Abstract
Histamine, serotonin and dopamine are biogenic amines involved in intercellular communication with multiple effects on human pathophysiology. They are products of two highly homologous enzymes, histidine decarboxylase and l-aromatic amino acid decarboxylase, and transmit their signals through different receptors and signal transduction mechanisms. Polyamines derived from ornithine (putrescine, spermidine and spermine) are mainly involved in intracellular effects related to cell proliferation and death mechanisms. This review summarizes structural and functional evidence for interactions between components of all these amine metabolic and signalling networks (decarboxylases, transporters, oxidases, receptors etc.) at cellular and tissue levels, distinct from nervous and neuroendocrine systems, where the crosstalk among these amine-related components can also have important pathophysiological consequences. The discussion highlights aspects that could help to predict and discuss the effects of intervention strategies.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: biogenic amines, biogenic polyamines, inflammation, cancer, parasites
The aim of the present review
Integration of biological information can reveal new properties and features that could contribute to progress in the characterization of complex systems; this is a fundamental principle of systems biology (Kitano, 2002). A human being can be considered as a system composed of different subsystems specialized in certain functions: interchange with the environment, defence, internal coordination (metabolic homeostasis, motor and cognitive capacities) and reproduction.
Biogenic amines and polyamines have relevant roles in all of these human functions, but the topic is too extensive to be the subject of a single review. Excellent reviews have been published recently on the pharmacological potential of biogenic amines (and related compounds) in neurotransmission (Nuutinen and Panula, 2010; Lin et al., 2011; Lodge and Grace, 2011; Sharp and Cowen, 2011; Tiligada et al., 2011; Deneris and Wyler, 2012; Fleck et al., 2012; and others within this volume).
In the present work, we focus our attention on a set of small physiological subsystems beyond neurotransmission, where components related to cationic amino acids, dopamine and serotonin are present and can interact (Figure 1). Here, we also discuss the potential consequences of these metabolic connections for pharmacology, as well as pointing out some important gaps of information that require further basic research in order to generate useful translational information.
Figure 1.
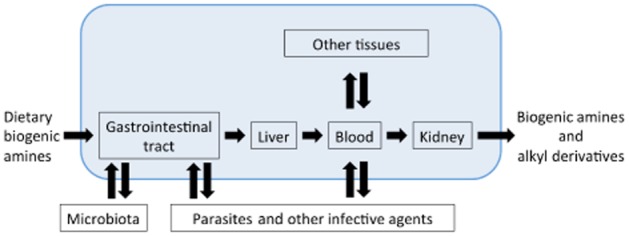
Schematic representation of the different scenarios of amine and amine analogues interactions in humans.
General biochemical and physiological features of biogenic amines and polyamines
Biogenic amines are produced by α-decarboxylation of amino acids. Most amino acid decarboxylases are pyridoxal phosphate-dependent enzymes with several pyruvoyl-dependent exceptions, such as histidine decarboxylase (HDC) of Gram-positive bacteria (Huynh and Snell, 1985) and arginine decarboxylase of hyperthermophilic methanogen bacteria (Graham et al., 2002). For human physiology, the most important amino acid-derived biogenic amines are those synthesized from cationic l-amino acids (putrescine from ornithine and histamine from histidine), from aromatic l-amino acids (serotonin from tryptophan, and dopamine, noradrenaline and adrenaline from tyrosine) and from glutamate (GABA) (Figure 2). An aminopropyl group derived from decarboxylated S-adenosyl methionine has to be added to putrescine to produce spermidine and another one to spermidine to produce spermine. Figure 2 summarizes the amine metabolic pathways in mammalian cells. Other biogenic amines such as cadaverine (decarboxylation product of lysine) and agmatine (decarboxylation product of arginine), present in the human body, mainly come from our diet or are produced by gut microbiota. Recent reviews provide a synthesis of the interspecies differences in biogenic amine metabolism and polyamine metabolism (Heby et al., 2007; Landete et al., 2008; Michael, 2011), which yield opportunities for specific intervention.
Figure 2.
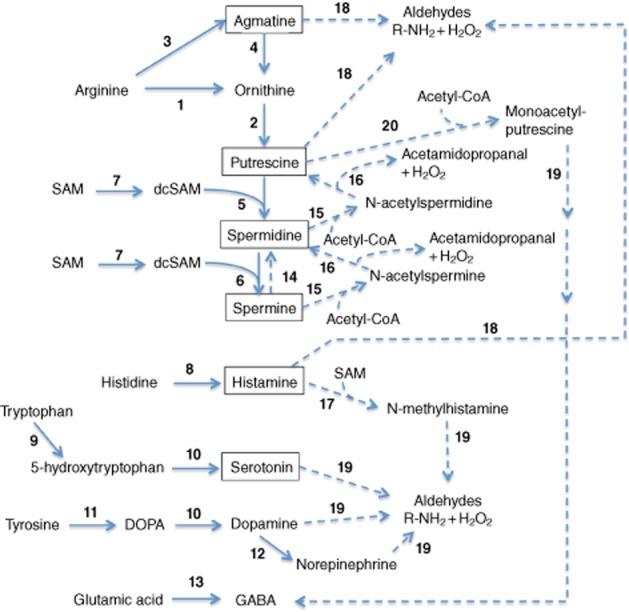
Overview of biogenic amine and polyamine metabolism in humans. Synthetic reactions are shown as solid lines, degradative reactions are shown as dashed lines. 1: arginase; 2: ornithine decarboxylase; 3: arginine decarboxylase (still controversial, Morris, 2007); 4: agmatinase; 5: spermidine synthase; 6: spermine synthase; 7: S-adenosylmethionine decarboxylase; 8: histidine decarboxylase; 9: tryptophan 5-monooxygenase; 10: aromatic L-amino acid decarboxylase (DDC); 11: tyrosine hydrolase; 12: dopamine monooxygenase; 13: glutamate decarboxylase; 14: spermine oxidases; 15: spermidine/spermine N1-acetyltransferase; 16: polyamine oxidases; 17: histamine N-methtyltransferase 18: amine oxidases; 19: aldehyde dehydrogenases.
Most biogenic amines and polyamines are degraded by flavin-dependent or copper-dependent amino oxidases; for example, MAOs, MAO-A and MAO-B (Mitchell, 2008; Wang et al., 2012), and diamine oxidase (DAO)/histaminidase, polyamine oxidases (McGrath et al., 2009; Stevanato et al., 2011) respectively. Amine oxidases are located in different cellular levels (from extracellular to mitochondrial locations, globally referred to as reaction 18 in Figure 2) (Edmondson et al., 2009). Methylation or acetylation steps can precede the action of oxidases. Accumulation of some amine degradation products, such as aldehydes and reactive oxygen species (ROS), can become toxic (Sinigaglia et al., 2012). Therefore, amine oxidases are well-established pharmacological targets, especially for neuroprotection and cardioprotection (Bortolato et al., 2008; Edmondson et al., 2009; Bolasco et al., 2010).
All amines are important signal transducers in mammalian physiology. Nevertheless, the major roles of polyamines (spermidine and spermine) are carried out inside the polyamine-producing cells, as they bind to chromatin and RNAs and are essential to maintain macromolecular synthesis and cell viability (Ruiz-Chica et al., 2001; Medina et al., 2003). Polyamine metabolism is very robust and strictly regulated, as both lack and excess of polyamine levels can be lethal for cells (Rodríguez-Caso et al., 2006). On the other hand, the other monoamines and diamines (histamine, serotonin, dopamine and their derivatives and GABA) are mainly involved in highly complex cell–cell communication networks. The involvement of histamine and serotonin in immune responses is well known (Chodaczek et al., 2009; Schneider et al., 2010). They are all considered neurotransmitters. These intercellular communication processes elicited by biogenic amines involve a numerous family of GPCRs, which have conserved rhodopsin-like structures (Katritch et al., 2012). In spite of the common structural features of the receptors, many questions remain to be answered about the signal transduction pathways of a given biogenic amine in the different mammalian/human cell types under different circumstances. Different arrangements of the polypeptide subunits of the G proteins and other associated macromolecules can even occur in a same cell type, leading to different intracellular effects depending on the stage of development and differentiation, concomitant external stimuli or metabolic status (Ferrada et al., 2009; Moreno et al., 2011). This complexity is in agreement with the ‘pleiotropy’ of these compounds driven by evolution to transmit information among different cell types, tissues and organs.
Biogenic mono-, di- and polyamines can also be transported through different biological membranes by common transport systems, such as the organic cation transporters 2 and 3 – SLC22A2/A3 – and the vesicular monoamine transporters – VMATs/SLC18 (Schütz et al., 1998; Ogasawara et al., 2006)]. Information on cell-specific expression of these and other amine-related proteins can be obtained from Gene Expression Atlas database (Kapushesky et al., 2010) and/or from BioGPS database (Wu et al., 2012). As these transporters are also responsible for the uptake and efflux of other metabolites and drugs; the amines can compete among themselves and with many other drugs, with physiological consequences (Ciarimboli, 2011). Recent reviews have been published on this issue, taking into account the tissue-dependent expression patterns of these transporters in the small intestine, liver, kidney (Müller and Fromm, 2011) and lung (Bosquillon, 2010). In addition, histamine and histamine analogues and polyamines can interfere with two mammalian polyamine-related transport systems: ornithine/arginine transport systems (Medina et al., 1991) and spermine transporter systems (Fajardo et al., 2001; Paz et al., 2001).
Other common macromolecular binding targets have been described for biogenic amines and polyamines that are clearly very important for human physiopathology, as well as for prevention of unwanted pharmacological side effects. For instance, biogenic amines and polyamines are able to bind to members of the cytochrome P450 (CYP) family, which constitute a major pathway for detoxification of chemical environmental hazards (Maréchal et al., 2008). The NMDA receptor also binds both histamine and polyamines (including agmatine and amine analogues), although the preferential binding modes are still under discussion (Watanabe et al., 2008; Mony et al., 2009; Burban et al., 2010). Both polyamines (spermidine and spermine) and diamines (putrescine and histamine) can also bind to nucleic acids (Ruiz-Chica et al., 1999; 2001). The preferential binding modes have been studied for all of them and the presence of histamine in mammalian cell nuclei has also been observed (Medina et al., 2006). In spite of the importance of amine and amine analogue-induced structural changes on nucleic acids (Medina et al., 1998 and; 2003; Ruiz-Chica et al., 1999), the characterization of specific amine effects on gene expression is very difficult because of the chemical nature of the interactions. These are weak but specific electrostatic interactions with ill-defined three-dimensional (3D) structures of nucleic acids, along with many other possibilities for non-specific interactions as organic cations (Igarashi and Kashiwagi, 2000). In any case, such interactions should not be overlooked during pre-clinical evaluation of amine analogues.
A very interesting discovery made more than 25 years ago (Fesus et al., 1985) has required technological development in order to be studied further. In mammalian cells, transglutaminases (EC 2.3.2.13; protein-glutamine γ-glutamyltransferase) are able to covalently bind biogenic amines and polyamines to proteins, including small GTPases (Tabolacci et al., 2012). In a recent review, Walther et al. (2011) summarized the potential regulatory roles of protein ‘histaminylation’, ‘serotonylation’, ‘dopaminylation’ and ‘norepinephrinylation’ on the function of small GTPases, heterotrimeric G-protein and lipid signalling, as well as for modulation of metabolic enzymes, with implications for many different pathophysiological processes, including mast cell activation, bleeding disorders, primary pulmonary hypertension and diabetes. The application of proteomic technologies advance the progress in this interesting topic with clear translational value (Grosso and Mouradian, 2012; Vowinckel et al., 2012).
Inputs and outputs of amines: a dynamic balance with many gaps of information
In its most simple abstraction, the dynamics of every living process can be defined as the balance between inputs and outputs of the participating components (Figure 1). Amine inputs come from uptake and endogenous synthesis (Figure 1). Amine outputs depend on endogenous degradation and excretion of amines or their derivatives.
Endogenous concomitant synthesis of histamine, serotonin and polyamines occurs in the stomach. The essential role of histamine for gastric secretion is well known. At least in rat, both ornithine decarboxylase (ODC) and HDC activities are affected by food intake, ranitidine, omeprazole and NaHCO3 (Ding et al., 1996). Histamine has a relevant role on Helicobacter pylori-induced inflammatory response, as studied in HDC−/− mice (Klausz et al., 2004). Spermine oxidase has also been recently related to carcinogenesis induced by H. pylori infection. Thus, dietary histamine and polyamine content should be restricted to patients with H. pylori infections (Chaturvedi et al., 2012).
Amino acid decarboxylases, and consequently amine biosynthetic pathways, can be found in almost all living organisms (Recsei and Snell, 1985; Medina et al., 2003; Moya-García et al., 2006, 2009), including gut microbiota. As concluded by previous COST Actions (European Science Foundation, http://www.cost.eu/) devoted to dietary amines (Eerola and Maijala, 2004), this could be a major problem for the control of amine availability, as gut microbiota could contribute a very important percentage to the pool, even when a low-amine diet is provided. Nevertheless, it is a highly complex and controversial issue that needs further research efforts. On one hand, anti-ageing and anti-inflammatory effects of microbiota-produced polyamines have been reported (Li et al., 2001; Matsumoto et al., 2011). On the other hand, pharmacological or dietary polyamine deprivation has been demonstrated to be effective in clinical trials for preventing the occurrence of multiple adenomas closely associated with the development of colon cancers (Gerner and Meyskens, 2009) and dietary polyamine input has been proposed as one of the major reasons for reducing effectiveness of anti-tumour strategies based on polyamine deprivation (Cipolla et al., 2007). In addition, polyamines are described as both growth and death promoters depending on their concentrations, the cell type and its environment (e.g. the presence of amino oxidases) (Coffino and Poznanski, 1991; Averill-Bates et al., 2008). The relationships between polyamine concentrations and the corresponding benefits and hazards caused by them in the gut must depend on a delicate balance influenced by both genetics and environment.
In the gastrointestinal tract, polyamine transport is mediated by both caveolar endocytosis and the solute carrier transporter SLC3A2 (Uemura et al., 2010). In rat intestine, histamine uptake is modified by other biogenic diamines such as cadaverine (Lyons et al., 1983).
Histamine deficiency can promote carcinogenesis through aberrant myeloid cell differentiation (Yang et al., 2011), but an excess of histamine uptake also becomes a problem for patients with inflammatory bowel diseases, in spite of the presence of amine catabolic enzymes in situ (Fogel et al., 2007). Taking into account the current information on the relationship between chronic inflammation and cancer (Demaria et al., 2010), the question mentioned above arises again; are amine-containing diets and amine-producing probiotics ‘good or bad’ for inflammatory bowel disease patients in the long term? The answer probably depends on the genetic and epigenetic background of each person, and consequently it will require a more comprehensive view of the molecular networks established into and among the different involved cell types. Structural and functional genomics combined with systems biology could contribute greatly to the characterization of this complex system, which would help to develop more efficient personalized treatments (including preventive prebiotics or pharmacological treatments).
With respect to amine degradation, diamines such as putrescine and histamine are substrates of DAO (or histaminidase, EC1.4.3.22). The enzyme is the product of the human abp1 (amiloride binding protein 1) gene, mainly expressed in placenta, kidney, colon, prostate and lymphoblasts (see BioGPS database, Wu et al., 2012).
N-methylation and/or N-acetylation of amines facilitate their transport through biological membranes, as these derivatives have a reduced polarity. Histamine can be N-methylated by histamine N-methyl transferase before its degradation by MAOs (La Regina et al., 2007), and spermidine and spermine are acetylated by spermidine/spermine acetyl-transferase before their degradation by polyamine oxidases or spermine oxidase (Figure 2; Cervelli et al., 2012; Polticelli et al., 2012). These processes consume two energy-consuming compounds with wide metabolic importance: S-adenosylmethionine (SAM) and acetyl-CoA respectively (Figure 2). Therefore, active amine degradation could alter the levels of these key metabolic components, thus spreading the metabolic consequences of amine pool alteration (Correa-Fiz et al., 2012). As amine alkyl derivatives can behave as substrates of MAOs (Figure 2) and these enzymes play a relevant role in detoxification mechanisms for xenobiotics (Strolin Benedetti, 2011), we suggest that the potential interferences of those compounds on drug metabolism should be carefully studied.
Agmatine [1-(4-aminobutyl) guanidine] is a precursor of polyamine metabolism and a cell growth modulator that has been claimed to be involved in several neurological responses and disorders (Agostinelli et al., 2010; Winter et al., 2011). Agmatine, histamine and putrescine uptake is carried out by the organic cation transport systems OCT2/SLC22A2 in renal cells (Ogasawara et al., 2006; Winter et al., 2011). Agmatine is also a substrate of the multidrug and toxin extrusion transporter-1 (MATE1/SLC47A1) in kidney and liver, among other cell types and tissues (Winter et al., 2011; see BioGPS database, Wu et al., 2012). This system also participates in the clearance of many different drugs and toxins; for example, the histamine H2 receptor antagonist, cimetidine (Kurata et al., 2010). Therefore, competition for the transport systems could have consequences in renal clearance of biogenic amines or analogues, and consequently in the physiological processes in which the amines are involved (including neurological functions). In a recent work, Meyer zu Schwabedissen et al. (2010) concluded that the coordinated function of MATE1 with OCT2 probably contributes to the renal elimination of organic cationic drugs. Thus, altered MATE1 activity, whether by drugs or polymorphisms, could be considered an important determinant of renal cationic drug elimination.
Much is currently known regarding the input and output of biogenic amine–polyamines but much more remains to be discovered in order to provide hints with real effects on clinical conditions. On one hand, biogenic amine–polyamine uptake seems to be dependent on gut microbiota activity and is probably modulated by not fully characterized metabolic interferences among different amines. In order to get a deeper knowledge of the biological bases of these processes, it would be extremely useful to design and carry out new and more systematic strategies and approaches. The results would help analysis of pathologies such as cancer and inflammatory diseases, especially those located in the gastrointestinal tract. On the other hand, biogenic amine–polyamine catabolism and elimination involves – among others – changes in global redox state and methylation pattern with potential effects on the general health of the organism. This is an additional area of research with many current uncertainties and requires further experimental efforts. In summary, as in other areas of biomedicine, much more additional basic knowledge is required in this area for future effective translational initiatives. However, more accumulated knowledge is not enough and there is also an urgent need of additional efforts for integrative analysis of all the available data. We believe that the present and future ‘omics’ analyses of both gut microbiota and patients could provide highly valuable data. New information derived from systematic analyses of these data could be applied to a wide spectrum of emergent and rare human pathologies.
Biogenic amines and analogues in the liver: the major controller of homeostasis
The liver is the major organ for the control of human homeostasis and detoxification. It buffers most of the dietary and pharmacological input via the gut (Figure 1). Consequently, its biochemical characteristics in terms of biogenic amine–polyamine metabolism are important in order to explain the final levels of circulating amines and amine analogues. Under healthy conditions, the adult liver synthesizes only low levels of biogenic amines and growth-related polyamines. An increase in hepatic polyamine biosynthesis can be indicative of, and could take part in, a neoplastic process (Smirnova et al., 2012). Histamine and serotonin synthesis can be observed in the liver as a consequence of macrophage infiltration (Correa-Fiz et al., 2012).
It is well known that CYP comprises a large superfamily of proteins (from more than 50 genes in the human genome) (Nelson, 2009). Members of CYP 1–3 families play important roles in liver drug detoxification. A recent review on hepatocytes summarised the role of the phase I enzymes, including CYP isoforms in this task (Sevior et al., 2012). Both histamine and polyamines and analogues of both amine families, including anti-histaminic drugs and other histamine receptor agonists and antagonists, and polyamine analogues, have been described as CYP ligands (Sharma and Hamelin, 2003; Ghosal et al., 2009; Gaertner et al., 2012) and this property may explain the growth regulatory effects of these amines (LaBella and Brandes, 2000). Serotonin and dopamine are also described as CYP isoform regulators with clinical relevance for neuropathology (Wójcikowski and Daniel, 2009). For instance, altered levels of brain or plasma serotonin (caused by a tryptophan-free diet) modulated the hepatic CYP isozymic pattern in rats, and consequently the global hepatic CYP activity, suggesting that such modulations may also be exhibited by drugs acting via the serotonergic system (Kot et al., 2012). MAO inhibitors inactivate human drug-metabolizing CYP enzymes, suggesting that impaired metabolic clearance of biogenic amines or their analogues may contribute to undesired amine and drug–drug interactions (Polasek et al., 2006). The histamine receptor antagonist, cimetidine, inhibits cytochrome isoforms CYP2D6, CYP2C19 and CYP3A4, and such inhibition could enhance serotonin toxicity (serotonin syndrome) (Talarico et al., 2011). All this information emphasizes that these CYP isoforms could be important factors in amine system pharmacology.
Biocomputational techniques may promise efficient progress in the knowledge of 3D structures of mammalian CYP enzymes and their interactions with biomolecules and drugs. There are some recent studies applying quantitative structure–activity relationship methods (Sridhar et al., 2012), molecular dynamics and virtual screening methods (Mo et al., 2011; Vass et al., 2012) to the structural characterization of CYP-ligand interactions. These approaches have also been successfully applied for another amine-related receptor, the NMDA receptor (Krueger et al., 2009), among others. Such approaches could help to predict in silico and to prevent unwanted amine and drug–drug interactions involving biogenic amines and/or their analogues. In addition, there are also animal models (transgenic mice expressing hepatic human CYP isoforms) that could provide interesting experimental platforms for pre-clinical validation of the predicted results (Sevior et al., 2012).
This strategy would also benefit the characterization of the binding details of biogenic amines and polyamines within these enzymes and their putative interferences with drug detoxification processes. In addition, in silico metabolic modelling approaches could help evaluate the metabolic flux changes induced by drugs under different hepatic circumstances. A lot of information on human hepatic elements and their affinity and kinetic constants has been collected in public databases (Kanehisa et al., 2010; Takarabe et al., 2011), and predictive mathematical models exist for hepatic cationic amino acid metabolism (Reyes-Palomares et al., 2012).
In addition, amine metabolic pathways are SAM-consuming processes as mentioned before (Figure 2); consequently, changes in amine metabolic fluxes can be transmitted to sulphur-containing amino acid metabolic pathways (such as the folate cycle, homocysteine and glutathione synthesis), with important consequences for gene methylation and/or redox status (Reyes-Palomares et al., 2012) and vice versa (Karouzakis et al., 2012). In mice a long-term diet supplemented with methionine altered SAM, polyamine and histamine levels in liver with important consequences on other hepatic stress markers (Correa-Fiz et al., 2012).
In summary, interactions between amino acid and amine metabolism and primary hepatic functions should be considered during pharmacokinetic studies and evaluation of pharmacological treatments in general (Funayama et al., 2010).
Amines and mast cells: an interesting model of amine metabolism organization
The role of histamine and serotonin in the immune system has recently been thoroughly analysed by several excellent reviews (Ringvall et al., 2008; O'Mahony et al., 2011; Simon et al., 2011; Ferstl et al., 2012; Gibbs and Levi-Schaffer, 2012). Among the different cell types involved in immune responses, the mast cell can be a particularly interesting model to study amine metabolism crosstalk as mast cells are able to synthesize polyamines, histamine and serotonin. Results from different research groups (including ours) suggest that regulatory mechanisms have evolved to avoid the simultaneous synthesis of these amines. For instance, during differentiation of cultured mouse bone marrow-derived mast cells (BMMC), the maximum of polyamine content preceded the maximum of histamine synthesis, while the peak of serotonin was attained after the maximum of histamine production (García-Faroldi et al., 2009, 2010). In vivo, mature mast cells are specialized in storing mainly histamine or serotonin depending on their differentiation program and environment. As will be explained below, an interaction between polyamine synthesis and serotonin secretion has also been observed in serotonin-producing mast cells (Kanerva et al., 2009).
The active enzyme responsible for histamine synthesis, HDC, (EC 4.1.1.22), is a 110 kDa homodimer. The monomers are translated as 74 kDa polypeptide precursors that need to be processed by proteolytic removal of their carboxy-terminal fragments to give rise to the fully active enzyme (Fleming et al., 2004). This C-terminal portion of the full-length polypeptide contains the signal to drive the HDC monomers to the endoplasmic reticulum before their maturation (Suzuki et al., 1998; Fleming et al., 2004; Furuta et al., 2006). These facts indicate that preventing cytosolic HDC activity is important for mammalian histamine-producing cell homeostasis or function. On the contrary, the other two decarboxylases producing amines in mast cells, ODC and dopamine decarboxylase (DDC), are located in the cytosol. The primary sequence of human DDC is highly homologous to the human HDC active monomer sequence. However, human ODC seems to have another evolutionary origin (Sandmeier et al., 1994). Their separate syntheses do not preclude the final spatial convergence of the products during mast cell differentiation. In fact, as mentioned earlier, BMMCs are able to produce histamine, serotonin and polyamines during in vitro differentiation, the latter also being important for granule homeostasis (García-Faroldi et al., 2010).
ODC activity is a major determinant for polyamine biosynthesis. Its activity, as well as the uptake of exogenous polyamines, is regulated by inhibitory ODC-binding proteins named antizymes. Two polypeptides homologous to ODC (antizyme inhibitors I and II) have been detected in different human cell types. They bind antizymes with higher affinity than the ODC monomer, so acting as indirect ODC activators (Kahana, 2009). Expression of the antizyme inhibitor II has been detected in mast cells and is related to exocytosis of serotonin-containing mast cell granules (Kanerva et al., 2009; López-Contreras et al., 2010). These findings indicate a key role for polyamines in the structure and dynamics of mast cell granules, a key process for many immune responses.
It is also tempting to think that spatial and temporal compartmentalization of amine synthesis is a mechanism evolved to avoid an excess of SAM consumption and/or aldehydes and ROS derived from concomitant amine metabolism. Polyamine deficit has been described as deleterious for both mast cell homeostasis and correct development of the immune system in general (Jolois et al., 2002; García-Faroldi et al., 2010). However, both polyamine uptake and synthesis are inhibited by histamine or their analogues (Fajardo et al., 2001; Paz et al., 2001). These crosstalk mechanisms lead us to suggest that amine metabolic pathways have important roles as part of a coordinated programme between immune cell proliferation and immune cell differentiation. Thus, pharmacological intervention in one of these pathways or processes could affect the others.
Amines as double agents during anti-parasitic wars
The relationships among host and parasites are also an interesting scenario when studying the pathophysiological effects of amines (Figure 1). Typical immune histamine-producing cells, mast cells and basophils, evolved as part of the adaptive immune system with the physiological mission, among others, to protect animals against parasitic infections. Histamine is one of the key mediators secreted by these cells that participate in the defence response against parasites (Karasuyama et al., 2011; Specht et al., 2011; Ferstl et al., 2012). Nevertheless, it could depend on a delicate equilibrium, as a dysregulated balance of the inflammatory response (including histamine release) has recently been proposed as a major factor for malaria pathogenesis and parasite (Plasmodium falciparum) transmission (Mecheri, 2012).
Parasite proliferation, as almost any other living cell/organism, needs higher polyamines (spermidine/spermine). Some parasitic protozoa, such as Trypanosoma cruzi, are unable to synthesize putrescine by themselves, so they are strictly dependent on the polyamine supply from the host. Even when they can produce polyamines to some extent, as in T. brucei and Leishmania, their requirements are higher than the endogenous synthetic capacity as they expend important quantities of spermidine in the production of trypanothione [bis(glutathionyl)spermidine], a natural redox buffer that confers an advantage to the parasite (Krauth-Siegel and Comini, 2008). The absolute dependence of the parasite progression on polyamines, together with the structural and regulatory differences found among the key enzymes and transporters of host and parasite polyamine metabolism, encourage the development of anti-parasite strategies based on these features (Birkholtz et al., 2011). In fact, the first ODC inhibitor, difluoromethyl ornithine (DFMO), is effective as a anti-parasitic treatment, together with other polyamine analogues and this strategy has shown promise against several parasite infections, especially in combined therapies (Müller et al., 2008). Another potential lead is that P. falciparum growth in vitro was inhibited by the putrescine analogue, 1-amino-oxy-3-aminopropane (Das Gupta et al., 2005), and with inhibitors of spermidine synthesis (Blavid et al., 2010). However, P. berghei seems to be more resistant than P. falciparum to intervention with polyamine synthesis analogues or inhibitors (Wright et al., 1991). It is also worth mentioning that some diamines (1,4- and 1–5-diamines) are able to reverse the cytostatic effects of DFMO on P. falciparum (Assaraf et al., 1987).
Nature provides evidence to support the hypothesis that interference with biogenic amines during parasite infection could be beneficial. Polypeptides able to scavenge biogenic amines are used as generalized anti-inflammatory mechanisms by haematophagous arachnids (soft ticks), assassin bugs and mosquitoes. In the case of soft ticks, these polypeptides belong to the class of polypeptide named lipocalins, as monomine and monotonin, acting as scavengers of histamine and serotonin respectively. They confer advantage to the parasite by preventing the immune and haemostatic response of the host (Mans et al., 2008). Structural and docking data of these polypeptides (and maybe other amine binding proteins) could provide the basis for the design of new biogenic amine scavengers with potential pharmacological applications.
Amines and combined therapies: the risk of ‘pleiotropy’
Histamine can be considered the most ‘pleiotropic’ amine, affecting most of the important biological functions of humans: defence, nutrition, neurological activity, growth, development and fertility. In this sense, it can be considered an important link between different modules of human (mammalian) physiology. A recent review shows genetic and biochemical evidence supporting the involvement of histamine in more than 20 rare diseases (Pino-Ángeles et al., 2012). For neurological and neuroinflammatory diseases in particular, clear evidence of interaction between histamine-related and dopamine and serotonin-related components was found.
The molecular bases of the histamine ‘pleiotropy’ are mainly explained by the existence of four types of histamine GPCRs that can, in turn, be promiscuous in their protein–protein interactions (Ferrada et al., 2009). In addition, histamine can also bind to NMDA receptors (Watanabe et al., 2008), as can many other biogenic amines, polyamines and analogues. The existence of histamine-linked chloride-like channels in the mammalian brain has also been proposed (Fleck et al., 2012). These findings would suggest that histamine could interfere with the metabolic and signalling pathways of other amines and related compounds. It could therefore affect pharmacological interventions of many pathological processes. In a recent study of drug interactions in palliative care (Gaertner et al., 2012), the authors identify drugs (inter-) acting via histamine receptors as among the best candidates for drug–drug interactions, which is a logical consequence of histamine ‘pleiotropy’. Taking into account that patients with palliative care treatments usually suffer pain, weakness and other phenotypic features related to histamine, these drug–drug interactions deserve further pharmacological interest.
In general, the ‘pleiotropy’ of biogenic amines could contribute to many unexpected interactions, thus making pharmacovigilance advisable and even necessary. The multiple advertisements concerning the use of MAO inhibitors in combined therapies against neurological, neuroinflammatory and immune treatments are good examples of this (Montastruc et al., 2000; Sommet et al., 2007).
Amines and human reproduction
We would like to mention some recent observations that should be taken into account not only during pregnancy but also during preparation for fertility treatments. Spermine was discovered in seminal fluids more than a century ago, as it is essential for correct spermatogenesis. Seminal fluid is rich in both polyamines spermidine and spermine and the indirect activator of ODC, the antizyme inhibitor II, is preferentially expressed in testes.(López-Contreras et al., 2010). Polyamines play roles in embryo implantation, in decidualization and in placental formation and function (Lefèvre et al., 2011). Serotonin and other monoamines also play important roles in decidualization and embryo implantation and different biogenic amine transport activities have been observed in the endometrium (Hansson et al., 2009). Monoamine transporters may serve as a protective mechanism preventing vasoconstriction in the placental vascular bed and thereby securing a stable blood flow to the foetus (Bottalico et al., 2004). High levels of HDC expression in placenta has also been reported, suggesting that histamine contributes to embryo-uterine interactions due to its vasoactive, differentiation and/or growth-promoting properties (Brew et al., 2007). However, its benefits on successful pregnancy seem to be dependent on a delicate equilibrium between histamine synthesis and degradation because reduced DAO activity correlated with abortion risk and trophoblastic disorders (Maintz et al., 2008). DAO can also degrade putrescine (Figure 2). Results obtained with HDC−/− mice indicated that peripheral histamine was an important regulatory factor of male gonadal development during embryogenesis and of sex steroid metabolism later in adulthood (Pap et al., 2002). These data imply that human reproduction needs biogenic amines at different stages of the process. Perhaps, as in other developmental processes (i.e. mast cell differentiation), the requirement for the different amines during reproduction is a part of a coordinated programme. More efforts should be made to clarify their roles during different stages of reproduction and human embryogenesis in order to control the process as they appear to be important for correct human reproduction and differentiation.
Concluding remarks and future prospects
It is clear that crosstalk among biogenic amines, including polyamines, plays an important role in neurology and the neuroendocrine system (data not discussed here). Nevertheless, in other physiological subsystems, interactions between components related to biogenic amines such as histamine, serotonin and arginine/ornithine-derived polyamines can also be involved in and even control, other processes essential for human health, homeostatic control, defence and reproduction.
The four main steps followed by any drug provided to an organism (for instance, a patient) are summarized in the ADME concept, that is, absorption, distribution, metabolism and excretion. Based on the previously mentioned ‘pleiotropy’ of biogenic amine actions and their metabolic crosstalk, we would predict that the metabolism of exogenous and endogenous biogenic amines (Figure 1) could interfere with any of these four key steps of drug ADME, thus reinforcing our previous claim of appropriate pharmacovigilance. Furthermore, we would also expect that drugs targeted against specific steps of the metabolism of one of the biogenic amines could also affect the metabolism and signalling of the others. This review provides several examples of such possibilities which have been recently revealed. The gaps in our knowledge are still considerable and further efforts to fill them are necessary, as this information may contribute to the development of new and more efficient strategies of prevention and therapy for many biomedical problems over the medium or long term, after the appropriate pre-clinical validation and clinical trial phases (Table 1).
Table 1.
Physiological subsystems for amine interplay beyond neurotransmission, their major current basic knowledge gaps and their potential interest for pharmacology
| Subsystem | Further basic research efforts are required on: | Potential interest for Pharmacologya |
|---|---|---|
| Amine uptake | • Integrative efforts for characterization of microbiota amine metabolism. | • Gastrointestinal epithelia protection; probiotics and chemoprevention. |
| • Integrative efforts for characterization of amine transport systems in the different gastrointestinal epithelia and their modulation by amines and drugs. | • New possibilities for treatment of gastrointestinal inflammatory diseases. New combined therapy protocols. | |
| • Integrative analysis of patient functional genomic data and microbiota properties. | ||
| Amine efflux | • Systematic characterization of amine–amine and amine–drug interferences during renal filtration on animal models (e.g. humanized mice). | • Improvement of pharmacovigilance actions. |
| Amines in liver | • Molecular dynamic studies of CYP-amine and CYP-drug preferential binding modes. | • Prevention/Intervention of hepatic degeneration and homeostatic imbalance. |
| • Metabolic interferences of amines with redox metabolism and gene methylation. Metabolomic and proteomic approaches, and systemic analyses. | • Improvement of drug pharmacokinetic and pharmacodynamic properties. | |
| • Systemic evaluation of CYP 1-3 ligand co-occurrence (amines and drugs) in cell and/or humanized animal models. | • Improvement of pharmacovigilance actions. | |
| Multiamineb producing cells and tissues | • Systemic studies using transgenic models. | • Multiple potential applications in diagnosis, prevention and treatment of neurological, inflammatory and neuroinflammatory emergent and rare diseases, including neoplasms. |
| • Identification of the major amine crosstalk responsible elements in the different cell types/tissues assisted by in silico simulations. Experimental validation. | ||
| Amines in host-parasite interactions | • Detailed structural characterization of docking events among amine-related elements important for host–parasite interactions and compounds with anti-parasitic potential. Combination of in silico (i.e. molecular dynamics) and experimental approaches. | • Selective inhibition of parasite infection and/or proliferation. |
| • Modulation of the immune response. | ||
| • Chemoprevention. | ||
| Amines in reproduction | • Generation of genetically manipulated cell lines and animal models. | • Improvement of pharmacovigilance actions during pregnancy. |
| • Integrative characterization on the expression pattern evolution of amine-related genes during the different fertilization and gestation phases. | • Abortion prevention. | |
| • Increased success in assisted fertilization therapies |
This column mentions several possibilities for application of the knowledge obtained from basic results at medium and long term. Of course, as any other translational process in pharmacology, they would need to be submitted to the appropriate phases of pre-clinical validations and clinical trials before they could be approved for clinical use.
Multiamine producing cell and tissue. This describes those cells and tissues able to synthesize biogenic amines and polyamines as part of their physiological mission, such as mast cells and other immune cells, enterochromaffin-like cells and salivary gland cells.
In cases where targets have been located and well characterized, in silico approaches (for instance, molecular modelling and virtual screening) could efficiently provide new pharmacological solutions, as has already occurred in some cases.
The wide spectrum of physiological scenarios for biogenic amine interactions and their pathological consequences is a reflection of the highly complex biochemical interaction networks among the components involved. In fact, biogenic amines participate in all types of biochemical networks: metabolic pathways, internal signal transduction mechanisms and intercellular communication. A current challenge for analysis of biological and biomedical networks is how to manage highly connected components of a given network. In the case of theoretical metabolic models, a risky but frequently useful option to simplify the analysis is to remove the most frequent components (Ma and Zeng, 2003). In the field of amine physiology and pharmacology, this solution makes no sense, as the pharmacological targets could be included in the group of the most interconnected components in some of the involved networks. Thus, further efforts are necessary to analyse these complex metabolic networks, as well as to develop better ADME-TOX evaluators.
In summary, amine pharmacology is a perfect candidate to benefit from the advances in network theory and systems biology technologies, in general, as the most efficient way to progress towards more personalized solutions for a wide range of pathological circumstances. Multidisciplinary groups or R&D networks working in a coordinated way are needed to fill gaps in basic, clinical and pharmacological information, as well as providing new bioinformatic solutions, making the analysis of the integrated amine information possible. Some ongoing initiatives should be potentiated as the field is clearly included in the current world-wide R&D priorities (in terms of both concept and technology).
Acknowledgments
This work has been funded by Grants SAF2011-26518 (MEyC, Spain) and CVI-06585 (PAIDI, Andalusia, Spain). MVRP is part of the ‘Programa para la Formación del Profesorado Universitario’ funded by ‘Ministerio de Educación, Cultura y Deporte’. Thanks are due to Norma McVeigh and James Perkins for helpful English text editing. This work can be considered as part of COST Action BM0806 activities.
Glossary
- CYP
cytochrome P450
- DAO
diamine oxidase
- DDC
dopamine decarboxylase
- DFMO
difluoromethyl ornithine
- HDC
histidine decarboxylase
- MATE1
multidrug and toxin extrusion transporter-1
- ODC
ornithine decarboxylase
- SAM
S-adenosyl methionine
- sVMATs
vesicular monoamine transporters
Conflict of interest
No conflict of interests has been stated.
References
- Agostinelli E, Marques MP, Calheiros R, Gil FP, Tempera G, Viceconte N, et al. Polyamines: fundamental characters in chemistry and biology. Amino Acids. 2010;38:393–403. doi: 10.1007/s00726-009-0396-7. [DOI] [PubMed] [Google Scholar]
- Assaraf YG, Golenser J, Spira DT, Messer G, Bachrach U. Cytostatic effect of DL-alpha-difluoromethylornithine against Plasmodium falciparum and its reversal by diamines and spermidine. Parasitol Res. 1987;73:313–318. doi: 10.1007/BF00531084. [DOI] [PubMed] [Google Scholar]
- Averill-Bates DA, Ke Q, Tanel A, Roy J, Fortier G, Agostinelli E. Mechanism of cell death induced by spermine and amine oxidase in mouse melanoma cells. Int J Oncol. 2008;32:79–88. [PubMed] [Google Scholar]
- Birkholtz LM, Williams M, Niemand J, Louw AI, Persson L, Heby O. Polyamine homeostasis as a drug target in pathogenic protozoa: peculiarities and possibilities. Biochem J. 2011;438:229–244. doi: 10.1042/BJ20110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavid R, Kusch P, Hauber J, Eschweiler U, Sarite SR, Specht S, et al. Down-regulation of hypusine biosynthesis in plasmodium by inhibition of S-adenosyl-methionine-decarboxylase. Amino Acids. 2010;38:461–469. doi: 10.1007/s00726-009-0405-x. [DOI] [PubMed] [Google Scholar]
- Bolasco A, Carradori S, Fioravanti R. Focusing on new monoamine oxidase inhibitors. Expert Opin Ther Pat. 2010;20:909–939. doi: 10.1517/13543776.2010.495716. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquillon C. Drug transporters in the lung – do they play a role in the biopharmaceutics of inhaled drugs? J Pharm Sci. 2010;99:2240–2255. doi: 10.1002/jps.21995. [DOI] [PubMed] [Google Scholar]
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, et al. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Brew O, Lakasing L, Sullivan M. Differenctial activity of histidine decarboxylase in normal and pre-eclamptic placentae. Placenta. 2007;28:585–587. doi: 10.1016/j.placenta.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Burban A, Faucard R, Armand V, Bayard C, Vorobjev V, Arrang JM. Histamine potentiates N-methyl-D-aspartate receptors by interacting with an allosteric site distinct from the polyamine binding site. J Pharmacol Exp Ther. 2010;332:912–921. doi: 10.1124/jpet.109.158543. [DOI] [PubMed] [Google Scholar]
- Cervelli M, Amendola R, Polticelli F, Mariottini P. Spermine oxidase: ten years after. Amino Acids. 2012;42:441–450. doi: 10.1007/s00726-011-1014-z. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, de Sablet T, Peek R, Wilson K. Spermine oxidase, a polyamine catabolic enzyme that links Helicobacter pylori CagA and gastric cancer risk. Gut Microbes. 2012;3:48–56. doi: 10.4161/gmic.19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodaczek G, Bacsi A, Dharajiya N, Sur S, Hazra TK, Boldogh I. Ragweed pollen-mediated IgE-independent release of biogenic amines from mast cells via induction of mitochondrial dysfunction. Mol Immunol. 2009;46:2505–2514. doi: 10.1016/j.molimm.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7:159–174. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- Cipolla BG, Havouis R, Moulinoux JP. Polyamine contents in current foods: a basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids. 2007;33:203–312. doi: 10.1007/s00726-007-0524-1. [DOI] [PubMed] [Google Scholar]
- Coffino P, Poznanski A. Killer polyamines? J Cell Biochem. 1991;45:54–58. doi: 10.1002/jcb.240450112. [DOI] [PubMed] [Google Scholar]
- Correa-Fiz F, Reyes-Palomares A, Fajardo I, Melgarejo E, Gutiérrez A, García-Ranea JA, et al. Regulatory cross-talk of mouse liver polyamine and methionine metabolic pathways: a systemic approach to its physiopathological consequences. Amino Acids. 2012;42:577–595. doi: 10.1007/s00726-011-1044-6. [DOI] [PubMed] [Google Scholar]
- Das Gupta R, Krause-Ihle T, Bergmann B, Müller IB, Khomutov A, Müller S, et al. 3-Aminooxy-1-aminopropane and derivatives have an antiproliferative effect on cultured Plasmodium falciparum by decreasing intracellular polyamine concentrations. Antimicrob Agents Chemother. 2005;49:2857–2864. doi: 10.1128/AAC.49.7.2857-2864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Wyler SC. Serotonergic transcriptional networks and potential importance to mental health. Nat Neurosci. 2012;15:519–527. doi: 10.1038/nn.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Chen D, Rosengren E, Persson L, Hakanson R. Comparison between activation of ornithine decarboxylase and histidine decarboxylase in rat stomach. Am J Physiol. 1996;270:G476–G486. doi: 10.1152/ajpgi.1996.270.3.G476. [DOI] [PubMed] [Google Scholar]
- Edmondson DE, Binda C, Wang J, Upadhyay AK, Mettevi A. Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry. 2009;48:4220–4230. doi: 10.1021/bi900413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerola S, Maijala R. Biogenic amines: overview of food safety. In: Wallace HM, Huges A, editors. COST Action 922. Health Implications of Dietary Amines. I. Luxembourg, Belgium: European Communities; 2004. pp. 27–33. [Google Scholar]
- Fajardo I, Urdiales JL, Paz JC, Chavarría T, Sánchez-Jiménez F, Medina MA. Histamine prevents polyamine accumulation in mouse C57.1 mast cell cultures. Eur J Biochem. 2001;268:768–773. doi: 10.1046/j.1432-1327.2001.01930.x. [DOI] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casadó V, Bongers G, Cortés A, Mallol J, et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl R, Akdis CA, O'Mahony L. Histamine regulation of innate and adaptive immunity. Front Biosci. 2012;17:40–53. doi: 10.2741/3914. [DOI] [PubMed] [Google Scholar]
- Fesus L, Szucs EF, Barrett KE, Metcalfe DD, Folk JE. Activation of transglutaminase and production of protein-bound gamma-glutamylhistamine in stimulated mouse mast cells. J Biol Chem. 1985;260:13771–13778. [PubMed] [Google Scholar]
- Fleck MW, Thomson JL, Hough LB. Histamine-gated ion channels in mammals? Biochem Pharmacol. 2012;83:1127–1135. doi: 10.1016/j.bcp.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Fleming JV, Fajardo I, Langlois MR, Sánchez-Jiménez F, Wang TC. The C-terminus of rat L-histidine decarboxylase specifically inhibits enzymic activity and disrupts pyridoxal phosphate-dependent interactions with L-histidine substrate analogues. Biochem J. 2004;381:769–778. doi: 10.1042/BJ20031553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel WA, Lewinski A, Jochem J. Histamine in food: is there anything to worry about? Biochem Soc Trans. 2007;35:349–352. doi: 10.1042/BST0350349. [DOI] [PubMed] [Google Scholar]
- Funayama H, Huang L, Asada Y, Endo Y, Takada H. Enhanced induction of a histamine-forming enzyme, histidine decarboxylase, in mice primed with NOD1 or NOD2 ligand in response to various Toll-like receptor agonists. Innate Immun. 2010;16:265–272. doi: 10.1177/1753425909341070. [DOI] [PubMed] [Google Scholar]
- Furuta K, Ichikawa A, Nakayama K, Tanaka S. Membrane orientation of the precursor 74-kDa form of L-histidine decarboxylase. Inflamm Res. 2006;55:185–191. doi: 10.1007/s00011-006-0069-x. [DOI] [PubMed] [Google Scholar]
- Gaertner J, Ruberg K, Schlesiger G, Frechen S, Voltz R. Drug interactions in palliative care – it's more than cytochrome P450. Palliat Med. 2012;26:813–825. doi: 10.1177/0269216311412231. [DOI] [PubMed] [Google Scholar]
- García-Faroldi G, Correa-Fiz F, Abrighach H, Berdasco M, Fraga MF, Esteller M, et al. Polyamines affect histamine synthesis during early stages of IL-3-induced bone marrow cell differentiation. J Cell Biochem. 2009;108:261–271. doi: 10.1002/jcb.22246. [DOI] [PubMed] [Google Scholar]
- García-Faroldi G, Rodríguez CE, Urdiales JL, Pérez-Pomares JM, Dávila JC, Pejler G, et al. Polyamines are present in mast cell secretory granules and are important for granule homeostasis. Plos One. 2010;5:e15071. doi: 10.1371/journal.pone.0015071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal A, Gupta S, Ramanathan R, Yuan Y, Lu X, Su AD, et al. Metabolism of loratadine and further characterization of its in vitro metabolites. Drug Metab Lett. 2009;3:162–170. doi: 10.2174/187231209789352067. [DOI] [PubMed] [Google Scholar]
- Gibbs BF, Levi-Schaffer F. H4 receptors in mast cells and basophils: a new therapeutic target for allergy? Front Biosci. 2012;17:430–437. doi: 10.2741/3936. [DOI] [PubMed] [Google Scholar]
- Graham DE, Xu H, White RH. Methanococcus jannaschii uses a pyruvoyl-dependent arginine decarboxylase in polyamine biosynthesis. J Biol Chem. 2002;277:23500–23507. doi: 10.1074/jbc.M203467200. [DOI] [PubMed] [Google Scholar]
- Grosso H, Mouradian MM. Transglutaminase 2: biology, relevance to neurodegenerative diseases and therapeutic implications. Pharmacol Ther. 2012;133:392–410. doi: 10.1016/j.pharmthera.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Bottalico B, Noskova V, Casslén B. Monoamine transporters in human endometrium and decidua. Hum Reprod Update. 2009;15:249–260. doi: 10.1093/humupd/dmn048. [DOI] [PubMed] [Google Scholar]
- Heby O, Persson L, Rentala M. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids. 2007;33:359–366. doi: 10.1007/s00726-007-0537-9. [DOI] [PubMed] [Google Scholar]
- Huynh QK, Snell EE. Pyruvoyl-dependent histidine decarboxylases. Preparation and amino acid sequences of the beta chains of histidine decarboxylase from Clostridium perfringens and Lactobacillus buchneri. J Biol Chem. 1985;260:2798–2803. [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000;271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- Jolois O, Peulen O, Collin S, Simons M, Dandrifosse G, Heinen E. Spermine induces precocious development of the spleen in mice. Exp Physiol. 2002;87:69–75. doi: 10.1113/eph8702277. [DOI] [PubMed] [Google Scholar]
- Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays Biochem. 2009;46:47–61. doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–D360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva K, Lappalainen J, Mäkitie LT, Virolainen S, Kovanen PT, Andersson LC. Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One. 2009;4:e6858. doi: 10.1371/journal.pone.0006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapushesky M, Emam I, Holloway E, Kurnosov P, Zorin A, Malone J, et al. Gene expression atlas at the European bioinformatics institute. Nucleic Acids Res. 2010;38:D690–D698. doi: 10.1093/nar/gkp936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H, Obata K, Wada T, Tsujimura Y, Mukai K. Newly appreciated roles for basophils in allergy and protective immunity. Allergy. 2011;66:1133–1141. doi: 10.1111/j.1398-9995.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- Karouzakis E, Gay RE, Gay S, Neidhart M. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2012;64:1809–1817. doi: 10.1002/art.34340. [DOI] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. Systems Biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Klausz G, Buzás E, Scharek P, Tiszlavicz L, Gyulai Z, Fülöp AK, et al. Effects of Helicobacter pylori infection on gastric inflammation and local cytokine production in histamine-deficient (histidine decarboxylase knock-out) mice. Immunol Lett. 2004;94:223–228. doi: 10.1016/j.imlet.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Kot M, Pilc A, Daniel WA. Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacol Res. 2012;66:292–299. doi: 10.1016/j.phrs.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel RL, Comini MA. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Krueger BA, Weil T, Schneider G. Comparative virtual screening and novelty detection for NMDA-GlycineB antagonists. J Comput Aided Mol Des. 2009;23:869–881. doi: 10.1007/s10822-009-9304-1. [DOI] [PubMed] [Google Scholar]
- Kurata T, Muraki Y, Mizutani H, Iwamoto T, Okuda M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet. 2010;25:328–334. doi: 10.2133/dmpk.dmpk-10-rg-004. [DOI] [PubMed] [Google Scholar]
- La Regina G, Silvestri R, Artico M, Lavecchia A, Novellino E, Befani O, et al. New pyrrole inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. J Med Chem. 2007;50:922–931. doi: 10.1021/jm060882y. [DOI] [PubMed] [Google Scholar]
- LaBella FS, Brandes LJ. Interaction of histamine and other bioamines with cytochromes P450: implications for cell growth modulation and chemopotentiation by drugs. Semin Cancer Biol. 2000;10:47–53. doi: 10.1006/scbi.2000.0307. [DOI] [PubMed] [Google Scholar]
- Landete JM, De las Rivas B, Marcobal A, Muñoz R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit Rev Food Sci Nutr. 2008;48:697–714. doi: 10.1080/10408390701639041. [DOI] [PubMed] [Google Scholar]
- Lefèvre PL, Palin MF, Murphy BD. Polyamines on the reproductive landscape. Endocr Rev. 2011;32:694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- Li L, Rao JN, Bass BL, Wang JY. NF-kappaB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G992–G1004. doi: 10.1152/ajpgi.2001.280.5.G992. [DOI] [PubMed] [Google Scholar]
- Lin JS, Anaclet C, Sergeeva OA, Haas HL. The waking brain: an update. Cell Mol Life Sci. 2011;68:2499–2512. doi: 10.1007/s00018-011-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Contreras AJ, Ramos-Molina B, Cremades A, Peñafiel R. Antizyme inhibitor 2: molecular, cellular and physiological aspects. Amino Acids. 2010;38:603–611. doi: 10.1007/s00726-009-0419-4. [DOI] [PubMed] [Google Scholar]
- Lyons DE, Beery JT, Lyons SA, Taylor SL. Cadaverine and aminoguanidine potentiate the uptake of histamine in vitro in perfused intestinal segments of rats. Toxicol Appl Pharmacol. 1983;70:445–458. doi: 10.1016/0041-008x(83)90162-x. [DOI] [PubMed] [Google Scholar]
- Ma H, Zeng AP. Reconstruction of metabolic networks from genome data and analysis of their global structure for various organisms. Bioinformatics. 2003;19:270–277. doi: 10.1093/bioinformatics/19.2.270. [DOI] [PubMed] [Google Scholar]
- McGrath AP, Hilmer KM, Collyer CA, Shepard EM, Elmore BO, Brown DE, et al. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maintz L, Schwarzer V, Bieber T, van der Ven K, Novak N. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update. 2008;14:485–495. doi: 10.1093/humupd/dmn014. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Ribeiro JM, Andersen JF. Structure, function, and evolution of biogenic amine-binding proteins in soft ticks. J Biol Chem. 2008;283:18721–18733. doi: 10.1074/jbc.M800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal JD, Kemp CA, Roberts GC, Paine MJ, Wolf CR, Sutcliffe MJ. Insights into drug metabolism by cytochromes P450 from modelling studies of CYP2D6-drug interactions. Br J Pharmacol. 2008;153:S82–S89. doi: 10.1038/sj.bjp.0707570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One. 2011;6:e23652. doi: 10.1371/journal.pone.0023652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecheri S. Contribution of allergic inflammatory response to the pathogenesis of malaria disease. Biochim Biophys Acta. 2012;1822:49–56. doi: 10.1016/j.bbadis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Medina MA, Urdiales JL, Núñez de Castro I, Sánchez-Jiménez F. Diamines interfere with the transport of L-ornithine in Ehrlich-cell plasma-membrane vesicles. Biochem J. 1991;280:825–827. doi: 10.1042/bj2800825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina MA, Ramírez FJ, Ruiz-Chica J, Chavarría T, López-Navarrete JT, Sánchez-Jiménez F. DNA-chlorpheniramine interaction studied by spectroscopic techniques. Biochim Biophys Acta. 1998;1379:129–133. doi: 10.1016/s0304-4165(97)00093-7. [DOI] [PubMed] [Google Scholar]
- Medina MA, Urdiales JL, Rodríguez-Caso C, Ramírez FJ, Sánchez-Jiménez F. Biogenic amines and polyamines: similar biochemistry for different physiological missions and biomedical applications. Crit Rev Biochem Mol Biol. 2003;38:23–59. doi: 10.1080/713609209. [DOI] [PubMed] [Google Scholar]
- Medina V, Cricco G, Nuñez M, Matrín G, Mohamad N, Correa-Fiz F, et al. Histamine-mediated signaling processes in human malignant mammary cells. Cancer Biol Ther. 2006;5:1462–1471. doi: 10.4161/cbt.5.11.3273. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol. 2010;298:F997–F1005. doi: 10.1152/ajprenal.00431.2009. [DOI] [PubMed] [Google Scholar]
- Michael AJ. Exploring polyamine biosynthetic diversity through comparative and functional genomics. In: Pegg AE, Casero RA Jr, editors. Polyamines: Methods and Protocols. Methods in Molecular Biology. Vol. 720. New York: Humana Press; 2011. pp. 39–50. [DOI] [PubMed] [Google Scholar]
- Mitchell SC. Flavin mono-oxygenase (FMO): the ‘other’ oxidase. Curr Drug Metab. 2008;9:280–284. doi: 10.2174/138920008784220682. [DOI] [PubMed] [Google Scholar]
- Mo SL, Liu WF, Li CG, Zhou ZW, Luo HB, Chew H, et al. Pharmacophore, QSAR, and binding mode studies of substrates of human cytochrome p450 2D6 (CYP2D6) using molecular docking and virtual mutations and an application to Chinese herbal medicine screening. Curr Pharm Biotechnol. 2011;13:1640–1704. doi: 10.2174/138920112800958779. [DOI] [PubMed] [Google Scholar]
- Montastruc JL, Chaumerliac C, Desboeuf K, Manika M, Bagheri H, Rascol O, et al. Adverse drug reactions to selegiline: a review of the French pharmacovigilance database. Clin Neuropharmacol. 2000;23:271–275. doi: 10.1097/00002826-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Mony L, Kew JNC, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Vaz SH, Cai NS, Ferrada C, Quiroz C, Barodia SK, et al. Dopamine-galanin receptor heteromers modulate cholinergic neurotransmission in the rat ventral hippocampus. J Neurosci. 2011;31:7412–7423. doi: 10.1523/JNEUROSCI.0191-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Moya-García AA, Pino-Angeles A, Sánchez-Jiménez F. New structural insights to help in the search for selective inhibitors of mammalian pyridoxal 5'-phosphate-dependent histidine decarboxylase. Inflamm Res. 2006;55:S55–S56. doi: 10.1007/s00011-005-0040-2. [DOI] [PubMed] [Google Scholar]
- Moya-García AA, Pino-Ángeles A, Gil-Redondo R, Morreale A, Sánchez-Jiménez F. Structural features of mammalian histidine decarboxylase reveal the basis for specific inhibition. Br J Pharmacol. 2009;157:4–13. doi: 10.1111/j.1476-5381.2009.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Fromm MF. Transporter-mediated drug-drug interactions. Pharmacogenomics. 2011;12:1017–1037. doi: 10.2217/pgs.11.44. [DOI] [PubMed] [Google Scholar]
- Müller IB, Das Gupta RG, Lüersen K, Wrenger C, Walter RD. Assessing the polyamine metabolism of Plasmodium falciparum as chemotherapeutic target. Mol Biochem Parasitol. 2008;160:1–7. doi: 10.1016/j.molbiopara.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Nelson DR. The cytochrome P450 homepage. Hum Genomics. 2009;4:59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- O'Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128:1153–1162. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Yamauchi K, Satoh Y, Yamaji R, Inui K, Jonker JW, et al. Recent advances in molecular pharmacology of the histamine systems: organic cation transporters as a histamine transporter and histamine metabolism. J Pharmacol Sci. 2006;101:24–30. doi: 10.1254/jphs.fmj06001x6. [DOI] [PubMed] [Google Scholar]
- Pap E, Ráck K, Kovács JK, Varga I, Buzás E, Madarász B, et al. Histidine decarboxylase deficiency in gene knockout mice elevates male sex steroid production. J Endocrinol. 2002;175:193–199. doi: 10.1677/joe.0.1750193. [DOI] [PubMed] [Google Scholar]
- Paz JC, Sánchez-Jiménez F, Medina MA. Characterization of spermine uptake by Ehrlich tumour cells in culture. Amino Acids. 2001;21:271–279. doi: 10.1007/s007260170013. [DOI] [PubMed] [Google Scholar]
- Pino-Ángeles A, Reyes-Palomares A, Melgarejo E, Sánchez-Jiménez F. Histamine: an undercover agent in multiple rare diseases? J Cell Mol Med. 2012;16:1947–1960. doi: 10.1111/j.1582-4934.2012.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polasek TM, Elliot DJ, Somogyi AA, Gillam EM, Lewis BC, Miners JO. An evaluation of potential mechanism-based inactivation of human drug metabolizing cytochromes P450 by monoamine oxidase inhibitors, including isoniazid. Br J Clin Pharmacol. 2006;61:570–584. doi: 10.1111/j.1365-2125.2006.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polticelli F, Salvi D, Mariottini P, Amendola R, Cervelli M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol Biol. 2012;12:90. doi: 10.1186/1471-2148-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei PA, Snell EE. Pyruvoyl-dependent histidine decarboxylases. Mechanism of cleavage of the proenzyme from Lactobacillus buchneri. J Biol Chem. 1985;260:2804–2806. [PubMed] [Google Scholar]
- Reyes-Palomares A, Montañez R, Sánchez-Jiménez F, Medina MA. A combined model of hepatic polyamine and sulfur amino acid metabolism to analyze S-adenosyl methionine availability. Amino Acids. 2012;42:597–610. doi: 10.1007/s00726-011-1035-7. [DOI] [PubMed] [Google Scholar]
- Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, Abrink M, et al. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008;121:1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA. Mathematical modeling of polyamine metabolism in mammals. J Biol Chem. 2006;281:21799–21812. doi: 10.1074/jbc.M602756200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Chica J, Medina MA, Sánchez-Jiménez F, Ramírez FJ. Raman study of the effects of polyamines on DNA: spermine and histamine. J Mol Structure. 1999;480:455–458. [Google Scholar]
- Ruiz-Chica J, Medina MA, Sánchez-Jiménez F, Ramírez FJ. Fourier transform Raman study of the structural specificities on the interaction between DNA and biogenic polyamines. Biophys J. 2001;80:443–454. doi: 10.1016/S0006-3495(01)76027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeier E, Hale TI, Christen P. Multiple evolutionary origin of pyridoxal-5'-phosphate-dependent amino acid decarboxylases. Eur J Biochem. 1994;221:997–1002. doi: 10.1111/j.1432-1033.1994.tb18816.x. [DOI] [PubMed] [Google Scholar]
- Schneider E, Leite-de-Moraes M, Dy M. Histamine, immune cells and autoimmunity. In: Thurmond RL, editor. Histamine in Inflammation. New York: Springer US; 2010. pp. 81–94. [DOI] [PubMed] [Google Scholar]
- Schütz B, Schäfer MK, Eiden LE, Weihe E. Vesicular amine transporter expression and isoform selection in developing brain, peripheral nervous system and gut. Brain Res Dev Brain Res. 1998;106:181–204. doi: 10.1016/s0165-3806(97)00196-x. [DOI] [PubMed] [Google Scholar]
- Sevior DK, Pelkonen O, Ahokas JT. Hepatocytes: the powerhouse of biotransformation. Int J Biochem Cell Biol. 2012;44:257–261. doi: 10.1016/j.biocel.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hamelin BA. Classic histamine H1 receptor antagonists: a critical review of their metabolic and pharmacokinetic fate from a bird's eye view. Curr Drug Metab. 2003;4:105–129. doi: 10.2174/1389200033489523. [DOI] [PubMed] [Google Scholar]
- Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Curr Opin Pharmacol. 2011;11:45–51. doi: 10.1016/j.coph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Simon T, László V, Falus A. Impact of histamine on dendritic cell functions. Cell Biol Int. 2011;35:997–1000. doi: 10.1042/CBI20100844. [DOI] [PubMed] [Google Scholar]
- Sinigaglia G, Magro M, Miotto G, Cardillo S, Agostinelli E, Zboril R, et al. Catalytically active bovine serum amine oxidase bound to fluorescent and magnetically drivable nanoparticles. Int J Nanomedicine. 2012;7:2249–2259. doi: 10.2147/IJN.S28237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova OA, Isaguliants MG, Hyvonen MT, Keinanen TA, Tunitskaya VL, Vepsalainen J, et al. Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N(1)-acetyltransferase in human hepatoma HUH7 cells. Biochimie. 2012;94:1876–1883. doi: 10.1016/j.biochi.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Sommet A, Desplas M, Lapeyre-Mestre M, Montastruc JL French Network of Pharmacovigilance Centers. Drug-induced yawning: a review of the French pharmacovigilance database. Drug Saf. 2007;30:327–331. doi: 10.2165/00002018-200730040-00005. [DOI] [PubMed] [Google Scholar]
- Specht S, Frank JK, Alferink J, Dubben B, Layland LE, Denece G, et al. CCL17 controls mast cells for the defense against filarial larval entry. J Immunol. 2011;186:4845–4852. doi: 10.4049/jimmunol.1000612. [DOI] [PubMed] [Google Scholar]
- Sridhar J, Liu J, Foroozesh M, Stevens CL. Insights on cytochrome p450 enzymes and inhibitors obtained through QSAR studies. Molecules. 2012;17:9283–9305. doi: 10.3390/molecules17089283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato R, Cardillo S, Braga M, De Iuliis A, Battaglia V, Toninello A, et al. Preliminary kinetic characterization of a copper amine oxidase from rat liver mitochondria matrix. Amino Acids. 2011;40:713–720. doi: 10.1007/s00726-010-0708-y. [DOI] [PubMed] [Google Scholar]
- Strolin Benedetti M. FAD-dependent enzymes involved in the metabolic oxidation of xenobiotics. Ann Pharm Fr. 2011;69:45–52. doi: 10.1016/j.pharma.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka S, Nemoto K, Ichikawa A. Membrane targeting and binding of the 74-kDa form of mouse L-histidine decarboxylase via its carboxyl-terminal sequence. FEBS Lett. 1998;437:44–48. doi: 10.1016/s0014-5793(98)01195-8. [DOI] [PubMed] [Google Scholar]
- Tabolacci C, Lentini A, Provenzano B, Beninati S. Evidences for a role of protein cross-links in transglutaminase-related disease. Amino Acids. 2012;42:975–986. doi: 10.1007/s00726-011-1011-2. [DOI] [PubMed] [Google Scholar]
- Takarabe M, Shigemizu D, Kotera M, Goto S, Kanehisa M. Network-based analysis and characterization of adverse drug-drug interactions. J Chem Inf Model. 2011;51:2977–2985. doi: 10.1021/ci200367w. [DOI] [PubMed] [Google Scholar]
- Talarico G, Tosto G, Pietracupa S, Piacentini E, Canevelli M, Lenzi GL, et al. Serotonin toxicity: a short review of the literature and two case reports involving citalopram. Neurol Sci. 2011;32:507–509. doi: 10.1007/s10072-011-0546-z. [DOI] [PubMed] [Google Scholar]
- Tiligada E, Kyriakidis K, Chazot PL, Passani MB. Histamine pharmacology and new CNS drug targets. CNS Neurosci Ther. 2011;17:620–628. doi: 10.1111/j.1755-5949.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Stringer DE, Blohm-Mangone KA, Gerner EW. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2010;299:G517–G522. doi: 10.1152/ajpgi.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass M, Tarcsay A, Keserü GM. Multiple ligand docking by glide: implications for virtual second-site screening. J Comput Aided Mol Des. 2012;26:821–834. doi: 10.1007/s10822-012-9578-6. [DOI] [PubMed] [Google Scholar]
- Vowinckel J, Stahlberg S, Paulmann N, Bluemlein K, Grohmann M, Ralser M. Histaminylation of glutamine residues is a novel posttranslational modification implicated in G-protein signaling. FEBS Lett. 2012;586:3819–3824. doi: 10.1016/j.febslet.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Stahlberg S, Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 2011;278:4740–4755. doi: 10.1111/j.1742-4658.2011.08347.x. [DOI] [PubMed] [Google Scholar]
- Wang CC, Billett E, Borchert A, Kuhn H, Ufer C. Monoamine oxidases in development. Cell Mol Life Sci. 2012;70:599–630. doi: 10.1007/s00018-012-1065-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Orito T, Watanabe H, Mizoguchi H, Yonezawa A, Yanai A, et al. Intrathecal high-dose histamine induces spinally-mediated nociceptive behavioral responses through a polyamine site of NMDA receptors. Eur J Pharmacol. 2008;581:54–63. doi: 10.1016/j.ejphar.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Winter TN, Elmquist WF, Fairbanks CA. OCT2 and MATE1 provide bidirectional agmatine transport. Mol Pharm. 2011;8:133–142. doi: 10.1021/mp100180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcikowski J, Daniel WA. The brain dopaminergic system as an important center regulating liver cytochrome P450 in the rat. Expert Opin Drug Metab Toxicol. 2009;5:631–645. doi: 10.1517/17425250902973703. [DOI] [PubMed] [Google Scholar]
- Wright PS, Byers TL, Cross-Doersen DE, McCann PP, Bitonti AJ. Irreversible inhibition of S-adenosylmethionine decarboxylase in Plasmodium falciparum-infected erythrocytes: growth inhibition in vitro. Biochem Pharmacol. 1991;41:1713–1718. doi: 10.1016/0006-2952(91)90174-4. [DOI] [PubMed] [Google Scholar]
- Wu C, Macleod I, Su AI. BioGPS and MyGene.info: organizing online, gene-centric information. Nucleic Acids Res. 2012;41:D561–D565. doi: 10.1093/nar/gks1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]


