Background: The dopamine D4 receptors in prefrontal cortex (PFC) play a key role in mental health and disorders.
Results: D4 activation caused a bi-directional, homeostatic regulation of glutamatergic responses in rats exposed to acute or chronic stress.
Conclusion: The altered synaptic excitation in stress conditions is restored by D4 signaling in PFC.
Significance: It provides a potential mechanism for the role of D4 in stress-related neuropsychiatric disorders.
Keywords: Calcium/Calmodulin-dependent Protein Kinase (CaMK); Dopamine Receptors; Glutamate Receptors, Ionotropic (AMPA, NMDA); Neurophysiology; Stress
Abstract
The prefrontal cortex (PFC), a key brain region for cognitive and emotional processes, is highly regulated by dopaminergic inputs. The dopamine D4 receptor, which is enriched in PFC, has been implicated in mental disorders, such as attention deficit-hyperactivity disorder and schizophrenia. Recently we have found homeostatic regulation of AMPA receptor-mediated synaptic transmission in PFC pyramidal neurons by the D4 receptor, providing a potential mechanism for D4 in stabilizing cortical excitability. Because stress is tightly linked to adaptive and maladaptive changes associated with mental health and disorders, we examined the synaptic actions of D4 in stressed rats. We found that neural excitability was elevated by acute stress and dampened by repeated stress. D4 activation produced a potent reduction of excitatory transmission in acutely stressed animals and a marked increase of excitatory transmission in repeatedly stressed animals. These effects of D4 targeted GluA2-lacking AMPA receptors and relied on the bi-directional regulation of calcium/calmodulin kinase II activity. The restoration of PFC glutamatergic transmission in stress conditions may enable D4 receptors to serve as a synaptic stabilizer in normal and pathological conditions.
Introduction
The dopaminergic system in prefrontal cortex (PFC)2 plays a key role in regulating high level cognitive functions, such as working memory (1, 2). Dopamine D4 receptors, which are largely restricted to PFC neurons (3, 4), are critically involved in PFC functions both under normal condition and in many neuropsychiatric disorders (5, 6). For example, attention deficit hyperactivity disorder and the responsiveness to its treatment have been associated with a D4 gene polymorphism that weakens D4 receptor function (7–10). The highly effective antipsychotic drug used in schizophrenia treatment, clozapine, has a high affinity to D4 receptors (11, 12). Preclinical studies also indicate that D4 receptor antagonists alleviate stress-induced working memory problems in monkeys (13) and ameliorate cognitive deficits caused by psychotomimetic drugs (14). Mice lacking D4 receptors exhibit supersensitivity to psychomotor stimulants, reduced exploration of novel stimuli, cortical hyperexcitability, and juvenile hyperactivity and impulsive behaviors associated with attention deficit hyperactivity disorder (15–18).
To understand the role of D4 in mental health and disorders, it is important to elucidate the molecular and cellular mechanisms underlying the impact of D4 on cortical excitability and working memory. It has been proposed that glutamate receptor-mediated synaptic transmission that controls PFC neuronal activity is crucial for working memory (19). Dysfunction of glutamatergic transmission is considered as the core feature and fundamental pathology of mental disorders (20–22). Recently we have demonstrated that D4 stimulation causes a profound depression of AMPA receptor (AMPAR) responses in PFC pyramidal neurons when their activity is elevated in vitro and causes a marked potentiation of AMPAR responses when their activity is dampened in vitro (23, 24). It suggests that D4 receptors may use the homeostatic control of glutamatergic transmission to stabilize the activity of PFC circuits.
The PFC is one of the primary targets of stress hormones (25). Stress has profound and divergent effects on the body and the brain. Acute stress is essential for adaptation and maintenance of homeostasis, whereas chronic stress often exacerbates deficiencies in emotional and cognitive processes associated with psychiatric disorders, such as depression, anxiety, and posttraumatic stress disorder (25, 26). In this study, we sought to determine whether animals exposed to acute or repeated behavioral stress have altered neuronal activity in vivo and whether D4 exerts an activity-dependent regulation of glutamatergic transmission in PFC pyramidal neurons from stressed animals. Knowledge gained from this study should help us understand the role of D4 in the adaptive and maladaptive changes linked to stress.
EXPERIMENTAL PROCEDURES
Stress Paradigm
All experiments were conducted in accordance to the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo. Juvenile (3–4 weeks old) Sprague-Dawley male rats were used in this study. For acute stress, rats were restrained in air-accessible cylinders for 2 h (10:00 a.m. to 12:00 p.m.) as described previously (27, 28). For repeated stress, rats were restrained daily (2 h) for 5–7 days.
Electrophysiological Recording in Slices
PFC slice preparation procedures were similar to what was described previously (29). In brief, animals were anesthetized by inhaling 2-bromo-2-chloro-1,1,1-trifluoroethane (1 ml/100 g; Sigma) and decapitated. Brains were quickly removed and sliced (300 μm) with a Leica VP1000S Vibrotome while bathed in a HEPES-buffered salt solution. Slices were then incubated for 1–5 h at room temperature (22–24 °C) in artificial cerebrospinal fluid (ACSF) (130 mm NaCl, 26 mm NaHCO3, 1 mm CaCl2, 5 mm MgCl2, 3 mm KCl, 10 mm glucose, 1.25 mm NaH2PO4) bubbled with 95% O2, 5% CO2.
Standard voltage clamp recording techniques were used to measure AMPAR-EPSC in layer V PFC pyramidal neurons as described before (23, 28). Neurons were visualized with a ×40 water-immersion lens and illuminated with near infrared (IR) light, and the image was captured with an IR-sensitive CCD camera. Recordings were obtained using a Multiclamp 700A amplifier (Axon Instruments). Tight seals (2–10 gigohms) were obtained by applying negative pressure. The membrane was disrupted with additional suction, and the whole-cell configuration was obtained. Neurons were held at −70 mV, and EPSCs were evoked by stimulating the neighboring neurons with a bipolar tungsten electrode (FHC, Inc.). The internal solution contained 130 mm cesium methanesulfonate, 10 mm CsCl, 4 mm NaCl, 1 mm MgCl2, 10 mm HEPES, 5 mm EGTA, 2.2 mm QX-314, 12 mm phosphocreatine, 5 mm MgATP, 0.5 mm Na2GTP, 0.1 mm leupeptin, pH 7.2–7.3, 265–270 mosm. To record miniature EPSCs (mEPSCs) in the slice, 1 mm MgCl2-containing ACSF (tetrodotoxin added) was used.
Whole-cell current clamp techniques were used to measure action potential firing (30). During the recording of spontaneous firing, PFC slices were bathed in a modified ACSF with reduced Mg2+ (0.5 mm) and slightly elevated K+ (3.5 mm) at room temperature. This modified ACSF is more similar to in vivo rodent CSF than standard ACSF (31). The internal solution contained 100 mm potassium gluconate, 20 mm KCl, 10 mm HEPES, 4 mm MgATP, 0.3 mm NaGTP, 10 mm phosphocreatine, and 0.1 mm leupeptin, pH 7.2–7.3, 265–270 mosm. A small depolarizing current was applied to adjust the interspike potential to −60 mV.
Dopamine receptor ligands PD168077 maleate (Tocris) and the second messenger reagents KN-93, KN-92, purified active CaMKIIα protein (74 kDa; Abcam ab60899) and CaM proteins (Calbiochem) were made up as concentrated stocks in water or dimethyl sulfoxide and stored at −20 °C or −80 °C. Stocks were thawed and diluted immediately before use. Reagents were dialyzed into neurons through the patch electrode for 20 min before electrophysiological recordings were started. Data analyses were performed with the Clampfit software (Axon Instruments). Experiments with two groups were analyzed statistically using Student's t tests. Experiments with more than two groups were subjected to one-way ANOVA followed by post hoc Tukey tests. Miniature synaptic currents were analyzed with Mini Analysis Program (Synaptosoft, Leonia, NJ). Statistical comparisons of mEPSCs were made using the Kolmogorov-Smirnov test.
Western Blotting
After treatment, slices were homogenized in boiling 1% SDS, followed by centrifugation (13,000 × g, 20 min). The supernatant fractions were subjected to 7.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk for 1 h at room temperature followed by incubation with various primary antibodies of CaMKII (1:1000; Santa Cruz Biotechnology sc-9035) and Thr(P)286-CaMKII (1:1000; Santa Cruz Biotechnology sc-12886). After incubation with horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich), the blots were exposed to the enhanced chemiluminescence substrate (Amersham Biosciences). Quantification was obtained from densitometric measurements of immunoreactive bands on film using ImageJ software.
RESULTS
Neuronal Excitability, Which Is Elevated by Acute Stress and Dampened by Repeated Stress, Is Restored by D4 Activation
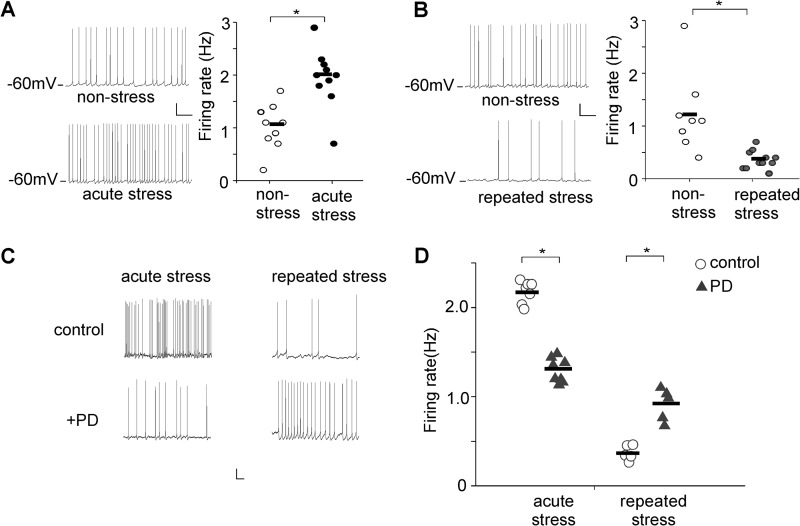
To compare overall excitability of PFC circuits in control versus stressed animals, we measured the spontaneous action potential firing, which helps to reveal the circuit excitability changes induced by altered synaptic drive onto pyramidal neurons (32). As shown in Fig. 1A, compared with neurons from nonstressed (NS) control rats, the firing rate was significantly increased in neurons from acutely stressed (AS) animals (NS: 1.14 ± 0.25 Hz, n = 9; AS: 2.04 ± 0.31 Hz, n = 10, p < 0.01, t test). On the other hand, a significant reduction in the firing rate was observed in repeatedly stressed (RS) animals (Fig. 1B, NS: 1.25 ± 0.41 Hz, n = 8; RS: 0.37 ± 0.10 Hz, n = 11, p < 0.01, t test). These data indicate that the excitability of PFC pyramidal neurons is elevated by acute stress and dampened by chronic stress. Thus, stress provides a physiological condition that has bi-directional changes in neuronal activity in vivo.
FIGURE 1.
In vivo stress alters the excitability of PFC pyramidal neurons. A and B, representative spontaneous firing recordings and scatter plots of firing rates of PFC pyramidal neurons from nonstressed versus acutely stressed (A) or repeatedly stressed (B) rats. Scale bars, 20 mV, 2 s. *, p < 0.01, t test. C and D, representative spontaneous firing recordings and scatter plots of firing rates of PFC pyramidal neurons showing the effect of PD168077 (40 μm) in stressed (acutely or repeatedly) rats. Scale bars, 20 mV, 2 s. *, p < 0.01, t test.
We then examined the impact of D4 receptor activation on the excitability of PFC pyramidal neurons from stressed animals. As shown in Fig. 1, C and D, application of the D4 receptor agonist PD168077 (40 μm) produced a significant reducing effect on the firing rate in acutely stressed animals (control: 2.14 ± 0.04 Hz, PD: 1.33 ± 0.03 Hz, n = 7). On the other hand, PD168077 significantly enhanced the firing rate in repeatedly stressed animals (control: 0.39 ± 0.02 Hz, PD: 0.97 ± 0.04 Hz, n = 5). Thus, the PFC neuronal excitability has been restored by D4 in stress conditions.
D4 Restores the Excitatory Synaptic Transmission in Stressed Animals
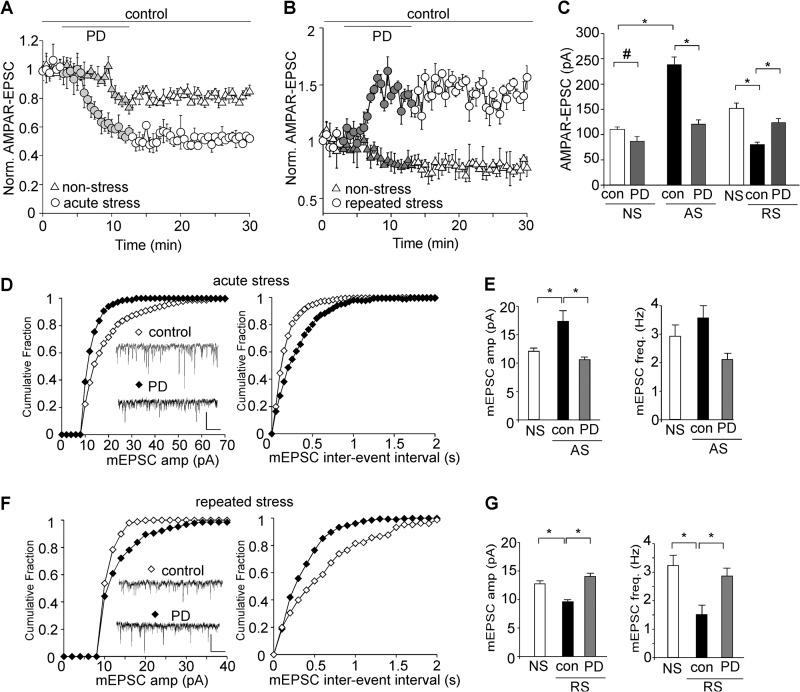
Because D4 stimulation induces an activity-dependent regulation of glutamatergic transmission in vitro (23), we next examined the effect of D4 in PFC neurons from animals whose neural activity has been perturbed by in vivo stressors. As shown in Fig. 2, A–C, the basal evoked AMPAR-EPSC amplitude was markedly increased by acute stress (NS: 109.8 ± 5.1 pA, n = 12; AS: 237.9 ± 15.1 pA, n = 16; p < 0.001, ANOVA) and was substantially decreased by repeated stress (NS: 151.4 ± 10.5 pA, n = 16; RS: 80.4 ± 4.4 pA, n = 19, p < 0.001, ANOVA). Application of PD168077 (40 μm) slightly reduced AMPAR-EPSC in nonstressed animals (23.0 ± 3.1% reduction, n = 7, p < 0.05, ANOVA), and this reducing effect of PD168077 was significantly augmented in acutely stressed animals (49.2 ± 4.5% reduction, n = 16, p < 0.001, ANOVA). On the other hand, PD168077 significantly enhanced AMPAR-EPSC in repeatedly stressed animals (53.6 ± 6.5% enhancement, n = 14, p < 0.001, ANOVA). Thus, the excitatory synaptic strength has been brought back to the control level by D4 in stress conditions (AS+PD: 120.8 ± 8.3 pA, n = 16; RS+PD: 123.6 ± 7.3 pA, n = 14).
FIGURE 2.
The stress-induced alteration of glutamatergic transmission is restored by D4 activation. A and B, plot of normalized AMPAR-EPSC showing the effect of PD168077 (40 μm) in nonstressed versus animals exposed to acute stress (A) or repeated stress (B). C, Bar graphs (mean ± S.E. (error bars)) showing the amplitude of EPSC in PFC neurons from nonstressed, acutely stressed, or repeatedly stressed animals before and after PD168077 application. #, p < 0.05; *, p < 0.001, ANOVA. D and F, cumulative distribution plot showing the effect of PD168077 (40 μm) on miniature EPSC amplitude and interevent interval in animals exposed to acute stress (D) or repeated stress (F). Inset, representative mEPSC traces. Scale bar, 20 pA, 1 s. E and G, bar graphs (mean ± S.E.) showing the amplitude and frequency of mEPSC in PFC neurons from different groups (NS, AS, RS) before and after PD168077 application. *, p < 0.001, ANOVA.
We next measured AMPAR-mediated mEPSC, a response from quantal release of single glutamate vesicles. Acute stress caused a significant increase of the mEPSC amplitude (NS: 12.1 ± 0.6 pA; AS: 17.2 ± 2.0 pA, p < 0.001, ANOVA), which was recovered to the nonstressed level by PD168077 (10.9 ± 0.3 pA, 2.1 ± 0.2 Hz, n = 8, Fig. 2, D and E). The mEPSC frequency was not significantly changed by acute stress, consistent with our previous report (27). On the other hand, the mEPSC amplitude and frequency were significantly reduced in animals exposed to repeated stress (9.5 ± 0.3 pA, 1.5 ± 0.3 Hz, n = 7), and PD168077 restored mEPSC similar to that observed in nonstressed animals (14 ± 0.5 pA, 2.8 ± 0.3 Hz, n = 7, Fig. 2, F and G). Taken together, these results suggest that D4 activation provides a homeostatic regulation of excitatory synaptic transmission in stressed animals.
The Homeostatic Effect of D4 Targets Mainly GluA2-lacking AMPA Receptors
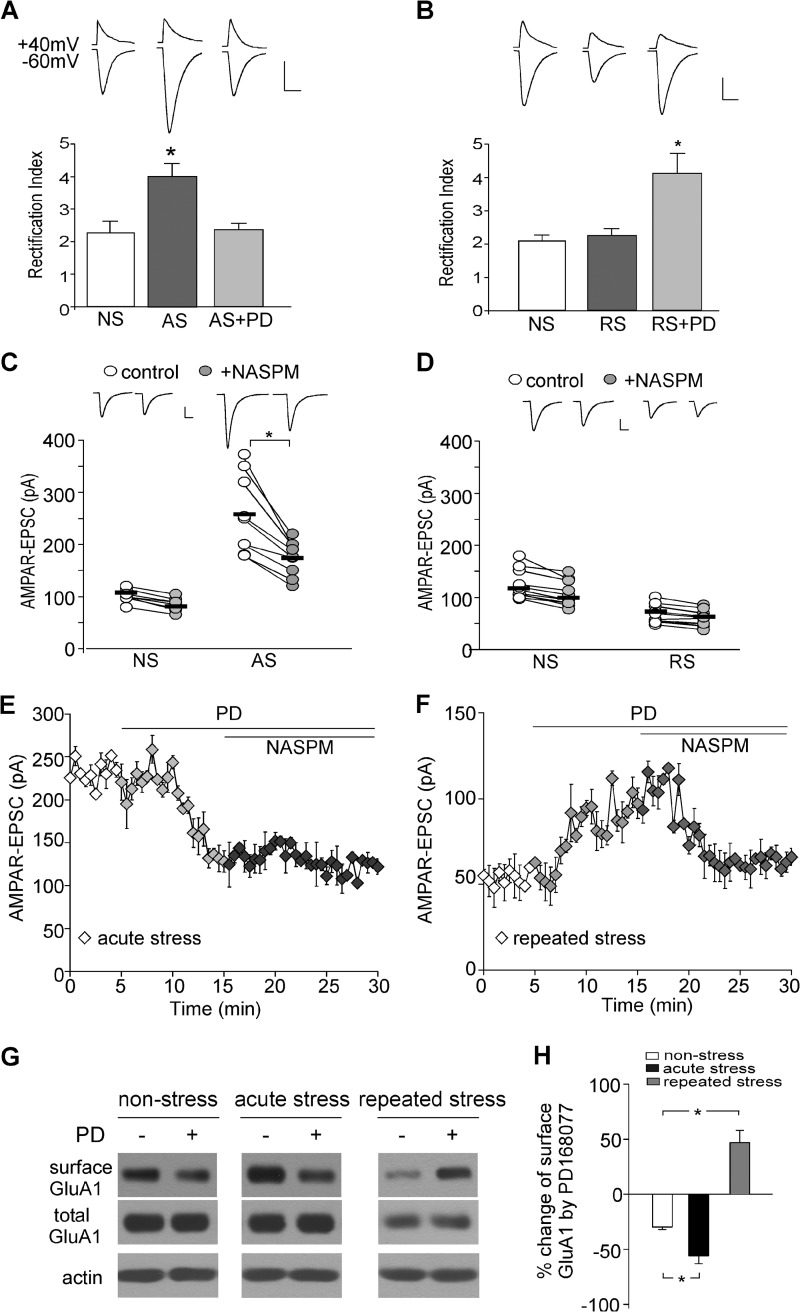
Because GluA2-containing and GluA2-lacking AMPAR channels have distinct channel conductance, open probability, Ca2+ permeability and rectification, we examined whether D4 receptors differentially affect AMPAR subunits in stress conditions. It has been found that GluA2-lacking AMPARs have prominent rectification (33), and changes in GluA2-lacking AMPARs alter the inward rectification due to voltage-dependent blockade of intracellular polyamine (34). Thus, rectification index (RI) of AMPAR responses (ratio of the current amplitude at −60 mV to that at +40 mV) was measured with a spermine (100 μm)-containing intracellular solution (35). As shown in Fig. 3A, the RI in acutely stressed animals was much bigger than that in nonstressed control animals (NS: 2.3 ± 0.34, n = 7; AS: 4.0 ± 0.39, n = 11; p < 0.001, ANOVA), suggesting that acute stress may predominantly increase GluA2-lacking AMPARs at the synapse. Application of PD168077 (40 μm) reduced the RI back to the control level (2.4 ± 0.2, n = 11), indicating that D4 activation removes the synaptic GluA2-lacking AMPARs that are previously delivered by acute stress. On the other hand, in repeatedly stressed animals, the RI was unchanged (NS: 2.1 ± 0.2, n = 9; RS: 2.2 ± 0.2, n = 9, p > 0.05, ANOVA) but was markedly increased by PD168077 (4.1 ± 0.6, n = 8), suggesting that D4 increases the synaptic recruitment of GluA2-lacking AMPARs.
FIGURE 3.
D4 targets GluA2-lacking AMPARs in stressed animals. A and B, bar graphs showing the rectification index of AMPAR-EPSC in PFC neurons from nonstressed, acutely stressed (A), or repeatedly stressed (B) animals before and after PD168077 (40 μm) application. Insets, representative EPSC traces at +40 mV and −60 mV. Scale bar, 50 pA, 20 ms, p < 0.001, ANOVA. C and D, dots plots showing the amplitude of AMPAR-EPSC in PFC neurons from nonstressed, acutely stressed (C) or repeatedly stressed (D) animals before and after NASPM (100 μm) application. Insets, representative EPSC traces. Scale bar, 50 pA, 20 ms. *, p < 0.001, t test. E and F, plot of AMPAR-EPSC showing the effect of PD168077 and subsequent NASPM in animals exposed to acute stress (E) or repeated stress (F). G and H, immunoblots of surface and total GluA1 subunit and quantification of the surface GluA1 in PFC slices from nonstressed, acutely stressed, or repeatedly stressed animals without or with PD168077 treatment. *, p < 0.01, ANOVA. Error bars indicate mean ± S.E.
To investigate further the target of D4, we used NASPM (100 μm), a selective blocker of GluA2-lacking AMPA receptors (36). As shown in Fig. 3, C and D, NASPM had a minimal effect on basal AMPAR-EPSC in nonstressed rats (−12.9 ± 4.2%, n = 8, p > 0.05, t test), consistent with the low level of synaptic GluA2-lacking AMPARs at the base-line condition as reported previously (37). The NASPM sensitivity was significantly increased in acutely stressed animals (−30.3 ± 3.2%, n = 9, p < 0.001, t test) and was unchanged in repeatedly stressed animals (−11.2 ± 2.2%, n = 11, p > 0.05, t test), consistent with the delivery of GluA2-lacking AMPARs by acute stress.
In acutely stressed animals, PD168077 significantly reduced AMPAR-EPSC (Fig. 3E, 52.4 ± 7.2% reduction, n = 9), and subsequent application of NASPM failed to alter AMPAR-EPSC (−11.1 ± 1.7%, n = 9). On the other hand, in repeatedly stressed animals, PD168077 significantly enhanced AMPAR-EPSC (Fig. 3F, 47.7 ± 9.4% enhancement, n = 11), and subsequent application of NASPM markedly reduced AMPAR-EPSC (−37.9 ± 5.1%, n = 11). This suggests that the D4-sensitive EPSC is mediated by GluA2-lacking AMPARs in both stress conditions.
To test further whether the change in AMPAR trafficking is involved in D4 regulation of synaptic transmission at stress conditions, we carried out surface biotinylation assays. As shown in Fig. 3, G and H, PD168077 caused a marked reduction of surface GluA1 expression in PFC slices from acutely stressed animals, but significantly increased the level of surface GluA1 in PFC slices from repeatedly stressed animals (NS: −29.6 ± 2.6%, n = 11; AS: −55.9 ± 7.0%, n = 7; RS: 46.9 ± 11.2%, n = 10; p < 0.01, ANOVA). The biochemical evidence is consistent with what was observed in electrophysiological recordings.
CaMKII Is Involved in the D4 Regulation of AMPAR Transmission in Stressed Animals
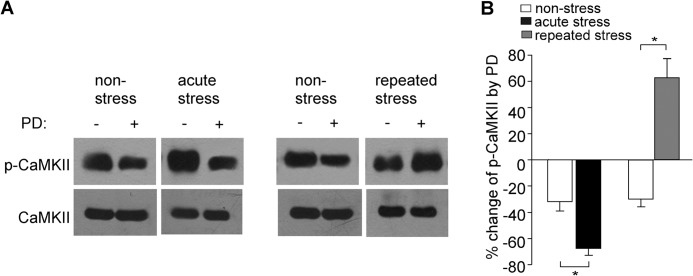
Our previous studies have shown that D4 exerts an activity-dependent bi-directional regulation of CaMKII (38), which is critical for the D4-induced homeostatic regulation of AMPARs in vitro (23, 24). Thus, we tested the role of CaMKII in the D4 actions in stressed animals. As shown in Fig. 4, A and B, in PFC slices from nonstressed animals, application of PD168077 (40 μm, 10 min) reduced the level of activated (Thr286-phosphorylated) CaMKII (32.1 ± 7.1% reduction, n = 5). In acutely stressed animals, the basal level of activated CaMKII was elevated (1.59 ± 0.19-fold of control, n = 5), and the reducing effect of PD168077 was significantly augmented (71.4 ± 7.3% reduction, n = 5). Conversely, in repeatedly stressed animals, the basal level of activated CaMKII was unchanged (1.12 ± 0.12-fold of control, n = 6), whereas PD168077 significantly increased the level of activated CaMKII (61.4 ± 10.5% increase, n = 6). This bi-directional regulation of CaMKII activity by D4 receptors provides a basis for the dual effects of D4 on AMPAR transmission in PFC pyramidal neurons from acutely versus chronically stressed animals.
FIGURE 4.
D4 activation exerts a bi-directional regulation of CaMKII activity in stressed animals. A, representative immunoblots of p-CaMKII and total CaMKII in lysates of PFC slices incubated without or with PD168077 (40 μm, 10 min) from nonstressed versus acutely or repeatedly stressed animals. B, quantification showing the percentage change of p-CaMKII by PD168077 in PFC slices from nonstressed versus stressed animals. *, p < 0.001, ANOVA. Error bars indicate mean ± S.E.
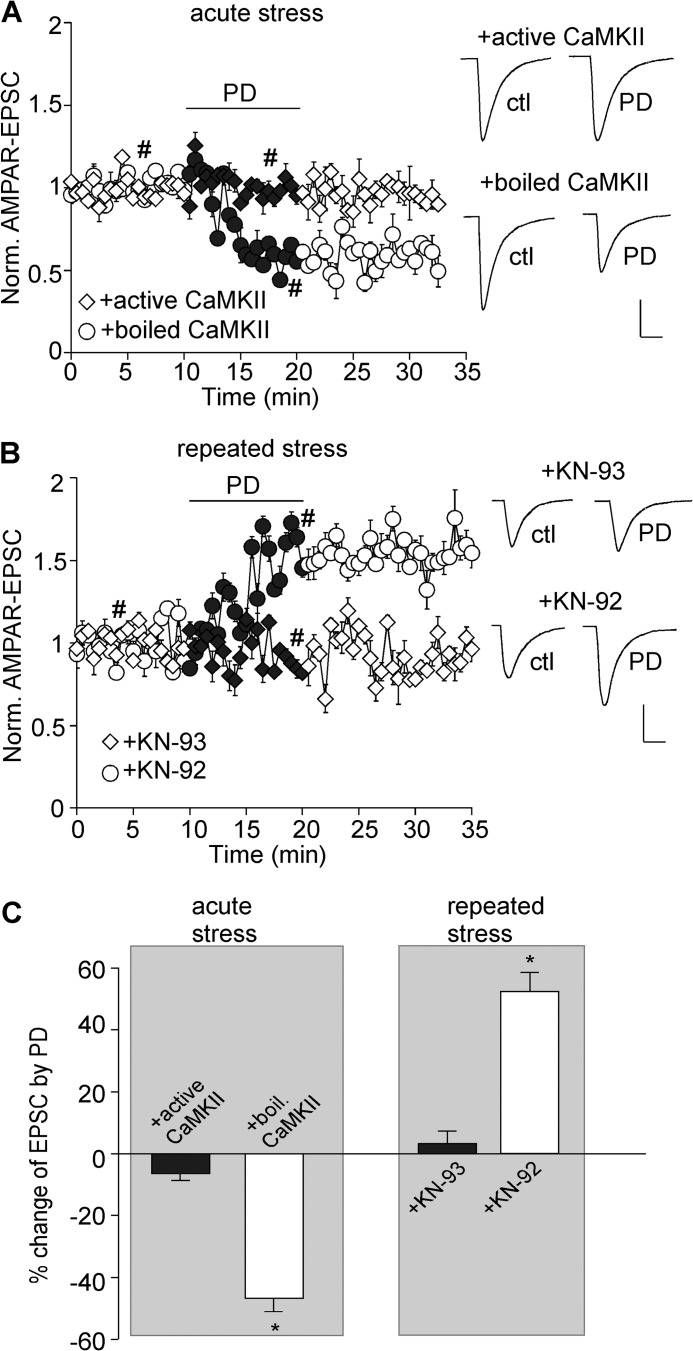
To examine whether the D4 reduction of AMPAR-EPSC in acutely stressed animals is through suppression of CaMKII, we dialyzed neurons with an EGTA-free internal solution containing purified active CaMKIIα protein (0.6 μg/ml), calmodulin (30 μg/ml) and CaCl2 (0.3 mm). The active CaMKII protein itself did not significantly alter the basal EPSC (−7.8 ± 1.7%, n = 6), which is likely due to the high level of CaMKII in these cells. As shown in Fig. 5A, the reducing effect of PD168077 on AMPAR-EPSC was largely blocked by intracellular infusion of the active CaMKII protein (6.2 ± 2.1% reduction, n = 9, Fig. 5C), but not the heat-inactivated CaMKII protein (46.4 ± 3.9% reduction, n = 11; p < 0.001, t test, Fig. 5C).
FIGURE 5.
Bi-directional regulation of CaMKII activity underlies the homeostatic regulation of AMPAR-EPSC by D4 in stressed animals. A and B, plot of normalized AMPAR-EPSC showing the effect of PD168077 (40 μm) in PFC neurons infused with active versus inactive CaMKII from acutely stressed animals (A) or KN-92 versus KN-93 from repeatedly stressed animals (B). Insets, representative EPSC traces taken from the time course denoted by #. Scale bar, 50 pA, 20 ms. C, bar graphs (mean ± S.E. (error bars)) showing the percentage modulation of AMPAR-EPSC by PD168077 in PFC neurons from stressed (acute or chronic) animals in the presence of various reagents affecting CaMKII activity. *, p < 0.001, t test.
To examine whether the D4 enhancement of AMPAR-EPSC in repeatedly stressed animals is through stimulation of CaMKII, we dialyzed the CaMKII inhibitor, KN-93 (20 μm). KN-93 itself did not significantly alter the basal EPSC (−7 ± 1.4%, n = 7), which is likely due to the low level of CaMKII in these cells. As shown in Fig. 5B, the enhancing effect of PD168077 on AMPAR-EPSC was blocked by KN-93 (3.0 ± 3.9% enhancement, n = 9, Fig. 5C), but not the inactive analog KN-92 (20 μm, 52.3 ± 6.1% enhancement, n = 9; p < 0.001, t test, Fig. 5C).
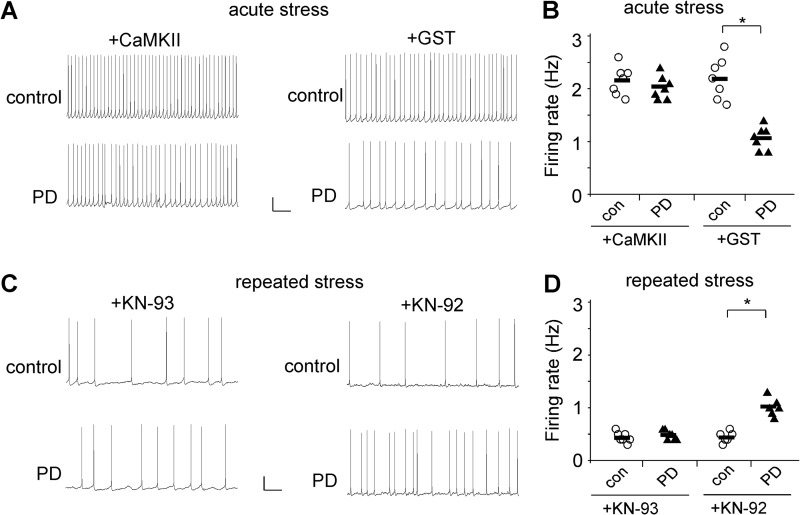
Finally, we examined the role of CaMKII in D4 regulation of neuronal excitability in stressed animals. As shown in Fig. 6, in animals exposed to acute stress, the reducing effect of PD168077 on spontaneous action potential firing was largely blocked by intracellular infusion of the active CaMKII protein (0.6 μg/ml, control: 2.16 ± 0.1 Hz, PD: 2.03 ± 0.08 Hz, n = 7), but not the GST control (control: 2.18 ± 0.14 Hz, PD: 1.07 ± 0.08 Hz, n = 7; p < 0.01, t test). On the other hand, in animals exposed to repeated stress, the enhancing effect of PD168077 on the firing rate was blocked by KN-93 (20 μm, control: 0.44 ± 0.03 Hz, PD: 0.49 ± 0.04 Hz, n = 7), but not the inactive analog KN-92 (control: 0.45 ± 0.04 Hz, PD: 1.02 ± 0.07 Hz, n = 6; p < 0.01, t test).
FIGURE 6.
The restoration of neuronal excitability by D4 in stressed animals depends on the bi-directional regulation of CaMKII activity. A and C, representative spontaneous action potential recordings showing the effect of PD168077 (40 μm) in PFC neurons infused with active CaMKII versus GST from acutely stressed rats (A) or KN-93 versus KN-92 from repeatedly stressed rats (C). Scale bars, 20 mV, 2 s. B and D, scatter plots of firing rates before (con) and after PD168077 in the presence of various reagents affecting CaMKII activity in PFC pyramidal neurons from acutely stressed (B) or repeatedly stressed (D) rats. *, p < 0.01, t test.
Taken together, these results suggest that D4 restores glutamatergic transmission in stress conditions through bi-directional regulation of CaMKII activity, which contributes the dual regulation of neuronal excitability by D4 receptors.
DISCUSSION
Stress serves as a key controller for neuronal responses that underlie behavioral adaptation, as well as maladaptive changes that lead to cognitive and emotional disturbances in stress-related mental disorders (26). Corticosterone, the major stress hormone, has been found to exert a complex effect on PFC excitatory synapses and PFC-mediated behaviors (25, 39). Acute stress induces synaptic potentiation by increasing surface delivery of AMPARs and NMDARs via glucocorticoid/SGK/Rab4 signaling, resulting in enhanced working memory performance (27, 28, 40). Conversely, chronic stress induces dendritic shortening, spine loss, and impairment in cognitive flexibility and perceptual attention (41–43). Our recent study has found that the detrimental effect of repeated stress on cognition is causally linked to the ubiquitin/proteasome-mediated degradation of AMPAR and NMDAR subunits and the suppression of glutamatergic transmission in PFC (44). In addition, the alteration of RNA editing of AMPAR subunits (45, 46) is potentially another mechanism underlying the effects of stress.
In this study, we have found that the spontaneous action potential firing is bi-directionally changed by acute versus repeated stress, suggesting that the synaptic drive onto PFC pyramidal neurons is altered by stress, leading to the change in PFC circuit excitability. Activation of D4 reduces the potentiated AMPAR responses in acutely stressed animals and enhances the depressed AMPAR responses in repeatedly stressed animals. The restoration of synaptic strength to the control level by D4 in stress conditions supports the notion that D4 serves as a homeostatic synaptic factor to stabilize cortical excitability (23, 24).
AMPA receptors are tetramers assembled by GluA1–4 subunits. Different populations of AMPARs have distinct properties and regulatory mechanisms. GluA2-containing AMPARs are dominant in glutamatergic synapses of neocortex and hippocampus (47, 48), whereasGluA2-lacking AMPARs can be synaptically recruited under certain conditions, such as neuronal activity blockade (49), sensory deprivation (50), or ischemia (51). GluA2-lacking AMPARs have prominent inward rectifying property (34), Ca2+ permeability (52) and larger single channel conductance (53), compared with GluA2-containing AMPARs. The unique Ca2+ permeability of GluA2-lacking AMPARs plays an important role in homeostatic plasticity (49) and LTP induction in hippocampus (35). Here, we show that GluA2-lacking (GluA1 homomeric) AMPARs is the major target of D4. D4 reduces the GluA2-lacking AMPARs that are delivered by acute stress, and D4 delivers more GluA2-lacking AMPARs to the synapse in repeatedly stressed animals. Thus, by redistributing GluA2-lacking AMPARs, D4 restores glutamatergic transmission in stress conditions. It is expected that GluA2 knock-out animals will show differential effects of D4R activation when acutely stressed and repeatedly stressed animals are compared.
One of the most important regulators of AMPARs and synaptic plasticity is CaMKII (54). CaMKII activation has been found to increase channel conductance (55) and promote synaptic insertion of AMPARs (56). In this study, we demonstrate that D4 markedly decreases CaMKII activity in acutely stressed animals, which is important for the D4 reduction of AMPAR responses. On the other hand, D4 potently increases CaMKII activity in repeatedly stressed animals, which is important for the D4 enhancement of AMPAR responses. It further confirms that the D4-induced homeostatic regulation of AMPARs depends on the activity-dependent bi-directional regulation of CaMKII (23, 24, 38).
Our previous study has shown that at the high activity state, D4 suppresses AMPAR responses by disrupting the kinesin motor-based transport of GluA2 along microtubules via a mechanism dependent on CaMKII inhibition. On the other hand, at the low activity state, D4 potentiates AMPAR responses by facilitating synaptic targeting of GluA1 through the scaffold protein SAP97 via a mechanism dependent on CaMKII stimulation (24). Thus, the major mechanism underlying the actions of D4 in stress conditions is likely to be CaMKII-mediated regulation of AMPAR trafficking, not phosphorylation-dependent changes on channel conductance.
In summary, we conclude that D4 is a synaptic gatekeeper that stabilizes glutamatergic transmission in prefrontal cortex. When neuronal activity changes in situations such as acute or repeated stress, D4 redistributes GluA2-lacking AMPARs at synapses through the bi-directional control of CaMKII. This homeostatic action of D4 provides a potential mechanism for the unique role of D4 in many stress-related neuropsychiatric disorders.
Acknowledgment
We thank Xiaoqing Chen for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants MH084233 and MH085774 (to Z. Y.).
- PFC
- prefrontal cortex
- ACSF
- artificial CSF
- AMPAR
- AMPA receptor
- ANOVA
- analysis of variance
- AS
- acutely stressed
- CaMKII
- calcium/calmodulin-dependent protein kinase II
- GluA2
- AMPA receptor subunit GluR2
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature EPSC
- NS
- nonstressed
- RI
- rectification index
- RS
- repeatedly stressed.
REFERENCES
- 1. Brozoski T. J., Brown R. M., Rosvold H. E., Goldman P. S. (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205, 929–932 [DOI] [PubMed] [Google Scholar]
- 2. Marié R. M., Defer G. L. (2003) Working memory and dopamine: clinical and experimental clues. Curr. Opin. Neurol. 16, S29–35 [DOI] [PubMed] [Google Scholar]
- 3. Mrzljak L., Bergson C., Pappy M., Huff R., Levenson R., Goldman-Rakic P. S. (1996) Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381, 245–248 [DOI] [PubMed] [Google Scholar]
- 4. Wedzony K., Chocyk A., Maćkowiak M., Fijał K., Czyrak A. (2000) Cortical localization of dopamine D4 receptors in the rat brain: immunocytochemical study. J. Physiol. Pharmacol. 51, 205–221 [PubMed] [Google Scholar]
- 5. Oak J. N., Oldenhof J., Van Tol H. H. (2000) The dopamine D4 receptor: one decade of research. Eur. J. Pharmacol. 405, 303–327 [DOI] [PubMed] [Google Scholar]
- 6. Tarazi F. I., Zhang K., Baldessarini R. J. (2004) Dopamine D4 receptors: beyond schizophrenia. J. Recept. Signal Transduct. Res. 24, 131–147 [DOI] [PubMed] [Google Scholar]
- 7. Van Tol H. H., Wu C. M., Guan H. C., Ohara K., Bunzow J. R., Civelli O., Kennedy J., Seeman P., Niznik H. B., Jovanovic V. (1992) Multiple dopamine D4 receptor variants in the human population. Nature 358, 149–152 [DOI] [PubMed] [Google Scholar]
- 8. LaHoste G. J., Swanson J. M., Wigal S. B., Glabe C., Wigal T., King N., Kennedy J. L. (1996) Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol. Psychiatry 1, 121–124 [PubMed] [Google Scholar]
- 9. Rowe D. C., Stever C., Giedinghagen L. N., Gard J. M., Cleveland H. H., Terris S. T., Mohr J. H., Sherman S., Abramowitz A., Waldman I. D. (1998) Dopamine DRD4 receptor polymorphism and attention deficit hyperactivity disorder. Mol. Psychiatry 3, 419–426 [DOI] [PubMed] [Google Scholar]
- 10. Cheon K. A., Kim B. N., Cho S. C. (2007) Association of 4-repeat allele of the dopamine D4 receptor gene exon III polymorphism and response to methylphenidate treatment in Korean ADHD children. Neuropsychopharmacology 32, 1377–1383 [DOI] [PubMed] [Google Scholar]
- 11. Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350, 610–614 [DOI] [PubMed] [Google Scholar]
- 12. Kapur S., Remington G. (2001) Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu. Rev. Med. 52, 503–517 [DOI] [PubMed] [Google Scholar]
- 13. Murphy B. L., Arnsten A. F., Goldman-Rakic P. S., Roth R. H. (1996) Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U.S.A. 93, 1325–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jentsch J. D., Taylor J. R., Redmond D. E., Jr., Elsworth J. D., Youngren K. D., Roth R. H. (1999) Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology 142, 78–84 [DOI] [PubMed] [Google Scholar]
- 15. Rubinstein M., Phillips T. J., Bunzow J. R., Falzone T. L., Dziewczapolski G., Zhang G., Fang Y., Larson J. L., McDougall J. A., Chester J. A., Saez C., Pugsley T. A., Gershanik O., Low M. J., Grandy D. K. (1997) Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90, 991–1001 [DOI] [PubMed] [Google Scholar]
- 16. Rubinstein M., Cepeda C., Hurst R. S., Flores-Hernandez J., Ariano M. A., Falzone T. L., Kozell L. B., Meshul C. K., Bunzow J. R., Low M. J., Levine M. S., Grandy D. K. (2001) Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J. Neurosci. 21, 3756–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dulawa S. C., Grandy D. K., Low M. J., Paulus M. P., Geyer M. A. (1999) Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 19, 9550–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avale M. E., Falzone T. L., Gelman D. M., Low M. J., Grandy D. K., Rubinstein M. (2004) The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol. Psychiatry 9, 718–726 [DOI] [PubMed] [Google Scholar]
- 19. Goldman-Rakic P. S. (1995) Cellular basis of working memory. Neuron 14, 477–485 [DOI] [PubMed] [Google Scholar]
- 20. Tsai G., Coyle J. T. (2002) Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 42, 165–179 [DOI] [PubMed] [Google Scholar]
- 21. Moghaddam B. (2003) Bringing order to the glutamate chaos in schizophrenia. Neuron 40, 881–884 [DOI] [PubMed] [Google Scholar]
- 22. Frankle W. G., Lerma J., Laruelle M. (2003) The synaptic hypothesis of schizophrenia. Neuron 39, 205–216 [DOI] [PubMed] [Google Scholar]
- 23. Yuen E. Y., Zhong P., Yan Z. (2010) Homeostatic regulation of glutamatergic transmission by dopamine D4 receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 22308–22313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuen E. Y., Yan Z. (2011) Cellular mechanisms for the dopamine D4 receptor-induced homeostatic regulation of AMPA receptors. J. Biol. Chem. 286, 24957–24965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McEwen B. S. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 [DOI] [PubMed] [Google Scholar]
- 26. de Kloet E. R., Joëls M., Holsboer F. (2005) Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 [DOI] [PubMed] [Google Scholar]
- 27. Yuen E. Y., Liu W., Karatsoreos I. N., Feng J., McEwen B. S., Yan Z. (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. U.S.A. 106, 14075–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuen E. Y., Liu W., Karatsoreos I. N., Ren Y., Feng J., McEwen B. S., Yan Z. (2011) Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry 16, 156–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuen E. Y., Jiang Q., Chen P., Gu Z., Feng J., Yan Z. (2005) Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J. Neurosci. 25, 5488–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong P., Yuen E. Y., Yan Z. (2008) Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex. Mol. Cell. Neurosci. 38, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang E. T., Hansen A. J., Wieloch T., Lauritzen M. (1990) Influence of MK-801 on brain extracellular calcium and potassium activities in severe hypoglycemia. J. Cereb. Blood Flow Metab. 10, 136–139 [DOI] [PubMed] [Google Scholar]
- 32. Maffei A., Nelson S. B., Turrigiano G. G. (2004) Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat. Neurosci. 7, 1353–1359 [DOI] [PubMed] [Google Scholar]
- 33. Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. (1991) Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252, 1715–1718 [DOI] [PubMed] [Google Scholar]
- 34. Bowie D., Mayer M. L. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 [DOI] [PubMed] [Google Scholar]
- 35. Plant K., Pelkey K. A., Bortolotto Z. A., Morita D., Terashima A., McBain C. J., Collingridge G. L., Isaac J. T. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 9, 602–604 [DOI] [PubMed] [Google Scholar]
- 36. Tsubokawa H., Oguro K., Masuzawa T., Nakaima T., Kawai N. (1995) Effects of a spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia. J. Neurophysiol. 74, 218–225 [DOI] [PubMed] [Google Scholar]
- 37. Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204 [DOI] [PubMed] [Google Scholar]
- 38. Gu Z., Yan Z. (2004) Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol. Pharmacol. 66, 948–955 [DOI] [PubMed] [Google Scholar]
- 39. Popoli M., Yan Z., McEwen B. S., Sanacora G. (2012) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Groc L., Choquet D., Chaouloff F. (2008) The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat. Neurosci. 11, 868–870 [DOI] [PubMed] [Google Scholar]
- 41. Radley J. J., Rocher A. B., Miller M., Janssen W. G., Liston C., Hof P. R., McEwen B. S., Morrison J. H. (2006) Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex 16, 313–320 [DOI] [PubMed] [Google Scholar]
- 42. Cerqueira J. J., Mailliet F., Almeida O. F., Jay T. M., Sousa N. (2007) The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 27, 2781–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liston C., Miller M. M., Goldwater D. S., Radley J. J., Rocher A. B., Hof P. R., Morrison J. H., McEwen B. S. (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 26, 7870–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yuen E. Y., Wei J., Liu W., Zhong P., Li X., Yan Z. (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbon A., Popoli M., La Via L., Moraschi S., Vallini I., Tardito D., Tiraboschi E., Musazzi L., Giambelli R., Gennarelli M., Racagni G., Barlati S. (2006) Regulation of editing and expression of glutamate α-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol. Psychiatry 59, 713–720 [DOI] [PubMed] [Google Scholar]
- 46. Caracciolo L., Barbon A., Palumbo S., Mora C., Toscano C. D., Bosetti F., Barlati S. (2011) Altered mRNA editing and expression of ionotropic glutamate receptors after kainic acid exposure in cyclooxygenase-2-deficient mice. PLoS One 6, e19398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Greger I. H., Khatri L., Ziff E. B. (2002) RNA editing at Arg-607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34, 759–772 [DOI] [PubMed] [Google Scholar]
- 48. Wenthold R. J., Petralia R. S., Blahos J., II, Niedzielski A. S. (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sutton M. A., Ito H. T., Cressy P., Kempf C., Woo J. C., Schuman E. M. (2006) Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125, 785–799 [DOI] [PubMed] [Google Scholar]
- 50. Goel A., Jiang B., Xu L. W., Song L., Kirkwood A., Lee H. K. (2006) Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat. Neurosci. 9, 1001–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu B., Liao M., Mielke J. G., Ning K., Chen Y., Li L., El-Hayek Y. H., Gomez E., Zukin R. S., Fehlings M. G., Wan Q. (2006) Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J. Neurosci. 26, 5309–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. (1994) Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 12, 1281–1289 [DOI] [PubMed] [Google Scholar]
- 53. Swanson G. T., Kamboj S. K., Cull-Candy S. G. (1997) Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci. 17, 58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Derkach V. A., Oh M. C., Guire E. S., Soderling T. R. (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 8, 101–113 [DOI] [PubMed] [Google Scholar]
- 55. Derkach V., Barria A., Soderling T. R. (1999) Ca2+/calmodulin kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hayashi Y., Shi S. H., Esteban J. A., Piccini A., Poncer J. C., Malinow R. (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 [DOI] [PubMed] [Google Scholar]