Abstract
The term ‘neurogenic inflammation’ has been adopted to describe the local release of inflammatory mediators, such as substance P and calcitonin gene-related peptide, from neurons. Once released, these neuropeptides induce the release of histamine from adjacent mast cells. In turn, histamine evokes the release of substance P and calcitonin gene-related peptide; thus, a bidirectional link between histamine and neuropeptides in neurogenic inflammation is established. The aim of this review is to summarize the most recent findings on the role of histamine in neurogenic inflammation, with particular regard to nociceptive pain, as well as neurogenic inflammation in the skin, airways and bladder.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: histamine, neurogenic inflammation, histamine receptors, neuropathic pain, skin, airways, bladder
Introduction
The term ‘neurogenic inflammation’ describes the local release of inflammatory mediators, such as substance P (SP) and calcitonin gene-related peptide (CGRP), from afferent neurons. The initial evidence for neurogenic vasodilatation in response to noxious stimuli was obtained in the skin of humans and other mammals (Schmelz and Petersen, 2001), but now it is recognized that neurogenic inflammation also occurs in visceral organs. The inflammatory response evoked by the activation of the sensory nerve fibres, which includes local vasodilatation, plasma extravasation, leukocyte and platelet adhesion, and mast cell degranulation, is brought about by neuropeptides released from the peripheral endings of sensory neurons upon stimulation of the primary sensory terminals. Both SP in neurons and histamine in mast cells have a dual mediator role in neurogenic inflammation (Figure 1). In fact, as demonstrated by Foreman and Jordan (1984), injury causes activation of sensory nerve endings either directly or through the release of histamine from the adjacent mast cells. The action potential generated travels orthodromically to the dorsal horn of the spinal cord and spreads to other branches of the same neurons, which will then release SP from their terminals or varicosities. The released SP, as well as contributing itself to local vasodilatation, induces histamine release from the adjacent mast cells, which produces flare and further activates other sensory nerve endings (Foreman et al., 1983).
Figure 1.
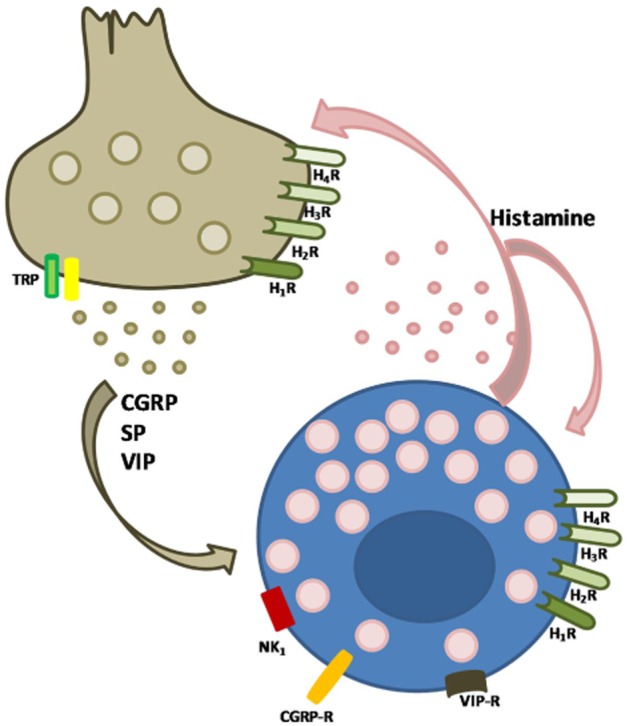
The dual mediator role of mast cells: bidirectional interaction of nerves with mast cells. Neuropeptides released from sensory nerve endings stimulate the adjacent mast cells in a receptor-dependent manner. Mediators released from mast cells act in both a paracrine and an autocrine fashion.
Neutrophils, whose responsiveness to SP has been repeatedly demonstrated, contribute to the inflammatory soup following neurogenic inflammation. In fact, neutrophils express the SP receptors NK1, NK2 and NK3, and when stimulated with SP induce the expression of COX-2 and PGE2 release in the nanomolar range (Gallicchio et al., 2008; 2009). Furthermore, SP at micromolar concentrations primes neutrophils, thus enhancing O2– production evoked by platelet-activating factor (Brunelleschi et al., 1991). In the picomolar range, SP is still able to induce neutrophil adhesion to HUVEC (Dianzani et al., 2003).
Together with histamine, other mast cell-derived mediators (such as 5-HT, renin, adenosine, heparin, tryptase, chymase, elastase, carboxypeptidase A and B, cathepsin, β-galactosidase, β-glucoronidase, MMP, chemotatactic factors for eosinophils and neutrophils, platelet-derived factor, PGD2, LT B4, C4 and D4, TXA2 and B2, NO, TNF-α and other cytokines and neuropeptides) contribute to the functional picture of neurogenic inflammation (Tore and Tuncel, 2009). Experimental evidence supports the role of these mediators in inflammation, demonstrating the presence of their neuronal receptors.
Histamine and neurogenic inflammation: experimental evidence
The evidence for the involvement of a neurogenic mechanism in the SP-induced flare response stems from data showing that a local anaesthetic injection into human skin reduced the spread of the flare response, but without affecting the development of the wheal response (Foreman et al., 1983). Likewise, the role of histamine has been investigated mostly using specific receptor-orientated approaches. The effects of SP on the flare response in human skin can only be mimicked by its neuropeptide fragments that are able to degranulate rat mast cells in vitro (Fewtrell et al., 1982). Also, the H1 histamine antagonist chlorpheniramine (20 mg, i.v.) has been shown to prevent the spread of the flare response to SP in the human skin (Foreman et al., 1983). Thanks to immunohistochemical studies demonstrating the proximity of mast cells and nerve fibres containing neuropeptides, these initial observations on the role of histamine in the neurogenic inflammation have been further elucidated. Preliminary studies were performed in the dura mater and gut, whereas now most of the evidence comes from experiments using skin (Nakanishi and Furuno, 2008). Moreover, Janiszewski et al. (1990) demonstrated, using a co-culture system, that the activation of peritoneal mast cells induces depolarization and decreases membrane resistance in sympathetic neurons (Janiszewski et al., 1990). Furthermore, the notion of a bidirectional communication between nerves and mast cells is supported by the presence of receptors for numerous neuropeptides on the surface of mast cells; these include vasoactive intestinal peptide (VIP), neuropeptide Y, SP, CGRP, pro-opiomelanocortin, galanin, neuromedin U, pituitary adenylate cyclase-activating polypeptide, neurotensin and corticotropin-releasing factor. Thus, these mediators can exert both paracrine and autocrine effects on mast cells (Figure 1). Similarly, histamine also exerts autocrine effects on mast cells as mast cells have been shown to express histamine receptors H1–4 (Tore and Tuncel, 2009).
Notably, the interactions between mast cells and adjacent nerves are also accomplished by adhesion molecules such as N-cadherin or cell adhesion molecule-1, a calcium-dependent adhesion protein that can be cleaved by the metalloproteinase MT5-MMP of the peptide receptors in the dorsal root ganglion (Nakanishi and Furuno, 2008; Guillot et al., 2012).
Histamine, neurogenic inflammation and nociceptive pain
While the contribution of neurogenic inflammation to nociception was elucidated some time ago (Julius and Basbaum, 2001), more recently, detailed analysis of the role of histamine receptors has been investigated.
Mechano-insensitive C-fibres are known to be activated by histamine and are responsible for the neuropeptide release, for example, in the skin they have been shown to induce the axon reflex flare response (Groetzner and Weidner, 2010). Histamine-immunoreactive nerve fibres have been found in the superficial laminae of the dorsal horn, an important site for nociceptive transmission. The mRNA of histamine H1 receptor genes has been detected in many SP and CGRP immunoreactive neurons following peripheral nerve injuries (Kashiba et al., 1999), and histamine has been shown to mediate the release of SP and glutamate in inflammatory conditions (Riedel and Neeck, 2001). Moreover, a bidirectional relationship between CGRP and histamine has been proposed. This is based on data showing that (i) CGRP induces histamine release from mast cells and potentiates histamine effects in the rat (Mobarakeh et al., 2006); (ii) an i.p. injection of histamine induces CGRP release into the CSF (Bileviciute et al., 1994) and (iii) histamine administered to the nasal mucosa causes CGRP release from the peripheral terminals of trigeminal ganglion in the guinea pig (Tani et al., 1990). Further support for this link between histamine and CGRP is attained from the observed co-localization of the histamine H3 receptor with CGRP on Aδ fibres, with both mediators contributing to a high-threshold mechanical nociceptive effect (Cannon et al., 2007).
More recently, it has been demonstrated that both the H1 receptor antagonist pyrilamine and the H2 receptor antagonist ranitidine produce dose-dependent antinociceptive responses in the formalin test, and that hyperalgesia induced by intrathecal administration of histamine is attenuated by the GCRP antagonist CGRP 8–37 (Mobarakeh et al., 2011).
Studies with histamine H1 receptor knockout mice have demonstrated that both the receptor and its natural ligand are needed to facilitate pain transmission at both the peripheral and central levels (Mobarakeh et al., 2000; 2002). In addition, using histidine decarboxylase (HDC) gene knockout mice, it was shown that NK1 receptors in the spinal cord mediate the histamine-induced hyperalgesic responses (Yoshida et al., 2005). The antinociceptive efficacy of antihistamines have been evaluated in several in vivo studies. The administration of H1 and H2 receptor antagonists, chlorpheniramine and cimetidine, respectively, was found to inhibit the development of both thermal and mechanical hyperalgesia, although chlorpheniramine was more potent (Zuo et al., 2003). Even the most recently discovered histamine receptors, the H3 and H4 receptors, have been shown to be involved in mediating nociception. Pretreatment with the dual H3/H4 receptor antagonist thioperamide attenuated c48/80-induced thermal pain 30 min after the challenge without causing analgesia (Chatterjea et al., 2012). Moreover, it was reported that H3 receptor antagonists, such as GSK-189254 and ABT-239, are effective in reducing allodynia and hyperalgesia in models of neuropathic and inflammatory pain (Medhurst et al., 2007; 2008; Hsieh et al., 2010b).
Focusing on H4 receptors, both the experimental antagonists JNJ7777120 (10 and 30 mg·kg−1, s.c.) and VUF6002 (10 mg·kg−1, s.c.) significantly reduced the paw oedema and hyperalgesia induced by a subplantar injection of carrageenan (Coruzzi et al., 2007). According to these data, JNJ7777120 reversed hyperalgesia in both acute and chronic pain models (Hsieh et al., 2010a). Notably, in the same study it was found that the H1 receptor antagonist diphenhydramine, H2 receptor antagonist ranitidine or H3 receptor antagonist ABT-239 had no effect on this hyperalgesia, suggesting a dominant role for the H4 receptor in animal models of inflammatory and neuropathic pain.
Taken together, these data from animal models may be regarded as indicative of a potential efficacy of antihistamines in the clinical setting. However, clinical evidence is still lacking and previous trials obtained negative results (Raffa, 2001).
Histamine and neurogenic inflammation in the skin
The involvement of histamine in cutaneous neurogenic inflammation stems from the observation that this amine triggers the so-called triple response. Nowadays, local erythema is know to result from the axon reflex and antidromic sensory nerve stimulation-induced release of different vasoactive mediators, not only histamine but also SP, histamine, purines and CGRP. The itching sensation is mediated by dedicated afferent non-myelinated C-type nerve fibres, which are different from the polymodal C-fibres as they are unresponsive to mechanical stimulation. The so-called pruriceptors are differentiated in histamine-sensitive and histamine-independent itch-specific C-fibres. In particular, histamine-sensitive C-fibres, which constitute about 5% of the afferent C-fibres in human cutaneous nerves, are characterized by slow conduction velocities and extensive terminal branching (Shim and Oh, 2008; Raap et al., 2011). When activated by histamine, they transmit electrical signals to the superficial layer of the dorsal horn of the spinal cord. These signals then ascend to the thalamus through contralateral spinothalamic tracts and are eventually conducted to the somatosensory and cingulated cortex (Figure 2). All of the histamine receptors are involved in mediating the histamine signal in neurons, but to different extents. The failure of cimetidine to suppress histamine-induced itch in Balb/C mice (Bell et al., 2004) indicates that H2 receptors are not involved in itch. The H1 receptor is the most extensively histamine receptor studied. Indeed, H1 receptor blockers have an established and valued place in the treatment of itching of allergic and non-allergic origin (Skidgel et al., 2011).
Figure 2.
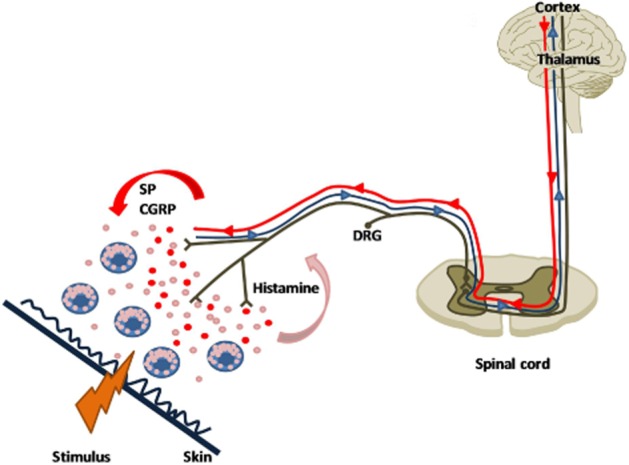
The itch system. Histamine released from mast cells after a local stimulus activates histamine-sensitive pruriceptors, thus generating an action potential that orthodromically travels through to the dorsal horn of the spinal cord and the thalamus, and to the cortex (blue line). The following antidromic stimulation induces the release of different mediators from sensory endings, SP and CGRP. The SP and CGRP released causes further mast cell degranulation, resulting in vasodilatation (flare) and the recruitment of other pruriceptors.
However, intriguing data on the H3 receptor seem to contradict the aforementioned histamine-induced itch pathway (Summey and Yosipovitch, 2005); antagonists such as thioperamide and AQ0145 were found to increase significantly the incidence of scratching behaviour in mice (Sugimoto et al., 2004). It is possible that these histamine H3 receptor antagonists block the modulatory role of the presynaptic H3 receptor, thus favouring the release of neuropeptides from sensory endings (Cannon et al., 2007). More recently, data obtained with clobenproprit, an H3 receptor antagonist and H4 receptor agonist, suggest that the H4 receptor is also involved in itch. Clobenproprit induced scratching responses in the mouse that were attenuated by pretreating the animals with the H4 receptor antagonist, JNJ7777120 (Dunford et al., 2007). Moreover, it has been reported that H4 receptor agonists are able to induce itch through a direct action on the peripheral nerves (Bell et al., 2004), and that H4 antagonists inhibit SP-induced itch, which has been found to be resistant to H1 receptor antagonists (Yamaura et al., 2009). More recently, Suwa et al. (2011) demonstrated that JNJ7777120 (10 and 30 mg·kg−1 day), but not the histamine H1 receptor antagonist fexofenadine (30 mg·kg−1 day), reduces the scratching behaviour and ameliorates the skin lesions induced by repeated challenge with 2,4,6-trinitrochlorobenzene in hairless mice, in a dose-dependent manner (Suwa et al., 2011).
Interestingly, in mice, blocking both the H1 receptors and the H4 receptors with a dual antagonist or a combination of drugs gave the maximum response; it almost completely abolished the itch response (Dunford et al., 2007).
In addition, other published data indicate that H1 and H4 receptors share a similar pathway, in that they stimulate neurons by increasing intracellular Ca2+ levels (Shim and Oh, 2008). The histamine-induced increase in calcium levels is mediated through H1 receptors in rat cultured sensory neurons, and this effect is blocked by the PLC inhibitor U73122 (Nicolson et al., 2002).
Moreover, in mouse sensory neurons stimulated by capsaicin, histamine induces inward currents and calcium influx in a TRPV1-dependent manner, as demonstrated by the inhibitory effects of TRPV1 antagonists. In keeping with these results, histamine-induced scratching is significantly lower in TRPV1-deficient mice (Shim et al., 2007; Kajihara et al., 2010).
All these data explain, at least in part, the antipruritic effects of mirtazapine and doxepin, two antidepressant that also have additional antihistamine effects (Raap et al., 2011). Moreover, the well-recognized risk-benefit profile of H1 antihistamines, especially the non-sedative second generation ones, has lead the European Academy of Allergy and Clinical Immunology to recommend, in its guidelines, the use of a maximum dose of non-sedating antihistamines up to fourfold higher in patients with chronic urticaria. Clinical studies on H4 receptor antagonists are currently ongoing (Engelhardt et al., 2009).
Histamine and neurogenic inflammation in the airways
Misery (2008) approached the intriguing parallelism between itch/scratching and cough starting from a common pathophysiology that involves the C fibres, the mast cells, histamine, SP and other tachykinins. Indeed, the close proximity between mast cells and the nerve endings in the lung suggests a similar neuroimmuno crosstalk (Misery, 2008), as that found in the skin. Sensory nerve endings release neuropeptides such as SP and CGRP, which induce mast cell activation and degranulation. The antidromic release of neuropeptides from nociceptors in the airways causes vasodilatation and oedema, which are associated with nasal obstruction in the upper airways and bronchoconstriction in the lower airways, as well as plasma protein exudation, mucus secretion and inflammatory cell recruitment. As a proof of neurogenic inflammation in the airways, it was shown that local anaesthesia with lidocaine improved airway hyperreactivity and reduced capsaicin-induced cough (Muraki et al., 2008).
The first evidence of a relationship between SP and histamine in the airways came in 1994, when Heaney et al. demonstrated that SP is able to stimulate human mast cells obtained from bronchoalveolar lavage (Heaney et al., 1994). More recently, sputum SP and mast cell tryptase concentrations were found to be markedly increased in patients with chronic cough, and were even elevated in those patients with cough due to gastro-oesophageal reflux disease (GERD). These latter data suggest that GERD-induced cough may be related to a cough reflex hypersensitivity caused by neurogenic airway inflammation (Qiu et al., 2011) and are in keeping with the results obtained by Birring et al. (2004), who measured an increased histamine content in the sputum of patients with idiopathic chronic cough and cough variant asthma/eosinophilic bronchitis in comparison with normal subjects. Rat lung mast cells have been found to release histamine in response to high doses of SP in vitro; moreover, it has been shown that the NK1 receptor-mediated mast cell activation partly accounts for the airway plasma leakage in F344-, but not in BDE-rats, exposed to SP and capsaicin. The airway responsiveness to tachykinins differentiates these two inbred strains, and, in fact only the F344 strain presents NK1 receptor mast cells that display a pro-releasing effect (Pauwels et al., 1995). More recently, it has been reported that CP-99 994 (5 mg·kg−1, i.v.), an NK1 receptor antagonist, abolishes the microvascular leakage elicited in rat airways by a single inhalation of toluene-2,4-diisocyanate. In contrast, ketotifen (1 mg·kg−1, i.v.), an H1 antagonist with mast cell-stabilizing properties, did not have any effect in this model (Sakamoto et al., 2012). In a guinea pig model of microvascular leakage where hypersensitivity was induced in the airways of guinea-pigs treated with aerosolized histamine, it was demonstrated that, while NKB and the NK3 receptor agonist senktide, enhanced airway hypersensitivity to histamine, both the tachykinin NK3 receptor antagonist osanetant and the NK1 receptor antagonist nolpitantium were able to abolish the histamine-induced microvascular leakage. In the same model, NK2 ligands were found to be ineffective (Daoui et al., 2001). Moreover, it has been reported that toluene-2,4-diisocyanate exposure causes an increase in histamine content, HDC activity and gene expression in the nasal mucosa of sensitized rats (Kitamura et al., 2004). Olopatadine hydrochloride, an H1 receptor antagonist, besides inhibiting the capsaicin-induced sneezing response, has been found to inhibit antigen-induced sneeze and nasal rubbing responses in both wild-type and H1 receptor-deficient mice, although at very high doses (Tamura and Komai, 2008), thus suggesting it has an effect that is not dependent on H1 receptors. Indeed, these data, together with the failure of cetirizine to inhibit completely these responses in H1 receptor-deficient mice (Kayasuga et al., 2002; Sugimoto et al., 2004), indicate that other receptors may be involved in these responses.
Histamine and neurogenic inflammation in the bladder
Interstitial cystitis (IC) and painful bladder syndrome (PBS) are both causes of neurogenic inflammation; in fact, an increased density of nerve fibres has been reported in these conditions (Peeker et al., 2000). Evidence from both rodent and humans suggest that mast cells play an important role in these conditions. It has been shown that activated mast cells are associated with rodent neurogenic cystitis induced by the Bartha strain of pseudorabies virus (PRV) (Chen et al., 2006; Jasmin et al., 2000). Moreover, clinical studies have demonstrated an elevated number of mast cells in the lamina propria of IC bladder biopsies (Leiby et al., 2007) and increased urinary histamine metabolites (el-Mansoury et al., 1994). Although the central role of mast cells in PBS/IC is still unclear, results obtained in an in vivo model of IC pathogenesis suggest the existence of a positive feedback loop with SP containing peripheral nerves and mast cells: activation of the bladder-associated circuits in the CNS initiates SP release by peripheral nerves in the bladder, leading to SP-mediated mast cell activation. Consequently, mast cell degranulation induces bladder inflammation by acting on urothelium. Histamine contributes to the symptoms of IC/PBS, not only by evoking an inflammatory response, but is also associated with the pelvic pain. In fact, pain responses mediated by histamine and histamine receptors have been described in both animal and human models (Thilagarajah et al., 2001; Mobarakeh et al., 2006). Although the specific cell type (it could be mast cells, basophils, neutrophils or dendritic cells) or histaminergic neurons regulating histamine-mediated pain have not been identified, it has been demonstrated, using a model of PRV-induced pelvic pain in mast cell-deficient KitW-sh/KitW-sh mice, that mast cells are required for histamine-mediated pelvic pain (Rudick et al., 2008). However, the same authors demonstrated that KitW-sh/KitW-sh mice reconstituted with HDC-/- bone marrow exhibited diminished pain, thus suggesting that non-mast cell sources of histamine may also contribute to this pain response. Moreover, Rudick et al. (2008) demonstrated that PRV-induced pelvic pain is not dependent on TNF-mediated effects but is mediated by mast cell histamine, which then induces pain by stimulating histamine H1 and H2 receptors. Thus, it has been suggested that antagonists of histamine H1 and H2 receptors are suitable candidates in clinical trials for the treatment of chronic pain conditions, such as IC-related pelvic pain. Indeed, the findings from pilot clinical studies suggest that antihistamine therapy could be effective in IC-related pelvic pain. In particular, a symptomatic improvement has been reported in 30% of patients treated with the H1 receptor antagonist hydroxyzine hydrochloride at doses ranging from 25 mg day-1 at night to 50 mg at night +25 mg in the morning over a 2 week period (Theoharides et al., 1997). Although these positive results suggest a therapeutic role for histamine H1 receptor antagonists in the treatment of IC/PBS, further studies with the newer generation of histamine H1 receptor antagonists, which have fewer sedative effects, need to be conducted. Moreover, in a limited trial of PBS patients, the histamine H2 receptor antagonist cimetidine produced significant improvements in the pain and nocturia associated with this condition (Thilagarajah et al., 2001). It has to be stressed that approximately 90% of patients with PBS/IC are women, thus suggesting that the process involving mast cells may be influenced by fluctuations in hormone levels. In fact, oestrogen receptors (ERs) are expressed on human mast cells and can induce them to degranulate, an effect inhibited by the ER antagonist tamoxifen (Rudick et al., 2012).
Taken together, these findings suggest that reproductive hormones may modulate IC symptoms at the mast cell level. This hypothesis has recently been tested by Rudick et al. (2012), who assessed the basis of gender-specific pelvic pain in the murine model of PRV-induced neurogenic cystitis. The data obtained suggest that pelvic pain in mice with murine neurogenic cystitis is mediated by mast cells; in contrast, the severity of the pelvic pain is affected by genetic factors (Rudick et al., 2012).
Conclusion
In conclusion, the experimental data described in this review generally show that histamine plays a key role in the complex physiopathological mechanism known as neurogenic inflammation (Table 1). We have summarized recent findings on the role of histamine and the contribution of its four receptors, identified so far, to this condition, with particular focus on nociceptive pain, neurogenic inflammation in the skin, airways and interstitial cystitis. The experimental evidence, although not conclusive, indicates that several of the newly available histamine ligands have potential for clinical development as treatments for the pain and other symptoms associated with neurogenic inflammation.
Table 1.
Effect of selective histamine receptor antagonists on neurogenic inflammation
| Histamine receptor subtype | ||||
|---|---|---|---|---|
| Effect | H1R | H2R | H3R | H4R |
| Pain | ↓ | ↓ | ↓ | ↓ |
| Hyperalgesiaa | ↓ | ↓ | ↓ | ↓ |
| Allodynia | ? | ? | ↓ | ? |
| Itch | ↓ | ↔ | ↑ | ↓ |
| Sneezing response | ↓b | ? | ? | ? |
The differential involvement of histamine receptor subtypes has been demonstrated in different experimental models.
Only at high doses.
Acknowledgments
This work was supported by the University of Turin, Royal College of Anaesthesia/BJA and COST Action BM0806.
Glossary
- CGRP
calcitonin gene-related peptide
- ERs
oestrogen receptors
- GERD
gastro-oesophageal reflux disease
- HDC
histidine decarboxylase
- IC
interstitial cystitis
- PBS
painful bladder syndrome
- SP
substance P
- VIP
vasoactive intestinal peptide
Conflict of interest
The authors declared that they have no conflict of interest.
References
- Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bileviciute I, Lundeberg T, Ekblom A, Theodorsson E. Substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity (-LI) in rat knee joint synovial fluid during acute monoarthritis is not correlated with concentrations of neuropeptide-LI in cerebrospinal fluid and plasma. Neurosci Lett. 1994;167:145–148. doi: 10.1016/0304-3940(94)91048-0. [DOI] [PubMed] [Google Scholar]
- Birring SS, Parker D, Brightling CE, Bradding P, Wardlaw AJ, Pavord ID. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med. 2004;169:15–19. doi: 10.1164/rccm.200308-1092OC. [DOI] [PubMed] [Google Scholar]
- Brunelleschi S, Tarli S, Giotti A, Fantozzi R. Priming effects of mammalian tachykinins on human neutrophils. Life Sci. 1991;48:PL1–PL5. doi: 10.1016/0024-3205(91)90416-9. [DOI] [PubMed] [Google Scholar]
- Cannon KE, Chazot PL, Hann V, Shenton F, Hough LB, Rice FL. Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain. 2007;129:76–92. doi: 10.1016/j.pain.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjea D, Wetzel A, Mack M, Engblom C, Allen J, Mora-Solano C, et al. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem Biophys Res Commun. 2012;425:237–243. doi: 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol. 2006;175:754–759. doi: 10.1016/S0022-5347(05)00171-0. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Guaita E, de Esch IJ, Leurs R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur J Pharmacol. 2007;563:240–244. doi: 10.1016/j.ejphar.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Daoui S, Ahnaou A, Naline E, Emonds-Alt X, Lagente V, Advenier C. Tachykinin NK(3) receptor agonists induced microvascular leakage hypersensitivity in the guinea-pig airways. Eur J Pharmacol. 2001;433:199–207. doi: 10.1016/s0014-2999(01)01505-9. [DOI] [PubMed] [Google Scholar]
- Dianzani C, Collino M, Lombardi G, Garbarino G, Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:1103–1110. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- el-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:350–353. doi: 10.1016/s0022-5347(17)32737-4. [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Smits RA, Leurs R, Haaksma E, de Esch IJ. A new generation of anti-histamines: histamine H4 receptor antagonists on their way to the clinic. Curr Opin Drug Discov Devel. 2009;12:628–643. [PubMed] [Google Scholar]
- Fewtrell CM, Foreman JC, Jordan CC, Oehme P, Renner H, Stewart JM. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman JC, Jordan CC. Neurogenic inflammation. Trends Pharmacol Sci. 1984;5:116–119. [Google Scholar]
- Foreman JC, Jordan CC, Oehme P, Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio M, Benetti E, Rosa AC, Fantozzi R. Substance P-induced cycloxygenase-2 expression in polymorphonuclear cells. Inflamm Res. 2008;57(Suppl. 1):S17–S18. doi: 10.1007/s00011-007-0608-0. [DOI] [PubMed] [Google Scholar]
- Gallicchio M, Benetti E, Rosa AC, Fantozzi R. Tachykinin receptor modulation of cyclooxygenase-2 expression in human polymorphonuclear leucocytes. Br J Pharmacol. 2009;156:486–496. doi: 10.1111/j.1476-5381.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groetzner P, Weidner C. The human vasodilator axon reflex – an exclusively peripheral phenomenon? Pain. 2010;149:71–75. doi: 10.1016/j.pain.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Guillot X, Semerano L, Decker P, Falgarone G, Boissier MC. Pain and immunity. Joint Bone Spine. 2012;79:228–236. doi: 10.1016/j.jbspin.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Heaney LG, Cross LJ, Stanford CF, Ennis M. Differential reactivity of human bronchoalveolar lavage mast cells to substance P. Agents Actions. 1994;41:C19–C21. doi: 10.1007/BF02007748. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Chandran P, Salyers AK, Pai M, Zhu CZ, Wensink EJ, et al. H4 receptor antagonism exhibits anti-nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol Biochem Behav. 2010a;95:41–50. doi: 10.1016/j.pbb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Honore P, Pai M, Wensink EJ, Chandran P, Salyers AK, et al. Antinociceptive effects of histamine H3 receptor antagonist in the preclinical models of pain in rats and the involvement of central noradrenergic systems. Brain Res. 2010b;1354:74–84. doi: 10.1016/j.brainres.2010.07.083. [DOI] [PubMed] [Google Scholar]
- Janiszewski J, Bienenstock J, Blennerhassett MG. Activation of rat peritoneal mast cells in coculture with sympathetic neurons alters neuronal physiology. Brain Behav Immun. 1990;4:139–150. doi: 10.1016/0889-1591(90)90016-j. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Janni G, Ohara PT, Rabkin SD. CNS induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J Urol. 2000;164:852–855. doi: 10.1097/00005392-200009010-00061. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kajihara Y, Murakami M, Imagawa T, Otsuguro K, Ito S, Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience. 2010;166:292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Fukui H, Morikawa Y, Senba E. Gene expression of histamine H1 receptor in guinea pig primary sensory neurons: a relationship between H1 receptor mRNA-expressing neurons and peptidergic neurons. Brain Res Mol Brain Res. 1999;66:24–34. doi: 10.1016/s0169-328x(98)00346-5. [DOI] [PubMed] [Google Scholar]
- Kayasuga R, Sugimoto Y, Watanabe T, Kamei C. Participation of chemical mediators other than histamine in nasal allergy signs: a study using mice lacking histamine H(1) receptors. Eur J Pharmacol. 2002;449:287–291. doi: 10.1016/s0014-2999(02)02005-8. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Miyoshi A, Murata Y, Kalubi B, Fukui H, Takeda N. Effect of glucocorticoid on upregulation of histamine H1 receptor mRNA in nasal mucosa of rats sensitized by exposure to toluene diisocyanate. Acta Otolaryngol. 2004;124:1053–1058. doi: 10.1080/00016480410022525. [DOI] [PubMed] [Google Scholar]
- Leiby BE, Landis JR, Propert KJ, Tomaszewski JE. Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol. 2007;177:142–148. doi: 10.1016/j.juro.2006.08.096. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Collins SD, Billinton A, Bingham S, Dalziel RG, Brass A, et al. Novel histamine H3 receptor antagonists GSK189254 and GSK334429 are efficacious in surgically-induced and virally-induced rat models of neuropathic pain. Pain. 2008;138:61–69. doi: 10.1016/j.pain.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Misery L. Are pruritus and scratching the cough of the skin? Dermatology. 2008;216:3–5. doi: 10.1159/000109351. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Sakurada S, Katsuyama S, Kutsuwa M, Kuramasu A, Lin ZY, et al. Role of histamine H(1) receptor in pain perception: a study of the receptor gene knockout mice. Eur J Pharmacol. 2000;391:81–89. doi: 10.1016/s0014-2999(00)00060-1. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Sakurada S, Hayashi T, Orito T, Okuyama K, Sakurada T, et al. Enhanced antinociception by intrathecally-administered morphine in histamine H1 receptor gene knockout mice. Neuropharmacology. 2002;42:1079–1088. doi: 10.1016/s0028-3908(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Takahashi K, Sakurada S, Kuramasu A, Yanai K. Enhanced antinociceptive effects of morphine in histamine H2 receptor gene knockout mice. Neuropharmacology. 2006;51:612–622. doi: 10.1016/j.neuropharm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Torkaman-Boutorabi A, Rahimi AA, Ghasri S, Nezhad RM, Hamzely A, et al. Interaction of histamine and calcitonin gene-related peptide in the formalin induced pain perception in rats. Biomed Res. 2011;32:195–201. doi: 10.2220/biomedres.32.195. [DOI] [PubMed] [Google Scholar]
- Muraki M, Iwanaga T, Haraguchi R, Kubo H, Tohda Y. Continued inhalation of lidocaine suppresses antigen-induced airway hyperreactivity and airway inflammation in ovalbumin-sensitized guinea pigs. Int Immunopharmacol. 2008;8:725–731. doi: 10.1016/j.intimp.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Furuno T. Molecular basis of neuroimmune interaction in an in vitro coculture approach. Cell Mol Immunol. 2008;5:249–259. doi: 10.1038/cmi.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience. 2002;110:329–338. doi: 10.1016/s0306-4522(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Germonpre PR, Kips JC, Joos GF. Genetic control of indirect airway responsiveness in the rat. Clin Exp Allergy. 1995;25(Suppl. 2):55–60. doi: 10.1111/j.1365-2222.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Peeker R, Aldenborg F, Dahlstrom A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol. 2000;163:1112–1115. [PubMed] [Google Scholar]
- Qiu Z, Yu L, Xu S, Liu B, Zhao T, Lu H. Cough reflex sensitivity and airway inflammation in patients with chronic cough due to non-acid gastro-oesophageal reflux. Respirology. 2011;16:645–652. doi: 10.1111/j.1440-1843.2011.01952.x. [DOI] [PubMed] [Google Scholar]
- Raap U, Stander S, Metz M. Pathophysiology of itch and new treatments. Curr Opin Allergy Clin Immunol. 2011;11:420–427. doi: 10.1097/ACI.0b013e32834a41c2. [DOI] [PubMed] [Google Scholar]
- Raffa RB. Antihistamines as analgesics. J Clin Pharm Ther. 2001;26:81–85. doi: 10.1046/j.1365-2710.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- Riedel W, Neeck G. Nociception, pain, and antinociception: current concepts. Z Rheumatol. 2001;60:404–415. doi: 10.1007/s003930170003. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PloS ONE. 2008;3:e2096. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Pavlov VI, Chen MC, Klumpp DJ. Gender specific pelvic pain severity in neurogenic cystitis. J Urol. 2012;187:715–724. doi: 10.1016/j.juro.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kamijima M, Miyake M. Neurogenic airway microvascular leakage induced by toluene inhalation in rats. Eur J Pharmacol. 2012;685:180–185. doi: 10.1016/j.ejphar.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Petersen LJ. Neurogenic inflammation in human and rodent skin. News Physiol Sci. 2001;16:33–37. doi: 10.1152/physiologyonline.2001.16.1.33. [DOI] [PubMed] [Google Scholar]
- Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA, Kaplan AP, Erdös EG. Histamine, bradykinin, and their antagonists. In: Brunton LL, editor. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 12th edn. Columbus: The McGraw-Hill Companies, Inc; 2011. pp. 911–935. [Google Scholar]
- Sugimoto Y, Iba Y, Nakamura Y, Kayasuga R, Kamei C. Pruritus-associated response mediated by cutaneous histamine H3 receptors. Clin Exp Allergy. 2004;34:456–459. doi: 10.1111/j.1365-2222.2004.01876.x. [DOI] [PubMed] [Google Scholar]
- Summey BT, Yosipovitch G. Pharmacologic advances in the systemic treatment of itch. Dermatol Ther. 2005;18:328–332. doi: 10.1111/j.1529-8019.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- Suwa E, Yamaura K, Oda M, Namiki T, Ueno K. Histamine H(4) receptor antagonist reduces dermal inflammation and pruritus in a hapten-induced experimental model. Eur J Pharmacol. 2011;667:383–388. doi: 10.1016/j.ejphar.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Tamura T, Komai M. Effect of olopatadine hydrochloride, an anti-histamine drug, on rhinitis induced by intranasal instillation of toluene-2,4-diisocyanate in rats. Int Immunopharmacol. 2008;8:916–921. doi: 10.1016/j.intimp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Tani E, Senba E, Kokumai S, Masuyama K, Ishikawa T, Tohyama M. Histamine application to the nasal mucosa induces release of calcitonin gene-related peptide and substance P from peripheral terminals of trigeminal ganglion: a morphological study in the guinea pig. Neurosci Lett. 1990;112:1–6. doi: 10.1016/0304-3940(90)90312-w. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49:108–110. doi: 10.1016/s0090-4295(97)00182-9. [DOI] [PubMed] [Google Scholar]
- Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87:207–212. doi: 10.1046/j.1464-410x.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- Tore F, Tuncel N. Mast cells: target and source of neuropeptides. Curr Pharm Des. 2009;15:3433–3445. doi: 10.2174/138161209789105036. [DOI] [PubMed] [Google Scholar]
- Yamaura K, Oda M, Suwa E, Suzuki M, Sato H, Ueno K. Expression of histamine H4 receptor in human epidermal tissues and attenuation of experimental pruritus using H4 receptor antagonist. J Toxicol Sci. 2009;34:427–431. doi: 10.2131/jts.34.427. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Mobarakeh JI, Sakurai E, Sakurada S, Orito T, Kuramasu A, et al. Intrathecally-administered histamine facilitates nociception through tachykinin NK1 and histamine H1 receptors: a study in histidine decarboxylase gene knockout mice. Eur J Pharmacol. 2005;522:55–62. doi: 10.1016/j.ejphar.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain. 2003;105:467–479. doi: 10.1016/S0304-3959(03)00261-6. [DOI] [PubMed] [Google Scholar]


