Background: Expression of Ror2 leads to increased tumorigenicity in RCC.
Results: Ror2 expression stabilizes an increased pool of soluble β-catenin, which enhances the response to Wnt stimulation.
Conclusion: Ror2 promotes basal and canonical Wnt3a ligand-enhanced β-catenin signaling in RCC.
Significance: This work demonstrates a novel Ror2-dependent tiered state of canonical Wnt signaling, promoting basal signals and priming RCC cells for a heightened response to the ligand.
Keywords: β-Catenin, Cancer, Receptor Tyrosine Kinase, Signaling, Wnt Signaling, RCC, Ror2, Wnt3a, Renal Cell Carcinoma
Abstract
Expression of the receptor tyrosine kinase-like orphan receptor 2 (Ror2) has been identified in an increasing array of tumor types and is known to play a role as an important mediator of Wnt signaling cascades. In this study, we aimed to clarify Ror2 interactions with the Wnt pathways within the context of renal cell carcinoma (RCC). An examination of Ror2 expression in primary human RCC tumors showed a significant correlation with several Wnt signaling genes, including the classical feedback target gene Axin2. We provide evidence that Ror2 expression results in a partially activated state for canonical Wnt signaling through an increased signaling pool of β-catenin, leading to an enhancement of downstream target genes following Wnt3a stimulation in both renal and renal carcinoma-derived cells. Additionally, inhibition of low-density lipoprotein receptor-related protein 6 (LRP6) with either siRNA or dickkopf decreased the response to Wnt3a stimulation, but no change was seen in the increased β-catenin pool associated with Ror2 expression, suggesting that LRP6 cofactor recruitment is necessary for a Wnt3a-induced signal but that it does not participate in the Ror2 effect on β-catenin signaling. These results highlight a new role for Ror2 in conveying a tonic signal to stabilize soluble β-catenin and create a poised state of enhanced responsiveness to Wnt3a exogenous signals in RCC.
Introduction
Cancer cells usurp many normal signaling processes in the course of transformation or acquisition of transformed cell traits. The Wnt signaling pathway is a growth and differentiation control pathway central to embryogenesis and cancer (1). The canonical Wnt pathway is characterized by secreted Wnt factors, with Wnt3a being the most widely studied, engaging the receptor complex consisting of low-density lipoprotein receptor-related protein (LRP)2 and a member of the Frizzled (Fzd) family of receptors, halting the basal state of ubiquitination and degradation of β-catenin within the destruction complex. As β-catenin becomes stabilized, it results in the saturation of the destruction complex, leading to an increase of cytosolic β-catenin and localization to the nucleus (2). Nuclear β-catenin drives the transcription of genes involved in promoting cell proliferation, maintenance of a primitive state, or inducing differentiation dependent upon cellular context. Wnts can initiate other signaling processes, known as the non-canonical Wnt signaling pathways and promote migration, establishment of polarity, or programming of morphogenesis (3). In tumors, these pathways provide signals for cancer cells to proliferate, acquire stem cell-like properties, and secure invasive characteristics as a result of mutations or differential expression of key members of these pathways.
One of the recently described non-canonical Wnt receptors, the receptor tyrosine kinase-like orphan receptor 2 (Ror2), has been implicated in a diverse set of cancers (4–9), including renal cell carcinoma (RCC), where the aberrant expression occurs as a result of the constitutive deregulation of the hypoxia response pathway resulting from loss of the von Hippel-Lindau (VHL) gene (4, 10). Ror2 is best known for its role in bone morphogenesis because mutation or loss of this protein in early embryogenesis results in limb foreshortening in humans and mouse models (11–13). The expression of Ror2 in mice is restricted to early gestation and mesenchymal stem cell niches (14, 15). Ror2 is emerging as an intriguing mediator of various context-specific Wnt signals in developmental processes as well as cancer (16–18). Ror2 has been shown to engage both canonical and noncanonical Wnts, and it has been shown to transmit signals related to convergent extension movements, planar cell polarity, and proliferation (19, 20). Ror2 engagement of Wnt5a has been shown to inhibit canonical signaling. However, recent work utilizing primary cell lines showed this effect to be Ror2-independent (18, 21). The ability of Ror2 to convey situation-appropriate signals likely involves the availability of cofactors, binding partners, or downstream substrates to affect the signal and functional outcome and may contribute to cell type- or situation-specific effects.
Although we have shown previously that Ror2 expression is associated with cellular migration and invasion phenotypes in renal carcinoma cells, growth of RCC is also facilitated by β-catenin transcription-mediated canonical signals (22). The mediators of this signal are thought to be dependent on the lost expression of the von Hippel-Lindau tumor suppressor (23, 24). Therefore, we decided to explore the signaling pathways related to Ror2 expression in renal carcinoma- and renal-derived cellular systems. Here, we demonstrate that human RCC tumors and cell lines show concordance of Ror2 expression with a large number of established canonical Wnt pathway targets, suggestive of a role of Ror2 in mediating β-catenin-dependent signaling. Expression of Ror2 increased the stabilization of β-catenin as well as the transcriptional activity of canonical Wnt target genes, independently of exogenously added Wnt3a, and led to the enhancement of the canonical signal when exogenous Wnt3a was supplied. Inhibition of the canonical coreceptor LRP6 reduced the responsiveness to Wnt3a. However, it did not ablate the Wnt3a-independent increase in β-catenin. Mutations in the kinase domain of Ror2 abrogated the stabilization of β-catenin and Axin2 transcription observed with expression of wild-type protein. These findings demonstrate a novel LRP-independent function of Ror2 to maintain an increased signaling pool of stable β-catenin in renal cells while priming cells for additional responsiveness to Wnt3a activation of the β-catenin transcriptional pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
786-0 and the derivative cell lines 786-0 RC3 (25) and 786-0 pRS, stable monoclonal knockdown 786-0 shRor2.1 and shRor2.2 (4) cells, 786-0 Tap-hRor2 cells, and HEK293T cells were grown in DMEM with 10% FBS, nonessential amino acids, l-glutamine, and penicillin/streptomycin. L cells and Wnt3a-producing L cells were grown in DMEM with 10% FBS. Control and Wnt3a-conditioned medium (CM) was collected at 24 and 48 h after cells reached confluency. 786-0 cells were transfected with TAP-hRor2 and selected with 1 μg/ml puromycin to generate polyclonal stable lines. HEK293T and 786-0 cells were transduced with lentiviruses carrying the eGFP control, TRE(tight)-eGFP-pEF1a-rtTA-IRES-Puro or hRor2-eGFP, TRE(tight)-hRor2eGFP-pEF1a-rtTA-IRES-Puro, with stable lines generated following selection with 1 μg/ml puromycin.
Plasmids
The TAP-hRor2 was generated with hRor2 cloned into the pIRESpuro-GLUE backbone (26). The control TRE(tight)-eGFP-pEF1a-rtTA-IRES-Puro and TRE-tight-hRor2-eGFP-eF1a-rtTA-IRES-Puro vectors were generated with eGFP alone or the fusion hRor2-eGFP being cloned into a PHAGE6 lentiviral backbone (27). Expression of eGFP or hRor2-eGFP was confirmed visually 48 h after induction with doxycycline (500 ng/ml). Site-directed mutagenesis using a QuikChange II site-directed mutagenesis kit (Agilent Technologies, La Jolla CA) was used to introduce the amino acid substitutions K507M and D633A for hRor2-eGFP-DM.
siRNA
Stealth siRNA for LRP6 (5′-ACGCAGCAUUGAGCGUGCCAACAAA-3′ and 5′-GAUCCCAUGGUUGGGUACAUGU AUU-3′) were pooled, and Stealth negative control siRNA (Stealth RNAi negative control low GC duplex) was introduced into cells using Lipofectamine RNAiMAX (Invitrogen) according to the instructions of the manufacturer and allowed to incubate for 48 h.
Quantitative RT-PCR and Human Wnt Signaling RT2 Profiler PCR Array
Total RNA was extracted from cells using a Qiagen RNeasy mini kit (Valencia, CA). cDNA was made from 500 ng of total RNA using random primers (Invitrogen) and Superscript II RT-PCR reagents (Invitrogen) and analyzed using the ABI 7900HT fast real-time PCR system with the following proprietary FAM-labeled primers: Ror2, Axin2, Fzd1, Jun, 18S, and β-actin (Applied Biosystems, Foster City, CA). Wnt-related gene expression was examined with the human Wnt signaling RT2 Profiler PCR array (SABiosciences, Frederick, MD) using cDNA from 786-0 +TAP-hRor2, 786-0, and 786-0 shRor2.2 cells with the ABI 7500 real-time PCR system. Changes in gene expression were calculated following normalization to an internal pool of five housekeeping genes. Axin2 was not available in the RT2 Profiler PCR array.
Immunoblotting
Cells were lysed in 10 mm Tris (pH 7.4), 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm NaF, 20 mm Na4P2O7, 2 mm Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mm PMSF, and a protease inhibitor mixture (Roche Diagnostics, Indianapolis IN) and quantitated using Bradford reagent to measure absorbance at 595 nm. Lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes (GE Healthcare, Pittsburg, PA), and evaluated using the following antibodies: Ror2 (catalog no. AF2064, R&D Systems, Minneapolis, MN); DVL2 (catalog no. 3216), DVL3 (catalog no. 3218), LRP6 (catalog no. 3395), and pLRP6 (catalog no. 2568, Cell Signaling Technology, Danvers, MA); Ku80 used as loading control (catalog no. ab1273, Abcam, Cambridge, MA); and β-catenin (catalog no. C2206, Sigma-Aldrich, St. Louis, MO). For all immunoblot analyses, following incubation with corresponding secondary antibodies conjugated to IR dye 680 or 800, membranes were scanned using the Odyssey IR imager (LI-COR Biosciences, Lincoln, NE) with densitometric analysis performed using Odyssey v3.0.21.
Concanavalin-A Separation of Cytosolic β-Catenin
Cells were incubated for 1 h with control-conditioned medium, Wnt3a-conditioned medium (1:2 dilution), or DKK1 (400 ng/ml, R&D Systems). 50 μg of protein lysates from each sample as determined by Bradford assay were rotated with 10 μl of prepared concanavalin A-Sepharose 4b beads (GE Healthcare) overnight at 4 °C. Supernatants were removed following a brief centrifugation and analyzed by SDS-PAGE.
Luciferase Assays
To determine the activity of canonical Wnt signaling in the RCC cells, 786-0 and HEK293T cells were transfected with TOPFlash and constitutive pHRG-Renilla luciferase reporters. 24 h post-transfection, cells were seeded in triplicate, with medium being replaced after 8 h with serum-free medium and incubated for 20 h. Post-serum starvation, cells were treated with control PBS or recombinant human Wnt3a (100 ng/ml, R&D Systems) and incubated for 24 h. Luciferase activity was measured from lysed samples using a luminometer and the Dual-Luciferase reporter assay system (Promega, Madison, WI). Relative luciferase units were calculated by the ratio of TOPFlash luciferase activity to internal Renilla luciferase activity for each sample.
Microarray Analysis
Gene expression for 95 human RCC tumors was downloaded and prepared as described previously (28). Collection and analysis of these tumors was approved by the institutional biomedical ethics review committee. Significance of analysis of microarrays was used to determine genes that significantly correlated with Ror2 expression among the 95 human RCC tumors with a false discovery rate < 0.043% and analyzed using Database for Annotation, Visualization and Integrated Discovery (DAVID) to identify significantly enriched gene ontologies.
Statistical Analysis
One-way ANOVA was used to generate p values in the comparison of each experimental condition with the control. A p value of <0.05 was considered to be significant and < 0.001 to be highly significant. All error bars shown are the calculated S.D. or S.E. across duplicate or triplicate experiments.
RESULTS
Expression of Ror2 in Primary Human RCC Tumors Correlates with Expression of Wnt Signaling Component Genes
We established previously that Ror2 is expressed in RCC and contributes to three-dimensional growth, migration, and in vivo tumor growth (4). However, the role of Ror2 as a Wnt receptor in RCC remains largely unknown. Ror2 has been shown to engage both canonical and non-canonical Wnt ligands (16–18). We first sought to elucidate the dominant pattern of interaction of Ror2 with signaling networks through examination of gene expression patterns from 95 human clear cell RCC tumors. An analysis of transcript diversity was performed using Agilent microarray data using significance of microarrays. This evaluation yielded 723 genes (supplemental Table 1) that exhibited a significant correlation or anticorrelation with Ror2 (false discovery rate < 0.043%). A heat map of these genes following an unsupervised clustering exhibited two cluster patterns correlating with the level of Ror2 transcript in these tumors (Fig. 1A). A variety of significantly enriched gene ontologies (supplemental Table 2) were identified, using DAVID, from the genes exhibiting a positive correlation with Ror2. Similar to previous results (4), Ror2 expression correlated with many genes playing a role in skeletal system development (GO (Gene Ontology database): 0001501, p = 6.11 × 10−08) (Fig. 1B). In addition, enrichment for genes in the Wnt receptor canonical signaling pathway (GO:0016055, p = 2.6 × 10−4) was also observed (Fig. 1C). These results suggest that the role of Ror2 as a Wnt receptor in RCC tumors may not be limited to non-canonical Wnt pathways.
FIGURE 1.
Expression of Ror2 in primary human RCC tumors correlates with expression of Wnt signaling component genes. A, heat map of 721 probes exhibiting significant (false discovery rate < 0.043%) correlation and anticorrelation with Ror2 expression, as determined by significance of analysis of microarrays in a panel of 95 primary human RCC tumors. Shown are magnified heat maps for the following significantly enriched gene ontologies identified using DAVID from genes positively correlating with Ror2 expression, skeletal development (B), and the Wnt signaling pathway (C).
Expression of Ror2 in RCC Cells Results in Changes of Canonical Wnt Regulators and Target Genes
Building on our findings in our human RCC tumors, we examined the effects of Ror2 expression on gene expression using the human Wnt signaling RT2 Profiler PCR array (SABiosciences). The RCC-derived cell line 786-0, which exhibits endogenous Ror2 expression, a derived shRNA Ror2 knockdown cell line, 786-0 shRor2, and an overexpression cell line, 786-0 TAP-hRor2, were used to determine alterations in gene expression under basal conditions (Fig. 2A). We observed a large number of canonical genes displaying concordant expression with Ror2 (Fig. 2B), suggesting a potential association between Ror2 expression and the activity of canonical β-catenin transcriptional activity independent of Wnt3a stimulation with exogenous ligand. This concordant expression pattern with Ror2 was verified independently for targets Fzd1 and Jun using independent samples and primer pairs (Fig. 2, C and D).
FIGURE 2.
Expression of Ror2 in RCC cells results in changes of canonical Wnt regulators and target genes. A, RNA expression was paneled across various Wnt component and target genes using the human Wnt signaling RT2 Profiler PCR array with cDNA from 786-0 cells that endogenously express Ror2, 786-0 shRor2 where Ror2 is stably suppressed, and the Ror2 overexpression cell line 786-0 + TAP-hRor2. Transcript values for each sample were normalized to the geometric mean of five internal housekeeping genes. Changes in the level of expression correlating with Ror2 expression are displayed for the indicated 24 genes, with fold change calculated relative to 786-0. B, representative quantitative RT-PCR showing Ror2 expression levels for all 786-0 cell lines relative to 786-0. Quantitative RT-PCR shows a decrease in 786-0 shRNA Ror2 knockdown relative to 786-0 cells for FZD1 (C) and JUN (D). For all quantitative RT-PCR assays, transcript values were normalized to the β-actin RNA internal standard. Error bars represent S.E. across triplicates in triplicate experiments. p values were calculated using one-way ANOVA. *, p < 0.05; **, < 0.001.
Suppression of Ror2 Results in Decreased β-Catenin-mediated Transcription in RCC Cells
To more fully determine whether the aberrant expression of Ror2 in RCC cells contributes to β-catenin-dependent signaling, we used 786-0 cells, which endogenously express Ror2. To directly examine canonical Wnt target gene activation in these cells, we first examined the mRNA expression of a classic canonical Wnt target gene, Axin2, using quantitative RT-PCR. Using two independent shRNAs targeting unique domains of Ror2, we observed a significant suppression of Ror2 (Fig. 3A) and of Axin2 transcription following treatment with Wnt3a (B) in comparison with the control empty vector (786-0 RC3), which also exhibited an increase in Ror2 expression with Wnt3a stimulation. These results were corroborated using the TOPFlash luciferase-reporter, where a 786-0 pRS (pRetroSuper) control showed a significant induction of signal in response to Wnt3a, but no induction was observed with shRor2 knockdown cell lines (Fig. 3C).
FIGURE 3.
Suppression of Ror2 results in decreased β-catenin-mediated transcription in RCC cells. Quantitative RT-PCR in 786-0 cells for Ror2 (A) shows a significant increase in control RC3 cells with Wnt3a (100 ng/ml) stimulation, whereas levels were reduced significantly in both shRNA Ror2 knockdown cell lines. B, likewise, a significant attenuation in Axin2 transcription in response to Wnt3a (100 ng/ml) is seen in both shRNA Ror2 knockdowns using quantitative RT-PCR. For all quantitative RT-PCR assays, transcript values were normalized to an 18 S RNA internal standard, with fold change calculated in reference to unstimulated 786-0 RC3. C, a significant reduction of β-catenin-mediated transcriptional response following Wnt3a (100 ng/ml) stimulation with the TOPFlash luciferase reporter in 786-0 Ror2-suppressed cells in comparison to control 786-0 pRS cells. Relative luciferase units (RLU) were calculated by normalizing TOPFlash luciferase to internal Renilla luciferase activity. Error bars represent S.E. across triplicates in duplicate experiments. p values were calculated using one-way ANOVA. *, p < 0.05; **, < 0.001.
Overexpression of Ror2 Enhances β-Catenin-mediated Transcription in Renal and RCC Cells
Prior studies expressing Ror2 in HEK293 cells have focused on concomitant treatment of Wnt3a and Wnt5a but have not elaborated how Ror2 expression may also potentiate Wnt3a canonical signaling or examine the potential for differential signaling (18, 29). To further examine Ror2 contributions as a mediator of canonical β-catenin signaling in RCC, we utilized 786-0 and HEK293T cells expressing either a tetracycline-dependent fusion protein, Ror2-GFP, or a GFP control. Robust expression of Ror2 mRNA was observed in 786-0 Ror2-overexpressing cells, relative to control GFP cells, following the addition of doxycycline and remained unaltered by treatment with Wnt3a (Fig. 4A). Upon expression of Ror2, there was a significant increase in basal Axin2 mRNA as well as a heightened response to Wnt3a treatment (Fig. 4B). This enhancement of β-catenin-mediated transcription in the absence of Wnt3a with expression of Ror2 and further potentiation was also observed in 786-0 cells transfected with a TOPFlash luciferase reporter (Fig. 4D).
FIGURE 4.
Overexpression of Ror2 enhances β-catenin-mediated transcription in renal and RCC cells. Quantitative RT-PCR for Ror2 in 786-0 (A) and HEK293Tcells (E) shows a strong induction of Ror2 following treatment with doxycycline (500 ng/ml) and concordant significant increase in basal expression of the canonical Wnt target gene Axin2 that is further heightened upon the addition of Wnt3a (100 ng/ml) relative to the control cells (B and F). Quantitative RT-PCR in 786-0 (C) and 293T (G) cells for Axin2 again shows an increase in basal levels with Ror2 expression and a significant increase in response to treatment with LiCl (10 mm). For all quantitative RT-PCR assays, transcript values were normalized to the β-actin RNA internal standard, with fold change calculated in reference to unstimulated GFP-expressing cells. D, the expression of Ror2 in 786-0 transfected with a TOPFlash luciferase reporter exhibited a significant increase in activity basally, with Ror2 expression leading to a significant increase in response to Wnt3a (100 ng/ml) stimulation. H, HEK293T cells transfected with a TOPFlash luciferase reporter displayed a significantly enhanced response to Wnt3a (100 ng/ml) stimulation. Error bars represent S.E. across triplicates in duplicate experiments. p values were calculated using one-way ANOVA. *, p < 0.05; **, p < 0.001.
To validate these findings in an additional renal-derived cell background, we used HEK293T cells expressing either GFP or Ror2. Upon the induction of Ror2 in HEK293T cells, we again observed a significant enhancement of Axin2 transcription and a further increase following stimulation with Wnt3a (Fig. 4F). Likewise, β-catenin mediated transcription measured using the TOPFlash luciferase reporter showed an enhanced response to Wnt3a stimulation, although no significant change was detected under basal conditions (Fig. 4H). Inhibition of GSK3-β with LiCl resulted in an additive effect of Axin2 transcription with Ror2 expression, suggesting that Ror2 effects are mediated through β-catenin (Fig. 4, C and G).
Ror2 Expression Results in an Increased Pool of Stable β-Catenin Independent of Exogenous Wnt Stimulation
Both TCF-luciferase reporters and target gene expression of Axin2 demonstrated that Ror2 expression is associated with heightened β-catenin target gene transcription in response to Wnt3a stimulation. In addition, expression of Ror2 resulted in an enhanced transcriptional activity in the absence of exogenous ligand. Therefore, we sought to determine whether these findings also correlated with the availability of β-catenin for transcriptional activation.
To examine the pool of cytosolic β-catenin available for signaling, protein lysates were incubated with concanavalin-A-Sepharose beads. Immunoblot analysis of 786-0 cell lysates revealed an increase of free β-catenin levels (indicated with an asterisk, compared with total levels) upon expression of Ror2, with a further increase following stimulation with Wnt3a CM (Fig. 5A). These results were corroborated in HEK293T cells, demonstrating increased stabilized β-catenin upon Ror2 expression that is enhanced with the addition of Wnt3a CM in comparison to GFP expressing cells (Fig. 5B). Quantification of stabilized β-catenin relative to total β-catenin is shown below the corresponding bands in each immunoblot analysis.
FIGURE 5.
Ror2 expression results in an increased pool of stable β-catenin independent of exogenous Wnt stimulation. Immunoblot analysis for the indicated antibodies of whole cell lysates or concanavalin A-treated lysates (asterisk) following stimulation with either control or Wnt3a CM (dilution 1:2) for 1 h. 786-0 (A) and HEK293T (B) cells show increased levels of phosphorylated DVL2, DVL3, and stable β-catenin with Ror2 expression and an additional increase of stable β-catenin following treatment with Wnt3a CM. C, 786-0 shRor2 knockdown cell lines exhibited attenuated levels of stable β-catenin basally and in response to Wnt3a CM stimulation in comparison to 786-0 RC3 control cells. Changes in pDVL2, pDVL3, and stable β-catenin relative to unphosphorylated DVL or total β-catenin are shown with densitometric quantification below the corresponding bands. LC, loading control.
To understand the mechanism Ror2 could be using to initiate this poised signaling state, we examined upstream pathway components. Dishevelled proteins (DVL2 and DVL3) play an important role in canonical Wnt signaling, and DVL protein activation via phosphorylation has been reported previously as a mediator of Ror2 signaling (21, 32, 33). Expression of Ror2 in 786-0 and HEK293T cells resulted in increased levels of phosphorylated DVL2 and DVL3, detected as a shift from the faster-migrating band to a slower-migrating phosphorylated form indicative of activation (Fig. 5, A and B). Quantitation of pDVL signal relative to total DVL demonstrates increased activation seen with Ror2 expression and, additionally, in response to Wnt3a.
We also examined the receptor LRP6 established previously to be required for Wnt ligand induced β-catenin signaling in mice and Xenopus (30, 31). However, although we observed the expression of LRP6 and its phosphorylation in response to Wnt3a stimulation, no effect was observed related to Ror2 expression (Fig. 5, A–C). These data suggest that Ror2 expression mediates an LRP6-independent signal, resulting in DVL2/3 activation and subsequent stabilization of β-catenin. However, the Wnt-ligand-enhanced signal proceeds via LRP6 phosphorylation.
When we examined suppression of Ror2, 786-0 cells demonstrated no effect on LRP activation in response to Wnt3a, consistent with the previous overexpression studies (Fig. 5C). However, Ror2 shRNA abrogated the stabilized β-catenin both basally and upon Wnt3a CM stimulation (Fig. 5C). The extent of suppression of Ror2 protein differs between the two shRNAs, targeting different sequences in Ror2, with the more potent hairpin, shRor2.2, eliciting the most significant reduction in β-catenin stabilization with or without Wnt3a. This is demonstrated visually as well as quantitatively.
Ror2-dependent Stabilization of β-Catenin Is Independent of LRP6
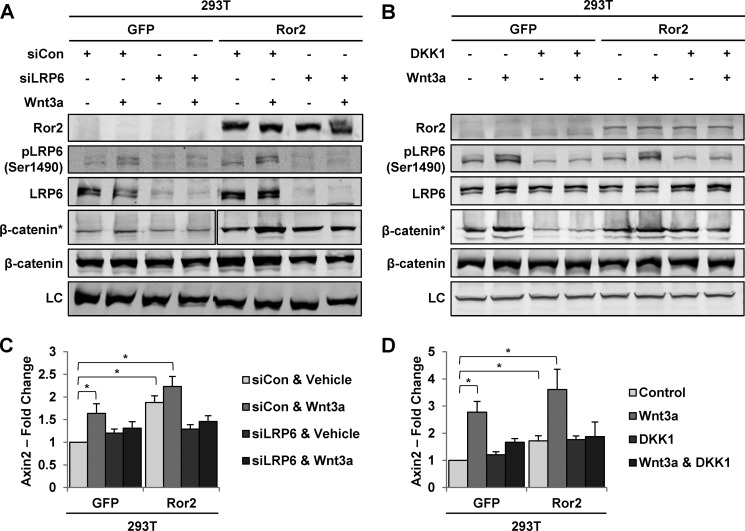
To further determine what contribution the coreceptor LRP6 has on the Ror2 effects in β-catenin-dependent signaling, we utilized siRNA targeting to deplete cells of LRP6. Suppression of the coreceptor LRP6 resulted in the loss of β-catenin stabilization following treatment with Wnt3a CM in GFP control cells. However, the increased basal β-catenin concordant with Ror2 expression remained unaltered (Fig. 6A). We did observe repression of the stimulated signal at the level of Axin2 transcription with siLRP6 in both GFP and Ror2-expressing cells, although in HEK293T cells, the basal increased level of Axin2 transcript was lost on LRP6 suppression (Fig. 6C).
FIGURE 6.
Ror2-dependent stabilization of β-catenin is independent of LRP6. A, immunoblot analysis for the indicated antibodies of either whole cell lysates or concanavalin A bead-treated lysates (asterisk) from 293T cells 48 h post-transfection with siCon (nonspecific control) or siLRP6 and stimulated with either control or Wnt3a CM (dilution 1:2) for 1 h shows that increased levels of β-catenin are maintained with Ror2 expression. B, immunoblot analysis for the indicated antibodies of whole cell lysates or concanavalin A-treated lysates (asterisk) following stimulation with either Wnt3a CM (dilution 1:2) or DKK1 (400 ng/ml) for 1 h followed by Wnt3a CM stimulation showed increased levels of β-catenin concurrent with Ror2 expression. LC, loading control. Quantitative RT-PCR for Axin2 in HEK293T cells (C) transfected with siCon or siLRP6 and stimulated 48 h post transfection with Wnt3a (100 ng/ml) show that LRP6 is needed for mediating Wnt3a/β-catenin-dependent transcription. D, Axin2 expression is shown in 293T cells stimulated with either Wnt3a (100 ng/ml) or DKK1 (400 ng/ml) for 1 h followed by Wnt3a stimulation, with high DKK1 inhibiting the response to Wnt3a stimulation in both GFP- and Ror2-expressing cells. For all quantitative RT-PCR assays, transcript values were normalized to the β-Actin RNA internal standard, with fold change calculated in reference to unstimulated GFP-expressing cells. Error bars represent S.E. across triplicates in duplicate experiments. p values were calculated using one-way ANOVA. *, p < 0.05; *, p < 0.001.
Dickkopf 1 (DKK1) is an established Wnt antagonist that binds to LRP5/6, leading to its internalization from the cell surface. Pretreatment of HEK293T cells with recombinant human DKK1 prior to the addition of Wnt3a CM resulted in decreased stabilization of β-catenin in GFP control cells. However, this addition of DKK1 preceding Wnt3a CM had no bearing on the increased β-catenin pool associated with Ror2 expression (Fig. 6B). Quantitative RT-PCR for Axin2 in DKK1-treated cells, likewise, showed maintenance of higher basal levels of transcription than control cells but with a loss of response to Wnt3a stimulation (Fig. 6D). Together, these data suggest that the LRP6 cofactor is important primarily to transduce the Wnt3a ligand-driven enhanced signal. However, the reduction of the basal target gene transcript by LRP6 siRNA indicates that LRP6 may retain a minor role in mediating this signal.
An Intact Ror2 Kinase Domain Is Required for Poised Wnt Signaling State
To determine whether Ror2 is directly contributing to these changes in canonical Wnt signaling, site-directed mutagenesis was used to disrupt the putative Ror2 kinase domain. Ror2-DM contains mutations in two key residues of the catalytic site, lysine 507 within the putative ATP binding pocket and aspartate 633 of the DFG (found as DLG in Ror2) motif. This mutant version, despite being expressed at similar levels in 786-0 cells as wild-type Ror2, failed to effectively stabilize β-catenin (Fig. 7A). A similar pattern of increased stable β-catenin levels is seen in HEK293T cells with overexpression of wild-type Ror2 but not with expression of the DM mutant (Fig. 7B). This effect is also shown quantitatively below the corresponding immunoblot analyses (Fig. 7, A and B). Quantitative RT-PCR for Axin2 also shows a significant decrease with the expression of mutant Ror2 (Ror2-DM) in comparison with wild-type Ror2 in both 786-0 (Fig. 7C) and HEK293T cells (D). Thus, an intact Ror2 kinase domain is necessary for achieving the increased pool of stabilized β-catenin and for canonical transcript activity independent of Wnt3a stimulation.
FIGURE 7.
Ror2 activity is required for a poised Wnt signaling state. Immunoblot analysis of whole cell lysates or concanavalin A-treated lysates (asterisk) from 786-0 (A) or HEK293T cells (B) expressing GFP, Ror2, or the double mutant Ror2-DM 48 h post induction with doxycycline (500 ng/ml) show increased levels of stable β-catenin with wild-type Ror2 compared with Ror2-DM. Densitometry quantification of stabilized β-catenin relative to total β-catenin is provided below the corresponding bands. LC, loading control. Quantitative RT-PCR for Ror2 in 786-0 (C) or HEK293T (D) cells shows expression of Ror2 and Ror2-DM cells 24 h post-induction but a significant decrease of Axin2 expression in Ror2-DM expression in both cell lines. For all quantitative RT-PCR assays, transcript values were normalized to the β-actin RNA internal standard, with fold change calculated in reference to unstimulated GFP-expressing cells. Error bars represent S.E. across triplicates of a representative duplicated experiment. p values were calculated using one-way ANOVA. *, p < 0.05; **, p < 0.001.
DISCUSSION
Renal cell carcinoma has historically been a cancer that challenges conventional paradigms. Canonical Wnt signaling disregulation is associated with tumorigenesis and progression of RCC, transduced by a previously unknown set of mediating factors (22–24, 34) Furthermore, increased expression of various Wnt ligands, including Wnt3a, have been observed in RCC tumors (34). We sought to identify what role Ror2 played as a Wnt signaling mediator in RCC because it is capable of engaging a variety of canonical and noncanonical Wnt ligands (17). In kidney tumors, we show that canonical β-catenin target genes, including Axin2, positively correlate with Ror2 expression. A translational validation of these results is seen with the multitude of target and canonical component genes showing correlated expression with Ror2 in RCC cells and primary human tumors, including PYGO1, SFRP1, and SFRP4.
Our data with both kidney-derived cells and kidney cancer cell lines show that Ror2 expression results in a state of basally enhanced canonical transcriptional signaling because of DVL activation and an increased pool of stabilized, soluble β-catenin. Stimulation of Ror2-expressing cells with Wnt3a further enhances the β-catenin pool and transcriptional response (Fig. 8).
FIGURE 8.

Ror2/Wnt3a signaling in RCC cells. Schematic overview of Ror2/Wnt3a signaling in RCC cells. A, in canonical Wnt signaling in the absence of either Wnt ligand or Ror2 expression, β-catenin is degraded by the proteasome. B, however, with the expression of Ror2, DVL becomes phosphorylated, leading to stabilization of β-catenin and its translocation into the nucleus, driving transcription of target genes (i.e. Axin2). C, in cells expressing Ror2 and stimulated with Wnt3a, a further enhancement of β-catenin stabilization and amplified transcription of target genes is observed.
The canonical signal cascade requires the canonical coreceptor LRP6 for pathway activation in response to Wnt3a because we observed consistent inhibition with siLRP6 or DKK1 of β-catenin stabilization as well as Axin2 transcription with the concurrent addition of Wnt3a. Inhibition or knockdown of LRP6, however, showed little effect on the increased pool of stabilized β-catenin associated with Ror2 expression in the absence of Wnt3a. This result would suggest that the Ror2-dependent tonic signal to stabilize β-catenin and enhance transcriptional signals is independent of LRP6. However, siRNA targeting of LRP6, but not inhibition with DKK1, inhibited the enhanced basal levels of Axin2 expression seen with Ror2, allowing the possibility that Ror2 also involves LRP6 in maintaining this tonic state of canonical Wnt signaling. Recent work has demonstrated that β-catenin remains bound in the destruction complex and that Wnt stimulation inhibits β-catenin degradation, leading to saturation of the complex. As a result, β-catenin accumulates in the cytosolic pool, is then transported into the nucleus, and binds to various target genes (2). These data suggest that Ror2 can play a role in regulating this available and active pool of β-catenin. The increase in measurable soluble β-catenin appears to occur independently of LRP6 cofactor recruitment. However, LRP6 may contribute to the transcriptional activation of target genes, perhaps via a nuclear influx of β-catenin that is not reflected in the soluble pool in the absence of LRP6 or an alternate mechanism.
Ror2 has been shown to mediate activation of various homologs of DVL, key signaling members in multiple Wnt pathways (21, 32, 33). Our data show that Ror2 expression results in an increase in activated (phosphorylated) DVL2/3 in both 786-0 and HEK293T cells. These results, combined with the previous findings in the field, suggest DVL molecules as possible substrates for the kinase activity of Ror2. However, the potential for intermediate substrates or activators of DVL family members cannot be excluded on the basis of these findings.
These findings highlight several points relevant to Wnt signaling and tumor biology. First, this model demonstrates that Ror2 activity may be multidimensional. We observed substantial changes in canonical signaling with Ror2 expression in RCC and renal-derived cell lines, in contrast to previous studies with Ror2 expression having no effect on β-catenin accumulation in response to Wnt3a (33). These expanding cell line-specific roles of Ror2 can likely be attributed, at least in part, to available coreceptors as well as access to Wnt ligands. LRP5/6 is well established as part of the heterodimer responding to Wnt ligand binding. Although Ror2 expression results in Wnt3a-independent, β-catenin-dependent stabilization and signaling, the dependence on the expression of LRP6 for Wnt induction exhibits the need for additional coreceptors for amplifying this signaling cascade from the poised state. Fzd2, which was previously reported to cooperate with Ror2 enhancement of canonical Wnt signaling (16), correlated with Ror2 expression in our set of human RCC tumors, represents a candidate cofactor in this regard, highlighting the future need for a comprehensive understanding of Ror2 interactions with possible coreceptors within the greater cellular context of cancer signaling.
On the basis of these observations, it is essential to examine cell signaling effects with an emphasis toward differential states of activation. Signals emanating from Wnt receptors such as Ror2 may produce partial or incomplete activation of the β-catenin transcriptional response repertoire, a feature we have labeled as a poised state of signaling. Engagement of the canonical Wnt3a ligand, expressed in RCC tumors, produces a robust enhancement that is dependent on the availability of the coreceptor LRP6 and exceeds the Wnt3a induced signal in the absence of Ror2. Together, this model system sheds light on the specific activity of Ror2 signaling in renal cancer and on the complex nature of this signaling pathway, which may be critical for dissecting these events in understanding cancer progression. Furthermore, this model highlights the potential for a tumor modulator to effect moderate pathway activation in a way that primes the pathway for a more intense response when ligand or other sources of stimulation become available.
Ultimately, it is increasingly apparent that Ror2 expression in cancers advances signals that provide a variety of advantages for tumor progression. With Ror2 serving as a mediator of β-catenin-dependent signaling in RCC cells and tumors, it provides a highly promising therapeutic target for RCC.
Acknowledgments
We thank the members of the Rathmell laboratory, the University of North Carolina Lineberger Tissue Procurement Facility; the University of North Carolina Lineberger Tissue Culture Facility, and the University of North Carolina Lineberger Genomics Core Facility.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA121781 (to W. K. R.), T32 ES007126 (to S. A. B.), and T32 GM008719 (to K. E. H.); by National Institutes of Health Vascular Biology Training Grant T32 HL069768-09 (to N. R. R.); by National Institutes of Health/UNC Research Training Service Award T32-CA009156 (to Z. D.); and National Institutes of Health Initiative for Maximizing Student Diversity Training Grants R25GM055336 and NCI-F31CA132543 (to T. M. W.). This work was also supported by American Cancer Society Research Scholar Grant RSG-10-192-01 (to W. K. R.), by the Howard Hughes Medical Institute Translational Medicine Program (to S. A. B.), and by Medical Scientist Training Program T32 GM008719 (to K. E. H.).

This article contains supplemental Tables 1 and 2.
- LRP
- low-density lipoprotein receptor-related protein
- RCC
- renal cell carcinoma
- CM
- conditioned medium
- DVL
- Dishevelled
- ANOVA
- analysis of variance.
REFERENCES
- 1. Majid S., Saini S., Dahiya R. (2012) Wnt signaling pathways in urological cancers. Past decades and still growing. Mol. Cancer 11, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li V. S., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., Gerlach J. P., Mohammed S., Heck A. J., Maurice M. M., Mahmoudi T., Clevers H. (2012) Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 [DOI] [PubMed] [Google Scholar]
- 3. Angers S., Moon R. T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 4. Wright T. M., Brannon A. R., Gordan J. D., Mikels A. J., Mitchell C., Chen S., Espinosa I., van de Rijn M., Pruthi R., Wallen E., Edwards L., Nusse R., Rathmell W. K. (2009) Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28, 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edris B., Espinosa I., Mühlenberg T., Mikels A., Lee C. H., Steigen S. E., Zhu S., Montgomery K. D., Lazar A. J., Lev D., Fletcher J. A., Beck A. H., West R. B., Nusse R., van de Rijn M. (2012) ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J. Pathol. 227, 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu B. J., Wang Y. Q., Wei X. J., Rong L. Q., Wei D., Yan C. M., Wang D. J., Sun J. Y. (2012) Expression of WNT-5a and ROR2 correlates with disease severity in osteosarcoma. Mol. Med. Rep. 5, 1033–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connell M. P., Fiori J. L., Xu M., Carter A. D., Frank B. P., Camilli T. C., French A. D., Dissanayake S. K., Indig F. E., Bernier M., Taub D. D., Hewitt S. M., Weeraratna A. T. (2010) The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi M., Shibuya Y., Takeuchi J., Murata M., Suzuki H., Yokoo S., Umeda M., Minami Y., Komori T. (2009) Ror2 expression in squamous cell carcinoma and epithelial dysplasia of the oral cavity. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 107, 398–406 [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto H., Oue N., Sato A., Hasegawa Y., Yamamoto H., Matsubara A., Yasui W., Kikuchi A. (2010) Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 29, 2036–2046 [DOI] [PubMed] [Google Scholar]
- 10. Wright T. M., Rathmell W. K. (2010) Identification of Ror2 as a hypoxia-inducible factor target in von Hippel-Lindau-associated renal cell carcinoma. J. Biol. Chem. 285, 12916–12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeuchi S., Takeda K., Oishi I., Nomi M., Ikeya M., Itoh K., Tamura S., Ueda T., Hatta T., Otani H., Terashima T., Takada S., Yamamura H., Akira S., Minami Y. (2000) Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5, 71–78 [DOI] [PubMed] [Google Scholar]
- 12. Afzal A. R., Rajab A., Fenske C. D., Oldridge M., Elanko N., Ternes-Pereira E., Tüysüz B., Murday V. A., Patton M. A., Wilkie A. O., Jeffery S. (2000) Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat. Genet. 25, 419–422 [DOI] [PubMed] [Google Scholar]
- 13. Oldridge M., Fortuna A. M., Maringa M., Propping P., Mansour S., Pollitt C., DeChiara T. M., Kimble R. B., Valenzuela D. M., Yancopoulos G. D., Wilkie A. O. (2000) Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat. Genet. 24, 275–278 [DOI] [PubMed] [Google Scholar]
- 14. Al-Shawi R., Ashton S. V., Underwood C., Simons J. P. (2001) Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev. Genes Evol. 211, 161–171 [DOI] [PubMed] [Google Scholar]
- 15. Matsuda T., Nomi M., Ikeya M., Kani S., Oishi I., Terashima T., Takada S., Minami Y. (2001) Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech. Dev. 105, 153–156 [DOI] [PubMed] [Google Scholar]
- 16. Li C., Chen H., Hu L., Xing Y., Sasaki T., Villosis M. F., Li J., Nishita M., Minami Y., Minoo P. (2008) Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol. Biol. 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Billiard J., Way D. S., Seestaller-Wehr L. M., Moran R. A., Mangine A., Bodine P. V. (2005) The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol. Endocrinol. 19, 90–101 [DOI] [PubMed] [Google Scholar]
- 18. Mikels A. J., Nusse R. (2006) Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laird D. J., Altshuler-Keylin S., Kissner M. D., Zhou X., Anderson K. V. (2011) Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet. 7, e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schambony A., Wedlich D. (2007) Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12, 779–792 [DOI] [PubMed] [Google Scholar]
- 21. Ho H. Y., Susman M. W., Bikoff J. B., Ryu Y. K., Jonas A. M., Hu L., Kuruvilla R., Greenberg M. E. (2012) Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 109, 4044–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi H., Chun Y. S., Kim T. Y., Park J. W. (2010) HIF-2α enhances β-catenin/TCF-driven transcription by interacting with β-catenin. Cancer -Res. 70, 10101–10111 [DOI] [PubMed] [Google Scholar]
- 23. Chitalia V. C., Foy R. L., Bachschmid M. M., Zeng L., Panchenko M. V., Zhou M. I., Bharti A., Seldin D. C., Lecker S. H., Dominguez I., Cohen H. T. (2008) Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat. Cell Biol. 10, 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peruzzi B., Athauda G., Bottaro D. P. (2006) The von Hippel-Lindau tumor suppressor gene product represses oncogenic β-catenin signaling in renal carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 103, 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iliopoulos O., Kibel A., Gray S., Kaelin W. G., Jr. (1995) Tumour suppression by the human von Hippel-Lindau gene product. Nat. Med. 1, 822–826 [DOI] [PubMed] [Google Scholar]
- 26. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 27. O'Connell R. M., Balazs A. B., Rao D. S., Kivork C., Yang L., Baltimore D. (2010) Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS ONE 5, e12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teng B. L., Hacker K. E., Chen S., Means A. R., Rathmell W. K. (2011) Tumor suppressive activity of prolyl isomerase Pin1 in renal cell carcinoma. Mol. Oncol. 5, 465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mikels A., Minami Y., Nusse R. (2009) Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 284, 30167–30176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 [DOI] [PubMed] [Google Scholar]
- 31. Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 [DOI] [PubMed] [Google Scholar]
- 32. Yamagata K., Li X., Ikegaki S., Oneyama C., Okada M., Nishita M., Minami Y. (2012) Dissection of Wnt5a-Ror2 signaling leading to matrix metalloproteinase (MMP-13) expression. J. Biol. Chem. 287, 1588–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishita M., Itsukushima S., Nomachi A., Endo M., Wang Z., Inaba D., Qiao S., Takada S., Kikuchi A., Minami Y. (2010) Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol. Cell Biol. 30, 3610–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsu R. J., Ho J. Y., Cha T. L., Yu D. S., Wu C. L., Huang W. P., Chu P., Chen Y. H., Chen J. T., Yu C. P. (2012) WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway. PLoS ONE 7, e47649. [DOI] [PMC free article] [PubMed] [Google Scholar]