Abstract
Background and Purpose
Conflicting data have been published on whether histamine is inhibitory to the rewarding effects of abused drugs. The purpose of this study was to clarify the role of neuronal histamine and, in particular, H3 receptors in alcohol dependence-related behaviours, which represent the addictive effects of alcohol.
Experimental Approach
Alcohol-induced conditioned place preference (alcohol-CPP) was used to measure alcohol reward. Alcohol-induced locomotor stimulation, alcohol consumption and kinetics were also assessed. mRNA levels were quantified using radioactive in situ hybridization.
Key Results
Low doses of H3 receptor antagonists, JNJ-10181457 and JNJ-39220675, inhibited alcohol reward in wild-type (WT) mice. However, these H3 receptor antagonists did not inhibit alcohol reward in histidine decarboxylase knock-out (HDC KO) mice and a lack of histamine did not alter alcohol consumption. Thus H3 receptor antagonists inhibited alcohol reward in a histamine-dependent manner. Furthermore, WT and HDC KO mice were similarly stimulated by alcohol. The expression levels of dopamine D1 and D2 receptors, STEP61 and DARPP-32 mRNA in striatal subregions were unaltered in HDC KO mice. No differences were seen in alcohol kinetics in HDC KO compared to WT control animals. In addition, JNJ-39220675 had no effect on alcohol kinetics in WT mice.
Conclusions and Implications
These data suggest that histamine is required for the H3 receptor-mediated inhibition of alcohol-CPP and support the hypothesis that the brain histaminergic system has an inhibitory role in alcohol reward. Increasing neuronal histamine release via H3 receptor blockade could therefore be a novel way of treating alcohol dependence.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: histamine, alcohol, reward, H3 receptor antagonist, dopamine
Introduction
Neuronal histamine is important in several physiological and behavioural functions including sleep-wake cycle, feeding behaviour and cognition (Monnier et al., 1967; Sakata et al., 1988; Cacabelos et al., 1989; Haas and Panula, 2003). The histaminergic system is altered in several CNS disorders, such as Alzheimer's disease (Mazurkiewicz-Kwilecki and Nsonwah, 1989; Airaksinen et al., 1991a,b; Panula et al., 1998), schizophrenia (Nakai et al., 1991; Prell et al., 1995), Parkinson's disease (Anichtchik et al., 2000; 2001; Rinne et al., 2002) and Tourette syndrome (Ercan-Sencicek et al., 2010; Fernandez et al., 2012) highlighting the important modulatory role of histamine in the brain. Early studies have also established that the brain histaminergic system is involved in the regulation of reward (Olds and Milner, 1954) and behaviours related to addictive drugs (Henwood and Mazurikiewicz-Kwilecki, 1975; Wong, 1975; Mazurkiewicz-Kwilecki and Henwood, 1976) but the underlying mechanisms are yet to be discovered.
There is some evidence that the concentrations of histamine and its metabolite tele-methylhistamine are elevated in cortical grey matter of alcoholics compared to non-alcoholics (Alakarppa et al., 2002; 2003), but most studies on the role of histamine in alcohol dependence have been performed in rodents. Brain histamine and tele-methylhistamine levels are elevated in an alcohol-preferring ALKO alcohol (AA) rat line compared with the alcohol non-preferring ALKO non-alcohol (ANA) line. The AA rats also express lower levels of histamine H3 receptor radioligand binding in the brain than ANA rats (Lintunen et al., 2001). It was also found that H3 receptor antagonists decreased alcohol drinking in a dose-dependent manner. These studies suggested an association between an altered histaminergic system and a genetic predisposition to high alcohol preference.
Previously, we showed that mice lacking histamine (histidine decarboxylase knock-out; HDC KO) display stronger alcohol-induced conditioned place preference (Alcohol-CPP; Nuutinen et al., 2010), further supporting the inhibitory role of histamine in reward. In the present study, we also determined whether this stronger alcohol reward is due to alterations in dopamine signalling. The expression levels of dopamine receptors D1 and D2, striatal-enriched phosphatase 61 (STEP61) and dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) mRNA were analysed as important components of the dopamine-driven reward system.
Our previous studies demonstrated that both pharmacological blockade and genetic knock out of the H3 receptor lead to diminished alcohol reward, consumption and stimulation in mice (Nuutinen et al., 2011a,b). In alcohol-preferring rats, the H3 receptor antagonist JNJ-39220675 dose-dependently reduces both alcohol intake and preference (Galici et al., 2011), but the role of histamine in these findings was unclear. Hence, the aim of this study was to reveal the role of histamine in alcohol drinking and reward.
Methods
Animals
Inbred HDC KO mice and wild-type (WT) 129/Sv mice were used in a two-bottle choice test, locomotor stimulation, radioactive in situ hybridization and in plasma ethanol concentration measurements. HDC KO mice were used in conditioned place preference (CPP). After being backcrossed to the C57BL/6J background strain, HDC KO and WT mice were used in the drinking in the dark (DID) paradigm. The generation of HDC gene deletion has been described previously (Ohtsu et al., 2001). HDC KO mice were bred in heterozygous crosses and genotypes verified by PCR amplification. Male inbred WT JAX®DBA/2J mice were used in the CPP, locomotor stimulation tests and later for plasma ethanol concentration measurements. Mice were delivered from Charles River (France) at the age of 6–8 weeks. HDC KO and WT mice were naive to drug treatments in the alcohol drinking, CPP, locomotor activation and in situ hybridization experiments. DBA/2J mice used in the CPP tests were later used in the locomotor activity study after a 2-week break. The total number of animals used in these studies was 396. Animals were group-housed, except for the drinking paradigms where mice were housed singly. Standard food pellets (Scanbur, Sweden) and water were available ad libitum. Animal rooms were maintained on a 12–12 h light–dark cycle (lights on at 06 h). Temperature and humidity were controlled at 20 ± 1°C and 50 ± 10%, respectively. Behavioural experiments were carried out between 07 h and 13 h. The principles of the Finnish Act on the Use of Animals for Experimental Purposes were followed and all protocols were approved by the Animal Experiment Committee of the State Provincial Office of Southern Finland and by the Institutional Animal Care and Use Committee of Abo Akademi University. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Drug treatments
Alcohol drinking solutions were prepared from 99.5% alcohol (Altia, Rajamäki, Finland) and diluted to 3–20% solutions (v v-1) using tap water. Saccharin (0.033 and 0.066% w v-1), quinine (15 and 30 μM) and sucrose (3%, w v-1) were dissolved in tap water. Injected drugs including alcohol [10–20% (w v-1)], ciproxifan hydrochloride (Sigma-Aldrich, St Louis, MO, USA), JNJ-10181457 and JNJ-39220675 (both from Johnson & Johnson Pharmaceutical Research & Development, L.L.C., La Jolla, CA, USA; Bonaventure et al., 2007; Galici et al., 2009; 2011) were diluted with sterile 0.9% saline. All drug doses correspond to free bases. Injections were given i.p.
Two-bottle choice alcohol drinking
To measure alcohol self-administration and preference, animals (HDC KO and WT controls in 129/Sv background strain) were housed singly and trained to drink in a two-bottle choice procedure. First, mice were habituated to two water bottles for 1 week. They were then given access to both water and alcohol for 24 h. To avoid side preference, the positions of the bottles were alternated daily. Alcohol concentration was elevated every 14th day, increasing from 3 to 6 to 10 and finally to 20% (v v-1). Average alcohol consumption per body weight per day (g·kg−1·day−1) was calculated taking into account the density of alcohol (0.7894 g·L−1). The potential differences in taste preference were also examined. The same mice were tested for sweet saccharin and bitter quinine solution intake and preference by providing first 0.033 and then 0.066% saccharin in addition to water for 1 week. After a recovery of 1 week, the mice were given first 15 and then 30 μM quinine solutions in addition to water, both concentrations for 1 week. Throughout the experiment, fluid intake and body weight were monitored. Relative alcohol, saccharine or quinine preference was calculated (alcohol/total fluid consumption) at each concentration.
DID
Alcohol consumption in HDC KO and WT mice (C57BL/6J background) was studied using the DID procedure with minor modifications (Rhodes et al., 2005). The light–dark cycle was reversed 2 weeks before the experiment and the mice were housed singly for 1 week before the beginning of the experiment. In brief, 3 h after the beginning of the dark period, water bottles were replaced with a graduated tube containing 20% (v v-1) alcohol and left in place for 4 h. Control animals received 3% (w v-1) sucrose. The volume of alcohol and sucrose consumed was recorded after each drinking session.
Alcohol-CPP
The CPP paradigm was used as described previously (Nuutinen et al., 2010) and it followed the principles of an unbiased, fully counterbalanced conditioning schedule (Cunningham et al., 2006). Metal grid and plastic mat were used as tactile conditioning cues on the cage floors. The activity of the mice was recorded in each phase using a video camera attached to Ethovision Color-Pro 3.0 video-tracking software (Noldus Information Technology, Wageningen, The Netherlands). The trial consisted of three phases:
Habituation (day 1): animals were weighed and given a saline injection just before being placed in the centre of the empty conditioning cage without the conditioning cues for 5 min.
Conditionings (days 2–9): mice were randomly assigned to one of the two conditioning subgroups (metal or plastic cue). Mice in the metal cue subgroup received alcohol (2 g·kg−1, i.p.) paired with metal floor and saline paired with the plastic floor on alternating days. The pretreatment with H3 receptor ligands (ciproxifan, JNJ-10181457 or JNJ-39220675) was administered (i.p.) 30 min before the alcohol treatment. Mice in the plastic cue group received alcohol paired with the plastic floor and saline paired with the metal floor. Each mouse went through four conditioning trials (5 min) of both types on alternating days.
Place preference test (day 10): the place preference test was carried out 24 h after the last conditioning session; immediately after a saline injection mice were placed in the centre of the cage with both floor materials (half metal/half plastic cue). Time spent during a period of 15 min on different zones (metal or plastic cue) of the cage and the total distance moved were recorded. Time spent on the metal floor was used as a primary dependent variable in data analysis.
Alcohol stimulation
To investigate whether H3 receptor antagonists affect alcohol-induced stimulation of locomotor activity, we used ciproxifan and JNJ-39220675 (Kathmann et al., 1998; Letavic et al., 2010). Before the experiment, mice were placed in the plastic test cages for 60–90 min to let them habituate to the new environment. After habituation, animals were pretreated with a H3 receptor antagonist, after which they were immediately put back in the test cage. Alcohol injection (1.0 or 1.5 g·kg−1, i.p.) was given 30 min after the pretreatment. The activity of the mice was recorded using a video camera and Ethovision software.
Quantitative radioactive in situ hybridization
The method used was as described previously (Lintunen et al., 1998). Several 16 μm cryostat sections were cut from unfixed, freshly frozen HDC KO and WT mouse brains and kept at −80°C until the hybridization. Selective and specific oligonucleotide probes designed for mouse dopamine D1 and D2 receptors, striatal-enriched protein 61 (STEP61) and dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) were used to quantify the expression of their mRNAs. The length of the probes was 43 bases and the nucleotide sequences were as follows: D1 receptor (ATGGACTGCTGCCCTCTCCAAAGCTGAGATGCGCCGGATTTGC), D2 receptor (GCTTTCTTCTCCTTCTGCTGGGAGAGCTTCCTGCGGCTCATCG), STEP61 (AGGTATTCATGGGCTGACTCCTCTCGTGGGGACACCAGGTAGC) and DARPP-32 (two sequences were combined: CCACACTCACTGGCGATCCCCGGATGTCAACTTCTGTCAGACC and GCTGGCTCCTTGGGAATCCAGTGGTAGCATGTGGGCTGAAAGG). The probes were labelled with radioactive deoxyATP ([33P]-dATP) (Perkin Elmer, Boston, MA, USA) at their 3′-ends by using terminal deoxynucleotidyl transferase (Promega, Madison, WI, USA) and purified with Sephadex G-50 columns (Roche, Mannheim, Germany). Sections were covered with the hybridization mixture containing 10 000 000 cpm·mL−1 of the labelled probe, 10 μL·mL−1 of denatured single-strand salmon sperm DNA, 50 μL·mL−1 of tRNA and 94 μL·mL−1 hybridization solution [50% of deionized formamide, 4 X standard sodium citrate (SSC; 0.6 M sodium chloride, 0.06 M sodiumcitrate), 1 X Denhardt's solution, 1% sarcosyl, 0.02 M sodium phosphate and 10% dextran sulphate]. Hybridization was carried out at 45°C for 16 h and thereafter the slides were washed with 1 x SSC at 55°C. Next, the sections were dehydrated in a series of ethanol (60, 80 and 100%). Dried sections were then exposed to Kodak Biomax-MR films (Perkin Elmer, New York, NY, USA) for 5–7 days. Films were developed with a Kodak X-omat 1000 Processor and quantified with MCID4 Image Analysis Software. The person doing the analysis was blinded to the genotype of the mouse. Cresyl violet staining was carried out in order to select matching striatal sections (+0.98 mm from bregma).
Plasma alcohol concentration measurements
Plasma alcohol concentrations were determined in DBA/2J, HDC KO and WT control mice after acute alcohol administration (2.0 g·kg−1, at 10, 20, 100 and 150 min, i.p.). Terminal blood samples were collected via cardiac puncture immediately after the mice had been killed with CO2. Blood samples were transferred to cold lithium heparin 12.5 IU tubes (Terumo, Capiject, Leuven, Belgium) and centrifuged at 2000 g for 2 min. Plasma was transferred to microcentrifuge tubes and kept at −80°C. A commercial enzyme-based assay (Abcam, ab65343, Cambridge, UK) was used to measure the alcohol concentration.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4 statistical software. After two-way anova, one-way anova was used in the analysis of CPP studies. Repeated-measures (RM) anova was used for drinking paradigms and locomotor stimulation studies. Alcohol kinetics was assessed using a regular two-way anova. Student's two-tailed t-test was used to analyse data from the in situ hybridization. Values exceeding more than two SD from the group mean were excluded.
The nomenclature used for the receptors conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Results
Low doses of JNJ-10181457 and JNJ-39220675 inhibit alcohol-CPP in DBA/2J mice
The unbiased CPP paradigm was applied in order to determine whether the H3 receptor antagonists JNJ-10181457 and JNJ-39220675 affect alcohol-CPP. Data were first analysed by two-way anova in which there was no interaction between the two factors (cue and treatment), which allowed further analysis with one-way anova. The difference between the time spent on the metal and the plastic cue during the place preference test was significant in the control group (saline administered before alcohol) (P < 0.01, one-way anova, Tukey's post test, Figure 1A) indicative of the development of alcohol-induced place preference (Cunningham et al., 2006). Low doses of JNJ-10181457 (5 mg·kg−1) and JNJ-39220675 (0.3 mg·kg−1) inhibited alcohol-CPP as indicated by the lack of difference in the time spent on the two floor materials during the preference test (P > 0.05, Figure 1A). The inhibition of alcohol-CPP was substantially more robust with JNJ-39220675 (0.3 mg·kg−1) than with JNJ-10181457 (5 mg·kg−1). Interestingly, a higher dose of JNJ-10181457 (10 mg·kg−1) and two higher doses (3 and 10 mg·kg−1) of JNJ-39220675 had no effect on the development of CPP as indicated by the significant difference between the two subgroups on the time spent on the metal floor during preference test (P < 0.05–0.01 one-way anova, Tukey's post test, Figure 1A). The activity of mice, measured as distance moved during the 15 min preference test, was not significantly different between any of the treatment groups [1490 ± 520–1780 ± 260 cm (mean± SEM)]. Pretreatment with the H3 receptor antagonists had no effect on alcohol stimulation during the conditioning sessions (data not shown).
Figure 1.
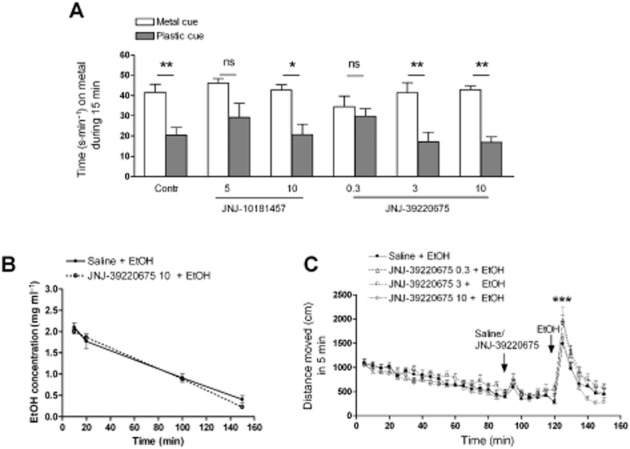
Only low doses of H3 receptor antagonists JNJ-10181457 and JNJ-39220675 inhibit alcohol-induced conditioned place preference (alcohol-CPP) in DBA/2J male mice. Mice develop alcohol-CPP (Contr), which is inhibited by a pretreatment with JNJ-10181457 (5 mg·kg−1, i.p.) or JNJ-39220675 (0.3 mg·kg−1) (A). Higher doses of JNJ-10181457 (10 mg·kg−1) and JNJ-39220675 (3 mg·kg−1 and 10 mg·kg−1) had no effect on alcohol-CPP. Columns indicate the subgroup that received alcohol paired with the metal floor and the subgroup that received alcohol paired with the plastic floor. Place preference is confirmed by the significant difference between the two subgroups of each conditioning group. n = 8–10 per subgroup **P > 0.01, *P > 0.05, ns P > 0.05, one-way anova. Data are expressed as mean time spent (s·min−1 ± SEM) on the metal cue side. H3 receptor antagonist JNJ-39220675 (10 mg·kg−1, i.p.) has no effect on plasma alcohol concentration at any measured time point (10, 20 100 and 150 min after 2 g·kg−1 alcohol injection) (B). n = 3–5 per group. P > 0.05, two-way anova. Data are expressed as mean ± SEM. JNJ-39220675 does not alter alcohol-induced locomotor stimulation (C). Pretreatment with saline or JNJ-39220675 (0.3, 3 or 10 mg·kg−1, i.p.) was given after a 90-min habituation period and 30 min before alcohol injection (1.0 g·kg−1, i.p.). Alcohol induces significant locomotor activation regardless of the JNJ-39220675 pretreatment; n = 13 per group. ***P < 0.0001, two-way RM anova. Data are expressed as mean ± SEM.
Alcohol kinetics and locomotor stimulation
Plasma alcohol concentrations were measured 10, 20, 100 and 150 min after alcohol (2 g·kg−1, i.p.) administration. Pretreatment with JNJ-39220675 (10 mg·kg−1, i.p.) had no effect on plasma alcohol concentrations (Figure 1B) confirmed by the lack of significant treatment effect by two-way anova (P > 0.05). JNJ-39220675 pretreatment (0.3, 3 and 10 mg·kg−1) had no effect on the stimulating effect of alcohol (Figure 1C). All groups were stimulated by alcohol which was confirmed by a significant time effect (F29,1479 = 54, P < 0.0001, two-way RM anova). Pretreatments with JNJ-39220675 did not alter alcohol stimulation, confirmed by the lack of significant treatment effect (F3,1479 = 1.88, P = 0.15, two-way RM anova).
H3 receptor antagonists have no effect on alcohol-CPP in HDC KO mice
The role of histamine in alcohol-CPP was tested using HDC KO mice. The H3 receptor antagonists (ciproxifan, JNJ-10181457 and JNJ-39220675) did not inhibit alcohol reward, as indicated by the significant difference between the time spent on the metal and the plastic floor during the place preference test in each group (P < 0.05–0.001, one-way anova, Tukey's post test, Figure 2A), which demonstrates the development of place preference (Cunningham et al., 2006). The activity of mice, measured as distance moved during the 15 min preference test, was similar between all treatment groups (1010 ± 370–1250 ± 170 cm, mean ± SEM). Pretreatments with the H3 receptor antagonists had no effect on alcohol stimulation during the conditioning sessions (data not shown).
Figure 2.
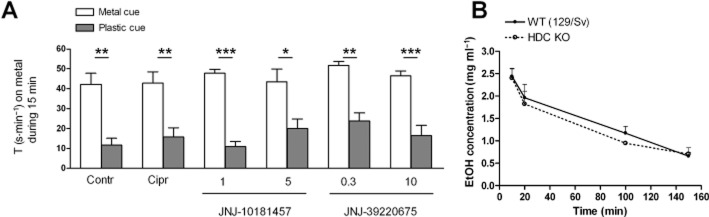
Alcohol-induced conditioned place preference in histidine decarboxylase knock-out (HDC KO) mice. HDC KO mice develop alcohol-CPP (Contr) which was unaffected by H3 receptor antagonist pretreatments (Cipr – ciproxifan 3 mg·kg−1, JNJ-10181457 1 or 5 mg·kg−1, JNJ-39220675 0.3 or 10 mg·kg−1) (A). Data are expressed as mean time spent (s·min−1 ± SEM) on the metal cue side. Columns indicate the subgroup that received alcohol paired with the metal floor and the subgroup that received alcohol paired with the plastic floor; n = 5–8 per subgroup. Place preference is confirmed by the significant difference between the two subgroups of each conditioning group ***P < 0.001, **P < 0.01 and *P < 0.05, one-way anova. Alcohol concentration in the mouse plasma in HDC KO and wild-type (WT) (129/Sv) control mice (B). Blood was collected 10, 20 100 and 150 min after the alcohol injection (2 g·kg−1, i.p) No differences were detected between the genotypes; n = 3–5. P > 0.05, two-way anova. Data are expressed as mean ± SEM.
HDC gene deletion does not affect alcohol kinetics
Plasma alcohol concentrations were measured 10, 20, 100 and 150 min after alcohol (2 g·kg−1, i.p.) administration. No differences in alcohol kinetics were detected between the HDC KO and WT 129/Sv mice (Figure 2B), verified by the lack of significant genotype effect by two-way anova (P > 0.05).
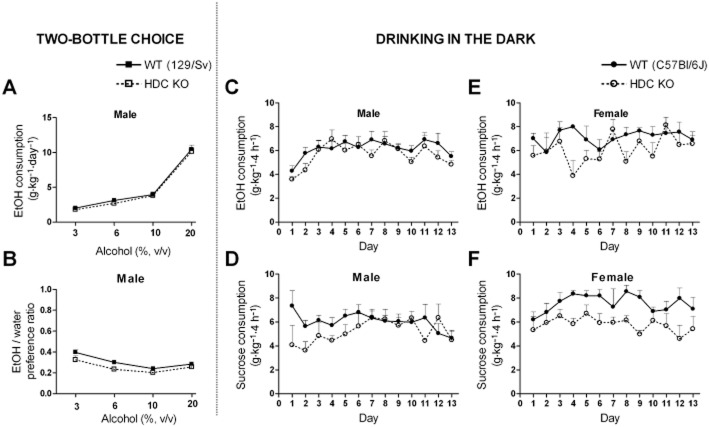
Voluntary or binge-like alcohol consumption are not affected by the lack of histamine
In the voluntary two-bottle choice paradigm where male 129/Sv mice could freely select to drink either from tap water or alcohol solution (3, 6, 10, or 20%, v v-1) bottle, HDC KO mice consumed as much alcohol (g·kg−1·day−1) as the WT control mice (Figure 3A), confirmed by the lack of genotype effect in the RM two-way anova (F1,78 = 1.06, P = 0.31, Figure 3A). Alcohol preference ratio (alcohol/total fluid consumption) was slightly different between the genotypes (F1,78 = 7.55, P = 0.011, Figure 3B). HDC KO mice had a tendency to drink more water throughout the trial, but this was not statistically different (data not shown). There was no significant difference between the genotypes in food consumption, preference for saccharine or aversion for quinine solutions (data not shown).
Figure 3.
Alcohol consumption of histidine decarboxylase knock-out (HDC KO) and wild-type (WT) mice in two-bottle choice and in the drinking in the dark experiments. In the two-bottle choice test, no differences were observed between male HDC KO and WT mice (in 129/Sv background strain) in total alcohol consumption (A) or water-alcohol preference ratio (B). P > 0.05, two-way repeated measures (RM) anova. n = 13–15 per genotype. In the drinking in the dark no differences were observed between male HDC KO and WT mice (in C57BL/6J background strain) in total alcohol (20%, v v-1, n = 7–8) (C) or sucrose (3%, w v-1, n = 5–7) (D) consumption. In female mice, no difference was observed in alcohol (20%, v v-1, n = 4–5) consumption (E) but HDC KO mice consumed less sucrose (3%, w v-1).P = 0.017, two-way RM anova, n = 4–6. (F). All data are expressed as mean ± SEM.
In the DID protocol, the consumption of 20% (v v-1) alcohol of WT (C57BL/6J) and HDC KO mice was measured for 13 days and no differences in consumption were observed either in male (F1,63 = 1.63, P = 0.23, Figure 3C) or female (F1,84 = 3.19, P = 0.12, Figure 3E) mice confirmed by the lack of significant genotype effect in two-way RM anova. No differences were detected between the genotypes in sucrose consumption in male mice confirmed by the lack of significant genotype effect (F1,120 = 1.89, P = 0.2, Figure 3D). In female mice, two-way RM anova revealed that HDC KO consumed less sucrose than WT mice (F1,96 = 9.08, P = 0.017, Figure 3F).
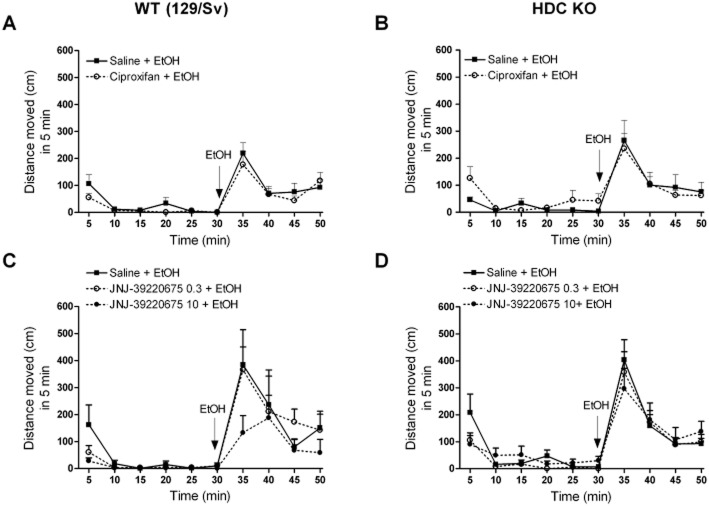
H3 receptor antagonism and alcohol-induced locomotor stimulation
Locomotor activities of HDC KO and WT mice during pretreatment (saline, ciproxifan or JNJ-39220675) and in response to alcohol (1.5 g·kg−1, i.p.) are shown in Figure 4A–D. Ciproxifan had no effect on alcohol stimulation in WT (time effect F9,216 = 16.82, P < 0.0001, treatment effect F1,216 = 0.98, P = 0.33, Figure 4A) or in KO mice (time effect F9,216 = 11.39, P < 0.0001, treatment effect F1,216 = 0.19, P = 0.67, Figure 4B), confirmed by two-way RM anova. Also, JNJ-39220675 had no effect on alcohol stimulation in WT (time effect F9,198 = 12.05 P < 0.0001, treatment effect F2,198 = 1.24, P = 0.31, Figure 4C) or KO mice (time effect F9,333 = 34.89, P < 0.0001, treatment effect F2,333 = 0.20, P = 0.82, Figure 4D), confirmed by two-way RM anova.
Figure 4.
H3 receptor antagonists do not alter alcohol-induced locomotor stimulation in wild-type (WT; 129/Sv) or in histidine decarboxylase knock-out (HDC KO) mice. Pretreatment (saline, ciproxifan 3 mg·kg−1, JNJ-39220675 0.3 or 10 mg·kg−1, i.p.) was given after a 60–90 min habituation period and alcohol (1.5 g·kg−1, i.p.) was injected 30 min after the pretreatment. Alcohol induces stimulation in both genotypes. Alcohol-induced stimulation is not affected by the pretreatment with ciproxifan in WT (A) or in HDC KO (B) mice; n = 12–14 per group. Alcohol-induced stimulation is also not affected by the pretreatment with JNJ-39220675 in WT (C) or in HDC KO (D) mice; n = 8–9 per group. Two-way repeated measures anova, no interaction or treatment effect, significant time effect in all groups (P < 0.0001) indicating similar alcohol-induced stimulation regardless of pretreatments with H3 receptor antagonist. All data are expressed as mean ± SEM.
Expression of dopamine receptor signalling components in the striatum
To determine whether there is a difference in the expression levels of D1 and D2 receptors, STEP61 and DARPP-32 mRNA in HDC KO and WT mice, we used quantitative radioactive in situ hybridization. We found no significant differences between the genotypes in the mRNA levels measured in any of the striatal subdivisions, confirmed by the lack of significant genotype effect by Student's two-tailed t-test (n = 7–8/genotype, P > 0.05, Table 1).
Table 1.
The mRNA expression levels (nCi·mg−1, average ± SEM) of D1 and D2 receptors, STEP61 and DARPP-32 following radioactive in situ hybridization in the striatal subdivisions of WT (129/Sv) and HDC KO mice brain
| D1receptor | D2receptor | STEP61 | DARPP-32 | |||||
|---|---|---|---|---|---|---|---|---|
| WT | HDC KO | WT | HDC KO | WT | HDC KO | WT | HDC KO | |
| DL Cpu | 214 ± 8 | 219 ± 11 | 343 ± 15 | 348 ± 14 | 335 ± 9 | 323 ± 8 | 21 ± 1 | 20 ± 1 |
| DM Cpu | 203 ± 8 | 204 ± 12 | 321 ± 11 | 327 ± 12 | 327 ± 7 | 313 ± 8 | 22 ± 1 | 22 ± 1 |
| V Cpu | 204 ± 6 | 201 ± 12 | 334 ± 12 | 342 ± 13 | 336 ± 10 | 328 ± 8 | 21 ± 1 | 21 ± 1 |
| NA Core | 155 ± 9 | 148 ± 8 | 264 ± 6 | 260 ± 7 | 296 ± 5 | 296 ± 9 | 22 ± 1 | 22 ± 1 |
| NA Shell | 169 ± 10 | 172 ± 9 | 271 ± 6 | 275 ± 6 | 293 ± 8 | 294 ± 7 | 22 ± 1 | 21 ± 1 |
Cpu, caudate putamen; DL, dorsolateral; DM, dorsomedial; NA, nucleus accumbens; V, ventral.
Discussion
Several studies have suggested that histamine is an important neuromodulator in the rewarding effects of addictive drugs (Brabant et al., 2010; Panula and Nuutinen, 2011; Nuutinen et al., 2012). There is, however, no consensus on the role of histamine in reward circuitry. Several studies have shown that histamine has an inhibitory role in reward. These include tuberomammillary nucleus (TMN) lesion studies (Huston et al., 1997) and experiments where it was demonstrated that histamine, injected discretely into the lateral hypothalamus, inhibits self-stimulation (Cohn et al., 1973). However, in contrast, it has also been shown that histamine-deficient mice are as responsive as WT mice to the psychostimulant effects of cocaine (Brabant et al., 2007). The purpose of this study was to clarify the role of neuronal histamine in alcohol dependence-related behaviours.
Here, we found that H3 receptor antagonist-mediated inhibition of alcohol-CPP is dose-dependent. Low doses of H3 receptor antagonists JNJ-10181457 and JNJ-39220675 inhibited the development of alcohol-CPP in DBA/2J mice whereas the higher doses had no effect. The underlying mechanisms remain unclear. Pharmacokinetic interactions are unlikely as JNJ-39220675 had no effect on the pharmacokinetic profile of alcohol. The possible different roles played by the different splice forms of the H3 receptor expressed in different parts of the circuits involved, including the striatum, cortex and midbrain ventral tegmental area (Drutel et al., 2001) may contribute to the findings. Both JNJ-10181457 and JNJ-39220675 penetrate easily through the blood-brain barrier in rats (Bonaventure et al., 2007; Galici et al., 2009; 2011), and the doses used here were chosen based on studies carried out in rats. When JNJ-39220675 is administered ex vivo, H3 receptor occupancy reaches the maximal level quickly at both 3 and 10 mg·kg−1 doses (Galici et al., 2011), suggesting that H3 receptor-dependent effects can be seen when using lower doses. Another reason for the low dose inhibition of alcohol-CPP might arise from the H3 receptors located both postsynaptically in dendrites and cell bodies of GABAergic medium spiny neurons and at autoreceptors in histaminergic terminals. H3 receptor antagonists acting at presynaptic H3 autoreceptors increase histamine release allowing more histamine to act on the postsynaptic histamine receptors, which include H3 receptors, perhaps most importantly on the GABAergic neurons which then indirectly inhibit dopaminergic neurons. Thus, the responses seen with different doses of H3 receptor ligands might in part be explained by opposing receptor effects.
Interestingly, in this study, we found that none of the tested receptor antagonists tested inhibited alcohol-CPP in HDC KO mice suggesting that the inhibition of alcohol reward, mediated by H3 receptor antagonists, is histamine-dependent. Furthermore, it has been reported that the expression of H3 receptors in the hippocampus is decreased in HDC KO mice, whereas the expression of H3 receptor mRNA is increased in the TMN (Chepkova et al., 2012). This could in part explain why H3 receptor antagonists did not block the rewarding effects of alcohol, however, the expression of H3 receptors in the striatum of HDC KO mice has not yet been reported. H3 receptors are expressed on both the somata and terminals of the GABAergic medium spiny neurons, which form the feedback loop to the midbrain dopaminergic neurons (Pillot et al., 2002). One possible mechanism by which H3 receptor antagonists might inhibit alcohol reward is via these H3 receptors on GABAergic neurons. Increased release of histamine by H3 receptor antagonists might increase the firing of GABAergic neurons (Korotkova et al., 2002; Ellender et al., 2011), which in turn would suppress the activity of dopaminergic neurons. On the other hand, HDC KO mice were of the 129/Sv background strain, which has a low baseline activity and that might partly explain these results. Correlation analyses have reflected stronger preferences in CPP in less active strains, such as 129/Sv (Gremel and Cunningham, 2007). Therefore, further studies with HDC KO mice using a different, more active background strain (e.g. C57BL/6J) are needed to clarify the role of histamine in the H3 receptor antagonist-evoked prevention of alcohol-CPP.
In support of an inhibitory role of histamine in alcohol reward, in our previous study we demonstrated that HDC KO mice develop a stronger alcohol-CPP than WT mice (Nuutinen et al., 2010), which led us to study whether some dopaminergic signalling molecules are altered in these mice. In the present study, we showed that the expression levels of D1 and D2 receptor, STEP61 and DARPP-32 mRNA in the HDC KO were similar to those of WT mice. Thus, the elevated alcohol reward in HDC KO mice cannot be explained by changes in the mRNA expression of these proteins. Nevertheless, dopaminergic signalling should be further studied in HDC KO mice. In addition, in the present study we showed that histamine-deficient mice consumed as much alcohol as the WT mice, demonstrating that a deficit of histamine has no effect on voluntary or binge-like alcohol consumption. Interestingly, our previous findings have demonstrated that H3 receptor KO mice consume less alcohol in both voluntary and binge-like drinking paradigms and that H3 receptor antagonists inhibit binge-like drinking (Nuutinen et al., 2011a). The present data suggest that a deficit of histamine per se fails to bring about such a marked effect on alcohol consumption.
Mesolimbic and nigrostriatal dopaminergic systems are known to be crucial in motivated behaviours and locomotion (Beninger, 1983; Mogenson and Yang, 1991; Vallone et al., 2000; Wise, 2009). Alcohol injected systemically or locally into the tegmental area increases extracellular dopamine in the nucleus accumbens (Di Chiara and Imperato, 1985; Yim et al., 1998) and produces a dose-dependent increase in the locomotor activity (Cunningham and Noble, 1992). In contrast to our previous findings in HDC KO mice (Nuutinen et al., 2010), in the present study we showed that HDC KO mice are as stimulated by alcohol as WT mice. In our previous study, we measured the cumulative distance moved during the 30 min experiment, whereas in the present study we looked at the effects on locomotor activity in greater detail. We now found that the activation peak is already present at 5 min after alcohol injection and declines quickly thereafter. It is therefore likely that cumulative effects over 30 min do not exclusively reflect the stimulating effect of alcohol.
In the present study, the DBA/2J mice were highly stimulated by alcohol, even more so than we previously observed (Nuutinen et al., 2011b). This is probably because the same mice were used in the CPP study previously (see Methods) and they were probably sensitized to the locomotor activating effect of alcohol (Cunningham and Noble, 1992). Regardless of the different doses used, JNJ-39220675 had no effect on the alcohol-induced stimulation in DBA/2J mice. This result was in contrast to that observed previously with ciproxifan, which was found to increase and prolong the locomotor activation (Nuutinen et al., 2011b). The lack of effect of JNJ-39220675 on alcohol-induced locomotion might be advantageous if this drug is to be used clinically. However, neither ciproxifan nor JNJ-39220675 altered the stimulant effects of alcohol in the 129/Sv mice, demonstrating robust differences in behavioural responsiveness between mouse strains.
H3 receptor activation inhibits dopamine synthesis (Molina-Hernandez et al., 2000) and D1 receptor-induced cAMP accumulation in rat striatum (Sanchez-Lemus and Arias-Montano, 2004). Dopamine signalling via D2 receptors is essential in alcohol preference and sensitivity (Phillips et al., 1998). Importantly, D1 and D2 receptors have been shown to heteromerize in the presynaptic terminals, for example, in the nucleus accumbens and globus pallidus (Perreault et al., 2010). Interestingly, the postsynaptic H3 receptors may also heteromerize with both D1 and D2 receptors leading to altered dopaminergic signalling (Ferrada et al., 2008; 2009; Moreno et al., 2011). All these findings highlight the close interaction between the histaminergic and dopaminergic signalling cascades. Accordingly, the results from the present study support the concept that the modulation of dopaminergic signalling requires both histamine and functional H3 receptors. However, our results also indicate that histamine is unlikely to regulate the mRNA expression of D1 or D2 receptors or other essential components of the dopamine signalling pathway. Studies on dopamine receptor radioligand binding and second messengers are needed to reveal the mechanisms of the histamine-dopamine interactions in the alcohol reward response.
Conclusions
The present data suggest that H3 receptor antagonist-mediated inhibition of alcohol-CPP is dose-dependent. The H3 receptor antagonists did not inhibit alcohol-CPP in mice lacking histamine suggesting that H3 receptor antagonist-mediated inhibition of alcohol reward is dependent on histamine. We also found that a total lack of histamine has no effect on alcohol consumption or stimulation. Altogether, these findings support the concept that brain histamine has an inhibitory role in alcohol reward. Increasing neuronal histamine via H3 receptor blockade could therefore potentially be a novel way of treating alcohol dependence. However, the dose-dependent effect of H3 receptor antagonists may be challenging in clinical practice.
Acknowledgments
This work was supported by EU COST BM806 and grants from the Academy of Finland, The Finnish Society of Sciences and Letters, The Finnish Foundation for Alcohol Studies and the Research Foundation of the University of Helsinki. We thank Johnson & Johnson Pharmaceutical Research & Development, L.L.C. (San Diego, CA, U.S.A) for JNJ-10181457 and JNJ-39220675.
Glossary
- Alcohol-CPP
alcohol-induced conditioned place preference
- DARPP-32
dopamine- and cAMP-regulated neuronal phosphoprotein
- DID
drinking in the dark
- HDC KO
histidine decarboxylase knock-out
- STEP61
striatal-enriched protein 61
- TMN
tuberomammillary nucleus
Conflict of interest
P. P. has received payments for lectures from Abbott Laboratories. The remaining authors declare no conflicts of interest.
References
- Alexander SPH, Mathie A, Peter JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. S1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, Suomalainen R, et al. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991a;44:465–481. doi: 10.1016/0306-4522(91)90070-5. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Reinikainen K, Riekkinen P, Panula P. Neurofibrillary tangles and histamine-containing neurons in Alzheimer hypothalamus. Agents Actions. 1991b;33:104–107. doi: 10.1007/BF01993139. [DOI] [PubMed] [Google Scholar]
- Alakarppa K, Tupala E, Mantere T, Sarkioja T, Rasanen P, Tarhanen J, et al. Effect of alcohol abuse on human brain histamine and tele-methylhistamine. Inflamm Res. 2002;51:40–41. doi: 10.1007/pl00022438. [DOI] [PubMed] [Google Scholar]
- Alakarppa K, Tupala E, Mantere T, Sarkioja T, Rasanen P, Tarhanen J, et al. Alcoholics show altered histaminergic neurotransmission in several cortical areas–preliminary report. Inflamm Res. 2003;52:37–38. doi: 10.1007/s000110300044. [DOI] [PubMed] [Google Scholar]
- Anichtchik OV, Rinne JO, Kalimo H, Panula P. An altered histaminergic innervation of the substantia nigra in Parkinson's disease. Exp Neurol. 2000;163:20–30. doi: 10.1006/exnr.2000.7362. [DOI] [PubMed] [Google Scholar]
- Anichtchik OV, Peitsaro N, Rinne JO, Kalimo H, Panula P. Distribution and modulation of histamine H(3) receptors in basal ganglia and frontal cortex of healthy controls and patients with Parkinson's disease. Neurobiol Dis. 2001;8:707–716. doi: 10.1006/nbdi.2001.0413. [DOI] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Letavic M, Dugovic C, Wilson S, Aluisio L, Pudiak C, et al. Histamine H3 receptor antagonists: from target identification to drug leads. Biochem Pharmacol. 2007;73:1084–1096. doi: 10.1016/j.bcp.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Anaclet C, Lin JS, Ohtsu H, Tirelli E. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology. 2007;190:251–263. doi: 10.1007/s00213-006-0603-0. [DOI] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Quertemont E, Tirelli E. Involvement of the brain histaminergic system in addiction and addiction-related behaviors: a comprehensive review with emphasis on the potential therapeutic use of histaminergic compounds in drug dependence. Prog Neurobiol. 2010;92:421–441. doi: 10.1016/j.pneurobio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Yamatodani A, Niigawa H, Hariguchi S, Tada K, Nishimura T, et al. Brain histamine in Alzheimer's disease. Methods Find Exp Clin Pharmacol. 1989;11:353–360. [PubMed] [Google Scholar]
- Chepkova A, Yanovsky E, Parmentier R, Ohtsu H, Haas HL, Lin J, et al. Histamine receptor expression, hippocampal plasticity and ammonia in histidine decarboxylase knockout mice. Cell Mol Neurobiol. 2012;32:17–25. doi: 10.1007/s10571-011-9730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn CK, Ball GG, Hirsch J. Histamine: effect on self stimulation. Science. 1973;180:757–758. doi: 10.1126/science.180.4087.757. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, Timmerman H, et al. Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol Pharmacol. 2001;59:1–8. [PubMed] [Google Scholar]
- Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Paul Bolam J. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci. 2011;31:15340–15351. doi: 10.1523/JNEUROSCI.3144-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette's syndrome. N Engl J Med. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan-Sencicek AG, Kim YS, Fishman DO, et al. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biol Psychiatry. 2012;71:392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casado V, Bongers G, Cortes A, Mallol J, et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Boggs JD, Aluisio L, Fraser IC, Bonaventure P, Lord B, et al. JNJ-10181457, a selective non-imidazole histamine H(3) receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology. 2009;56:1131–1137. doi: 10.1016/j.neuropharm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Galici R, Rezvani AH, Aluisio L, Lord B, Levin ED, Fraser I, et al. JNJ-39220675, a novel selective histamine H3 receptor antagonist, reduces the abuse-related effects of alcohol in rats. Psychopharmacology. 2011;214:829–841. doi: 10.1007/s00213-010-2092-4. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Henwood RW, Mazurikiewicz-Kwilecki IM. Possible role of brain histamine in morphine addiction. Life Sci. 1975;17:55–56. doi: 10.1016/0024-3205(75)90234-9. [DOI] [PubMed] [Google Scholar]
- Huston JP, Wagner U, Hasenöhrl RU. The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: evidence for an inhibitory role. Behav Brain Res. 1997;83:97–105. doi: 10.1016/s0166-4328(97)86052-4. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Schlicker E, Marr I, Werthwein S, Stark H, Schunack W. Ciproxifan and chemically related compounds are highly potent and selective histamine H3-receptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:623–627. doi: 10.1007/pl00005303. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Haas HL, Brown RE. Histamine excites GABAergic cells in the rat substantia nigra and ventral tegmental area in vitro. Neurosci Lett. 2002;320:133–136. doi: 10.1016/s0304-3940(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Letavic MA, Aluisio L, Atack JR, Bonaventure P, Carruthers NI, Dugovic C, et al. Pre-clinical characterization of aryloxypyridine amides as histamine H3 receptor antagonists: identification of candidates for clinical development. Bioorg Med Chem Lett. 2010;20:4210–4214. doi: 10.1016/j.bmcl.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Lintunen M, Sallmen T, Karlstedt K, Fukui H, Eriksson KS, Panula P. Postnatal expression of H1-receptor mRNA in the rat brain: correlation to L-histidine decarboxylase expression and local upregulation in limbic seizures. Eur J Neurosci. 1998;10:2287–2301. doi: 10.1046/j.1460-9568.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- Lintunen M, Hyytia P, Sallmen T, Karlstedt K, Tuomisto L, Leurs R, et al. Increased brain histamine in an alcohol-preferring rat line and modulation of ethanol consumption by H(3) receptor mechanisms. FASEB J. 2001;15:1074–1076. doi: 10.1096/fj.00-0545fje. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurkiewicz-Kwilecki I, Henwood RW. Alterations in brain endogenous histamine levels in rats after chronic morphine treatment and morphine withdrawal. Agents Actions. 1976;6:402–408. doi: 10.1007/BF01973210. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz-Kwilecki IM, Nsonwah S. Changes in the regional brain histamine and histidine levels in postmortem brains of Alzheimer patients. Can J Physiol Pharmacol. 1989;67:75–78. doi: 10.1139/y89-013. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez A, Nunez A, Arias-Montano JA. Histamine H3-receptor activation inhibits dopamine synthesis in rat striatum. Neuroreport. 2000;11:163–166. doi: 10.1097/00001756-200001170-00032. [DOI] [PubMed] [Google Scholar]
- Monnier M, Fallert M, Battacharya IC. The waking action of histamine. Experientia. 1967;23:21–22. doi: 10.1007/BF02142244. [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepulveda M, Navarro G, Casado V, Cortes A, et al. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem. 2011;286:5846–5854. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T, Kitamura N, Hashimoto T, Kajimoto Y, Nishino N, Mita T, et al. Decreased histamine H1 receptors in the frontal cortex of brains from patients with chronic schizophrenia. Biol Psychiatry. 1991;30:349–356. doi: 10.1016/0006-3223(91)90290-3. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Karlstedt K, Aitta-Aho T, Korpi ER, Panula P. Histamine and H3 receptor-dependent mechanisms regulate ethanol stimulation and conditioned place preference in mice. Psychopharmacology. 2010;208:75–86. doi: 10.1007/s00213-009-1710-5. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Lintunen M, Vanhanen J, Ojala T, Rozov S, Panula P. Evidence for the role of histamine H3 receptor in alcohol consumption and alcohol reward in mice. Neuropsychopharmacology. 2011a;36:2030–2040. doi: 10.1038/npp.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Vanhanen J, Pigni MC, Panula P. Effects of histamine H3 receptor ligands on the rewarding, stimulant and motor-impairing effects of ethanol in DBA/2J mice. Neuropharmacology. 2011b;60:1193–1199. doi: 10.1016/j.neuropharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Vanhanen J, Mäki T, Panula P. Histamine H3 receptor: a novel therapeutic target in alcohol dependence? Front Syst Neurosci. 2012;6:1–7. doi: 10.3389/fnsys.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Panula P, Nuutinen S. Histamine and H3 receptor in alcohol-related behaviors. J Pharmacol Exp Ther. 2011;336:9–16. doi: 10.1124/jpet.110.170928. [DOI] [PubMed] [Google Scholar]
- Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, et al. Neuronal histamine deficit in Alzheimer's disease. Neuroscience. 1998;82:993–997. doi: 10.1016/s0306-4522(97)00353-9. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, et al. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamineandin schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, et al. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Prell GD, Green JP, Kaufmann CA, Khandelwal JK, Morrishow AM, Kirch DG, et al. Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr Res. 1995;14:93–104. doi: 10.1016/0920-9964(94)00034-6. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Anichtchik OV, Eriksson KS, Kaslin J, Tuomisto L, Kalimo H, et al. Increased brain histamine levels in Parkinson's disease but not in multiple system atrophy. J Neurochem. 2002;81:954–960. doi: 10.1046/j.1471-4159.2002.00871.x. [DOI] [PubMed] [Google Scholar]
- Sakata T, Fukagawa K, Ookuma K, Fujimoto K, Yoshimatsu H, Yamatodani A, et al. Modulation of neuronal histamine in control of food intake. Physiol Behav. 1988;44:539–543. doi: 10.1016/0031-9384(88)90316-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lemus E, Arias-Montano JA. Histamine H3 receptor activation inhibits dopamine D1 receptor-induced cAMP accumulation in rat striatal slices. Neurosci Lett. 2004;364:179–184. doi: 10.1016/j.neulet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal -not just mesocorticolimbic- dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CL. Possible role of brain histamine and H1 and H2 receptors in development of morphine-tolerance and physical-dependence in mice. Agents Actions. 1975;5:476–483. doi: 10.1007/BF01972684. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]