Background: The pathway to form green leaf volatiles is important for plant defense against pathogens and herbivores.
Results: Galactolipids with truncated oxoacids are formed as counterparts of green leaf volatiles.
Conclusion: Galactolipid-hydroperoxides are substrates for hydroperoxide lyase.
Significance: The biosynthetic pathway partly proceeds directly with galactolipids, without the involvement of lipases, to form free fatty acids.
Keywords: Glycolipids, Lipid Peroxidation, Lipoxygenase Pathway, Mass Spectrometry (MS), Plant Biochemistry, Green Leaf Volatiles, Hydroperoxide Lyase
Abstract
Green leaf volatiles (GLVs) consisting of six-carbon aldehydes, alcohols, and their esters, are biosynthesized through the action of fatty acid hydroperoxide lyase (HPL), which uses fatty acid hydroperoxides as substrates. GLVs form immediately after disruption of plant leaf tissues by herbivore attacks and mechanical wounding and play a role in defense against attackers that attempt to invade through the wounds. The fates and the physiological significance of the counterparts of the HPL reaction, the 12/10-carbon oxoacids that are formed from 18/16-carbon fatty acid 13-/11-hydroperoxides, respectively, are largely unknown. In this study, we detected monogalactosyl diacylglycerols (MGDGs) containing the 12/10-carbon HPL products in disrupted leaf tissues of Arabidopsis, cabbage, tobacco, tomato, and common bean. They were identified as an MGDG containing 12-oxo-9-hydroxy-(E)-10-dodecenoic acid and 10-oxo-7-hydroxy-(E)-8-decenoic acid and an MGDG containing two 12-oxo-9-hydroxy-(E)-10-dodecenoic acids as their acyl groups. Analyses of Arabidopsis mutants lacking HPL indicated that these MGDGs were formed enzymatically through an active HPL reaction. Thus, our results suggested that in disrupted leaf tissues, MGDG-hydroperoxides were cleaved by HPL to form volatile six-carbon aldehydes and non-volatile 12/10-carbon aldehyde-containing galactolipids. Based on these results, we propose a novel oxylipin pathway that does not require the lipase reaction to form GLVs.
Introduction
Green leaf volatiles (GLVs),2 which consist of six-carbon (C6) aldehydes, alcohols, and their esters, are ubiquitous in the leaves of most plants (1). GLVs form rapidly after the disruption of plant tissues (2, 3). Insecticidal, fungicidal, and bactericidal activities have been reported for (Z)-3-hexenal and its related aldehydes (4–7). A portion of the C6 aldehydes that form in disrupted tissues diffuses into adjacent intact tissues, where they are reduced to C6 alcohols and further acetylated to C6 acetates (8). They function as airborne infochemicals in specific plant-herbivore, plant-carnivore, and plant-plant relationships (9).
The biosynthetic pathway that produces GLVs (Fig. 1) is widespread in the plant kingdom. Lipoxygenase (LOX) adds dioxygen (O2) at position 13 of α-linolenic acid to produce α-linolenic acid 13-hydroperoxide (13-HPOT). The hydroperoxide (HPO) is cleaved by fatty acid hydroperoxide lyase (HPL) at the C12–C13 bond to produce two carbonyl compounds; (Z)-3-hexenal and 12-oxo-(Z)-9-dodecenoic acid ((9Z)-traumatin) (10). Hexadecatrienoic acid 11-hydroperoxide (11-HPHT) is also a substrate for HPL, yielding (Z)-3-hexenal and 10-oxo-(Z)-7-decenoic acid ((7Z)-dinortraumatin) as the primary products. If allene oxide synthase (AOS) acts on 13-HPOT, the hydroperoxide is diverted into the jasmonate pathway to form 12-oxophytodienoic acid and jasmonic acid (JA) after several enzymatic reaction steps (11).
FIGURE 1.
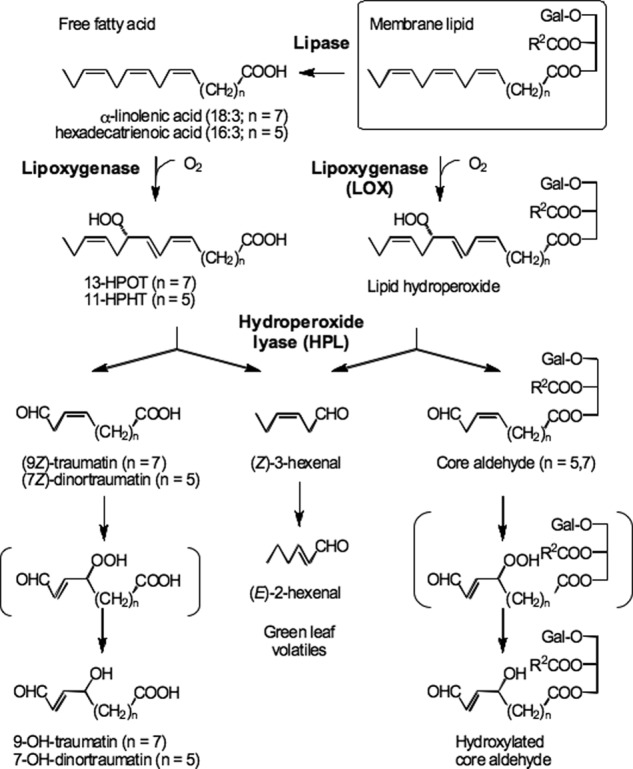
Proposed biosynthetic pathway for green leaf volatiles and their counterparts. Complex membrane lipids are hydrolyzed by lipases to form free fatty acids (18:3 and 16:3). Then the free fatty acid is oxygenated by lipoxygenase, and resulting hydroperoxides are cleaved to form (Z)-3-hexenal and free traumatin or dinortraumatin derivatives. In disrupted leaves of Arabidopsis and some other plant species, galactolipids are directly oxygenated by lipoxygenase to form galactolipid-hydroperoxides which are subsequently cleaved by hydroperoxide lyase to form (Z)-3-hexenal and traumatin- and dinortraumatin-containing galactolipids.
In general, GLVs and the corresponding traumatins, as well as JAs, are thought to be formed from free fatty acids that are released from glycerolipids by lipases; however, there are only a few reports on the identification of lipases involved in JA synthesis (11). Mono- and digalactosyldiacylglycerols (MGDGs and DGDGs) that contain 12-oxophytodienoic acid and/or 12-oxodinorphytodienoic acid (collectively known as arabidopsides) have been identified in Arabidopsis (Col-0) (12, 13). The oxidative modification of MGDG to yield arabidopsides occurs on esterified fatty acids (14). Thus, the first enzyme acting on the lipid may be LOX, especially AtLOX2 in Arabidopsis, because this LOX is known to contribute the major portion of arabidopsides formed after leaf wounding (15). The oxylipin galactolipids containing divinyl ether residues (linolipins) that are present in flax were thought to be biosynthesized without free fatty acid intermediates derived from the lipase reaction (16). Therefore, in some plant species, lipases are not essential to form oxylipins.
Nonenzymatic oxidation of lipids can also occur, especially in chloroplasts, because reactive oxygen species such as 1O2 are inevitably formed as by-products of photosynthesis (17). The chloroplast-abundant lipids, such as MGDGs and DGDGs, are prone to conversion into their peroxides, a portion of which are modified by radical-catalyzed fragmentation reactions to yield a wide variety of compounds (e.g. malondialdehyde (18), trioxygenated products, phytoprostanes, or even MGDGs harboring C12 or C9 oxoacids) (19–20).
Studies with purified HPL and 13-HPOT as substrate showed that the C6 and C12 aldehydes formed in an equimolar ratio, as expected from the reaction catalyzed by HPL (10). An isomer of (9Z)-traumatin, 12-oxo-(E)-10-dodecenoic acid ((10E)-traumatin), was found in runner bean (Phaseolus vulgaris) (21). Recently, Kallenbach et al. (22) quantified the C12 counterparts and their derivatives in wounded Nicotiana attenuata leaves. After mechanical wounding, they found only small amounts of (9Z)-traumatin but substantial amounts of its hydroxylated metabolite 12-oxo-9-hydroxy-(E)-10-dodecenoic acid (9-OH-traumatin), which was formed via peroxidation of traumatin either enzymatically by NaLOX2 or nonenzymatically, and its glutathione conjugate. However, they provided no data to precisely compare the amount of GLVs released with the amount of traumatin derivatives retained. Hence, it remains uncertain whether the C6 components and their corresponding C12 and C10 counterparts are formed in an equimolar ratio. Inspired by the findings by Kallenbach et al. (22), we tried to quantify C12/C10 compounds, including (9Z)-traumatin, (10E)-traumatin, and 9-OH-traumatin, (7Z)-dinortraumatin, 10-oxo-(E)-8-decenoic acid ((10E)-dinortraumatin), and 10-oxo-7-hydroxy-(E)-8-decenoic acid (7-OH-dinortraumatin) in wounded Arabidopsis leaves (No-0). We generally used the No-0 ecotype instead of the Col-0 ecotype, because the latter has a deletion in the HPL gene and thus lacks HPL activity (23). We found that the amounts of these C12/C10 compounds were still much lower than those expected based on the amounts of C6 volatiles released. Therefore, we assumed that the C12/C10 compounds were present as esterified galactolipids. Because MGDGs are the most abundant source for octadecatrienoic acid (18:3) and hexadecatrienoic acid (16:3) in Arabidopsis leaves (24), we focused on this lipid class to find the corresponding traumatin and dinortraumatin derivatives.
EXPERIMENTAL PROCEDURES
Plant Materials
Seeds of Arabidopsis (Arabidopsis thaliana (L.) Heynh.) wild-type ecotypes Col-0, Ler-0, No-0, and WS and the transfer DNA-inserted line aos::Col-6 (gl1) (25) were obtained from ABRC (Columbus, OH). aos::Col-6 (gl1) was crossed with Ler-0, and a line showing the aos (male-sterile) and HPL (ability to form GLVs) phenotypes was selected from the resultant F2 progenies. The line was denoted as aos::Ler-0. hpl1 has a Col-0-derived deletion of the HPL gene in the Ler-0 background; thus, it has no HPL activity (26). The seeds were germinated in soil (Metro-Mix, Sun Gro Horticulture Distribution Inc., Bellevue, WA) in plastic pots (6-cm inner diameter). Plants were cultivated in a growth chamber at 22 °C under fluorescent lights (60 μmol m−2 s−1) with a 14-h light/10-h dark photoperiod for 30 days until they reached the stage just before bolting. Seeds of Brassica oleracea, Nicotiana tabacum, Solanum lycopersicum, and Phaseolus vulgaris were purchased from a local market and were cultivated in a growth chamber at 25 °C under fluorescent lights (60 μmol m−2 s−1) with a 14-h light/10-h dark photoperiod for 30 days. Clover (Trifolium repens L.) leaves were harvested from the experimental farm at the Faculty of Agriculture, Yamaguchi University, in June 2009. Spinach (Spinacia oleracea L.) was purchased from a local market.
Chemicals
(9Z)-Traumatin was purchased from Larodan (Malmö, Sweden). 9-OH-traumatin was generated by nonenzymatic oxidation of (9Z)-traumatin at 60 °C for 80 min (22, 27). (7Z,10Z,13Z)-7,10,13-Hexadecatrienoic acid (16:3) was purified from MGDGs isolated from spinach leaves. Crude lipids were extracted from 1 kg of spinach leaves using the Bligh-Dyer method (28) and fractionated by column chromatography on silica gel (Wakogel C-300, Wako Pure Chemicals, Osaka, Japan) with a solvent system consisting of chloroform and acetone. The fractions eluted with chloroform/acetone (3:2, v/v) were collected. A portion (100 mg) of the purified MGDGs was submitted to alkaline hydrolysis with 50% ethanolic 3.5 m KOH in boiling water for 2 h. Then, after acidification of the reaction mixture, the fatty acids were extracted with diethyl ether. The fatty acid mixture was fractionated by preparative HPLC using a Mightysil RP-18 column (250 mm × 4.6-mm inner diameter, Kanto Chemicals, Tokyo, Japan) with acetonitrile as the elution solvent at a flow rate of 1 ml min−1. Compounds were detected by monitoring absorbance at 220 nm. Purified 16:3 eluting at ∼4 min was collected, and its identity was confirmed by GC-MS analysis after methyl esterification with 2.0 m trimethylsilyldiazomethane in hexane (Sigma-Aldrich).
Hexadecatrienoic acid was oxygenated by the activity of partially purified LOX-1 from soybean seeds. The reaction proceeded in 0.1 m sodium borate buffer at pH 9.5. LOX-1 was obtained from soybean seeds (Glycine max L., cv. Yumeyutaka) that contained only the LOX-1 isoform instead of the three isozymes found in normal soybean seeds (29). The resulting 11-HPHT was recovered with a 64% yield and was subjected to a cleavage reaction in 50 mm MES-KOH (pH 5.5) containing 0.1 mm diethylenetriamine-N,N,N′,N″,N″-pentaacetic acid (DETAPAC) using recombinant bell pepper hydroperoxide lyase (CaHPL) expressed in E. coli (30). The products were applied to a Sep-pak C18 Plus cartridge (Waters, Milford, MA) and eluted with methanol. The yield of (7Z)-dinortraumatin from 11-HPHT was 11%. 7-OH-Dinortraumatin was generated nonenzymatically by treating (7Z)-dinortraumatin at 60 °C for 80 min. The structure was assigned by LC-MS/MS analysis.
MGDG bis18:3 and MGDG 18:3/16:3 were purified from MGDG isolated from clover leaves and spinach leaves, respectively, by HPLC using a Mightysil RP-18 column (250 mm × 4.6-mm inner diameter) with methanol/water/acetonitrile (90.5:7.0:2.5, v/v/v; flow rate of 1 ml min−1). Compounds were detected by monitoring absorbance at 210 nm. Structures of the acyl groups were confirmed by GC-MS analysis after transesterification with 5% HCl/methanol at 80 °C for 2 h. According to the literature (24, 31), MGDG 18:3/16:3 was defined as sn1-O-C18:3-sn2-O-C16:3 MGDG.
MGDG-HPOs were prepared by treating purified MGDG bis18:3 and MGDG 18:3/16:3 with soybean LOX-1 (isolated as described above). Hydroperoxidation of both of the acyl groups, at the 13-position of 18:3 and the 11-position of 16:3, was confirmed by HPLC analysis as described previously (29). Bis-O-(13-hydroperoxy-(9Z,11E,15Z)-9,11,15-octadecatrienoyl)monogalactosyl diglyceride (MGDG bis13-HPOT) or O-(13-hydroperoxy-(9Z,11E,15Z)-9,11,15-octadecatrienoyl)-O-(11-hydroperoxy-(7Z,9E,13Z)-7,9,13-hexadecatrienoyl)monogalactosyl diglyceride (MGDG 13-HPOT/11-HPHT) (1 mg) was dissolved in 500 μl of methanol and diluted with 50 ml of 0.1 m MES-KOH (pH 5.5) containing 0.1 mm DETAPAC. After sonication to suspend the lipid-HPOs in the buffer, 50 μl of purified recombinant CaHPL (see above) was added to the mixture. The reaction was monitored by measuring absorbance at 234 nm after diluting a 50-μl aliquot of the reaction mixture with 950 μl of acetonitrile/water (1/1, v/v). After confirming consumption of the HPOs, the products were loaded onto a Sep-pak C18 (500 mg) cartridge that had been washed with methanol and subsequently equilibrated with 0.1 m MES-KOH (pH 5.5). The products were eluted from the cartridge with methanol. Their hydroxylated products were generated by spontaneous oxygenation at 60 °C for 80 min.
Extraction and Analysis of Free Traumatin Derivatives
Arabidopsis (No-0) leaves (2.5 g FW) were homogenized in 5 ml of 20 mm sodium phosphate buffer (pH 6.3) containing 0.1 mm DETAPAC. The homogenate was incubated for 5 min at 25 °C. Then the homogenate was acidified to pH 4.0 by adding HClO4, and 10 nmol each of 15-hydroxy-(11Z,13E)-11,13-icosadecadienoic acid (prepared from (11Z,14Z)-11,14-icosadienoic acid (Sigma-Aldrich) with soybean LOX-1) and 10-hydroxy-(E)-2-decenoic acid (Wako Pure Chemicals) were added as internal standards. Then 25 ml of methanol (containing 0.0025% butylated hydroxytoluene), 12.5 ml of chloroform, and 12.5 ml of 1% KCl solution were added to the mixture, and the aqueous and organic phases were allowed to separate. The aqueous phase was extracted again with chloroform (12.5 ml), and the combined organic phase was washed with 1% KCl solution. The solvent was removed under vacuum, and the remaining residue was dissolved in 0.7 ml of chloroform. To isolate lipids from intact leaves, the leaves (2.5 g FW) were homogenized with 10 ml of methanol containing 0.0025% butylated hydroxytoluene to avoid any enzymatic modifications of the lipid composition, and then extraction was conducted as described above.
A portion (∼1 mg) of the crude lipids was dried in a stream of N2 gas and suspended in 1 ml of 0.1 m Tris-HCl (pH 8.0) containing 0.01% sodium deoxycholate with the aid of an ultrasonic bath (US Cleaner, AS ONE Co., Osaka, Japan) under an N2 atmosphere. To the suspension 0.1 ml of 2.2% CaCl2 solution and 0.5 ml of 20 mg ml−1 pancreatin (Wako Pure Chemicals) were added. The mixture was incubated at 37 °C for 30 min. After hydrolysis, the mixture was acidified to pH 4.0 with HClO4 and applied to a Bond Elut C18 cartridge (200 mg, Agilent Technologies Inc., Santa Clara, CA) equilibrated with water. The hydrolyzed compounds were eluted with methanol.
Free forms of traumatin derivatives were analyzed by LC-MS/MS (3200 Q-TRAP LC/MS/MS System (AB Sciex, Framingham, MA) equipped with a Prominence UFLC (Shimadzu, Kyoto, Japan)). Products were separated on a Mightysil RP18 column (150 mm × 2-mm inner diameter) with a binary gradient consisting of water/formic acid (100:0.1, v/v, solvent A) and acetonitrile/formic acid (100:0.1, v/v, solvent B). The run consisted of a linear increase from 20% B to 90% B over 25 min (flow rate, 0.2 ml min−1). Compounds were detected with a photodiode array detector (SPD-M20A, Shimadzu) and by MS/MS using ESI in the negative ion mode (ion spray voltage, −4500 V; nitrogen as both the curtain gas (set to 40 arbitrary units) and collision gas (set to “high”); collision energy, −10 V; scan range, m/z 100–1200; scan speed, 4,000 Da s−1; declustering potential, −10 V) and multiple reaction monitoring (Table 1). The response curves of the traumatin derivatives versus the IS were determined and used to calculate the response factors (Table 1). To quantify traumatin derivatives, the areas of corresponding peaks in the chromatogram were integrated and divided by the peak area of the IS, and the values were corrected by the respective response factor. The peak with a signal/noise ratio of more than 10 (0.6 pmol for traumatin and dinortraumatin and 0.4 pmol for their hydroxylated derivatives; roughly corresponding to 0.15 and 0.10 nmol g FW−1, respectively) was used for calculation.
TABLE 1.
Parameters used for detection of traumatin and its derivatives
DP, declustering potential; EP, energy potential; CEP, collision energy potential; CE, collision energy.
| Compound | Q1 mass | Q3 mass | DP | EP | CEP | CE | Response factora |
|---|---|---|---|---|---|---|---|
| Da | Da | V | V | V | V | ||
| 10-Hydroxy-(2E)-decenoic acid (IS) | 184.86 | 139.0 | −30 | −9.0 | −12.00 | −22.0 | NAb |
| (7Z)-Dinortraumatin | 182.79 | 155.0 | −50 | −10.5 | −18.42 | −10.0 | 0.605 |
| 7-OH-dinortraumatin | 198.72 | 180.8 | −30 | −10.0 | −19.01 | −6.0 | 0.321 |
| (9Z)-Traumatin | 210.78 | 183.0 | −45 | −10.5 | −19.45 | −12.0 | 6.503 |
| 9-OH-traumatin | 226.79 | 208.8 | −45 | −10.5 | −20.05 | −12.0 | 3.161 |
| Formononetin (IS) | 266.96 | 252.0 | −50 | −8.5 | −16.00 | −16.0 | NA |
| MGDG-9-OH-traumatin-7-OH-dinortraumatin | 645.20 | 209.1 | −40 | −12.0 | −35.53 | −46.0 | 0.060 |
| MGDG-bis-9-OH-traumatin | 673.35 | 209.1 | −40 | −12.0 | −36.57 | −35.0 | 0.060 |
| Arabidopside A | 773.45 | 291.2 | −40 | −12.0 | −40.27 | −35.0 | NDc |
| Arabidopside B | 801.48 | 291.2 | −40 | −12.0 | −41.31 | −50.0 | ND |
a The values for free carboxylic acids were determined with 10-hydroxy-(2E)-decenoic acid, and those for MGDG-derivatives were determined with formononetin.
b NA, not applicable.
c ND, not determined.
Six-carbon aldehydes formed after complete disruption of Arabidopsis leaves were quantified by HPLC after derivatization of the aldehydes with 2,4-dinitrophenylhydrazine, as described previously (32). Under these experimental conditions, (Z)-3-hexenal was the main product, accounting for ∼85% of the GLVs formed (8).
Extraction and Analysis of Galactolipid Oxylipins
Plant leaves were thoroughly ground using a mortar and pestle, and a portion (0.1 g FW) of the slurry was mixed with 1 ml of acetone containing 0.1% butylated hydroxytoluene and 10 ng ml−1 formononetin (IS). The suspension was mixed vigorously for 10 min and then centrifuged at 17,000 × g for 10 min. The resultant green supernatant was analyzed by LC-MS/MS (see below). To determine the amounts in intact leaves, the leaves were frozen in liquid nitrogen immediately after harvest. The extraction was conducted as described above after powdering the frozen tissues.
MGDGs containing traumatin derivatives were qualitatively analyzed using the LC-PDA-MS/MS system described above. Multiple reaction monitoring analysis was conducted to quantify the compounds (Table 1). When the partially purified MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin were analyzed by LC-PDA-MS/MS, we found that there was no other compound with absorbance around 220 nm. Therefore, these compounds were tentatively quantified using the molar absorption coefficient (ϵ) reported for 4-hydroxy-(E)-2-nonenal (13,750) (33), which has the same chromophore. The response curves of MGDG 7-OH-dinortraumatin/9-OH-traumatin and MGDG bis-9-OH-traumatin versus those of IS (formononetin) were determined and used to calculate the response factors (Table 1). For quantification, the corresponding peak areas obtained by integration of the ion chromatograms were divided by that of the IS, and the values were corrected by the respective response factor. The detection limit for MGDG 7-OH-dinortraumatin/9-OH-traumatin and MGDG bis-9-OH-traumatin was 0.2 nmol g FW−1 with a signal/noise ratio on the multiple reaction monitoring chromatogram of more than 10. Arabidopsides A and B were identified from their MS and MS/MS spectra according to parameters reported by Buseman et al. (13). Because standard arabidopsides were unavailable, the ratios of their areas to that of the IS were calculated.
Purification of MDGD Bis-9-OH-traumatin and MGDG 9-OH-traumatin/7-OH-dinortraumatin
Arabidopsis (No-0) leaves (437 g) were completely disrupted with a Polytron homogenizer (PT-20sk, Kinematica AG, Luzern, Switzerland), and the homogenate was stirred vigorously for 5 min to facilitate the enzyme reaction. Crude lipids were extracted with the Bligh-Dyer method as described above. Total lipids were subjected to column chromatography on silica gel (Wakogel C-300, 220 × 20 mm inner diameter). The column was washed with chloroform/acetone (4:6, v/v), and the glycolipids were eluted with acetone/methanol (9:1, v/v). For further purification, the fraction was concentrated and subjected to preparative TLC (20 × 20 cm, silica gel 60 F254, Merck) using ethyl acetate/acetone/acetic acid (10:10:1, v/v/v) as the developing solvent. The zones containing the galactolipids of traumatin derivatives were visible under UV light and were scraped off the plates. The lipids were eluted in ethyl acetate. The fraction containing MGDGs with traumatin and its derivatives was further separated by RP-HPLC with a Mightysil RP18 column (250 × 4.6 mm) using a binary gradient consisting of acetonitrile/water/formic acid (20:80:0.1, v/v/v, solvent A) and acetonitrile/water/formic acid (50:50:0.1, v/v/v, solvent B). The run consisted of a linear increase from 100% A to 100% B over 40 min at a flow rate of 1 ml min−1. The MGDGs containing oxygenated traumatin derivatives were detected by monitoring UV absorbance at 220 nm and collected.
NMR Analysis of MDGD 9-OH-traumatin/7-OH-dinortraumatin
Fractions enriched in MDGD 9-OH-traumatin/7-OH-dinortraumatin were concentrated under vacuum. The residue was dissolved in 70 μl of CD3OD and transferred into a 2-mm NMR tube. NMR spectra were recorded using a Bruker Avance 500 NMR instrument (Bruker Biospin, Karlsruhe, Germany) operating at 500.13 MHz and equipped with a 5-mm TCI cryoprobe. The spectra were acquired at 300 K and a total of 5,000–7,000 transient signals were recorded as 32,000 data points with a spectral width of 20 ppm using a pulse sequence with water suppression (zgpurge). Selective COSY (SELCOSY) (selcogp) and selective TOCSY (SELTOCSY) (selmlgp.2) spectra were recorded using standard Bruker pulse sequences with a 10-ms excitation range and 200-ms mixing time. Data analysis was conducted using Topspin version 3.1 software (Bruker Biospin, Karlsruhe, Germany).
HPLC-HR-ESI-MS/MS Analysis of MDGD 9-OH-traumatin/7-OH-dinortraumatin
High resolution-ESI-MS analysis was performed using an Ultimate 3000 series RSLC (Dionex, Sunnyvale, CA) system and an Orbitrap XL mass spectrometer (Thermo Fisher Scientific) equipped with an ESI source. For HPLC, compounds were separated on an Acclaim C18 column (150 × 2.1-mm inner diameter, 2.2 μm; Dionex) at a constant flow rate of 300 μl min−1 with a linear gradient of 0.1% (v/v) formic acid in water to 0.1% formic acid in acetonitrile.
HR-ESI-MS spectra were measured in the negative ionization mode on the Orbitrap mass analyzer using 30,000 m/Δm resolving power. MS/MS spectra of the [M − H] ion were acquired at a collision gas energy of 35 arbitrary units. Data analysis was conducted using XCALIBUR software (Thermo Fisher Scientific).
RESULTS
Quantification of Free and Esterified Traumatin Derivatives
In extracts from intact leaf tissues of Arabidopsis (No-0), free C12/C10 oxoacids were undetectable. After complete disruption of the leaves, small amounts of traumatin and 9-OH-traumatin (∼5 and 10 nmol g FW−1, respectively) were detected (Fig. 2). Under these experimental conditions, 279 ± 47 nmol g FW−1 (Z)-3-hexenal was formed after disruption of the leaves. The markedly lower yield of free C12/C10 oxoacids prompted us to examine esterified C12/C10 oxoacid derivatives.
FIGURE 2.
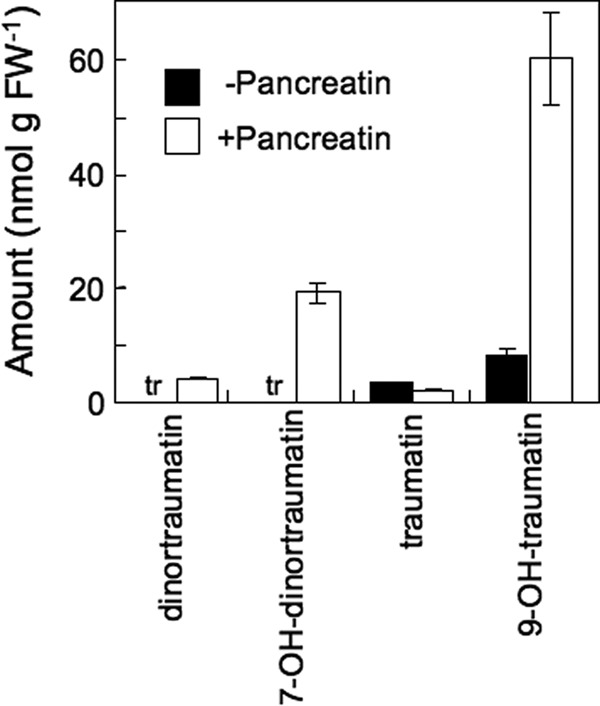
Amounts of free and esterified traumatin and its derivatives in leaves of Arabidopsis (No-0). Crude lipids prepared from disrupted Arabidopsis (No-0) leaves were treated with pancreatin or left untreated and then analyzed by LC-MS/MS. In intact leaves, the amounts of free and esterified traumatin and its derivatives were below detection limits. tr, trace. Mean values ± S.E. (error bars) are shown (n = 3).
To quantify the esterified forms of oxoacids, the crude lipid extract prepared from homogenized Arabidopsis (No-0) leaves was hydrolyzed with pancreatin, which has strong galactolipase activity and triacylglycerol lipase activity (34). The pancreatin treatment increased the amounts of 9-OH-traumatin, 7-OH-dinortraumatin, and (7Z)-dinortraumatin (Fig. 2). The amount of (9Z)-traumatin hardly increased after hydrolysis. This result indicated that the oxoacids were present as their esterified forms, probably as the acyl groups of lipids.
Detection of MGDGs Containing Traumatin Derivatives
Esterified oxylipins have been found predominantly in galactolipids (12, 13). The most abundant C6 compounds produced after wounding of Arabidopsis leaves are formed from octadecatrienoic acid (18:3) and hexadecatrienoic acid (16:3). Most 18:3 (∼65%) and 16:3 (∼96%) are found in MGDGs (24). We found that MGDGs were substrates for LOX to yield MGDG HPOs, which could be the substrates for HPL (29). Together, these findings led us to assume that a portion of esterified traumatin and its derivatives was present as the acyl moieties of MGDG. To explore this idea, we attempted to develop a method to analyze MGDGs containing oxoacids.
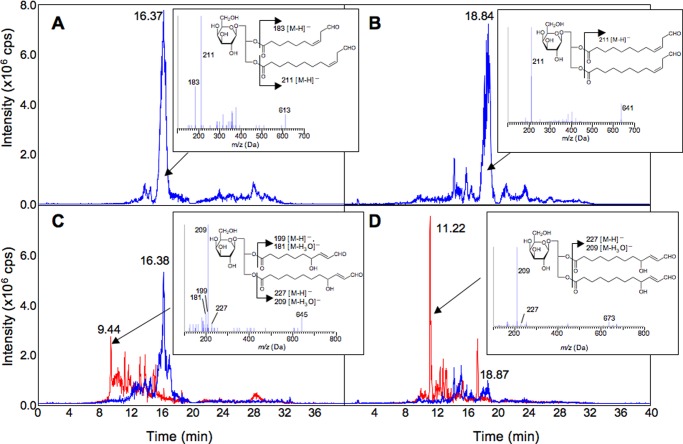
The recombinant CaHPL (30) showed significant activity toward either MGDG bis-13-HPOT or MGDG 13-HPOT/11-HPHT in the presence of deoxycholic acid. EMS scanning with LC-MS/MS showed the appearance of peaks with [M − H]− values corresponding to MGDGs containing oxoacids (Fig. 3). The products formed from MGDG 13-HPOT/11-HPHT and MGDG bis-13-HPOT were analyzed by enhanced product ion (EPI) analysis, which facilitates the detection of fragment ions corresponding to each acyl group of glycerolipids (35). This analysis suggested that the reaction products were MGDGs containing (9Z)-traumatin and/or (7Z)-dinortraumatin as their acyl groups. Incubation of MGDGs containing (9Z)-traumatin and/or (7Z)-dinortraumatin at 60 °C for 80 min resulted in decomposition of the reactants concomitant with the formation of compounds with MS profiles corresponding to MGDGs containing 9-OH-traumatin and/or 7-OH-dinortraumatin (Fig. 3). Although conclusive structural assignment required NMR analysis (as follows), we tentatively assigned the cleavage products of CaHPL with MGDG bis-13-HPOT or MGDG 13-HPOT/11-HPHT as MGDG harboring 9-OH-traumatin and 7-OH-dinortraumatin and MGDG harboring two 9-OH-traumatins, based on their MS/MS-EPI profiles. They were used as standards to detect those MGDGs formed endogenously in situ.
FIGURE 3.
Identification of HPL products formed from MGDG-bis-13-HPOT (A and C) or MGDG 13-HPOT/11-HPHT (B and D) by LC-MS/MS analysis. A portion of the reaction products was autooxidized (60 °C for 80 min) (C and D). LC-MS/MS analyses were carried out with EMS mode. The figure shows selected ion chromatograms with m/z = 613.3 ± 0.5 (blue chromatogram in A and C) (corresponding to [M − H]− of MGDG-(9Z)-traumatin-(7Z)-dinortraumatin), with m/z = 641.4 ± 0.5 (blue chromatogram in B and D) (corresponding to [M − H]− of MGDG-bis-(9Z)-traumatin), with m/z = 645.3 ± 0.5 (red chromatogram in C) (corresponding to [M − H]− of MGDG-9-OH-traumatin-7-OH-dinortraumatin), and with m/z = 673.4 ± 0.5 (red chromatogram in D) (corresponding to [M − H]− of MGDG-bis-9-OH-traumatin). The MS/MS profile (EPI mode) for each prominent peak marked with an arrow is shown in the inset. cps, counts/s.
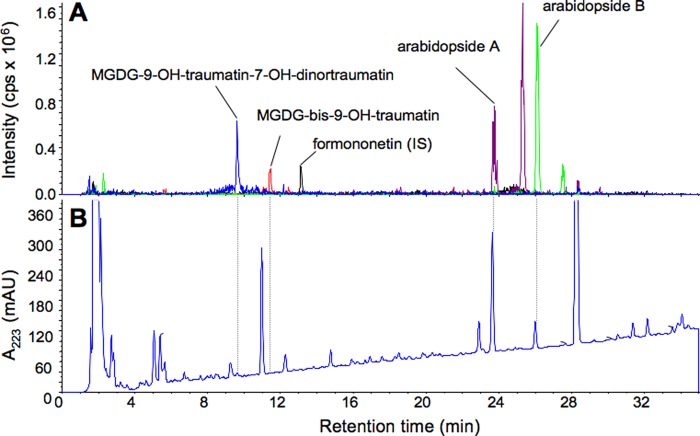
A crude lipid extract prepared from intact Arabidopsis (No-0) leaves was analyzed by LC-MS/MS to detect MGDGs containing C12 or C10 oxoacids. In intact leaf extracts, peaks corresponding to MGDGs containing C12/C10 acyl moieties were not detected. However, when the leaves were disrupted and incubated for 5 min to allow formation of GLVs, peaks corresponding to MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin were detected (Fig. 4). Neither MGDGs containing C12/C10 oxoacids without hydroxylation nor those with only one hydroxylation were detected under these experimental conditions. Arabidopsides A and B were present in very low quantities in intact leaves, but their quantities increased markedly after disruption of the leaves as reported previously (13) (Fig. 4).
FIGURE 4.
Representative chromatogram of acetone extract prepared from disrupted Arabidopsis (No-0) leaves. LC-MS/MS analyses were carried out with EMS mode. A, selected ion chromatograms with m/z = 645.3 ± 0.5 (blue) (corresponding to [M − H]− of MGDG-9-OH-traumatin-7-OH-dinortraumatin), m/z = 673.4 ± 0.5 (orange) (corresponding to [M − H]− of MGDG-bis-9-OH-traumatin), m/z = 773.5 ± 0.5 (purple) (corresponding to [M − H]− of arabidopside A), and m/z = 801.5 ± 0.5 (green) (corresponding to [M − H]− of arabidopside B). The peak at a retention time of 13.11 min corresponds to formononetin (internal standard; m/z = 267.0 ± 0.5; chromatogram in black). A trace with absorption at 223 nm is also shown at the bottom (B). cps, counts/s; mAU, milliabsorbance units.
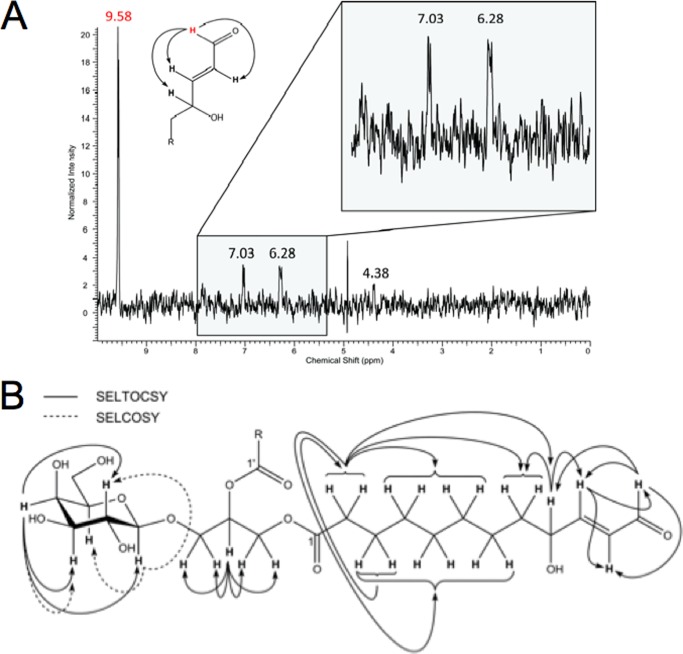
To confirm the tentative structural assignment of MGDG-9-OH-traumatin/7-OH-dinortraumatin, the compound was purified from crude lipids extracted from disrupted Arabidopsis (No-0) leaves and submitted to NMR analysis. Because of its instability, complete purification could not be accomplished. The small amount of enriched material rendered the sample unsuitable for two-dimensional NMR analysis. However, using a combination of one-dimensional SELTOCSY and SELCOSY experiments, all three structural units (β-galactose, glycerol, and fatty acid with a terminal γ-hydroxy-α,β-unsaturated aldehyde moiety) were confirmed (Fig. 5). Comparison of the 1H NMR data derived from SELTOCSY and SELCOSY spectra with those of a MDGD-derivative (36) and (E)-4-hydroxynon-2-enal (37) also confirmed the structural assignment. The molecular formula of C31H50O14 for MGDG-9-OH-traumatin/7-OH-dinortraumatin was unambiguously established by HR-ESI-MS. HR-MS/MS experiments further confirmed the loss of hydroxytraumatin or hydroxydinortraumatin fragments and revealed product ions corresponding to the glyceryl-galactoside moiety as well as dehydrated OH-traumatin and OH-dinortraumatin (Table 2). Accordingly, the structure was assigned as O-(9-hydroxy-12-oxo-(E)-10-dodecenyl)-O-(7-hydroxy-10-oxo-(E)-8-decenyl)-O-β-d-galactopyranosyl-glycerol (MGDG-9-OH-traumatin/7-OH-dinortraumatin). Based on the expected analogy in terms of reaction and structure, the MGDG with two 9-OH-traumatins was tentatively assigned as bis-O-(9-hydroxy-12-oxo-(E)-10-dodecenyl)-O-β-d-galactopyranosyl-glycerol (MGDG-bis-9-OH-traumatin).
FIGURE 5.
Structure determination with NMR. A, a representative spectrum used for structure determination. Shown is the SELTOCSY spectrum (500 MHz, CD3OD, irradiated at δ = 9.58 ppm, NS 5120) of enriched MDGD-9-OH-traumatin-7-OH-dinortraumatin, showing H,H-correlations between the aldehyde proton (δ = 9.58 ppm) and the adjacent double bond (δ = 7.03 and 6.28 ppm) as well as the hydroxymethine group (δ = 4.38 ppm). Further SELTOCSY and SELCOSY experiments were carried out with the other protons to confirm the structure. B, NMR correlations observed in MGDG-9-OH-traumatin-7-OH-dinortraumatin using SELTOCSY and SELCOSY experiments.
TABLE 2.
HPLC-HR-ESI-MS (negative ion mode) and MS/MS data of MDGD-9-OH-traumatin-7-OH-dinortraumatin
| Molecular formula | m/z calc. [M − H] | m/z obs. [M − H] | Δ | MS/MS @cid35 | Δ | Assignment |
|---|---|---|---|---|---|---|
| ppm | ppm | |||||
| C31H49O14 | 645.3122 | 645.3141 | 2.9 | [M − H] | ||
| C31H47O13 | 627.3017 | 627.3034 | 2.7 | 627.3008 | 1.4 | [M − H3O] |
| C31H45O12 | 609.2911 | 609.2928 | 2.8 | 609.2906 | 0.8 | [M − H3O − H2O] |
| C21H35O11 | 463.2179 | 463.2192 | 2.8 | [M − H − 7-HO dinortraumatin] | ||
| C21H33O10 | 445.2074 | 445.2084 | 2.2 | 445.2081 | 1.6 | [M − H3O − 7-HO dinortraumatin] |
| C19H29O10 | 417.1761 | 417.1763 | 0.5 | 417.1765 | 1.0 | [M − H3O − 9-HO traumatin] |
| C9H17O8 | 253.0923 | 253.0928 | 2.0 | Glyceryl-galactoside | ||
| C12H17O3 | 209.1178 | 209.1184 | 2.9 | 209.1182 | 1.9 | [9-OH traumatin − H3O] |
| C10H15O4 | 199.0970 | 199.0976 | 3.0 | [7-OH dinortraumatin − H] | ||
| C10H13O3 | 181.0865 | 181.0871 | 3.3 | 181.0873 | 4.4 | [7-OH dinortraumatin − H3O] |
In intact No-0 leaves, MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin were undetectable (Fig. 6). Upon disruption, they rapidly formed within 3 min, and their quantities gradually increased by 8.5 min. The amounts of MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin were almost equivalent. Detailed analysis of the extracts with EMS mode revealed that they were the sole HPL products formed after disruption, and MGDGs with non- or monohydroxylated C10 or C12 compounds were not detected.
FIGURE 6.
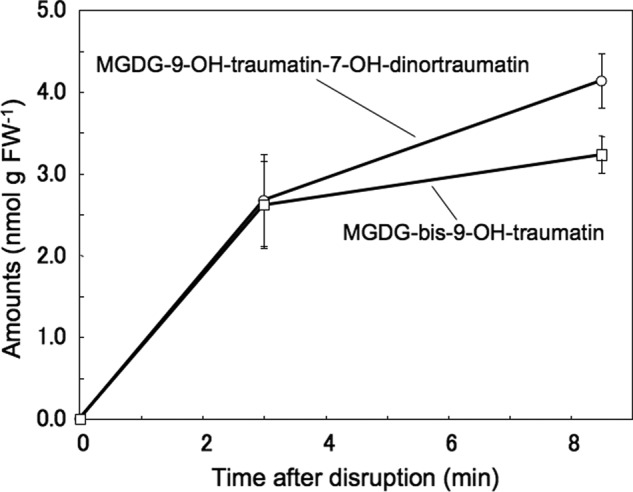
Formation of MGDG-9-OH-traumatin-7-OH-dinortraumatin (circle) and MGDG-bis-9-OH-traumatin (square) in disrupted leaves of Arabidopsis (No-0). Arabidopsis (No-0) leaves were completely disrupted with a mortar and pestle, and then a portion of the disrupted tissues was used to isolate and quantify MGDGs containing traumatin derivatives. In intact leaves at 0 min (leaves instantly frozen in liquid nitrogen), both compounds were undetectable. Mean values ± S.E. (error bars) are shown (n = 4).
Arabidopsis ecotype Col-0 has no HPL activity (23). As expected, formation of MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin was hardly observed after disruption of Col-0 leaves (Fig. 7). This result indicated that HPL activity was essential for the formation of MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin. This conclusion was confirmed by comparing their formation between hpl1 (a backcross progeny containing deleted HPL gene derived from Col-0 in Ler background) (26) and Ler lines. Again, formation of MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin was undetectable in disrupted hpl1 leaves, whereas significant amounts formed after disruption of Ler leaves.
FIGURE 7.
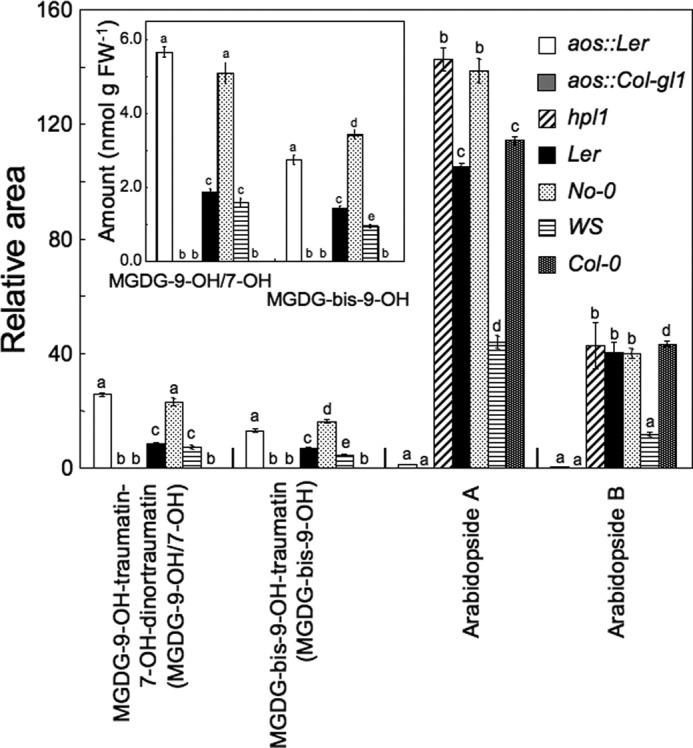
Formation of MGDG-9-OH-traumatin-7-OH-dinortraumatin, MGDG-bis-9-OH-traumatin, arabidopside A, and arabidopside B in Arabidopsis ecotypes and mutants. Leaves of each Arabidopsis line were completely disrupted, and then the oxylipin-containing MGDGs were extracted after 3 min. For arbitrary quantification, the ratio of each peak area to that of formononetin (IS) is shown. For MGDG-9-OH-traumatin-7-OH-dinortraumatin (MGDG-9-OH/7-OH) and MGDG-bis-9-OH-traumatin (MGDG-bis-9-OH), the amounts calculated based on response factors are shown in the inset. Mean values ± S.E. (error bars) are shown (n = 4). Different letters indicate significant difference (analysis of variance, Tukey's test, p < 0.01).
Arabidopsides are MGDGs containing 12-oxophytodienoic acid and/or 12-oxodinorphytodienoic acid formed via the AOS reaction (12, 13). It is assumed that arabidopsides are formed from HPOs of MGDGs; therefore, AOS and HPL may compete for the same substrates in disrupted leaf tissues. Massive formation of arabidopsides A and B was detected in the various Arabidopsis ecotypes/genotypes, whereas they were undetectable in the transfer DNA knock-out mutants, aos::Ler and aos::Col-gl1. The amounts of MGDG-9-OH-traumatin/7-OH-dinortraumatin and MGDG-bis-9-OH-traumatin formed after disruption of leaves were markedly lower in Ler leaves than in aos::Ler leaves (Fig. 7). This suggested that HPL and AOS compete at least in part for the same substrate (MGDG HPOs) to form oxylipin-containing MGDGs.
MGDG-oxoacids in Other Plant Species
It has been reported that arabidopsides occur only in A. thaliana and Arabidopsis arenosa but not in any other plant species examined so far (38). We examined whether MGDG-oxoacids were present in cabbage (B. oleracea, Brassicaceae), tobacco (N. tabacum, Solanaceae), tomato (S. lycopersicum, Solanaceae), and common bean (P. vulgaris, Fabaceae) (Table 3). As expected, arabidopsides were not detected in the disrupted leaf tissues of these four plant species. However, significant amounts of MGDG-9-OH-traumatin/7-OH-dinortraumatin and/or MGDG-bis-9-OH-traumatin were detected in all four species. The amounts varied, with lower amounts detected in tobacco than in Arabidopsis (No-0). Higher quantities formed in cabbage, tomato, and common bean leaves. The highest concentration detected was that of MGDG-bis-9-OH-traumatin (51 nmol g FW−1) in common bean leaves. Plants belonging to the Fabaceae have no 16:3, and accordingly, MGDG-9-OH-traumatin/7-OH-dinortraumatin was not detected.
TABLE 3.
Amounts of MGDG form and free form of HPL products in cabbage, tobacco, tomato, and common bean
| MGDG form |
Free form |
|||||
|---|---|---|---|---|---|---|
| (OH)C12/(OH)C10 | Bis(OH)C12 | 9-OH traumatin | Traumatin | 7-OH dinortraumatin | Dinortraumatin | |
| nmol g FW−1 | ||||||
| B. oleracea | 15.1 ± 0.65 | 8.42 ± 0.94 | 3.01 ± 0.39 | 0.17 ± 0.02 | 1.72 ± 0.20 | NDa |
| N. tabacum | 0.43 ± 0.07 | 2.28 ± 0.50 | 0.67 ± 0.02 | ND | ND | ND |
| S. lycopersicum | 3.82 ± 0.52 | 10.6 ± 1.30 | 5.84 ± 0.26 | 0.40 ± 0.03 | 1.62 ± 0.12 | ND |
| P. vulgaris | ND | 53.8 ± 2.21 | 37.9 ± 1.56 | 28.61 ± 1.08 | ND | ND |
a ND, not detected. The values are means ± S.E. (n = 4).
DISCUSSION
It has been believed that HPLs show higher affinities for free fatty acid-HPOs as substrates; thus, the C12/C10 counterparts of HPL products were assumed to be free oxoacids. In this study, we detected less than 10 nmol g FW−1 of (9Z)-traumatin and 9-OH-traumatin in their free forms, compared with ∼300 nmol g FW−1 of (Z)-3-hexenal in completely disrupted Arabidopsis (No-0) leaves. From this result, we propose that the C12/C10 counterparts are formed via a different mechanism from the canonical HPL pathway, in which free fatty acid HPOs are the substrates. It was reported that esterified (10E)-traumatin is present in runner bean (21); thus, we tried to identify esterified C12/C10 oxoacid derivatives.
Accordingly, we analyzed MGDGs containing 9-OH-traumatin and/or 7-OH-dinortraumatin not only in disrupted leaves of Arabidopsis but also those of cabbage, tobacco, tomato, and common bean. This is the first report of endogenous MGDGs containing traumatin and its derivatives in disrupted plant leaves. Previous reports have described MGDGs with 12-oxodinorphytodienoic acid and divinylether, which were thought to be formed by AOS and divinylether synthase, both of which are CYP74 enzymes closely related to HPL. However, these compounds were detected only in Arabidopsis sp. and flax plants (Linum usitatissimum), respectively (12–13, 16).
In Arabidopsis (No-0) leaves, MGDG-9-OH-traumatin/7-OH-dinortraumatin and/or MGDG-bis-9-OH-traumatin were formed quickly after disruption. This rapid formation coincided with the release of C6 aldehydes. The structures of their acyl moieties are those expected for the counterparts of canonical products of HPL activity toward free 13-HPOT and 11-HPHT, possibly followed by enzymatic or nonenzymatic peroxidation and subsequent reduction to yield hydroxylated oxo compounds (22, 27). The requirement for active HPL was confirmed by comparing formation of MGDGs containing traumatin derivatives between Arabidopsis ecotypes and mutants with/without an active HPL. We also found that recombinant CaHPL showed catalytic activity toward pure MGDG HPOs. These lines of evidence indicated that MGDG HPOs are substrates of HPL and that volatile C6 aldehydes and nonvolatile MGDG containing C12/C10 oxoacids are the products of HPL in disrupted Arabidopsis leaf tissues.
It has been widely believed that the most suitable substrates for HPL are free fatty acid HPOs. However, it was reported that the Arabidopsis HPL showed significant activity (80% of that toward the free fatty acid derivative) toward the methyl ester of 13-HPOT (39). Hughes et al. (40) reported that detergent micelles massively affect HPL and AOS activities. In leaf tissues disrupted by mechanical wounding or herbivore attack, the enzymes in the oxylipin pathway show catalytic activity toward substrates that co-exist with a wide array of biological molecules, including lipids. Thus, the reaction conditions of these enzymes in situ differ greatly from those in vitro. This study demonstrated that at least the HPLs examined here were able to utilize lipid HPOs as substrates. The substrate preferences of HPLs and related enzymes involved in oxylipin formation in disrupted leaf tissues should be reevaluated, taking these results into account.
The total amounts of C10/C12 compounds (the sum of their free and MGDG forms) detected in disrupted Arabidopsis (No-0) leaves were still substantially lower than those expected from the amounts of C6 aldehydes. It is possible that traumatin and its derivatives were esterified to other lipids. Greater amounts of traumatin derivatives than MGDG-containing traumatin derivatives were detected after pancreatin treatment of crude lipids extracted from disrupted Arabidopsis (No-0) leaves. Zoeller et al. (20) reported that in Arabidopsis (Col-0), in addition to MGDGs, also DGDGs, phosphatidylglycerols, and triacylglycerols contained significant amounts of 18:3 and 16:3 that were partially oxygenated or even fragmented under biotic stress conditions. In addition, it is likely that MGDG-hydroxylated oxoacids are further converted because 9-OH-traumatin and 7-OH-traumatin should be very reactive with various kinds of nucleophiles present in cells, such as glutathione, because such compounds contain a highly electrophilic γ-hydroxy-α,β-unsaturated aldehyde moiety (41–42). In the present study, the amounts of analytes detected may have been underestimated because of the spontaneous degradation of these reactive compounds, especially the hydroperoxides that may have formed as intermediates, during analysis. To match the reactions that occur in disrupted leaf tissues with the predicted stoichiometry between C6 and C12 products of HPL, an accurate system to quantify the possible metabolites must be established.
A comparison of the amounts of arabidopsides and MGDG with traumatin derivatives between aos::Ler and Ler suggested that AOS and HPL competed for the same substrates, MGDG-HPOs, in disrupted Arabidopsis leaf tissues. AtLOX2 is involved in supplying the substrate, at least for AOS (15). Because significant amounts of these lipid oxylipins formed rapidly, it was assumed that substantial amounts of both AOS and HPL were present in intact leaf cells in their latent forms or were located in different cellular compartments, separated from their substrates. Arabidopsides were not formed in leaves of cabbage, tobacco, tomato, and common bean, although these species formed significant amounts of MGDG containing traumatin derivatives as well as GLVs after disruption of leaf tissues. These findings suggested that the nature of the Arabidopsis AOS differed from that of AOSs in these plants. AOSs in leaves of cabbage, tobacco, tomato, and common bean may not be able to utilize MDGD-HPOs, or the amount of AOS in intact leaf cells might be too low to compete with HPL.
These findings do not exclude the possibility that GLVs can be formed via the lipase reaction with lipids to form 18:3 and 16:3. It is still possible that the free C12/C10 compounds are re-esterified; however, this possibility is unlikely because arabidopsides form on lipids without hydrolysis, as shown in a series of excellent experiments using isotopes (18). The formation of esterified and free oxylipins is regulated differently between natural and stress-induced senescence (43). The biosynthesis of free oxylipins definitely requires lipases, such as AtDAD1 (44) or NaGPA1 (45). GLV formation occurs even in intact plant tissues distant from the site of herbivore damage (46), after pathogen infection (47), under heat stress, after sudden darkness (48), or even after treating the tissues with pathogen-induced plant volatiles (49). In an analogous situation to the formation of free JAs, hydrolysis of free fatty acids may be required for the formation of GLVs in intact tissues under stress. This idea is partly supported by the fact that free JA production was enhanced in transgenic rice suppressing HPL (50).
γ-Hydroxy-α,β-unsaturated aldehydic esters of phosphatidylcholine, such as 1-palmitoyl-2-(5-hydroxy-8-oxooct-6-enoyl)-sn-glycero-3-phosphocholine, have been identified in human atherosclerotic lesions (51). The truncated, terminally oxidized phospholipids, which were formed through autooxidation and subsequent cleavage of phospholipids with polyunsaturated fatty acids, were proposed to be ligands for the macrophage scavenger receptor CD36 (52). Because of the high reactivity of γ-hydroxy-α,β-unsaturated aldehydes with biological molecules through the Michael addition reaction (42), MGDGs with γ-hydroxy-α,β-unsaturated aldehyde moieties might also have potent bioactivity. To gain further insights into the physiological and ecological significance of the formation of MGDGs with γ-hydroxy-α,β-unsaturated aldehydes, the fate of these lipid oxylipins must be identified.
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 23580151 and Core-to-Core Project 20004 and by the Yamaguchi University Yobimizu Project.
- GLV
- green leaf volatile
- HPL
- hydroperoxide lyase
- MGDG
- monogalactosyldiacylglycerol
- DGDG
- digalactosyldiacylglycerol
- 13-HPOT
- α-linolenic acid 13-hydroperoxide
- 11-HPHT
- hexadecatrienoic acid 11-hydroperoxide
- HPO
- hydroperoxide
- (9Z)-traumatin
- 12-oxo-(Z)-9-dodecenoic acid
- (10E)-traumatin
- 12-oxo-(E)-10-dodecenoic acid
- (7Z)-dinortraumatin
- 10-oxo-(Z)-7-decenoic acid
- 9-OH-traumatin
- 12-oxo-9-hydroxy-(E)-10-dodecenoic acid
- 7-OH-dinortraumatin
- 10-oxo-7-hydroxy-(E)-8-decenoic acid
- AOS
- allene oxide synthase
- JA
- jasmonic acid
- IS
- internal standard
- ESI
- electrospray ionization
- EMS
- enhanced mass spectrometry
- EPI
- enhanced product ion scan
- LOX
- lipoxygenase
- DETAPAC
- diethylenetriamine-N,N,N′,N″,N″-pentaacetic acid
- CaHPL
- bell pepper hydroperoxide lyase
- FW
- fresh weight
- HR
- high resolution
- SELCOSY and SELTOCSY
- selective COSY and TOCSY, respectively.
REFERENCES
- 1. Matsui K. (2006) Green leaf volatiles. Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9, 274–280 [DOI] [PubMed] [Google Scholar]
- 2. D'Auria J. C., Pichersky E., Schaub A., Hansel A., Gershenzon J. (2007) Characterization of BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 49, 194–207 [DOI] [PubMed] [Google Scholar]
- 3. Allmann S., Baldwin I. T. (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329, 1075–1078 [DOI] [PubMed] [Google Scholar]
- 4. Hammond D. G., Rangel S., Kubo I. (2000) Volatile aldehydes are promising broad-spectrum postharvest insecticides. J. Agric. Food Chem. 48, 4410–4417 [DOI] [PubMed] [Google Scholar]
- 5. Nakamura S., Hatanaka A. (2002) Green-leaf-derived C6 aroma compounds with potent antibacterial action that act on both gram-negative and gram-positive bacteria. J. Agric. Food Chem. 50, 7639–7644 [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto K., Matsui K., Ozawa R., Takabayashi J. (2008) Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 69, 2127–2132 [DOI] [PubMed] [Google Scholar]
- 7. Hubert J., Münzbergová Z., Santino A. (2008) Plant volatile aldehydes as natural insecticides against stored-product beetles. Pest Manag. Sci. 64, 57–64 [DOI] [PubMed] [Google Scholar]
- 8. Matsui K., Sugimoto K., Mano J., Ozawa R., Takabayashi J. (2012) Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS One 7, e36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arimura G., Matsui K., Takabayashi J. (2009) Chemical and molecular ecology of herbivore-induced plant volatiles. Proximate factors and their ultimate functions. Plant Cell Physiol. 50, 911–923 [DOI] [PubMed] [Google Scholar]
- 10. Grechkin A. N., Hamberg M. (2004) The “heterolytic hydroperoxide lyase” is an isomerase producing a short-lived fatty acid hemiacetal. Biochim. Biophys. Acta 1636, 47–58 [DOI] [PubMed] [Google Scholar]
- 11. Mosblech A., Feussner I., Heilmann I. (2009) Oxylipins. Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 47, 511–517 [DOI] [PubMed] [Google Scholar]
- 12. Stelmach B. A., Müller A., Hennig P., Gebhardt S., Schubert-Zsilavecz M., Weiler E. W. (2001) A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl diglyceride, from Arabidopsis thaliana. J. Biol. Chem. 276, 12832–12838 [DOI] [PubMed] [Google Scholar]
- 13. Buseman C. M., Tamura P., Sparks A. A., Baughman E. J., Maatta S., Zhao J., Roth M. R., Esch S. W., Shah J., Williams T. D., Welti R. (2006) Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol. 142, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsson A. K., Fahlberg P., Ellerström M., Andersson M. X. (2012) Oxo-phytodienoic acid (OPDA) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Lett. 586, 2483–2487 [DOI] [PubMed] [Google Scholar]
- 15. Glauser G., Dubugnon L., Mousavi S. A., Rudaz S., Wolfender J.-L., Farmer E. E. (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 284, 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chechetkin I. R., Mukhitova F. K., Blufard A. S., Yarin A. Y., Antsygina L. L., Grechkin A. N. (2009) Unprecedented pathogen-inducible complex oxylipins from flax. Linolipins A and B. FEBS J. 276, 4463–4472 [DOI] [PubMed] [Google Scholar]
- 17. Farmer E. E., Mueller M. J. (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 64, 429–450 [DOI] [PubMed] [Google Scholar]
- 18. Mène-Saffrané L., Dubugnon L., Chételat A., Stolz S., Gouhier-Darimont C., Farmer E. E. (2009) Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J. Biol. Chem. 284, 1702–1708 [DOI] [PubMed] [Google Scholar]
- 19. Ibrahim A., Schültz A.-L., Galano J.-M., Herrfurth C., Feussner K., Durand T., Brodhun F., Feussner I. (2011) The alphabet of galactolipids in Arabidopsis thaliana. Front. Plant Sci. 2, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zoeller M., Stingl N., Krischke M., Fekete A., Waller F., Berger S., Mueller M. J. (2012) Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes. Biogenesis of pimelic and azelaic acid. Plant Physiol. 160, 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zimmerman D. C., Coudron C. A. (1979) Identification of traumatin, a wound hormone, as 12-oxo-trans-10-dodecenoic acid. Plant Physiol. 63, 536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kallenbach M., Gilardoni P. A., Allmann S., Baldwin I. T., Bonaventure G. (2011) C12 derivatives of the hydroperoxide lyase pathway are produced by product recycling through lipoxygenase-2 in Nicotiana attenuata leaves. New Phytol. 191, 1054–1068 [DOI] [PubMed] [Google Scholar]
- 23. Duan H., Huang M. Y., Palacio K., Schuler M. A. (2005) Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol. 139, 1529–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li-Beisson Y., Shorrosh B., Beisson F., Andersson M. X., Arondel V., Bates P. D., Baud S., Bird D., Debono A., Durrett T. P., Franke R. B., Graham I. A., Katayama K., Kelly A. A., Larson T., Markham J. E., Miquel M., Molina I., Nishida I., Rowland O., Samuels L., Schmid K. M., Wada H., Welti R., Xu C., Zallot R., Ohlrogge J. (2010) Acyl-lipid Metabolism. Arabidopsis Book 8, e0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park J. H., Halitschke R., Kim H. B., Baldwin I. T., Feldmann K. A., Feyereisen R. (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 31, 1–12 [DOI] [PubMed] [Google Scholar]
- 26. Shiojiri K., Ozawa R., Matsui K., Sabelis M. W., Takabayashi J., (2012) Intermittent exposure to traces of green leaf volatiles triggers a plant response. Sci. Rep. 2, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noordermeer M. A., Feussner I., Kolbe A., Veldink G. A., Vliegenthart J. F. G. (2000) Oxygenation of (3Z)-alkenals to 4-hydroxy-(2E)-alkenals in plant extracts. A nonenzymatic process. Biochem. Biophys. Res. Commun. 277, 112–116 [DOI] [PubMed] [Google Scholar]
- 28. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 29. Nakashima A., Iijima Y., Aoki K., Shibata D., Sugimoto K., Takabayashi J., Matsui K. (2011) Monogalactosyl diacylglycerol is a substrate for lipoxygenase: Its implications for oxylipin formation directly from lipids. J. Plant Interact. 6, 93–97 [Google Scholar]
- 30. Matsui K., Shibutani M., Hase T., Kajiwara T. (1996) Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B). FEBS Lett. 394, 21–24 [DOI] [PubMed] [Google Scholar]
- 31. Sakaki T., Saito K., Kawaguchi A., Kondo N., Yamada M. (1990) Conversion of monogalactosyldiacylglycerols to triacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 94, 766–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsui K., Sugimoto K., Kakumyan P., Khorobrykh S. A., Mano J. (2009) Volatile oxylipins and related compounds formed under stress in plants. Methods Mol. Biol. 580, 17–28 [DOI] [PubMed] [Google Scholar]
- 33. Esterbauer H., Weger W. (1967) Über die Wirkungen von Aldehyden auf gesunde und maligne Zellen, 3. Mitt.: Synthese von homologen 4-Hydroxy-2-alkenalen, II. Monatsh. Chem. 98, 1994–2000 [Google Scholar]
- 34. Sias B., Ferrato F., Grandval P., Lafont D., Boullanger P., De Caro A., Leboeuf B., Verger R., Carrière F. (2004) Human pancreatic lipase-related protein 2 is a galactolipase. Biochemistry 43, 10138–10148 [DOI] [PubMed] [Google Scholar]
- 35. Nakanishi H., Iida Y., Shimizu T., Taguchi R. (2010) Separation and quantification of sn-1 and sn-2 fatty acid positional isomers in phosphatidylcholine by RPLC-ESIMS/MS. J. Biochem. 147, 245–256 [DOI] [PubMed] [Google Scholar]
- 36. Hisamatsu Y., Goto N., Hasegawa K., Shigemori H. (2006) A glycolipid involved in flower bud formation of Arabidopsis thaliana. Bot. Stud. 47, 45–50 [Google Scholar]
- 37. Komisarski M., Kaczmarska Z., Kuśmierek J. T. (2009) Practical highly enantioselective synthesis of (R)- and (S)-(E)-4-hydroxynon-2-enal. Acta Biochim. Pol. 56, 189–193 [PubMed] [Google Scholar]
- 38. Böttcher C., Weiler E. W. (2007) Cyclo-oxylipin-galactolipids in plants. occurrence and dynamics. Planta 226, 629–637 [DOI] [PubMed] [Google Scholar]
- 39. Kandzia R., Stumpe M., Berndt E., Szalata M., Matsui K., Feussner I., (2003) On the specificity of lipid hydroperoxide fragmentation by fatty acid hydroperoxide lyase from Arabidopsis thaliana. J. Plant Physiol. 160, 803–809 [DOI] [PubMed] [Google Scholar]
- 40. Hughes R. K., Belfield E. J., Casey R. (2006) CYP74C3 and CYP74A1, plant cytochrome P450 enzymes whose activity is regulated by detergent micelle association, and proposed new rules for the classification of CYP74 enzymes. Biochem. Soc. Trans. 34, 1223–1227 [DOI] [PubMed] [Google Scholar]
- 41. Bochkov V. N., Oskolkova O. V., Birukov K. G., Levonen A.-L., Binder C. J., Stöckl J. (2010) Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 12, 1009–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farmer E. E., Davoine C. (2007) Reactive electrophile species. Curr. Opin. Plant Biol. 10, 380–386 [DOI] [PubMed] [Google Scholar]
- 43. Seltmann M. A., Stingl N. E., Lautenschlaeger J. K., Krischke M., Mueller M. J., Berger S. (2010) Differential impact of lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis. Plant Physiol. 152, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishiguro S., Kawai-Oda A., Ueda J., Nishida I., Okada K. (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonaventure G., Schuck S., Baldwin I. T. (2011) Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis. A specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ. 34, 1507–1520 [DOI] [PubMed] [Google Scholar]
- 46. Rose U., Manukian A., Heath R. R., Tumlinson J. H. (1996) Volatile semiochemicals released from undamaged cotton leaves. A systemic response of living plants to caterpillar damage. Plant Physiol. 111, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Piesik D., Lemńczyk G., Skoczek A., Lamparski R., Bocianowski J., Kotwica K., Delaney K. J. (2011) Fusarium infection in maize. Volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. J. Plant Physiol. 168, 1534–1542 [DOI] [PubMed] [Google Scholar]
- 48. Loreto F., Barta C., Brilli F., Nogues I. (2006) On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 29, 1820–1828 [DOI] [PubMed] [Google Scholar]
- 49. Engelberth J., Alborn H. T., Schmelz E. A., Tumlinson J. H. (2004) Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. U.S.A. 101, 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu X., Li F., Tang J., Wang W., Zhang F., Wang G., Chu J., Yan C., Wang T., Chu C., Li C. (2012) Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice. PLoS One 7, e50089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng Y., Salomon R. G. (1998) Total synthesis of γ-hydroxy-α, β-unsaturated aldehydic esters of cholesterol and 2-lysophosphatidylcholine. J. Org. Chem. 63, 7779–7794 [Google Scholar]
- 52. Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., Silverstein R. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277, 38517–38523 [DOI] [PubMed] [Google Scholar]