Background: The quality of induced pluripotent stem (iPS) cells might be inherently worse than embryonic stem cells.
Results: Although the differentiation capacity of iPS cells is limited, it can be enhanced.
Conclusion: Improving their level of pluripotency alleviated the limited capacity of iPS cells.
Significance: This report offers an effective strategy for the development of iPS cell-based research.
Keywords: Cell Culture, Embryonic Stem Cell, Induced Pluripotent Stem (iPS) Cell, Neurodifferentiation, Oligodendrocytes, Naive, Primed, Rabbit
Abstract
Although induced pluripotent stem (iPS) cells are indistinguishable from ES cells in their expression of pluripotent markers, their differentiation into targeted cells is often limited. Here, we examined whether the limited capacity of iPS cells to differentiate into neural lineage cells could be mitigated by improving their base-line level of pluripotency, i.e. by converting them into the so-called “naive” state. In this study, we used rabbit iPS and ES cells because of the easy availability of both cell types and their typical primed state characters. Repeated passages of the iPS cells permitted their differentiation into early neural cell types (neural stem cells, neurons, and glial astrocytes) with efficiencies similar to ES cells. However, unlike ES cells, their ability to differentiate later into neural cells (oligodendrocytes) was severely compromised. In contrast, after these iPS cells had been converted to a naive-like state, they readily differentiated into mature oligodendrocytes developing characteristic ramified branches, which could not be attained even with ES cells. These results suggest that the naive-like conversion of iPS cells might endow them with a higher differentiation capacity.
Introduction
Mammalian cells can be directly reprogrammed into induced pluripotent stem (iPS)3 cells by the introduction of defined sets of transcription factors (1). A variety of differentiated somatic cells have been reprogrammed into iPS cells as cellular model systems for use in regenerative medicine, drug screening, and the exploration of early embryonic development (2, 3). The discovery that human somatic cells can be reprogrammed into iPS cells has given researchers a noncontroversial alternative source of pluripotent human cells. Moreover, iPS cell technology could overcome immune rejection after the transplantation of differentiated iPS cells (4–6). These iPS cells are similar to their embryo-derived counterparts, ES cells, in their phenotypic characteristics, including their cell morphology, their expression of pluripotency markers, and their capacity to differentiate into germ layers in vivo and in vitro. However, recent studies comparing their tumorigenic potential, DNA methylation status, expression of imprinted genes, and chromatin structures, have suggested that iPS cells are a subset of pluripotent stem cells that is influenced by the somatic cell of origin and cell culture conditions (2, 7–10). The differences observed between ES cells and iPS cells and among the iPS cells themselves could be explained by incomplete genomic reprogramming (8, 11, 12). However, there is little information about whether these differences between ES cells and iPS cells are actually reflected in their capacities to differentiate in vitro (12). Therefore, a careful analysis of pluripotent stem cells is necessary to evaluate their safety for use in human regenerative therapies.
To evaluate the safety of iPS cells, it is essential to develop translational research using several animal species. In this context, animal models are expected to play important roles before any clinical trials of iPS-based therapies can be ethically approved (13). iPS cells have been successfully established from several animal species other than the mouse and human, including the monkey, rat, pig, rabbit, horse, and sheep (14–19). The iPS cells from each species confer specific benefits on the development of translational research and the generation of genetically modified animals. For example, the laboratory rabbit (Oryctolagus cuniculus) is closer phylogenetically to primates than are rodents (20). It has a short gestation period (31 days) and shows high fecundity. For these reasons, a number of research groups have long used the rabbit in biomedical research, and it has served as a model for human diseases (21, 22). Many of these diseases are lifestyle-related, and their prevalence is increasing rapidly in developed countries. Rabbit models are important because the etiologies of some human diseases are more similar to those exhibited by rabbits than to those exhibited by mice. Several ES cell lines have been generated and characterized and share major important characteristics with human ES cells (23–28). We have successfully reprogrammed liver and stomach cells of adult rabbits, which are very similar to rabbit ES cells (15). These iPS cells of rabbits will probably be used as a laboratory model of human iPS cells. They fulfill all the requirements for the acquisition of a fully reprogrammed state, showing strong similarity to ES cell counterparts that we have recently generated. However, although the global gene expression profile of the rabbit iPS cells became closer to that of the rabbit ES cells as the number of cell passages increased, a slight but clear difference between the two types of rabbit pluripotent stem cells remained.
Here, we analyzed the efficiency of the in vitro neural differentiation of rabbit ES cells and iPS cells originating from different tissues (liver and stomach) and with different culture periods (early and late iPS cells), which might cause differences in their global gene expression profiles. The limited differentiation capacity of the iPS cells was improved with continuous passage and the conversion of the rabbit iPS cells to a more immature, naive-like state, like that of mouse ES cells, which exhibit unlimited self-renewal while retaining the attributes of preimplantation epiblasts in terms of their identity and potency. Thus, by using rabbits, we can effectively characterize these different pluripotent stem cells in parallel under the same experimental conditions to evaluate the ultimate feasibility of using them for pluripotent stem cell-based regenerative medicine in humans.
EXPERIMENTAL PROCEDURES
Cell Culture
The rabbit pluripotent stem cell lines used can be roughly divided into five categories as follows: liver-derived iPS (iPS-L); stomach-derived iPS (iPS-S); early passage (before passage number 7) iPS (e-iPS); late passage (after passage number 17) iPS (l-iPS); and ES cells. The Dutch rabbit ES cell lines (rdES2-1 and rdES6) and Dutch rabbit iPS cell lines (iPS-L1, iPS-L2, iPS-L3, iPS-S1, iPS-S2, and iPS-S3) were generated and maintained using established methods (15). Briefly, rabbit pluripotent stem cells were plated onto mitomycin-C-treated mouse embryonic fibroblasts at a concentration of 6 × 103/cm2 at 38 °C under 6% CO2 in air. The culture medium (embryonic stem cell medium) consisted of 78% DMEM/Ham's F-12 supplemented with 20% knock-out serum replacement (KSR) (Invitrogen), 1% nonessential amino acids, 0.1 mm β-mercaptoethanol, and 8 ng/ml human recombinant basic fibroblast growth factor (Wako, Osaka, Japan).
In Vitro Neural Differentiation
To induce neural differentiation, rabbit pluripotent stem cells were digested with trypsin, suspended in EB medium containing 78% DMEM/Ham's F-12, 20% KSR, 1% nonessential amino acids, 50 units/ml penicillin, 50 μg/ml streptomycin, 0.1 mm β-mercaptoethanol, 1% N-2 supplement (Invitrogen), 4 μm all-trans-retinoic acid (Sigma), and 10 μm SB431542 (Tocris Bioscience, Bristol, UK). The final cell concentration was adjusted to 1 × 104 cell/ml. To achieve single EBs of a uniform size, 1,000 ES cells in a volume of 100 μl were dispensed into the wells of low cell adhesion 96-well round-bottomed plates (Nunc, Thermo Fisher Scientific, Waltham, MA), tapped gently, and cultured at 37 °C under 6% CO2 in air. After 2 days, 100 μl of fresh medium was added to each well. The EBs were picked randomly, and their mean diameters were measured microscopically. Five days after EB formation, the EBs were transferred to Matrigel-coated (BD Biosciences) 4-well multidishes (Nunc) and allowed to attach to the bottoms of the wells. Twelve EBs were transferred to each well. The medium was then changed from EB medium to neural differentiation medium (the same formulation as EB medium, with the addition of 10% KSR). The cells were cultured for 10 days at 37 °C under 6% CO2. The medium was replaced with fresh medium every other day. To obtain differentiated oligodendrocytes, six EBs per well were placed on Matrigel-coated 4-well dishes 5 days after EB formation and cultured in neural differentiation medium. After 10 days, the medium was changed from neural differentiation medium to the culture medium without retinoic acid or SB431542 but with 100 ng/ml Noggin (Wako). The cells were cultured for a further 20 days, with fresh medium supplied every day. The appearance of each neural cell type was detected by immunocytostaining.
RT-PCR Analysis
Total RNA was isolated with ISOGEN (Nippon Gene, Toyama, Japan) from cells cultured under the appropriate conditions. After DNase treatment to prevent contamination with genomic DNA, the first strand cDNA was synthesized using a TaKaRa RNA PCR kit (TaKaRa Bio Inc., Shiga, Japan). The synthesized cDNA was amplified with PCR using the specific primers listed in supplemental Table S1, with a cycling program of 94 °C for 3 min and 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. For quantitative RT-PCR, a LightCycler 96 (Roche Applied Science) was used to determine mRNA expression levels using the Fast Start essential DNA green master (Roche Applied Science), with a program of 94 °C for 10 min, 40 cycles of 94 °C for 10 s, 60 °C for 10 s, and 72 °C for 10 s.
Immunocytochemical Analysis
Marker expression was analyzed by fixing the differentiated cells that had attached to the bottoms of the culture plates in 4% paraformaldehyde for 30 min at room temperature and then washing them three times (5 min each) with Tris-buffered saline containing 1% BSA (wash buffer). To permeabilize the cells, they were treated with 0.1% Triton X-100 in wash buffer for 10 min and then incubated in blocking solution (10% normal donkey serum and 1% BSA in wash buffer) for 30 min. The following primary antibodies were used: mouse anti-Tuj1 (TUJ1) and sheep anti-GFAP (R&D Systems, Minneapolis, MN); mouse anti-α-smooth muscle actin (SMA) (Abcam, Cambridge, MA); goat anti-GATA4, goat anti-nestin, and mouse anti-O1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and mouse anti-CNPase (Sigma). All antibodies were diluted in blocking solution and incubated with the samples overnight at 4 °C. The next day, the cells were washed three times with wash buffer and incubated with secondary antibodies at room temperature for 1 h. The cells were washed again three times with wash buffer and covered with 50% glycerol containing DAPI. The fluorescent signals were detected and analyzed quantitatively using a BZ-9000 Series All-in-One fluorescence microscope and BZII image analysis system (Keyence, Osaka, Japan).
Quantitative Assessment of Neural Differentiation
A quantitative immunocytochemical analysis of the differentiated immunopositive cells was performed after their differentiation was induced from the same numbers of uniformly sized EBs (6 or 12 per well) for the same duration. The immunopositive cells were analyzed quantitatively using a BZ-9000 image analyzer. Cells positive for nestin, TUJ1, GFAP, and α-SMA were analyzed. Because the core region of the EB attachment area constituted a large immunopositive cluster, it was not counted as a single immunopositive cell. Instead, the immunopositive area per unit area of the well was measured. The area of DAPI-labeled nuclei on each image is defined as the total cell area per unit area of the well. The immunopositive area/DAPI area is the “neural differentiation index.” To compare the neural differentiation efficiencies of each pluripotent stem cell line, the neural differentiation index in the presence of RA and SB431542 was normalized to that in the absence of RA and SB431542. To compare the capacities of the rabbit pluripotent stem cells to differentiate into oligodendrocytes, we compared the neural differentiation indices for cells cultured in the presence of RA and SB431542 calculated from the immunopositive signals for O1 and the merged signals for O1 and CNPase per unit area of each well.
Conversion of Rabbit iPS Cells to the Naive-like State
To convert rabbit iPS cells to the naive-like state, CSII-EF-hOct3/4-IRES-Venus, which drives the expression of human OCT3/4 and of GFP under the control of the EF1α promoter, was introduced into rabbit iPS-L1 and iPS-S1 cells using a lentivirus. Two days after transfection, the rabbit iPS cells were passaged to mouse embryonic fibroblasts at a concentration of 36 × 103/cm2. The culture medium consisted of 38% DMEM/Ham's F-12 and 38% Neurobasal Medium supplemented with 20% KSR, 1% N-2 supplement, 2% B27 supplement, 2 mm GlutaMax, 1% nonessential amino acids (Invitrogen), 0.1 mm β-mercaptoethanol, 10 μm forskolin (Sigma), 5 μm kenpaullone (Calbiochem), 3 μm CHIR99021 (Stemgent, Cambridge, MA), and 0.1% human leukemia inhibitory factor (Wako). Three days after passage, the GFP-positive colonies were picked and seeded into other culture plates. The rabbit iPS cells in the naive-like state were passaged by incubating the cells with 0.25% trypsin/EDTA for 3 min at 38 °C. After termination of the trypsin reaction by serum treatment, the cells were mechanically disaggregated into single cells. The cells were then counted in a hemocytometer, resuspended, and seeded into other culture plates.
Teratoma Formation
To generate teratomas, 1–2 × 106 rabbit iPS cells that had been converted to the naive-like state were injected under the kidney capsules of 5–8-week-old SCID mice. Four to 8 weeks after transplantation, the teratomas were dissected and fixed in paraformaldehyde, as described above. Paraffin wax sections were stained with hematoxylin and eosin.
Production of Chimeric Embryos
To evaluate whether the naive-like iPS cells could contribute to the inner cell masses (ICMs) of rabbit and mouse blastocysts to produce chimeric embryos, CSII-EF-hOct3/4-IRES-Venus was introduced into iPS-L1 and iPS-S1 cells, which were cultured under primed state or naive-like conditions, before being injected separately into rabbit and mouse 8-cell embryos. Naive-like iPS cells were trypsinized to dissociate them into single cells or small clumps. The recipient embryos were recovered from superovulated females at the 8-cell stage, following natural mating (for rabbit embryos) or after in vitro fertilization (for mouse embryos). The iPS cells (n = 10–20) were injected into the perivitelline spaces of the 8-cell embryos using a Piezo-driven micromanipulator. Two days after injection, the contribution of the injected cells to the ICM of each blastocyst was determined by the presence of GFP fluorescence.
DNA Microarray Analysis
The rabbit 60-mer oligonucleotide DNA microarray (G2519F, Agilent Technologies, Santa Clara, CA) was used in this study. DNase-treated total RNA was labeled with Cy3 dye (GE Healthcare) using a Quick Amp labeling kit (Agilent Technologies) and hybridized to the microarray slides for 17–18 h at 65 °C. The scanned images of microarray slides were processed using Feature Extraction software (version 10.5, Agilent Technologies). Clustering and principal component analyses of microarray data were performed with 16,000 genes stably detected in the samples by Gene Spring GX 12.5 (Agilent Technologies). The distance metric of clustering was calculated using Camberra.
Statistical Analysis
Mean values were compared using one-way analysis of variance. Where appropriate, the significance of differences between means was determined with Fisher's exact probability test; p < 0.05 was considered significant. All experiments were analyzed in triplicate at least.
RESULTS
In Vitro Differentiation of Rabbit ES Cells into Neural Lineage Cells Induced by RA and SB431542
Previously, we have detected in vitro differentiated neurons and astrocytes in spontaneously differentiated rabbit pluripotent stem cells (15, 25, 28). Using this method, rabbit pluripotent stem cells were first induced to form EBs by suspension culture and then allowed to differentiate in the presence of FBS. However, several other lineages of differentiated cells also appeared in this in vitro differentiation system. Because one of the critical issues to be resolved in the clinical application of human pluripotent stem cells is the precise control of cell differentiation to the expected cell types, we compared several rabbit pluripotent stem cell lines for their differentiation capacity under established neural differentiation conditions (29, 30). The schedule for neural differentiation is shown in Fig. 1A. RA and a TGFβ inhibitor, SB431542, are known to promote the neural differentiation of ES cells in EB cultures. Thus, treatment with RA and SB431542 triggers massive neural differentiation and represses mesoendodermal differentiation. Our previous method was based on a suspension culture of dissociated rabbit pluripotent stem cells that underwent spontaneous re-aggregation in 1–2 days. After 5 days of suspension culture, the aggregates were subjected to adhesion culture. In this original method, the rabbit ES cells formed EBs of several sizes. Here, we used an established set of conditions under which the formation of the aggregates was more tightly controlled (31). Dissociated rabbit ES cells (1,000 cells per well) were cultured in each well of a low cell adhesion 96-well (U-bottomed) plate. In this procedure, the cells reaggregated quickly (within a few hours) and formed a uniformly sized cell mass in each well. Although several sizes and shapes of EBs were observed in the uncontrolled suspension culture, uniform EBs were formed in the 96-well plates (Fig. 1, B and C). To evaluate this neural differentiation system, we examined the mRNA expression of the pluripotent stem cell markers OCT3/4 and NANOG and the neural stem cell marker NES, at 2 and 5 days after EB formation (Fig. 1D). The pluripotent stem cell markers had decreased or disappeared 2 days after EB formation. The expression of OCT3/4 disappeared completely in the presence of RA and SB431542. In contrast, the neural stem cell marker NES was detected 5 days after EB formation. NES expression was higher in the presence of RA and SB431542 than in their absence. These results suggest that treatment with RA and SB431542 not only causes the breakdown of pluripotency in rabbit ES cells but also effectively induces them to undergo neural differentiation.
FIGURE 1.
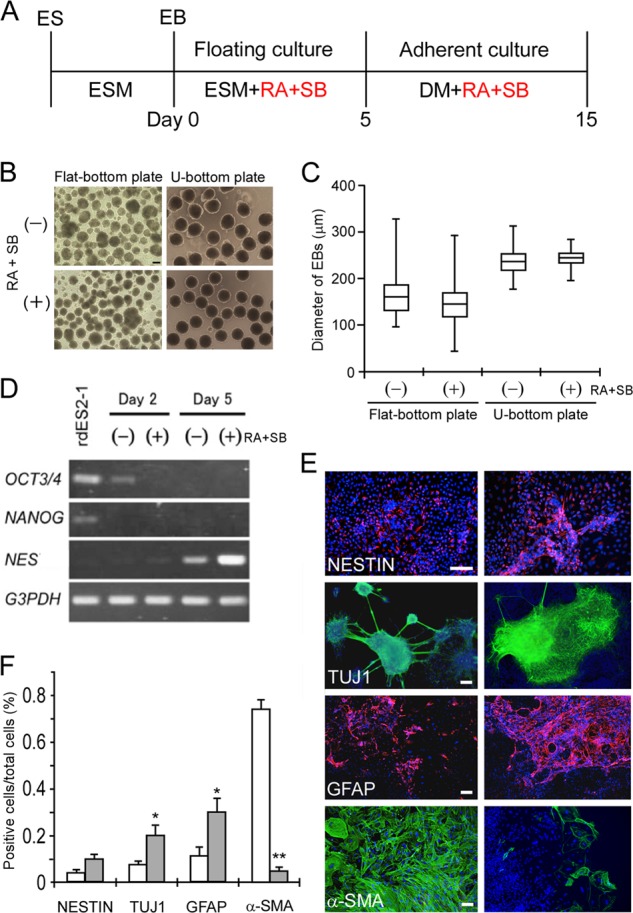
In vitro differentiation of rabbit ES cells (rdES2-1) into a neural lineage, induced by RA and SB431542 (SB). A, schematic representation of the early neural differentiation strategy. Red type shows the additional medium components. B, phase-contrast micrographs of EBs on day 5 of neural differentiation from rabbit ES cells treated with vehicle (upper panels) or RA and SB431542 (lower panels). Dissociated rabbit ES cells were cultured in each well of an untreated 24-well flat-bottomed plate (left panels) or a low cell adhesion 96-well U-bottomed plate (right panels). C, quantification of the sizes of EBs derived from rabbit ES cells. The box plot represents the diameters of EBs cultured in the individual wells of an untreated plate or a low cell adhesion plate. Depicted are the different conditions of EB formation in the absence (−) or presence (+) of RA and SB431542. Data are shown as the mean ± S.D. (randomly picked EBs were n = 92, 77, 66, and 81). D, RT-PCR analysis on days 2 and 5 after EB formation, after being treated with vehicle (−) or with RA and SB431542 (+). Pluripotent stem cell markers disappeared and the neural stem cell marker NES was expressed according to the state of neural differentiation. E, immunocytochemical analysis of rdES2-1 cells that had differentiated into early neural lineage cells, using antibodies directed against markers of neural stem cells (nestin), neurons (TUJ1), glial astrocytes (GFAP), and α-SMA in the absence (left panels) or presence of RA and SB431542 (right panels). Cell nuclei were stained with DAPI (blue). F, early neural differentiation efficiencies of EBs from rdES2-1 cells treated with RA and SB431542 (closed bars) were compared with those treated with vehicle (n = 8; open bars). *, p < 0.05; **, p < 0.01. ESM, embryonic stem cell medium; DM, differentiation medium; SB, SB431542. Scale bars, 100 μm.
Five days after EB formation in a 96-well low cell-adhesion plate in the presence or absence of RA and SB431542, the EBs were picked out and plated onto a Matrigel-coated 4-well plate at a density of 12 EBs/well. Ten days after attachment under neural differentiation conditions, the cells were analyzed immunocytochemically using early neural differentiation markers for neural stem cells (nestin), neurons (neuron-specific class III β-tubulin, TUJ1), and glial astrocytes (GFAP). A mesodermal marker, α-SMA, and an endodermal marker, GATA4, were also examined to assess other than neural differentiation (Fig. 1, E and F, and supplemental Fig. S1). In the absence of RA and SB431542, the rabbit ES cells partly differentiated to neural lineage cells, together with high densities of α-SMA-positive non-neural cells. Conversely, in the presence of RA and SB431542, the rabbit ES cells effectively differentiated into neural lineage cells, although the nestin signals increased only moderately. Importantly, α-SMA-positive cells were rarely detectable in the presence of RA and SB431542. The endodermal marker, GATA4, also disappeared in these conditions (supplemental Fig. S1). These results indicate that treatment with RA and SB431542 caused the rabbit ES cells to differentiate selectively into neural lineage cells.
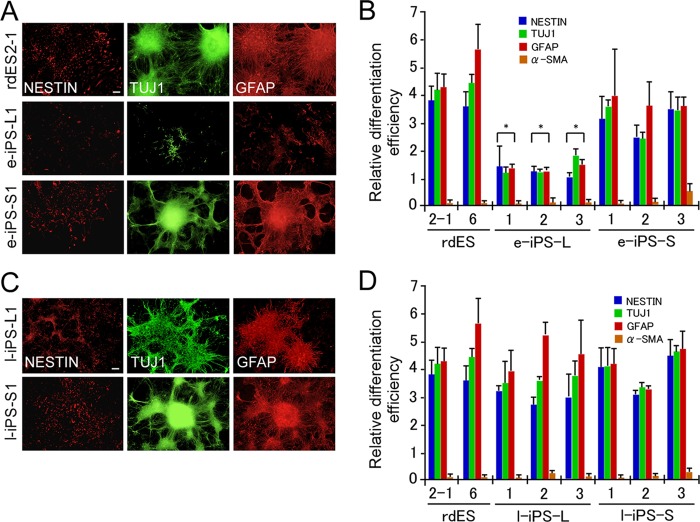
Comparison of the Early Neural Differentiation of Rabbit ES and iPS Cells
Our previous study demonstrated that the global gene expression profile of rabbit iPS cells became more similar to that of rabbit ES cells as the number of cell passages increased. However, a clear difference between the two types of rabbit pluripotent stem cells persisted (15). After we had established the system to evaluate the neural differentiation of rabbit pluripotent stem cells, we examined stomach- and liver-derived early iPS cell lines (e-iPS), in which introduced transgenes are incompletely silenced (15), in the neural differentiation system to compare their differentiation capacities with ES cells (Fig. 2, A and B). Although stomach-derived e-iPS cell lines (e-iPS-S1, e-iPS-S2, and e-iPS-S3) differentiated to neural stem cells, neurons, and astrocytes, with a degree of differentiation similar to that observed in rabbit ES cells, the liver-derived e-iPS cell lines (e-iPS-L1, e-iPS-L2, and e-iPS-L3) showed significantly limited ability to differentiate into all the neural lineage cells examined. In contrast, non-neural differentiation was effectively suppressed by treatment with RA and SB431542 in all the rabbit pluripotent stem cell lines examined. These results suggest that liver-derived e-iPS cell lines not only have a different global gene expression profile to that of ES cells (15) but also an inferior capacity for neural differentiation. However, despite the differences in the global gene expression profiles of the ES cells and stomach-derived e-iPS cells (15), these cells showed almost identical differentiation capacities when induced to differentiate into an early neural lineage.
FIGURE 2.
Comparison of the early neural differentiation of rabbit pluripotent stem cells. A, immunocytochemical analysis of neural marker proteins in differentiated neural stem cells (Nestin), neurons (TUJ1), and glial astrocytes (GFAP) derived from rabbit ES cells (rdES2-1, top panels) or early iPS cells (e-iPS-L1, middle panels; e-iPS-S1, bottom panels) in the presence of RA and SB431542. Because astroglia support neuronal migration, axonal guidance, and enable neural stem cells to differentiate into neurons, the immunoreactive pattern of GFAP resembled that of TUJ1. B, relative differentiation efficiency is shown (the absence of RA and SB431542 treatment is denoted “1”). Two rabbit ES cell lines and six e-iPS cell lines responded variably to RA and SB431542. The liver-derived e-iPS cell lines showed a worse capacity to differentiate than did the rdES2-1 cells. The data are shown as the mean ± S.D. (each rabbit ES cell line, n = 5; each rabbit e-iPS cell line, n = 3). *, p < 0.05. C, immunocytochemical analysis of neural marker proteins in RA- and SB431542-treated cells derived from l-iPS cells (liver-derived l-iPS-L1 cells, top panels; stomach-derived l-iPS-S1 cells, bottom panels). D, relative differentiation efficiency of the l-iPS cell lines was almost the same as that of the ES cells. Data are shown as the mean ± S.D. (each rabbit ES cell line, n = 5; each rabbit l-iPS cell line, n = 4). Scale bars, 100 μm.
To evaluate the early neural differentiation capacity of the late iPS (l-iPS) cell lines, e-iPS cell lines were cultured further to produce l-iPS cell lines and induced to undergo early neural differentiation (Fig. 2, C and D, and supplemental Fig. S2). Both the stomach-derived and liver-derived l-iPS cell lines showed differentiation activity similar to ES cells. These results suggest that the significantly lower early neural differentiation activity of the liver-derived e-iPS cell lines could be greatly improved by continuous passages, as far as the early phase of in vitro neural differentiation.
Rabbit iPS Cells Have a Limited Capacity to Differentiate into Oligodendrocytes
It is possible that the early neural differentiation system used in this study cannot identify differences in the global gene expression profiles of the rabbit ES cells and l-iPS cells. To clarify the differences in their capacities to differentiate in vitro, rabbit pluripotent stem cells were induced to undergo further neural differentiation into oligodendrocytes. These are a more mature neural cell type than other neural cells such as neural stem cells, neurons, or astrocytes (32, 33). Because the differentiation of pluripotent stem cells into oligodendrocytes was effectively induced by the addition of Noggin, further culture in the presence of Noggin was examined for 20 days (Fig. 3A). Differentiation to oligodendrocytes was evaluated with a marker for early oligodendrocytes, the monoclonal antibody O1, and a marker for mature oligodendrocytes, CNPase. In this experiment, one image was obtained (Fig. 3B) in which a CNPase-positive cell was merged with part of an O1-positive early oligodendrocyte. This suggests that a mixed population of early and moderately differentiated oligodendrocytes was generated under these culture conditions. When oligodendrocytes were induced to differentiate from rabbit ES cells, about 1,500 O1-positive cells were detected per well and about 30% of them were positive for CNPase. However, even if these culture periods were extended for over 60 days, no immunopositive cells with ramified branches, a morphological characteristic of the more mature form of oligodendrocytes, were detected. Although stomach-derived e-iPS cells and ES cells showed almost the same ability to differentiate into early neural cells (Fig. 2), the capacity of the stomach-derived e-iPS cells for differentiation into oligodendrocytes was significantly lower than that of the ES cells (Fig. 3, B and C, and supplemental Fig. S3). The differentiation capacity of liver-derived e-iPS cells into oligodendrocytes was worse than the stomach-derived e-iPS cells (Fig. 3C and supplemental Fig. S3). Furthermore, the low level differentiation into oligodendrocytes observed in the e-iPS cell lines was only slightly improved in the l-iPS cell lines, and neither could differentiate to the same degree as did the ES cell lines (Fig. 3, B and C). These results suggest that the differences observed in the global gene expression profiles of the ES cells and l-iPS cells reflect their different capacities to differentiate into more mature neural cells, such as oligodendrocytes.
FIGURE 3.
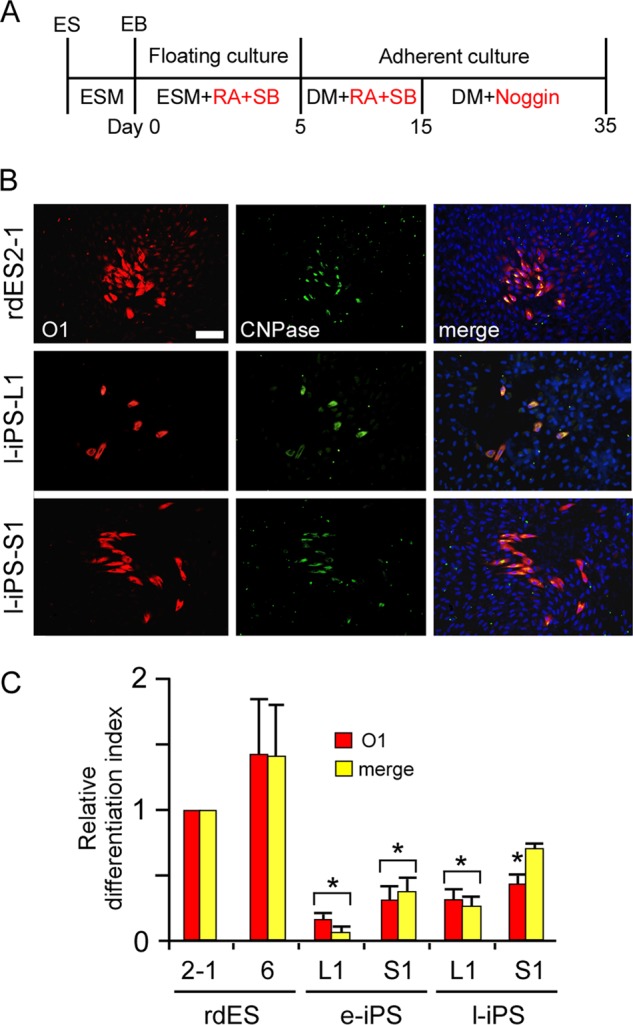
Comparison of the in vitro differentiation to oligodendrocytes by rabbit pluripotent stem cells derived from the liver or stomach. A, scheme of the strategy for oligodendrocyte differentiation. The induction of oligodendrocytes was examined after an additional 20 days in culture in the presence of Noggin. B, immunocytochemical analysis of the marker proteins for early oligodendrocytes (O1) and mature oligodendrocytes (CNPase) expressed by rabbit ES cells (rdES2–1, top panels) and l-iPS cells (liver-derived l-iPS-L1 cells, middle panels; stomach-derived l-iPS-S1 cells, bottom panels). C, quantification of the neural differentiation index of the iPS cell lines for O1 and the merged signals for O1 and CNPase compared with those of the rdES cells. Data are shown as the mean ± S.D. (each rabbit ES cell line, n = 5; each rabbit iPS cell line, n = 3). *, p < 0.05. Scale bars, 100 μm.
Naive-like Conversion Overcomes the Limited Neural Differentiation Capacity of Primed State iPS Cells
Pluripotent stem cells exist in naive or primed states, epitomized by mouse ES cells and the developmentally more advanced epiblast stem cells (EpiSCs) (34). Primed state EpiSCs are considered to represent a more advanced “differentiated” state than that shown by naive state ES cells. Actually, unlike naive state ES cells, primed state EpiSCs are highly inefficient in repopulating the ICM upon aggregation with or injection into host preimplantation embryos (35, 36). Because human and rabbit pluripotent stem cells so far have only assumed the primed state, their in vitro differentiation might be less effective than naive state pluripotent stem cells. However, several studies have demonstrated that primed state pluripotent stem cells can be converted to naive state cells by genetic manipulation or even by altering their culture conditions (10, 37–40). To overcome the limited differentiation capacity of iPS cells by naive-like conversion, primed state iPS cells (iPS-L1, L2, and L3 and iPS-S1, S2, and S3) were transfected with a lentivirus to introduce a transgene driving human (h)OCT3/4-GFP expression under the control of the EF1α promoter. After transfection with hOCT3/4-GFP, there were no obvious changes in their karyotype or the ability to form teratomas (data not shown). The transfected rabbit iPS cells were cultured in serum-free N2B27 medium in the presence of LIF, the glycogen synthase kinase 3 (GSK3) inhibitors, CHIR99021 and kenpaullone, and an activator of adenylate cyclase, forskolin. This naive-like conversion generated cells that were almost morphologically indistinguishable from mouse ES cells, which showed GFP and alkaline phosphatase activity (Fig. 4A). RT-PCR analysis also demonstrated expression of the endogenous stem cell marker genes OCT3/4, KLF4, SOX2, c-MYC, and NANOG (Fig. 4B). Moreover, the increased expression of the candidate genes, KLF4 and KLF5, which are responsible for maintaining a naive pluripotent state, were confirmed by quantitative RT-PCR (Fig. 4C). To assess the pluripotency of the naive-like state iPS cells after their naive-like conversion, they were transplanted into SCID mice, and the formation of teratomas was confirmed (data not shown). To distinguish these modified pluripotent stem cells, which overexpressed hOCT3/4-GFP and were subjected to primed or naive-specific culture conditions, we termed them PN- and N-type iPS cells, respectively. Although the EF1α-controlled expression of hOCT3/4-GFP was observed in the initial phase of in vitro differentiation, almost all the GFP signal was reduced during the early phase of neural differentiation (supplemental Fig. S4). To evaluate whether these N-type iPS cells contributed to the ICMs of blastocysts, PN- and N-type iPS cells were injected into rabbit 8-cell embryos and cultured in vitro to allow the blastocysts to develop. As reported by Tachibana et al. (41), the P-type iPS cells when injected into preimplantation embryos differentiated and could not integrate into the ICM. However, the N-type iPS cells readily colonized the ICMs of the blastocysts, maintaining homogeneous hOCT3/4-GFP activity (Fig. 4D). Moreover, the N-type iPS cells effectively contributed to the ICMs of mouse blastocysts to generate interspecific mouse/rabbit chimeric embryos (supplemental Fig. S5). Finally, we compared the capacities to differentiate into oligodendrocytes in vitro of rabbit ES cells (rdES2-1 and rdES6) and N-type iPS cells (Fig. 5A). The differentiation capacity of the N-type iPS cells increased more than that of the P-type iPS cells. Interestingly, stomach-derived naive-like iPS-S1, -S2, and -S3 cells showed a significantly enhanced differentiation capacity, equivalent to that of rabbit ES cells. Moreover, by naive-like conversion, all of the stomach-derived iPS cell lines effectively differentiated into the morphologically more mature form of oligodendrocytes with ramified branches, which were not observed even when ES cells differentiated (Fig. 5B). However, the liver-derived naive-like iPS cells could not be modified sufficiently to equal the degree of differentiation of the ES cells, and they rarely produced mature oligodendrocytes even after naive-like conversion.
FIGURE 4.
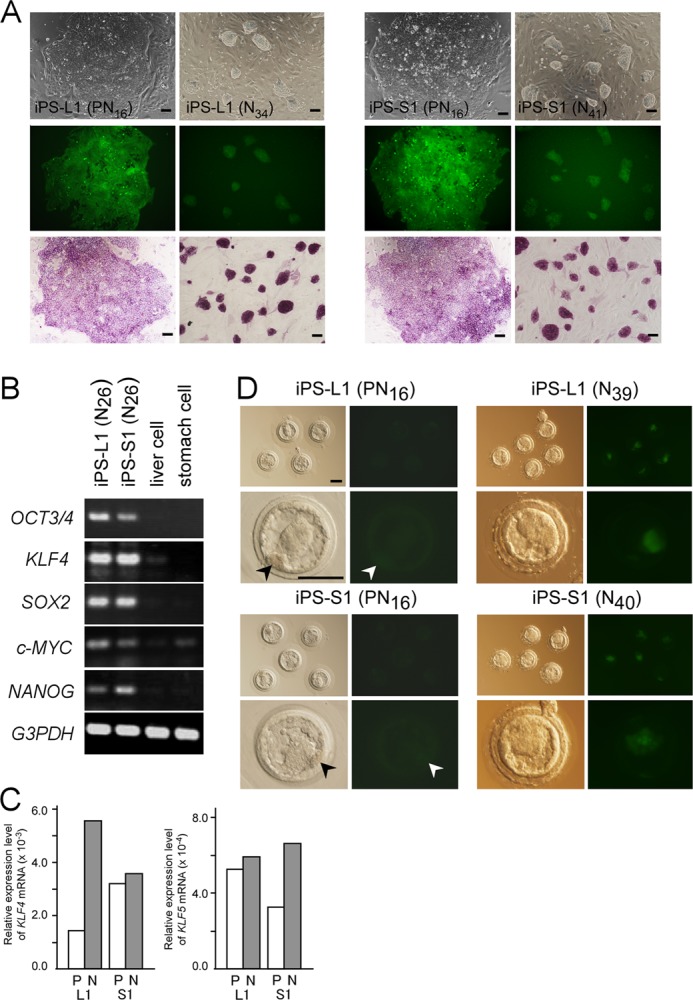
Evaluation of rabbit iPS cell characteristics after naive-like conversion of primed state (PN) or naive-like state (N) cells. A, colony morphology of iPS cell lines after EF1α-hOCT3/4-GFP transfection of primed state iPS-L1 (PN16) and primed state iPS-S1 (PN16) cells (left panels), and of the naive-like state iPS-L1 (N34) and iPS-S1 (N41) cell colonies (right panels), which all expressed EF1α and hOCT3/4-GFP (middle panels) and alkaline phosphatase activity (bottom panels). The passage number is denoted “PNnumber” or “Nnumber.” B, RT-PCR analysis of the expression of selected pluripotency-related genes in naive-like converted iPS cells. Endogenous pluripotent stem cell marker genes were still expressed after 26 passages following naive-like conversion (referred to as N26). C, increased expressions of rabbit KLF4 and KLF5 after naive-like conversion were confirmed by quantitative RT-PCR. D, EF1α-OCT3/4-GFP expression in rabbit blastocysts 48 h after an injection into 8-cell embryos of primed state iPS-L1 (PN16) cells and iPS-S1 (PN16) cells (left eight panels) or naive-like iPS-L1 (N39) and iPS-S1 (N40) cells (right eight panels). When primed state iPS cells were injected, putatively differentiated iPS cells with almost no hOCT3/4-GFP expression could not contribute to the ICM of blastocysts (arrowheads). Lower magnifications (top panels) and higher magnifications (bottom panels) are shown. Scale bars, 100 μm.
FIGURE 5.
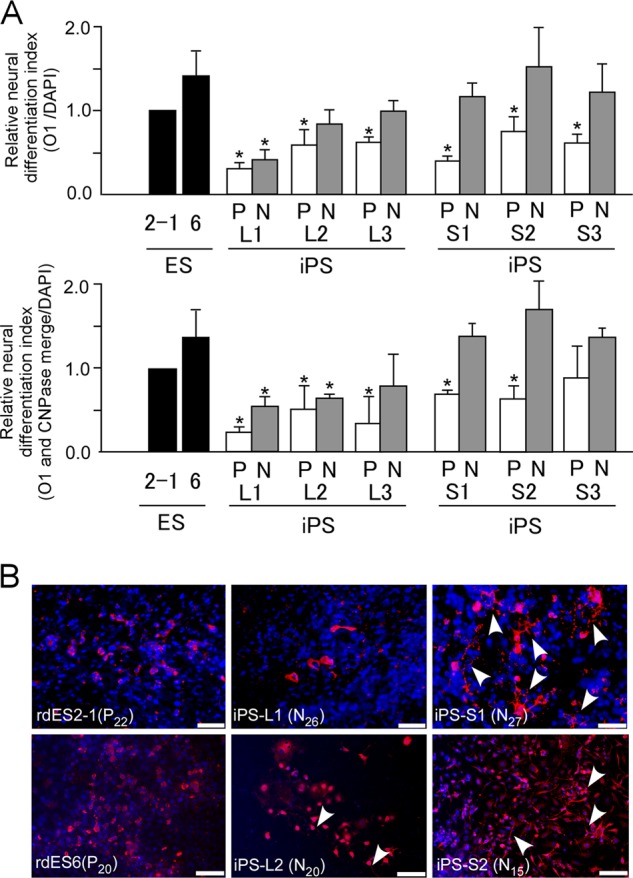
In vitro differentiation to oligodendrocytes of rabbit pluripotent stem cells after naive-like conversion. A, relative neural differentiation indices are shown for oligodendrocytes marked by O1 (left graph) and the merged signals for O1 and CNPase (right graph) derived from rabbit ES cells (rdES2-1 and rdES6) or primed state (P) and naive-like state (N) rabbit iPS cells (liver-derived iPS-L1, -L2, and -L3 cells and stomach-derived iPS-S1, -S2, and -S3 cells). Data are shown as the mean ± S.D. (rdES, n = 3; each rabbit iPS cell line, n = 3). *, p < 0.05. B, immunocytochemical detection of oligodendrocytes with ramified branches (arrowheads) using anti-O1 antibody (red) in in vitro-differentiated rabbit ES cells and naive-like-state iPS cells.
DISCUSSION
To compare several rabbit pluripotent stem cell lines precisely using an in vitro differentiation system, uniform EBs were formed in a low cell adhesion plate in the presence of RA and SB431542. Several reports have shown that EB-mediated differentiation efficiency is dependent on the EB size. EB formation from individual pluripotent stem cells through spontaneous aggregation was inefficient, resulting in a heterogeneous size distribution and uncontrolled differentiation lineages (42). The down-regulation of Wnt signaling has been shown to be one of the mechanisms involved in the RA-induced neural differentiation of mouse ES cells (43). The addition of RA promotes the development of neural progenitors in EBs from human ES cells, as does the simultaneous inhibition of activin/nodal and bone morphogenetic protein signaling (44, 45). The activin/nodal signaling pathway has been implicated in the inhibition of default neuroectodermal differentiation and in the maintenance of pluripotency in human ES cells (46). The drug SB431542 has been shown to enhance neural induction in EB-based human ES cells. SB431542 inhibits the TGFβ/activin/Lefty pathway by blocking the phosphorylation of ALK4, ALK5, and ALK7 receptors (47). Moreover, we have shown here that the addition of the bone morphogenetic protein antagonist Noggin, at a specific step in the neural induction of rabbit pluripotent stem cells, allows oligodendrocyte precursors to progress toward mature oligodendrocytes, as demonstrated previously in mouse and human ES cells (48). Thus, the effects of RA, SB431542, and Noggin on the neural induction of rabbit pluripotent stem cells have been confirmed, and we have emulated their effects on the pluripotent stem cells in mouse and human.
In our previous study, the global gene expression profile of stomach-derived e-iPS cells was more similar to that of ES cells than to that of liver-derived e-iPS cells (15). This study has demonstrated that stomach-derived iPS cells are better in their in vitro neural differentiation capacity than liver-derived iPS cells. Stomach-derived iPS cells have a similar capacity to differentiate in vitro into an early neural lineage of cells as do ES cells, even in early passages. This result implies that if the target cells to be generated by in vitro differentiation and the donor cells from which the iPS cell lines are derived are chosen carefully, differentiated tissues almost identical to those produced from ES cells can be obtained, even in the early stages of establishment. Furthermore, the limited capacity of liver-derived e-iPS cells to differentiate into early neural lineages is improved by repeated passages to l-iPS cells and equals the capacity of ES cells. Koehler et al. (49) reported that the efficiency of in vitro neuronal conversion depends on the complete reprogramming of iPS cells by extensive passaging. Our observations suggest that the advanced reprogramming induced by repeated passages could normalize the limited differentiation capacity of e-iPS cells to the point of early neural differentiation. In contrast to early neural differentiation, when iPS cells differentiated to oligodendrocytes, the ineradicable differences between the ES cells and iPS cells, which could not be overcome by repeated passages, were clarified (Fig. 3). Rabbit pluripotent stem cells were defined as EpiSC-type or primed state stem cells; these are rarely competent to contribute to blastocyst chimeras and are therefore developmentally and functionally distinct from naive state mouse ES cells (15, 25, 28).
In this study, we successfully generated and maintained rabbit iPS cells in a naive-like state by the continuous overexpression of hOCT3/4 under appropriate culture conditions. Hanna et al. (38) reported that human ES cells could be converted to the naive-like state by the overexpression of OCT3/4, KLF4, and KLF2 under the appropriate culture conditions. When exogenous Yamanaka factors, controlled by the CMV promoter, were effectively silenced in rabbit pluripotent stem cells (15), an additional human OCT3/4 gene was overexpressed under the EF1α promoter. The EF1α promoter is known to maintain the expression of exogenous genes in human ES cells (50). Although the genetically unmodified culture condition-dependent naive-like state rabbit iPS cells could not be maintained for longer than 2–3 passages, naive-like rabbit iPS cells were effectively maintained by the continuous overexpression of hOCT3/4. Hierarchical clustering and principal component analysis from DNA microarray studies demonstrated that the global gene expression profiles of the liver-derived iPS cells (iPS-L (P)) were shifted close to those of ES cells by overexpression of hOCT3/4 (iPS-L (PN)), and then elevated their expression profile to a naive-like state (iPS-L (N)).
However, the global gene expression profiles of stomach-derived iPS cells (iPS-S (P)), which showed comparatively similar profiles of ES cells, were shifted by hOCT3/4 overexpression as passing through that of iPS-S (PN) and then elevated to that of a naive-like state (iPS-S (N)) (supplemental Fig. S6, A and B). The putative stem cell qualities concerning in vitro neural differentiation capacity and chimeric contribution are summarized in supplemental Fig. S7. Because naive-like iPS cells could be differentiated more effectively into neural lineage cells than their primed state counterparts, naive-like conversion before the iPS cells are induced to differentiate should be a standard procedure for therapeutic applications. However, our research has also demonstrated that there is a limitation that cannot be overcome by naive-like conversion. Although primed state stomach-derived iPS cells effectively differentiated into oligodendrocytes after naive-like conversion, liver-derived iPS cells did not do so as effectively (Fig. 5). These results suggest that the donor cell type for iPS cells restricts their capacity for in vitro differentiation, even after naive-like conversion. Naive-like rabbit iPS cells could be effectively introduced into mouse embryos, as well as into rabbit embryos. It is well known that naive pluripotent stem cells from the mouse and rat can be incorporated into the ICMs of blastocysts to form interspecific chimeric embryos and chimeric offspring (51, 52). The production of an interspecific chimera that consists of a mouse preimplantation embryo and the pluripotent stem cells from another animal species could be used as an indicator of their pluripotency. However, because the GFP signal of the naive-like iPS cells generated in this study disappeared after differentiation (supplemental Fig. S4), it was difficult to evaluate chimerism in more developed embryos or in pups. It has been reported that although the EF1α promoter can drive the strong expression of exogenous genes in pluripotent stem cells, in vitro differentiation resulted in the loss of their expression (53). Our next objective is the derivation of rabbit naive pluripotent stem cells, which could be controlled via their hOCT3/4 expression by doxycycline and confirmed by their germ line transmission in chimeric rabbits, and then assessment of the in vitro differentiation capacity of these cells. Recently, new methods for establishing high quality pluripotent stem cells in a naive-like state have been developed in the human and pig (38, 54, 55), so naive-like conversion could be standardized in several research areas. Pluripotent stem cells are initially generated in rabbits in a primed state and can then be converted to the naive-like state. Moreover, the high fecundity and short gestation period of the rabbit and the lenient research ethics involved in its use make chimeric assays feasible. For these reasons, the rabbit offers a very valuable model system for the development of cell-based translational research.
In conclusion, we compared the differentiation capacities of rabbit pluripotent stem cell lines using an in vitro neural differentiation system. In the early phase of neural differentiation, although only a limited capacity to differentiate was observed in the liver-derived e-iPS cells, it was equivalent to the capacity of the ES cells when liver-derived l-iPS cells were used. Moreover, when these pluripotent stem cells were induced to differentiate to oligodendrocytes, which occurs in the mid or late phase of neural differentiation, none of the iPS cell lines examined could differentiate to the same degree as the ES cells. However, when the iPS cells were previously converted to a naive-like state, they readily differentiated into oligodendrocytes with characteristic ramified branches, which were not observed even when ES cells differentiated. These results indicate that although rabbit pluripotent stem cells vary in their gene expression profiles and in their neural differentiation capacities, the limited differentiation capacity of the primed state iPS cells can be boosted by continuous passages or naive-like conversion.
Shortly before the submission of this paper, naive-like conversion of rabbit pluripotent stem cells by Osteil et al. was published on line (56). This demonstrated the naive-like conversion of rabbit pluripotent stem cells and detailed characterization before and after naive-like conversion, as we also showed in this study. We stress here the possibility of naive-like conversion by other procedures by overexpression of hOCT3/4. Importantly, the main objective in our research is to elucidate the difference of targeted in vitro differentiation capacity and the possibility to improve the limited capacity of iPS cells by naive-like conversion.
This work was supported by PRESTO of the Japan Science and Technology Agency, a grant-in-aid for scientific research (B) and the Program to Disseminate Tenure Tracking System from the Ministry of Education, Culture, Sports, Science and Technology, and a grant for basic science research projects from Sumitomo Foundation.

This article contains supplemental Figs. S1–S7 and Table S1.
- iPS
- induced pluripotent stem
- GFAP
- glial fibrillary acidic protein
- EB
- embryoid body
- α-SMA
- α-smooth muscle actin
- RA
- retinoic acid
- KSR
- knock-out serum replacement
- ICM
- inner cell mass
- e-iPS
- early iPS
- l-iPS
- late iPS
- h
- human
- CNPase
- 2′,3′-cyclic nucleotide 3′-phosphohydrolase
- EpiSC
- epiblast stem cell.
REFERENCES
- 1. Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 2. Miura K., Okada Y., Aoi T., Okada A., Takahashi K., Okita K., Nakagawa M., Koyanagi M., Tanabe K., Ohnuki M., Ogawa D., Ikeda E., Okano H., Yamanaka S. (2009) Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 27, 743–745 [DOI] [PubMed] [Google Scholar]
- 3. Okita K., Yamanaka S. (2010) Induction of pluripotency by defined factors. Exp. Cell Res. 316, 2565–2570 [DOI] [PubMed] [Google Scholar]
- 4. Nishikawa S., Goldstein R. A., Nierras C. R. (2008) The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 9, 725–729 [DOI] [PubMed] [Google Scholar]
- 5. Sipp D. (2009) Gold standards in the diamond age: the commodification of pluripotency. Cell Stem Cell 5, 360–363 [DOI] [PubMed] [Google Scholar]
- 6. Okita K., Nagata N., Yamanaka S. (2011) Immunogenicity of induced pluripotent stem cells. Circ. Res. 109, 720–721 [DOI] [PubMed] [Google Scholar]
- 7. Lister R., Pelizzola M., Kida Y. S., Hawkins R. D., Nery J. R., Hon G., Antosiewicz-Bourget J., O'Malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomson J. A., Evans R. M., Ecker J. R. (2011) Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M. J., Ji H., Ehrlich L. I., Yabuuchi A., Takeuchi A., Cunniff K. C., Hongguang H., McKinney-Freeman S., Naveiras O., Yoon T. J., Irizarry R. A., Jung N., Seita J., Hanna J., Murakami P., Jaenisch R., Weissleder R., Orkin S. H., Weissman I. L., Feinberg A. P., Daley G. Q. (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guenther M. G., Frampton G. M., Soldner F., Hockemeyer D., Mitalipova M., Jaenisch R., Young R. A. (2010) Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell 7, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theunissen T. W., van Oosten A. L., Castelo-Branco G., Hall J., Smith A., Silva J. C. (2011) Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr. Biol. 21, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chin M. H., Mason M. J., Xie W., Volinia S., Singer M., Peterson C., Ambartsumyan G., Aimiuwu O., Richter L., Zhang J., Khvorostov I., Ott V., Grunstein M., Lavon N., Benvenisty N., Croce C. M., Clark A. T., Baxter T., Pyle A. D., Teitell M. A., Pelegrini M., Plath K., Lowry W. E. (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5, 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polo J. M., Liu S., Figueroa M. E., Kulalert W., Eminli S., Tan K. Y., Apostolou E., Stadtfeld M., Li Y., Shioda T., Natesan S., Wagers A. J., Melnick A., Evans T., Hochedlinger K. (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 28, 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Familari M., Selwood L. (2006) The potential for derivation of embryonic stem cells in vertebrates. Mol. Reprod. Dev. 73, 123–131 [DOI] [PubMed] [Google Scholar]
- 14. Martins-Taylor K., Xu R. H. (2010) Determinants of pluripotency: from avian, rodents, to primates. J. Cell. Biochem. 109, 16–25 [DOI] [PubMed] [Google Scholar]
- 15. Honda A., Hirose M., Hatori M., Matoba S., Miyoshi H., Inoue K., Ogura A. (2010) Generation of induced pluripotent stem cells in rabbits. Potential experimental models for human regenerative medicine. J. Biol. Chem. 285, 31362–31369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomioka I., Maeda T., Shimada H., Kawai K., Okada Y., Igarashi H., Oiwa R., Iwasaki T., Aoki M., Kimura T., Shiozawa S., Shinohara H., Suemizu H., Sasaki E., Okano H. (2010) Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells 15, 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagy K., Sung H. K., Zhang P., Laflamme S., Vincent P., Agha-Mohammadi S., Woltjen K., Monetti C., Michael I. P., Smith L. C., Nagy A. (2011) Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 7, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y., Cang M., Lee A. S., Zhang K., Liu D. (2011) Reprogramming of sheep fibroblasts into pluripotency under a drug-inducible expression of mouse-derived defined factors. PLoS One 6, e15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben-Nun I. F., Montague S. C., Houck M. L., Tran H. T., Garitaonandia I., Leonardo T. R., Wang Y. C., Charter S. J., Laurent L. C., Ryder O. A., Loring J. F. (2011) Induced pluripotent stem cells from highly endangered species. Nat. Methods 8, 829–831 [DOI] [PubMed] [Google Scholar]
- 20. Graur D., Duret L., Gouy M. (1996) Phylogenetic position of the order Lagomorpha (rabbits, hares, and allies). Nature 379, 333–335 [DOI] [PubMed] [Google Scholar]
- 21. Shiomi M., Ito T., Yamada S., Kawashima S., Fan J. (2004) Correlation of vulnerable coronary plaques to sudden cardiac events. Lessons from a myocardial infarction-prone animal model (the WHHLMI rabbit). J. Atheroscler. Thromb. 11, 184–189 [DOI] [PubMed] [Google Scholar]
- 22. Weekers F., Van Herck E., Coopmans W., Michalaki M., Bowers C. Y., Veldhuis J. D., Van den Berghe G. (2002) A novel in vivo rabbit model of hypercatabolic critical illness reveals a biphasic neuroendocrine stress response. Endocrinology 143, 764–774 [DOI] [PubMed] [Google Scholar]
- 23. Graves K. H., Moreadith R. W. (1993) Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 36, 424–433 [DOI] [PubMed] [Google Scholar]
- 24. Fang Z. F., Gai H., Huang Y. Z., Li S. G., Chen X. J., Shi J. J., Wu L., Liu A., Xu P., Sheng H. Z. (2006) Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic, or somatic cell nuclear transfer embryos. Exp. Cell Res. 312, 3669–3682 [DOI] [PubMed] [Google Scholar]
- 25. Honda A., Hirose M., Inoue K., Ogonuki N., Miki H., Shimozawa N., Hatori M., Shimizu N., Murata T., Hirose M., Katayama K., Wakisaka N., Miyoshi H., Yokoyama K. K., Sankai T., Ogura A. (2008) Stable embryonic stem cell lines in rabbits: potential small animal models for human research. Reprod. Biomed. Online 17, 706–715 [DOI] [PubMed] [Google Scholar]
- 26. Intawicha P., Ou Y. W., Lo N. W., Zhang S. C., Chen Y. Z., Lin T. A., Su H. L., Guu H. F., Chen M. J., Lee K. H., Chiu Y. T., Ju J. C. (2009) Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells 11, 27–38 [DOI] [PubMed] [Google Scholar]
- 27. Wang S., Shen Y., Yuan X., Chen K., Guo X., Chen Y., Niu Y., Li J., Xu R. H., Yan X., Zhou Q., Ji W. (2008) Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J. Biol. Chem. 283, 35929–35940 [DOI] [PubMed] [Google Scholar]
- 28. Honda A., Hirose M., Ogura A. (2009) Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp. Cell Res. 315, 2033–2042 [DOI] [PubMed] [Google Scholar]
- 29. Okada Y., Shimazaki T., Sobue G., Okano H. (2004) Retinoic acid concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 275, 124–142 [DOI] [PubMed] [Google Scholar]
- 30. Chambers S. M., Fasano C. A., Papapetrou E. P., Tomishima M., Sadelain M., Studer L. (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 [DOI] [PubMed] [Google Scholar]
- 32. Billon N., Jolicoeur C., Ying Q. L., Smith A., Raff M. (2002) Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J. Cell Sci. 115, 3657–3665 [DOI] [PubMed] [Google Scholar]
- 33. Tokumoto Y., Ogawa S., Nagamune T., Miyake J. (2010) Comparison of efficiency of terminal differentiation of oligodendrocytes from induced pluripotent stem cells versus embryonic stem cells in vitro. J. Biosci. Bioeng. 109, 622–628 [DOI] [PubMed] [Google Scholar]
- 34. Nichols J., Smith A. (2009) Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 [DOI] [PubMed] [Google Scholar]
- 35. Brons I. G., Smithers L. E., Trotter M. W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A., Vallier L. (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 [DOI] [PubMed] [Google Scholar]
- 36. Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., McKay R. D. (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 [DOI] [PubMed] [Google Scholar]
- 37. Xu Y., Zhu X., Hahm H. S., Wei W., Hao E., Hayek A., Ding S. (2010) Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. U.S.A. 107, 8129–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanna J., Cheng A. W., Saha K., Kim J., Lengner C. J., Soldner F., Cassady J. P., Muffat J., Carey B. W., Jaenisch R. (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. U.S.A. 107, 9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greber B., Wu G., Bernemann C., Joo J. Y., Han D. W., Ko K., Tapia N., Sabour D., Sterneckert J., Tesar P., Schöler H. R. (2010) Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226 [DOI] [PubMed] [Google Scholar]
- 40. Wang W., Yang J., Liu H., Lu D., Chen X., Zenonos Z., Campos L. S., Rad R., Guo G., Zhang S., Bradley A., Liu P. (2011) Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor γ and liver receptor homolog 1. Proc. Natl. Acad. Sci. U.S.A. 108, 18283–18288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tachibana M., Sparman M., Ramsey C., Ma H., Lee H. S., Penedo M. C., Mitalipov S. (2012) Generation of chimeric rhesus monkeys. Cell 148, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu F., Sridharan B., Wang S., Gurkan U. A., Syverud B., Demirci U. (2011) Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics 5, 22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aubert J., Dunstan H., Chambers I., Smith A. (2002) Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 20, 1240–1245 [DOI] [PubMed] [Google Scholar]
- 44. Schuldiner M., Eiges R., Eden A., Yanuka O., Itskovitz-Eldor J., Goldstein R. S., Benvenisty N. (2001) Induced neuronal differentiation of human embryonic stem cells. Brain Res. 913, 201–205 [DOI] [PubMed] [Google Scholar]
- 45. Kim D. S., Lee J. S., Leem J. W., Huh Y. J., Kim J. Y., Kim H. S., Park I. H., Daley G. Q., Hwang D. Y., Kim D. W. (2010) Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 6, 270–281 [DOI] [PubMed] [Google Scholar]
- 46. Vallier L., Reynolds D., Pedersen R. A. (2004) Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275, 403–421 [DOI] [PubMed] [Google Scholar]
- 47. Smith J. R., Vallier L., Lupo G., Alexander M., Harris W. A., Pedersen R. A. (2008) Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev. Biol. 313, 107–117 [DOI] [PubMed] [Google Scholar]
- 48. Izrael M., Zhang P., Kaufman R., Shinder V., Ella R., Amit M., Itskovitz-Eldor J., Chebath J., Revel M. (2007) Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol. Cell. Neurosci. 34, 310–323 [DOI] [PubMed] [Google Scholar]
- 49. Koehler K. R., Tropel P., Theile J. W., Kondo T., Cummins T. R., Viville S., Hashino E. (2011) Extended passaging increases the efficiency of neural differentiation from induced pluripotent stem cells. BMC Neurosci. 12, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim S., Kim G. J., Miyoshi H., Moon S. H., Ahn S. E., Lee J. H., Lee H. J., Cha K. Y., Chung H. M. (2007) Efficiency of the elongation factor-1α promoter in mammalian embryonic stem cells using lentiviral gene delivery systems. Stem Cells Dev. 16, 537–545 [DOI] [PubMed] [Google Scholar]
- 51. Kobayashi T., Yamaguchi T., Hamanaka S., Kato-Itoh M., Yamazaki Y., Ibata M., Sato H., Lee Y. S., Usui J., Knisely A. S., Hirabayashi M., Nakauchi H. (2010) Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799 [DOI] [PubMed] [Google Scholar]
- 52. Isotani A., Hatayama H., Kaseda K., Ikawa M., Okabe M. (2011) Formation of a thymus from rat ES cells in xenogeneic nude mouse↔rat ES chimeras. Genes Cells 16, 397–405 [DOI] [PubMed] [Google Scholar]
- 53. Vroemen M., Weidner N., Blesch A. (2005) Loss of gene expression in lentivirus- and retrovirus-transduced neural progenitor cells is correlated to migration and differentiation in the adult spinal cord. Exp. Neurol. 195, 127–139 [DOI] [PubMed] [Google Scholar]
- 54. Telugu B. P., Ezashi T., Sinha S., Alexenko A. P., Spate L., Prather R. S., Roberts R. M. (2011) Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J. Biol. Chem. 286, 28948–28953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fujishiro S. H., Nakano K., Mizukami Y., Azami T., Arai Y., Matsunari H., Ishino R., Nishimura T., Watanabe M., Abe T., Furukawa Y., Umeyama K., Yamanaka S., Ema M., Nagashima H., Hanazono Y. (2013) Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev. 22, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osteil P., Tapponnier Y., Markossian S., Godet M., Schmaltz-Panneau B., Jouneau L., Cabau C., Joly T., Blachère T., Gócza E., Bernat A., Yerle M., Acloque H., Hidot S., Bosze Z., Duranthon V., Savatier P., Afanassieff M. (2013) Induced pluripotent stem cells derived from rabbits exhibit some characteristics of naive pluripotency. Biol. Open 2, 613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]