Abstract
Background and Purpose
Since the identification of the histamine H4 receptor, several ligands activating this receptor have been described and more compounds are in development. These ligands are well characterized in pharmacological assays, including radioligand competition binding studies, GTPγS and GTPase assays. In most cases, these experiments are performed in transfected cell lines, expressing unnaturally high levels of target receptors and G-protein signalling components. In this study we investigated the specific properties of H4 receptor ligands in native cells.
Experimental Approach
Histamine and five different H4 receptor agonists – 4-methylhistamine, UR-PI376, clobenpropit, VUF8430 and ST-1006 – were characterized in freshly isolated human monocytes. The ligands (10 nM–10 μM) were tested as inhibitors of IL-12p70 secretion from human monocytes and the effects of the H2 receptor antagonist ranitidine and the H4 receptor antagonist JNJ7777120 on their action was investigated.
Key Results
Histamine and all the tested agonists reduced IL-12p70 secretion into monocyte supernatants by 40–70%. The potencies varied with pEC50 values ranging from 5.7 to 6.9, depending on the agonist used. All potencies were lower than those determined in the original investigations of the compounds. Pretreatment of monocytes with H2 or H4 receptor antagonists showed that some H4 receptor ligands also had low activity at the H2 receptor.
Conclusions and Implications
Our study demonstrates discrepancies between the potencies obtained from assays in transfected cell lines and assays in native human cells, indicating the importance of evaluating H4 receptor ligands in native cells.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: 4-methylhistamine, clobenpropit, histamine H4 receptor, histamine, ST-1006, UR-PI376, VUF8430
Introduction
The histamine H4 receptor subtype is the most recently identified (Nakamura et al., 2000; Oda et al., 2000) of the four known G-protein-coupled histamine receptor subtypes (receptor nomenclature follows Alexander et al., 2011). The H4 receptor is expressed in many different tissue types and particularly in those of the haematopoetic system. Although the main role of the H4 receptor is thought to be in inflammatory diseases such as asthma, atopic dermatitis, inflammatory pain and allergic rhinitis (Engelhardt et al., 2009; Gutzmer et al., 2011), newer studies suggest that H4 receptors could also be involved in pruritus, cancer, neuropathic pain, vestibular disorders and type 2 diabetes (Gutzmer et al., 2011; Kiss and Keseru, 2012). Because of its widespread expression and its potential as a target for therapeutic interventions, the function of the H4 receptor needs to be thoroughly characterized.
To accomplish this goal, several H4 receptor ligands have been developed in recent years for assessment in vitro, in animal models and as potential therapeutic agents. These H4 receptor agonists have been rigorously tested for their binding properties and potencies in binding-assays in cell lines (Lim et al., 2005; 2006; Igel et al., 2009; Sander et al., 2009). For these studies human neuroblastoma cells lines (Lim et al., 2005) or insect cells (Igel et al., 2009; Sander et al., 2009) are used, after transfection with the H4 receptor gene from different species and the relevant G-protein signalling components in order to induce signalling in response to receptor stimulation. With the use of these cell lines, histamine displacement assays and functional assays, for example GTPase assays, give information on the binding affinity and efficacy of H4 receptor ligands at this receptor derived from different species. These pharmacological assays allow the comparison of different ligands with a standardized and robust assay system. However, cell lines expressing high amounts of one single receptor and its signalling components represent artificial systems, which probably do not completely represent the situation in native cells. When comparing different H4 receptor ligands in in vitro assays on native cells, we and others have observed variations in their activity that were not predicted from the data derived from the cell-line assays. Therefore we decided to perform a more extensive study comparing the effect of histamine with a panel of different H4 receptor agonists, which have been described by different research groups: 4-methylhistamine (Lim et al., 2005), UR-PI376 (Igel et al., 2009), clobenpropit (Lim et al., 2005), VUF8430 (Lim et al., 2006) and ST-1006 (Sander et al., 2009). As a robust functional readout we selected the secretion of the cytokine IL-12p70 from human monocytes, which has been shown to be down-regulated by histamine and dependent on the activation of histamine H4 and H2 receptors (Gutzmer et al., 2005; Dijkstra et al., 2008).
Our study focused on the importance of evaluating H4 receptor ligands in a native cellular system, as distinct from a transfected cell line. After testing various H4 receptor ligands at several concentrations in such a native system, we found that the potency in down-regulating IL-12p70 production varied between the ligands and was somewhat less than their potency in mediating G-protein signalling in the cell-line assays. Furthermore the H4 receptor agonists seem to exhibit a different proportion of binding at H2 and H4 receptors, as identified by the corresponding receptor antagonists.
Methods
Isolation, culture, and stimulation of human monocytes
Buffy coats, after thrombocyte preparation from whole blood, were obtained from the local blood bank. According to the guidelines for blood donation, the anonymous donors were healthy and had not taken any medication for 4 weeks before giving blood. Peripheral blood mononuclear cells (PBMC) were separated from the buffy coats by density centrifugation on lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway), and erythrocytes were removed by incubation with Gey's lysis buffer. 1 × 108 PBMC were placed in a Petri dish (Ø 15 cm) for 1 h (37°C, 5% CO2, humidified atmosphere) and non-adherent cells were removed by vigorous washing with PBS. Adherent cells, which were monocytes with a purity of at least 85%, were cultured in RPMI 1640, supplemented with 2 mM l-glutamine, 100 mg·mL−1 penicillin/streptomycin, 12 mM HEPES and 5% v/v FCS (PAN-Biotech, Aidenbach, Germany; all other media components from Biochrom, Berlin, Germany) at 37°C in a humidified atmosphere containing 5% CO2. The monocytes were used within 24 h after isolation for the experiments.
Determination of pEC50 values
Monocytes were stimulated with different concentrations of histamine receptor agonists (10 nM–10 μM) for 24 h, the time shown in previous studies to be optimal for observing histamine-induced decrease in the production of cytokines of the IL-12 family on subsequent activation (Gutzmer et al., 2005; Gschwandtner et al., 2012). Then the cells were activated by addition of IFN-γ (200 ng·mL−1) and 2 h later LPS (50 ng·mL−1); this 2 h time gap was shown to be very effective in the induction of IL-12 (Wittmann et al., 1999) ). Supernatants were taken after 24 h and the secretion of IL-12p70 was measured by ELISA.
Histamine receptor blocking experiments
Blocking experiments were performed as described previously (Gschwandtner et al., 2012). Monocytes were treated with 10 μM of the receptor antagonists ranitidine and/or JNJ7777120. 30 min later monocytes were stimulated with histamine receptor agonists for 24 h, and then the cells were activated by addition of IFN-γ and 2 h later LPS. Supernatants were taken after 24 h and the secretion of IL-12p70 was measured by elisa.
Elisa
Cell free supernatants were taken after a total stimulation time of 48 h and the amount of IL-12p70 was determined with elisa according to the manufacturer's instructions (eBioscience, San Diego, CA, USA).
Statistical analysis
Data are represented as mean ± SEM and the program GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. For calculation of P-values, we used one-way anova with Bonferroni post test (Figures 1 and 3). The pEC50 values were calculated by non-linear regression analysis from dose-response curves (Figure 2). A P-value < 0.05 was regarded as significant.
Figure 1.
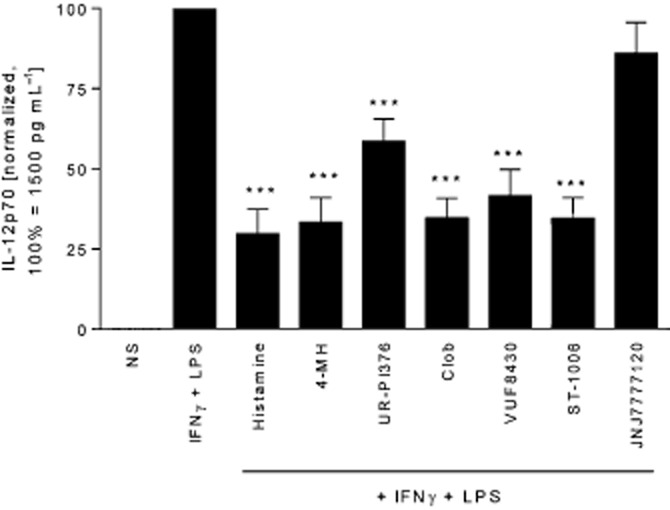
H4 receptor agonists down-regulate IL-12p70 production in human monocytes. Monocytes were stimulated for 24 h with histamine, followed by activation with IFN-γ and LPS for 24 h. The IL-12p70 content in the cell supernatant was determined by elisa. Histamine and the tested H4 receptor ligands decreased IFN-γ and LPS-induced IL-12p70, while JNJ7777120 did not affect IL-12p70 production. The absolute IL-12p70 production in IFN-γ and LPS stimulated monocytes was in the range of 70–5561 pg mL−1 with a mean of 1522 pg mL−1. Data shown are means ± SEM of eleven independent experiments. *P<0.05, significantly different from IFN-γ + LPS alone. 4-MH, 4-methylhistamine; Clob, clobenpropit.
Figure 3.
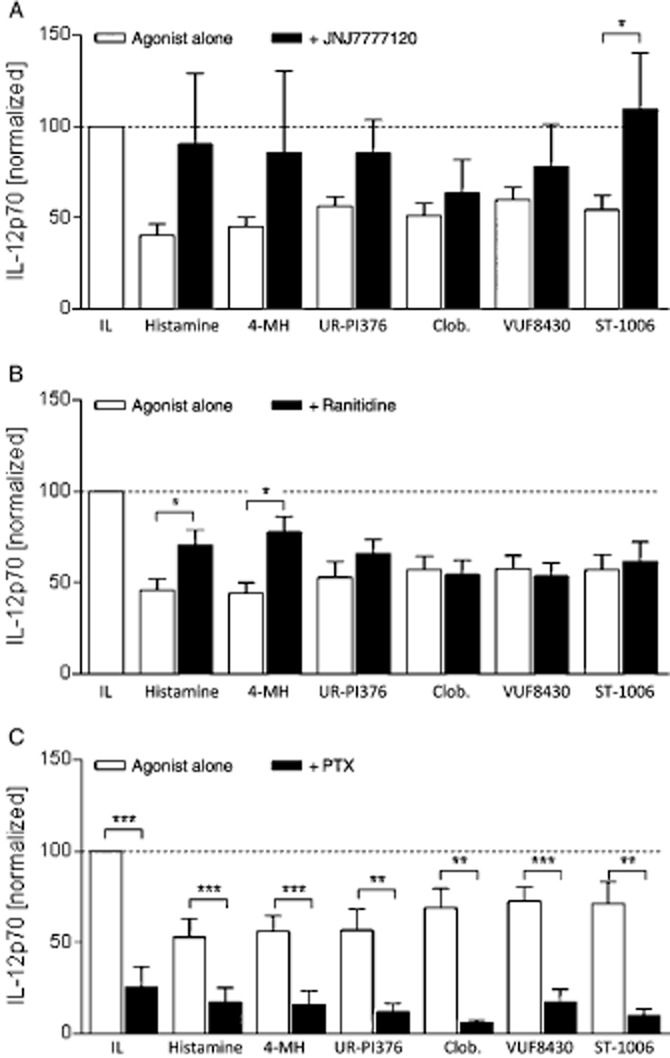
H4 receptor and H2 receptor antagonists and PTX have different actions on the effect of H4 receptor agonists. Monocytes were stimulated with 10 μM JNJ777120 (H4 receptor antagonist, A) or ranitidine (H2 receptor antagonist, B) for 30 min or with 100 ng mL−1 PTX for 20 min (C) and thereafter with H4 receptor agonists (10 μM) for 24 h, followed by activation with IFN-γ and LPS for 24 h. The IL-12p70 content in the cell supernatant was determined by elisa. The absolute IL-12p70 production in IFN-γ and LPS stimulated monocytes was in the range of 334–1236 pg mL−1 with a mean of 728 pg mL−1. Data shown are means ± SEM of 19, 16 and 7 independent experiments in a, b and c respectively. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from agonist alone. IL: IFN-γ and LPS; 4-MH, 4-methylhistamine; Clob, clobenpropit.
Figure 2.
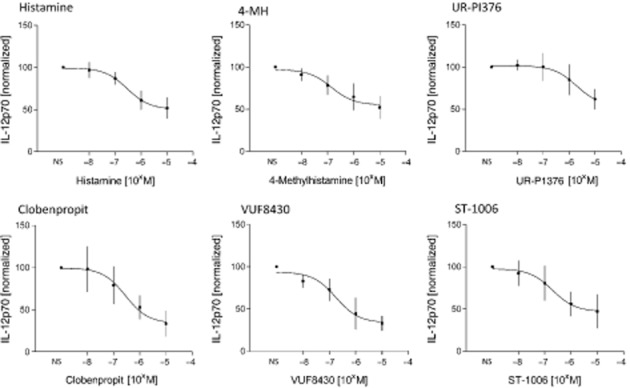
Dose titration and pEC50 determination for different H4 receptor agonists. Monocytes were stimulated for 24 h with H4 receptor agonists at the concentrations shown, followed by activation with IFN-γ and LPS for 24 h, and determination of IL-12p70 content in the cell supernatant by elisa. EC50 values are shown in Table 2 and were calculated by non-linear regression analysis from the sigmoidal dose response curves. The absolute IL-12p70 production in IFN-γ and LPS stimulated monocytes was in the range of 125–1697 pg mL−1 with a mean of 554 pg mL−1. Data shown are means ± SEM of six independent experiments. 4-MH, 4-methylhistamine.
Materials
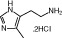
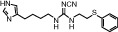
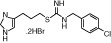
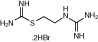
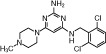
Various H4 receptor agonists were used in this study: histamine (Alk-Scherax, Wedel, Germany), 4-methylhistamine (Tocris Bioscience, Bristol, UK), described in (Lim et al., 2005)), UR-PI376 (Department of Pharmaceutical/Medicinal Chemistry, University, of Regensburg, Germany; (Igel et al. (2009)), clobenpropit (Sigma-Aldrich, Deisenhofen, Germany), described in Lim et al. (2005)), VUF8430 (Tocris Bioscience), described in Lim et al. (2006)) and ST-1006 (Institute of Pharmaceutical Chemistry, ZAFES/NeFF/OSF/CMP, Johann Wolfgang Goethe University Frankfurt/Main, Germany; (Sander et al., 2009)). Table 1 gives a summary of the chemical properties and characteristics of the H4 receptor agonists.
Table 1.
Properties of the tested H4 receptor ligands
| Histamine | 4-Methylhistamine | UR-PI376 | Clobenpropit | VUF8430 | ST-1006 | |
|---|---|---|---|---|---|---|
| Structure |  |
 |
 |
 |
 |
 |
| Molecular weight | 184 | 198 | 342 | 471 | 323 | 599 |
| Affinity pKi | 7.8 | 7.3 | - | 8.1 | 7.5 | 7.9 |
| pKi determination by | [3H]Histamine displacement assay on SK-N-MC cells stably expressing hH4R | [3H]Histamine displacement assay on SK-N-MC cells stably expressing hH4R | - | [3H]Histamine displacement assay on SK-N-MC cells stably expressing hH4R | [3H]Histamine displacement assay on SK-N-MC cells stably expressing hH4R | [3H]Histamine displacement on Sf9 cell membranes co-expressing hH4R, Gαi2 and Gβ1γ2 |
| Potency pEC50 | 7.7 | 7.4 | 7.5 | 7.7 | 7.3 | 8.95 |
| pEC50 determination by | Inhibition of 1 μM forskolin-induced CRE-β-galactosidase activity in SK-N-C/hH4R cells | Inhibition of 1 μM forskolin-induced CRE-β-galactosidase activity in SK-N-C/hH4R cells | Steady-state GTPase activity in Sf9 insect cell membranes expressing hH4R-RGS19 fusion protein + Giα2 + Gβ1γ2 | Inhibition of 1 μM forskolin-induced CRE-β-galactosidase activity in SK-N-C/hH4 cells | Inhibition of forskolin-induced cAMP-mediated increase in β-galactosidase activity | Binding assay with [35S]GTPγS / GTPase on membrane preparation of Sf9 cells expressing hH4R, co-expressed with Gαi2 and Gβ1γ2 subunits |
| Classification | Full agonist | Full agonist | - | Partial agonist | Full agonist | Partial agonist |
| Reference | Lim et al, 2005 | Lim et al, 2005 | Igel et al, 2009 | Lim et al, 2005 | Lim et al, 2006 | Sander et al, 2009 |
Chemical structure, molecular weight, affinity at the human H4 receptor (hH4R); potency at the human H4 receptor, classification and relevant literature reference for the tested H4 receptor agonists are given.
For blocking of histamine receptors, the following antagonists were used: ranitidine (selective H2 receptor antagonist; Biomol, Hamburg, Germany) and JNJ7777120 (selective H4 receptor antagonist, also shows low activity at H2 receptors (Jablonowski et al., 2003); Sigma-Aldrich). If not stated otherwise, histamine receptor ligands were used at a concentration of 10 μM. Other reagents for stimulation of human monocytes were used as follows: human recombinant IFN-γ (Peprotech Hamburg, Germany), LPS (Escherichia coli 055:B5; Sigma-Aldrich) and Pertussis toxin (PTX; Calbiochem, Darmstadt, Germany).
Results
All H4 receptor agonists tested down-regulate IL-12p70 production in monocytes
As described previously (Wittmann et al., 1999; Pflanz et al., 2002; Gschwandtner et al., 2012), stimulation of monocytes with sequential IFN-γ and LPS increased the secretion of IL-12 many-fold ( Figure 1). Here we used six different H4 receptor agonists (Table 1) for pre-stimulation of monocytes and evaluated the secretion of IL-12p70 in response to IFN-γ and LPS stimulation performed 24 h later. We used the ligands at a concentration known to induce effects in monocyte-derived dendritic cells, for some of the ligands, without inducing cellular toxicity (Gutzmer et al., 2005; Dijkstra et al., 2008). Pre-stimulation with all the tested H4 receptor agonists resulted in a significant decrease in IL-12p70 secretion, ranging from 40 to 70% suppression, depending on the agonist used. In contrast to the agonists, the H4 receptor antagonist JNJ7777120 (Thurmond et al., 2004) did not modulate the expression of IL-12p70 (Figure 1).
Differences in potency between the tested H4 receptor agonists
In order to calculate the potencies of the different H4 receptor agonists, we performed dose-response experiments over a range of concentrations (10 nM – 10 μM). The sigmoidal dose curves in Figure 2 showed that the different H4 receptor ligands exhibited different potencies, which were lower than the corresponding values determined in assays in transfected cell lines (Table 1 and Table 2).
Table 2.
Comparison of the properties of H4 receptor ligands in native human monocytes
| Histamine | 4-Methylhistamine | UR-PI376 | Clobenpropit | VUF8430 | ST-1006 | |
|---|---|---|---|---|---|---|
| IL-12 down-regulation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| pEC50 published | 7.7 ± 0.10 | 7.4 ± 0.10 | 7.5 ± 0.01 | 7.7 ± 0.10 | 7.3 ± 0.10 | 8.95 ± 0.29 |
| pEC50 determined in monocyte assay (see Figure 2 for data) | 6.5 ± 0.18 | 6.9 ± 0.24 | 5.7 ± 0.32 | 6.5 ± 0.28 | 6.8 ± 0.18 | 6.7 ± 0.29 |
| IL-12 down-regulation blocked by antagonism of | ||||||
| H4R | Partially | Partially | Partially | Partially | Partially | ✓ |
| H2R | Partially | Partially | X | X | X | X |
The potency of H4 receptor ligands with regard to IL-12p70 down-regulation and the modulation of their effect by H2 receptor (H2R) and H4 receptor (H4R) antagonists are shown.
Potencies are shown as pEC50 values and are expressed as means ± SEM.
Specificity of H4 receptor agonists
We used the established H4 receptor antagonist JNJ7777120 (Thurmond et al., 2004), to show specificity of the agonists for this histamine receptor. JNJ7777120 was used at 10 μM, a concentration shown previously to completely block cellular response to a H4 receptor agonist (Reher et al., 2012). The action of one out of six ligands – ST-1006 – was completely blocked by JNJ7777120 and IL-12p70 expression was around 100% (similar to the non-stimulated controls). In contrast, JNJ7777120 only partially blocked down-regulation of IL-12p70 induced by the other H4 receptor agonists tested (Figure 3A).
As some H4 receptor ligands are known to also bind to the H2 receptor (Sterk et al., 1986; Lim et al., 2005), we tested in addition the effect of the H2 receptor antagonist ranitidine (Daly et al., 1981) on the action of the H4 receptor ligands. Ranitidine partially blocked the effect of histamine and 4-methylhistamine and did not modulate the decreased secretion of IL-12p70 in response to UR-PI376, clobenpropit, VUF8430 and ST-1006 (Figure 3B). The antagonists alone did not modulate IL-12p70 production (data not shown).
To investigate the potential involvement of G–protein-independent signalling pathways, we performed experiments in the presence of PTX (100 ng·mL−1). IL-12p70 secretion from monocytes was significantly reduced by PTX alone (Figure 3c). In combination with the agonists, no further significant down-regulation of IL-12p70 was observed (Figure 3c). The concentration of PTX used in these experiments was not toxic to the cells, as the secretion of the chemokine CXCL10, as a control cytokine unrelated to IL-12, was not down-regulated by PTX (data not shown).
Discussion and conclusions
In the present study we compared the potencies of different H4 receptor agonists on a robust H4 receptor effect in freshly isolated, native human monocytes. All the tested agonists induced down-regulation of IL-12p70 secretion from monocytes in a magnitude similar to the endogenous ligand histamine. However, we found lower values for their potency (pEC50), compared with those from assays in transfected cell lines, performed with these agonists (Table 2). Plasma protein binding capacity of the ligands was not responsible for the discrepancy in potency between the GTPγS assay and monocyte assay (A. Buschauer, pers. comm.). Moreover, the observation was made for all tested H4 receptor agonists as well as for histamine, indicating that the lower potency values did not depend on the nature/structure of the various H4 receptor ligands, but probably resulted from differences between the present native test system, and that in the transfected cell lines used for the primary evaluation of the compounds (Lim et al., 2005; Igel et al., 2009; Sander et al., 2009). Possible differences in the test systems that might explain the variation in potency are:
The cell types used in the earlier studies were either derived from neoplastic cells (Lim et al., 2005) or represent insect cell lines (Igel et al., 2009; Sander et al., 2009). These cell lines are per se different from native human cells with regard to their characteristics, e.g. expression of different proteins, changed proliferative potential and growth at lower temperatures (especially insect cells).
Some pharmacological testing was performed with cell membranes (Igel et al., 2009) and not on intact cells. Clearly with cell membrane preparations, some potentially relevant influences present in the whole cell might be lost, especially in terms of the signalling cascade. This cascade is terminated at the membrane-bound G-proteins and cannot continue all the way into the nucleus in order to reach the relevant targets, as in whole cells.
The cell lines used in the earlier assays were usually selectively transfected to express high levels of one type of receptor and a limited range of G-protein subtypes. In contrast, native cells express lower levels of the desired receptor and in addition they express other types of receptors, as well as signalling components that might interfere with binding of a compound to the desired receptor.
Interestingly, the difference between the potency values obtained from our native test system in comparison with the cell-line assays is larger for H4 receptor agonists tested in Sf9 insect cells (UR-PI376 and ST-1006) than for other agonists tested in SK-N-MC cells. This difference might be explained by the fact that in the insect cell system, additional G-protein and regulators of G-protein signalling (RGS) fusions are required, which probably result in a further over-estimation of ligand potency. Therefore, when choosing a cellular system for functional assays one should always use a system with as few modifications as possible, relative to the native cells. In general, cell lines used for pharmacological assays represent an ideal model system, which helps in the initial characterization of pharmacologically active compounds. However, the results derived from these cells probably cannot be transferred without modification to native cells.
Recently another study investigated the effects of UR-PI376 on human native eosinophils (Reher et al., 2012). In this study, the potency of the H4 receptor agonist varied with the assay used. Thus for chemotaxis an EC50 of 8 nM (pEC50 of 8.1) was measured, a potency higher than that derived from the cell-line assays (pEC50 of 7.5) whereas in a Ca2+ influx assay, an EC50 of 320 nM (pEC50 of 6.5) was determined. In the same Ca2+ influx assay, histamine was also tested and exhibited a EC50 of 1 μM (pEC50 of 6.0). These potency values obtained from the Ca2+ influx assay are in the same range as the values determined in our monocyte assay. This study together with ours shows that the potency values of agonists in different native cellular systems are in a similar range, although there is, of course, some variation depending on the cellular context. Moreover, it has to be noted that compared with cell-line assays, the variation of results between different experiments is greater when working with native cells, due to the individual variations between the different human donors, whose blood was used for isolation of the monocytes.
Another important observation from the comparison of the two studies is that the potency seems to be similar for a readout that is measured within seconds after receptor stimulation (i.e. Ca2+ influx, pEC50 of 6.0 for histamine (Reher et al., 2012) ) and for a readout that is measured 48 h after receptor stimulation (i.e. IL-12p70 secretion measured in this study, pEC50 of 6.5 for histamine).
Based on the cell-line assays, the H4 receptor ligands were classified either as partial agonists (clobenpropit and ST-1006 (Lim et al., 2005; Sander et al., 2009) ) or as full agonists (4-methylhistamine and VUF8430 (Lim et al., 2005; 2006) ). This classification is based on the magnitude of their effect in relation to histamine, which is defined as a full agonist with maximum effectiveness. According to our assays, we would classify UR-PI376 as a partial agonist, because its maximum effect is half the effect of histamine. The other H4 receptor agonists tested show a slightly lower response than that of histamine, but are still comparable in effectiveness. The classification as partial or full agonist might be dependent on the readout system and probably is different in biological assays, where a submaximal activation of G-protein signalling by a ligand might still result in a full response in the final readout, such as cytokine secretion.
Some of the ligands that we used in our assays were shown to have also affinities to and activities at the histamine H2 receptor, such as 4-methylhistamine (Lim et al., 2005), VUF8430 (Sterk et al., 1986) and, of course, the endogenous ligand histamine, which binds to all histamine receptors. This is of particular importance because the H2 receptor is also involved in the down-regulation of IL-12p70 secretion from monocytes by histamine (Gutzmer et al., 2005). Therefore, we tested in our assay the effect of the H4 receptor antagonist JNJ7777120 (Thurmond et al., 2004) and the H2 receptor antagonist ranitidine (Daly et al., 1981) on the action of the agonists. The action of histamine and 4-methylhistamine was partially blocked by both the H4 receptor and the H2 receptor antagonist, whereas the effect of VUF8430 was partially blocked by the H4 receptor antagonist, but not by the H2 receptor antagonist. These results confirm that these substances have binding potential at both the H2 and H4 receptors.
Recently a study suggested that H4 receptor signalling can also proceed in a G-protein independent manner by the recruitment of β-arrestin to the H4 receptor, potentially initiating a signalling cascade, distinct from the G-protein mediated pathway (Rosethorn and Charlton, 2011). However, it is technically difficult to analyze β-arrestin recruitment in native primary cells. As an alternative approach, we performed experiments in presence of the G-protein uncoupling agent PTX. However, we observed that PTX alone down-regulated IL-12p70 production in monocytes, as has already been described (Bagley et al., 2002; Spensieri et al., 2006). Combination of PTX together with the H4 receptor agonists did not result in any greater inhibition of the secretion of IL-12p70, compared with PTX alone. Because of these difficulties when working with PTX with IL-12p70 as a readout, we can still only speculate that apart from Gi/o-dependent signalling, an alternative Gi/o-independent pathway might also be involved in histamine-mediated down-regulation of IL-12p70 in monocytes.
As shown by this study as well as others (Reher et al., 2012) there might be some paradoxical effects of H4 receptor ligands in native cells, which should be kept in mind. As already suggested (Seifert et al., 2011), the open and unbiased analysis of the effects of H4 receptor ligands in recombinant as well as native systems is necessary to fully understand H4 receptor signalling and the pathophysiological function of this histamine receptor. Moreover, an important aim in the future should be the development and functional characterization of H4 receptor ligands in systems closer to native cells and tissues. This would greatly improve the characterization of H4 receptor ligands as well as the understanding of the biological relevance of the H4 receptor in various tissues and diseases.
Acknowledgments
We thank Prof. Armin Buschauer from the Department of Pharmaceutical and Medicinal Chemistry II at the University of Regensburg, Germany, for critical discussions and for sharing his knowledge on H4 receptor ligand binding to plasma proteins. This study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG): Gu434/5-2 (RG and TW), the Austrian Science Fund (FWF): T545-B19 (MG) and the project Translational Research Innovation – Pharma (TRIP) (HS). We acknowledge the support from the European Community COST Action BM0806 (Recent Advances in histamine H4 receptor research).
Glossary
- PTX
Pertussis toxin
Conflicts of interest
The authors declare no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J Leukoc Biol. 2002;72:962–969. [PubMed] [Google Scholar]
- Daly MJ, Humphray JM, Stables R. Some in vitro and in vivo actions of the new histamine H2-receptor antagonist, ranitidine. Br J Pharmacol. 1981;72:49–54. doi: 10.1111/j.1476-5381.1981.tb09103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra D, Stark H, Chazot PL, Shenton FC, Leurs R, Werfel T, et al. Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol. 2008;128:1696–1703. doi: 10.1038/sj.jid.5701250. [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Smits RA, Leurs R, Haaksma E, de Esch IJ. A new generation of anti-histamines: histamine H4 receptor antagonists on their way to the clinic. Curr Opin Drug Discov Devel. 2009;12:628–643. [PubMed] [Google Scholar]
- Gschwandtner M, Bunk H, Kother B, Thurmond RL, Kietzmann M, Werfel T, et al. Histamine down-regulates IL-27 production in antigen-presenting cells. J Leukoc Biol. 2012;92:21–29. doi: 10.1189/jlb.0111017. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittmann M, et al. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Gschwandtner M, Rossbach K, Mommert S, Werfel T, Kietzmann M, et al. Pathogenetic and therapeutic implications of the histamine H4 receptor in inflammatory skin diseases and pruritus. Front Biosci (Schol Ed) 2011;3:985–994. doi: 10.2741/203. [DOI] [PubMed] [Google Scholar]
- Igel P, Geyer R, Strasser A, Dove S, Seifert R, Buschauer A. Synthesis and structure-activity relationships of cyanoguanidine-type and structurally related histamine H4 receptor agonists. J Med Chem. 2009;52:6297–6313. doi: 10.1021/jm900526h. [DOI] [PubMed] [Google Scholar]
- Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK, et al. The first potente and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- Kiss R, Keseru GM. Histamine H4 receptor ligands and their potential therapeutic applications: an update. Expert Opin Ther Pat. 2012;22:205–221. doi: 10.1517/13543776.2012.665447. [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Lim HD, Smits RA, Bakker RA, van Dam CM, de Esch IJ, Leurs R. Discovery of S-(2-guanidylethyl)-isothiourea (VUF 8430) as a potent nonimidazole histamine H4 receptor agonist. J Med Chem. 2006;49:6650–6651. doi: 10.1021/jm060880d. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279:615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Reher TM, Neumann D, Buschauer A, Seifert R. Incomplete activation of human eosinophils via the histamine H4-receptor: evidence for ligand-specific receptor conformations. Biochem Pharmacol. 2012;84:192–203. doi: 10.1016/j.bcp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Rosethorne EM, Charlton SJ. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- Sander K, Kottke T, Tanrikulu Y, Proschak E, Weizel L, Schneider EH, et al. 2,4-Diaminopyrimidines as histamine H4 receptor ligands – scaffold optimization and pharmacological characterization. Bioorg Med Chem. 2009;17:7186–7196. doi: 10.1016/j.bmc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- Seifert R, Schneider EH, Dove S, Brunskole I, Neumann D, Strasser A, et al. Paradoxical stimulatory effects of the ‘standard’ histamine H4-receptor antagonist JNJ7777120: the H4 receptor joins the club of 7 transmembrane domain receptors exhibiting functional selectivity. Mol Pharmacol. 2011;79:631–638. doi: 10.1124/mol.111.071266. [DOI] [PubMed] [Google Scholar]
- Spensieri F, Fedele G, Fazio C, Nasso M, Stefanelli P, Mastrantonio P, et al. Bordetella pertussis inhibition of interleukin-12 (IL-12) p70 in human monocyte-derived dendritic cells blocks IL-12 p35 through adenylate cyclase toxin-dependent cyclic AMP induction. Infect Immun. 2006;74:2831–2838. doi: 10.1128/IAI.74.5.2831-2838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk GJ, van der Goot H, Timmerman H. The influence of guanidino and isothiourea groups in histaminergic compounds on H2-activity. Agents Actions. 1986;18:137–140. doi: 10.1007/BF01988004. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Zwirner J, Larsson VA, Kirchhoff K, Begemann G, Kapp A, et al. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–6769. [PubMed] [Google Scholar]


