363, 327–339 [DOI] [PubMed] [Google Scholar]
- 27. Barkefors I., Le Jan S., Jakobsson L., Hejll E., Carlson G., Johansson H., Jarvius J., Park J. W., Li Jeon N., Kreuger J. (2008) Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J. Biol. Chem. 283, 13905–13912 [DOI] [PubMed] [Google Scholar]
- 28. Barkefors I., Fuchs P. F., Heldin J., Bergström T., Forsberg-Nilsson K., Kreuger J. (2011) Exocyst complex component 3-like 2 (EXOC3L2) associates with the exocyst complex and mediates directional migration of endothelial cells. J. Biol. Chem. 286, 24189–24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Q., Gallagher R., Ufret-Vincenty R., Li X., Olson E. N., Wang S. (2011) Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proc. Natl. Acad. Sci. U.S.A. 108, 8287–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan M. C., Hilyard A. C., Wu C., Davis B. N., Hill N. S., Lal A., Lieberman J., Lagna G., Hata A. (2010) Molecular basis for antagonism between PDGF and the TGFβ family of signalling pathways by control of miR-24 expression. EMBO J. 29, 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee Y., Kim M., Han J., Yeom K. H., Lee S., Baek S. H., Kim V. N. (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Presto J., Thuveson M., Carlsson P., Busse M., Wilén M., Eriksson I., Kusche-Gullberg M., Kjellén L. (2008) Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc. Natl. Acad. Sci. U.S.A. 105, 4751–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ledin J., Ringvall M., Thuveson M., Eriksson I., Wilén M., Kusche-Gullberg M., Forsberg E., Kjellén L. (2006) Enzymatically active N-deacetylase/N-sulfotransferase-2 is present in liver but does not contribute to heparan sulfate N-sulfation. J. Biol. Chem. 281, 35727–35734 [DOI] [PubMed] [Google Scholar]
- 34. Le Jan S., Hayashi M., Kasza Z., Eriksson I., Bishop J. R., Weibrecht I., Heldin J., Holmborn K., Jakobsson L., Söderberg O., Spillmann D., Esko J. D., Claesson-Welsh L., Kjellén L., Kreuger J. (2012) Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arterioscler. Thromb. Vasc. Biol. 32, 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindahl U., Li J. P. (2009) Interactions between heparan sulfate and proteins—design and functional implications. Int. Rev. Cell Mol. Biol. 276, 105–159 [DOI] [PubMed] [Google Scholar]
- 36. Fiedler J., Jazbutyte V., Kirchmaier B. C., Gupta S. K., Lorenzen J., Hartmann D., Galuppo P., Kneitz S., Pena J. T., Sohn-Lee C., Loyer X., Soutschek J., Brand T., Tuschl T., Heineke J., Martin U., Schulte-Merker S., Ertl G., Engelhardt S., Bauersachs J., Thum T. (2011) MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 124, 720–730 [DOI] [PubMed] [Google Scholar]
- 37. Pellegrini L., Burke D. F., von Delft F., Mulloy B., Blundell T. L. (2000) Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 407, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 38. Fransson L. A., Belting M., Cheng F., Jönsson M., Mani K., Sandgren S. (2004) Novel aspects of glypican glycobiology. Cell. Mol. Life Sci. 61, 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoeller J. J., Whitelock J. M., Iozzo R. V. (2009) Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 28, 284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lidholt K., Weinke J. L., Kiser C. S., Lugemwa F. N., Bame K. J., Cheifetz S., Massagué J., Lindahl U., Esko J. D. (1992) A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 89, 2267–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stickens D., Zak B. M., Rougier N., Esko J. D., Werb Z. (2005) Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132, 5055–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bengtsson J., Eriksson I., Kjellén L. (2003) Distinct effects on heparan sulfate structure by different active site mutations in NDST-1. Biochemistry 42, 2110–2115 [DOI] [PubMed] [Google Scholar]
- 43. van Rooij E., Sutherland L. B., Liu N., Williams A. H., McAnally J., Gerard R. D., Richardson J. A., Olson E. N. (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. U.S.A. 103, 18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chhabra R., Dubey R., Saini N. (2010) Cooperative and individualistic functions of the microRNAs in the miR-23a∼27a∼24–2 cluster and its implication in human diseases. Mol. Cancer 9, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calin G. A., Ferracin M., Cimmino A., Di Leva G., Shimizu M., Wojcik S. E., Iorio M. V., Visone R., Sever N. I., Fabbri M., Iuliano R., Palumbo T., Pichiorri F., Roldo C., Garzon R., Sevignani C., Rassenti L., Alder H., Volinia S., Liu C. G., Kipps T. J., Negrini M., Croce C. M. (2005) A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 353, 1793–1801 [DOI] [PubMed] [Google Scholar]
- 46. Szczyrba J., Löprich E., Wach S., Jung V., Unteregger G., Barth S., Grobholz R., Wieland W., Stöhr R., Hartmann A., Wullich B., Grässer F. (2010) The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol. Cancer Res. 8, 529–538 [DOI] [PubMed] [Google Scholar]
- 47. Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fuster M. M., Wang L., Castagnola J., Sikora L., Reddi K., Lee P. H., Radek K. A., Schuksz M., Bishop J. R., Gallo R. L., Sriramarao P., Esko J. D. (2007) Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J. Cell Biol. 177, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]