Abstract
Background and Purpose
The presence of the histamine H4 receptor (H4R) was previously reported in benign and malignant lesions and cell lines derived from the human mammary gland. The aim of this work was to evaluate the effects of H4R ligands on the survival, tumour growth rate and metastatic capacity of breast cancer in an experimental model.
Experimental Approach
Xenograft tumours of the highly invasive human breast cancer cell line MDA-MB-231 were established in immune deficient nude mice. The following H4R agonists were employed: histamine (5 mg kg−1), clozapine (1 mg kg−1) and the experimental compound JNJ28610244 (10 mg kg−1).
Results
Data indicate that developed tumours were highly undifferentiated, expressed H4R and exhibited high levels of histamine content and proliferation marker (PCNA) while displaying low apoptosis. Mice of the untreated group displayed a median survival of 60 days and a tumour doubling time of 7.4 ± 0.6 days. A significant decrease in tumour growth evidenced by an augment of the tumour doubling time was observed in the H4R agonist groups (13.1 ± 1.2, P < 0.01 in histamine group; 15.1 ± 1.1, P < 0.001 in clozapine group; 10.8 ± 0.7, P < 0.01 in JNJ28610244 group). This effect was associated with a decrease in the PCNA expression levels, and also reduced intratumoural vessels in histamine and clozapine treated mice. Histamine significantly increased median survival (78 days; Log rank Mantel-Cox Test, P = 0.0025; Gehan-Breslow-Wilcoxon Test, P = 0.0158) and tumoural apoptosis.
Conclusions and Implications
Histamine through the H4R exhibits a crucial role in tumour progression. Therefore, H4R ligands offer a novel therapeutic potential as adjuvants for breast cancer treatment.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: histamine, breast cancer, H4 receptor ligands, cell proliferation, apoptosis, clozapine, JNJ28610244
Introduction
Breast cancer is the second most common cancer worldwide after lung cancer, and the leading cause of cancer death in women (Jemal et al., 2010).
Breast cancer is a heterogeneous disease in terms of presentation, morphology, molecular profile and clinical behaviours to therapy. Triple-negative cancers are defined as tumours that lack oestrogen receptor (ER), progesterone receptor and epidermal growth factor receptor 2 expressions. These tumours account for 10–17% of all breast carcinomas and are associated with poor prognosis and lack the benefit of targeted systemic therapy (Perou et al., 2000; Sotiriou et al., 2003; Badve et al., 2011).
Advances in the understanding of the molecular basis of response and resistance to treatments are helping to develop novel drugs that can contribute to improve efficacy.
A huge number of molecules involved in cell proliferation, which is a key event in tumour development and progression, have been extensively investigated including histamine (Rivera et al., 2000; Darvas et al., 2003; Pós et al., 2004; Medina and Rivera, 2010a). Histamine [2-(4-imidazolyl)-ethylamine] is an endogenous biogenic amine widely distributed throughout the organism and is known to be a pleiotropic pathophysiological mediator (Kahlson and Rosengren, 1968; Hill et al., 1997; Pós et al., 2004; De Esch et al., 2005). Histamine exerts its effects through the activation of four different receptors H1, H2, H3 and H4 (H1R, H2R, H3R, H4R) (Medina and Rivera, 2010b). The discovery of the H4R with functional presence in a wide range of tissues including tumours, revealed novel functions for histamine leading to reconsideration of new perspectives in histamine pharmacology research (Huang and Thurmond, 2008; Leurs et al., 2009; Tiligada et al., 2009; Zampeli and Tiligada, 2009).
It was demonstrated that the four histamine receptor subtypes are expressed in cell lines derived from human mammary gland (Davio et al., 1993; Lemos et al., 1995; Medina et al., 2006; Medina and Rivera, 2010a). It has already been reported that histamine is capable of modulating cell proliferation in the triple-negative MDA-MB-231 breast cancer cells in a dose-dependent manner producing a significant decrease at 10 μmol L−1 concentration whereas at lower concentrations it increased proliferation moderately through the H3R. On the other hand, no effect on proliferation is observed in the non-tumourigenic HBL-100 cells (Medina et al., 2006; 2008). Furthermore, histamine acts as an anti-proliferative agent through the H4R in two different human breast cancer cells, MDA-MB-231 and MCF-7 (ERα+) (Medina et al., 2011). H4R ligands inhibited proliferation by 50%, increasing the exponential doubling time and the number of apoptotic and senescent cells. Furthermore, the H4R was expressed in human biopsies of breast cancer (Medina et al., 2008; 2011).
In the light of the above mentioned evidences, the aim of this work was to evaluate the effects of H4R ligands on the survival, tumour growth rate, metastatic capacity and molecular pattern of antigens expression related with the proliferative and apoptotic potential in a triple-negative breast cancer experimental model.
Materials and methods
Cell culture
The human breast cancer cell line MDA-MB-231 (American Type Tissue Culture Collection, VA, USA) was cultured in RPMI 1640 supplemented with 10% v/v FBS, 0.3 g L−1 glutamine and 0.04 g L−1 gentamicin (Gibco BRL, NY, USA). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cell proliferation assay
Proliferation was evaluated by the clonogenic assay. Twenty-four hours after seeding in 12-well plates (900 cells·well−1), cells were left untreated or were treated with histamine, clozapine, (kindly provided by Fabra Laboratories S.A., Buenos Aires, Argentina), JNJ28610244 in concentrations ranging from 0.01 to 50 μmol L−1 and/or JNJ7777120 (10 μmol L−1). Both compounds were generously given by Johnson & Johnson Pharmaceutical Research and Development, San Diego, CA, USA. The cells were incubated for 7 days and then fixed with 10% v/v formaldehyde in PBS and stained with 1% w/v toluidine blue in 70% v/v ethanol. The clonogenic proliferation was evaluated by counting the colonies containing 50 cells or more and was expressed as a percentage of the untreated wells.
Quantification of cellular DNA synthesis was performed by 5-bromo-2′-deoxyuridine (BrdU, Sigma Chemical Co., St Louis, MO, USA) incorporation as previously reported (Medina et al., 2011). Briefly, cells were seeded on glass coverslips into 12-well plates (50 000 cells·well−1) in culture medium. Cells were then treated for 48 h with 10 μmol L−1 histamine, clozapine, JNJ28610244 or were left untreated. After that, BrdU (30 μmol·L−1) was added for 2 h. The cells were then washed twice and fixed for 15 min in 4% v/v formaldehyde in PBS. To denature the DNA into single-stranded molecules, cells were incubated with 3 nmol·L−1 HCl, 1% Triton X-100 v/v in PBS for 15 min at room temperature. Cells were washed in 1 mL of 0.1 M Na2B4O7 (Sigma Chemical Co.), 1% v/v Triton X-100 in PBS, pH 8.5 to neutralize the acid. After inactivating the endogenous peroxidase activity with 3% v/v H2O2 in distilled water and blocking with 5% v/v FBS in PBS, cells were then incubated with anti-BrdU mouse monoclonal antibody diluted 1:100 in 1% BSA w/v in PBS (Sigma Chemical Co.). Cells were washed with PBS and further incubated for 1 h at room temperature with 1:100 horseradish peroxidase-conjugated anti-mouse IgG and visualized by diamino-benzidine staining (Sigma Chemical Co.). To evaluate subcellular localization of these proteins, nuclei were stained with haematoxylin. Light microscopy was performed on an Axiolab Karl Zeiss microscope (Göttingen, Germany). Photographs were taken at 630x magnifications using a Canon PowerShot G5 camera (Tokyo, Japan). At least 500 cells were scored for each determination.
Determination of apoptosis
Cells were left untreated or were treated with 10 μmol L−1 histamine, clozapine and JNJ28610244 for 48 h.
Apoptotic cells were determined by TUNEL assay. Cells were washed, fixed and the fragmented DNA was detected using ApoptagTM plus peroxidase in situ apoptosis Detection Kit (CHEMICON International, CA, USA) according to the manufacturer's instructions. Cells were visualized using Axiolab Karl Zeiss microscope (Germany). At least 500 cells were scored for each determination.
Small interfering RNA H4R silencing
Cells were seeded in 6-well plates (200 000 cells·well−1) or 24-well plates (35 000 cells·well) and cultured in RPMI 1640 supplemented with 10% v/v FBS and 0.3 g L−1 of glutamine. Cells were transfected according to the manufacturer's instructions using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), 8 μL (80 pmol) of human H4R small interfering RNA (siRNA) (sc-40025) pools of three to five target-specific 19–25 nucleotides siRNAs designed to knockdown H4R gene expression, 8 μL (80 pmol) of scrambled unconjugated control siRNA-A (sc-37007)-negative control that consists of a scrambled sequence that will not lead to the specific degradation of any cellular message (shared no homology to the human genome), (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Transfection was performed during 6 h and then cells were washed with PBS and fresh medium was added. Cells were then treated with 10 μmol·L−1 of histamine or were left untreated for 18 h. The extinction of H4R expression was ascertained by immunocytochemistry analysis and reverse transcription-polymerase chain reaction and was performed as previously described (Massari et al., 2011). Quantification of cellular DNA synthesis was performed by BrdU incorporation as described above.
Treatments and animals
Histamine was diluted in saline solution. Clozapine and JNJ28610244 were diluted in 0.1 N HCl, neutralized with 4 N NaOH and diluted with saline. JNJ10181457 and JNJ10191584 compounds were diluted with saline.
Specific pathogen-free athymic female nude (NIH nu/nu) mice were purchased from the Division of Laboratory Animal Production, School of Veterinary Sciences, University of La Plata, Buenos Aires (Argentina), and maintained in sterile isolated conditions. Five mice were kept per cage and maintained in our animal health care facility at 22 to 24°C and 50 to 60% humidity on a 12 h light/dark cycle with food and water available ad libitum. Animals used were 8–10 weeks old and had an average weight of 25–30 g. Animal procedures were in accordance with recommendations from the Guide for the Care and Use of Laboratory Animals of the National Research Council, USA, 1996, and protocols were approved by the Ethical Committee for the Use and Care of Laboratory Animals of the School of Pharmacy and Biochemistry and also by Ethical and Educational Committee of the Institute of Immunooncology. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals. (Kilkenny et al., 2010; McGrath et al., 2010).
MDA-MB-231 cells (1 × 107) were collected by centrifugation and resuspended in 100 μL RPMI-1640 (Gibco, Grand Island, NY, USA). Tumours were originally induced by s.c. injection of MDA-MB-231 cells into the right flank of two female athymic nude mice. When developed tumours reached a volume of 500 mm3, they were excised, cut into 25–30 mm3 pieces and grafted into the right flank of other nude mice. When the graft volumes reached 100–150 mm3, xenografted mice were separated in four groups and received a s.c. daily injection on the dorsal flank of saline solution (control group, n = 20), histamine (5 mg·kg−1, n = 14), clozapine (1 mg·kg−1, n = 14), JNJ28610244 (10 mg·kg−1, n = 14), JNJ10191584 (10 mg·kg−1, n = 12), JNJ10181457 (10 mg·kg−1, n = 12). The method consists in tenting the skin between the shoulder blades and inserting the needle bevel up in the pocket created.
Tumour growth and survival
Half of the animals in each group were killed by cervical dislocation 45 days after treatment to perform the tumour growth curve based on tumour size and the ex vivo histochemical and histopathological studies, while half were treated until spontaneous death.
The length and width of the subcutaneous tumours were measured using a calliper three times a week. The tumour size was calculated as sphere volume according to the following formula: Tumour volume [mm3] = 4/3π × [(large diameter + small diameter)/4]3. Tumour growth data were expressed as relative tumour volume (tumour volume measured with respect to initial tumour volume at the beginning of treatment) and analysis was carried out using GraphPad Prism version 5.00. The equation for exponential growth was Yt = Y0xe(kxt), where Y0 was the initial relative tumour volume that increased exponentially with a rate constant, k. The tumour doubling time was calculated as 0.69/k. Survival was evaluated in mice bearing xenografts until spontaneous death. Kaplan–Meier survival curves, median survival time of each group and P value were obtained using GraphPad Prism.
Histopathology and immunohistochemistry
Tumours were excised, fixed with 10% neutral buffered formalin, paraffin embedded and cut into 4 μm thick serial sections. Tumour morphology and histopathological characteristics were examined on tissue sections after haematoxylin-eosin staining.
Immunohistochemistry was done as previously described (Medina et al., 2008). Briefly, antigen retrieval was performed in citrate buffer (10 mmol L−1, pH 6.0) at boiling temperature while endogenous peroxidase activity was blocked with 3% H2O2 in distilled water. After blocking, tissues were incubated with primary rabbit anti-histamine (1:100, Sigma Chemical Co.), mouse anti-proliferating cell nuclear antigen (PCNA, 1:100, DakoCytomation, Glostrup, Denmark), rabbit anti-H4R (1:50, Millipore, Temecula, CA, USA), antibodies overnight in a humidified chamber at 4°C. Immunoreactivity was detected by using horseradish peroxidase-conjugated anti-mouse, or anti-rabbit antibodies, as appropriate, and visualized by diamino-benzidine staining (Sigma Chemical Co.). To evaluate subcellular localization of these proteins, nuclei were stained with haematoxylin. To control the signal specificity, serial sections were made from five selected positive cases which were subjected to the same staining procedure, with either a normal mouse or rabbit IgG or PBS to replace the first antibody. No signal was detected in this control staining.
Fragmented DNA in cells undergoing apoptosis was detected using Apoptag™ plus peroxidase in situ apoptosis Detection Kit (CHEMICON International, Temecula, CA, USA) according to the manufacturer's instructions.
Finally, vascularization was determined using Massons trichromic staining by screening trichrome stained sections at 50× magnification to identify the largest vascular areas around the tumour. In these hot spots, intratumoural vascularity was evaluated by counting vessels inside the tumour at 100× magnification in 10 random fields.
Light microscopy was performed on an Axiolab Karl Zeiss microscope (Göttingen, Germany). Photographs were taken at 100× and 630× magnifications using a Canon PowerShot G5 camera (Tokyo, Japan).
Statistical analysis
Results are presented as means ± SEM. Statistical evaluations were made by Unpaired t-test or ANOVA that was followed by Dunnet test or Newman–Keuls Multiple Comparison Test. Log rank test and Gehan-Breslow-Wilcoxon test were performed for Kaplan–Meier survival. All statistical analyses were performed with GraphPad Prism version 5.00 (San Diego, CA, USA).
Results
In vitro anti-proliferative properties of H4R agonists
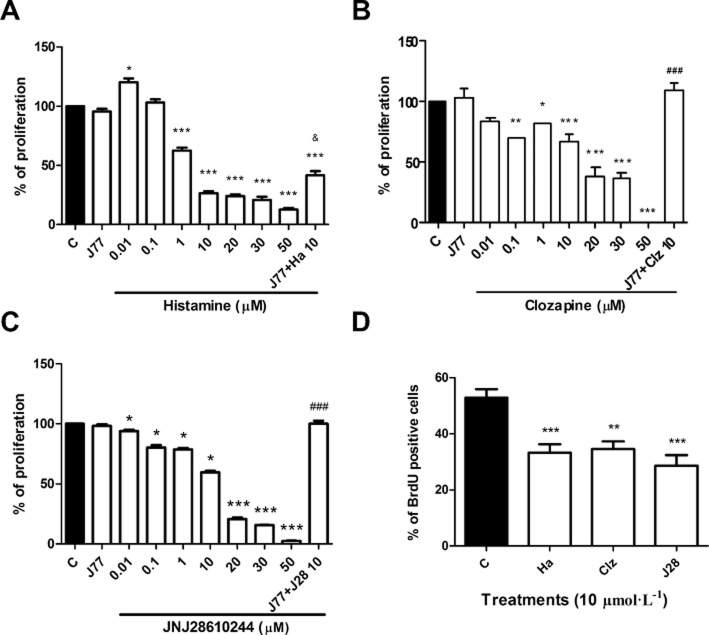
To determine the role of H4R ligands in MDA-MB-231 cell growth, proliferation was evaluated through the clonogenic assay. Results demonstrated that histamine modulated MDA-MB-231 cell proliferation in a dose-dependent manner, exhibiting an IC50 of 0.64 μmol L−1. The inhibitory effect on proliferation produced by 10 μmol L−1 concentration was partially reversed when cells were treated with the specific H4R antagonist, JNJ7777120 (Figure 1A).
Figure 1.
Effect of H4R ligands on in vitro MDA-MB-231 cell proliferation. Proliferation was evaluated by the clonogenic assay in a human breast adenocarcinoma cell line. Cells were left untreated or were treated with (A) histamine (Ha), (B) clozapine (Clz), (C) JNJ28610244 (J28) (0.01–50 μmol L−1) and/or 10 μmol L−1 JNJ7777120 (J77). (D) Incorporation of BrdU. Cells were left untreated or were treated with 10 μmol L−1 histamine (Ha), clozapine (Clz), JNJ28610244 (J28) for 48 h. Error bars represent the means ± SEM. (**P < 0.01, ***P < 0.001 vs. Control; &P < 0.05 versus 10 μmol L−1 histamine; ###P < 0.001 versus 10 μmol L−1 H4R agonist. ANOVA and Newman–Keuls Multiple Comparison Test).
Treatments with clozapine or JNJ28610244 also produced a concentration dependent inhibitory effect on proliferation, showing an IC50 of 11.5 and 13.4 μmol L−1, respectively (Figure 1B,C). The antiproliferative effect of these H4R agonists was fully blocked with the combined treatment with the H4R antagonist JNJ7777120 (Figure 1B,C). In agreement with these results, H4R ligands reduced the incorporation of BrdU (Figure 1D). To confirm the role of H4R in cell proliferation, siRNA specific for H4R messenger RNA was used to knockdown its expression in MDA-MB-231 cells. Results showed that histamine reduced the incorporation of BrdU, effect that was blocked in H4R siRNA transfected MDA-MB-231 cells (Figure 2A).
Figure 2.
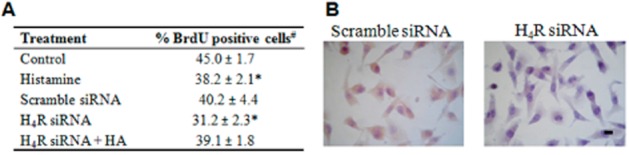
Incorporation of BrdU in MDA-MB-231 transfected cells. (A)# Error bars represent the mean ± SEM of two independent experiments performed in triplicates. Control, untreated cells; Histamine, cells treated with 10 μmol L−1 of histamine for 18 h; Scrambled siRNA (negative control), cells transfected with scrambled siRNA; H4R siRNA, cells transfected with specific sequences siRNA designed to knockdown H4R gene expression; H4R siRNA+HA, transfected cells with H4R siRNA and treated with 10 μmol L−1 of histamine for 18 h. (*P < 0.05 vs. Control; P = ns vs. Scrambled siRNA; ANOVA and Dunnett's Multiple Comparison Test). (B) H4R protein expression in MDA-MB-231 cells was determined by immunocytochemistry. Representative images of H4R stained cells are shown. x630 original magnification. Scale bar = 20 μm.
Furthermore, the H4R agonists increased the percentage of apoptotic cells evaluated by the TUNEL assay (Table 1).
Table 1.
Determination of apoptotic cells by the TUNEL assay
| Treatment | % apoptotic cellsa |
|---|---|
| Control | 4.3 ± 0.7 |
| Histamine | 13.4 ± 1.6*** |
| Clozapine | 7.7 ± 1.8** |
| JNJ28610244 | 6.3 ± 0.6* |
*P < 0.05, **P < 0.01, ***P < 0.001 versus Control. ANOVA and Newman–Keuls Multiple Comparison Test.
Error bars represent the mean ± SEM of two independent experiments performed in triplicates. Cells were left untreated or were treated with 10 μmol L−1 histamine, clozapine, or JNJ28610244 for 48 h. Error bars represent the means ± SEM.
Effect of H4R agonists on the MDA-MB-231 xenografted tumour into nude mice
In order to evaluate whether H4R agonists modulated the growth of the human breast cancer cell line in vivo, xenografted tumours of MDA-MB-231 were inoculated into nude mice.
The histological analysis demonstrated that tumours presented undifferentiated adenocarcinoma cells, which were highly invasive with high grade of atypia and marked anisokaryosis and anisocytosis. The histopathological characteristics of these tumours were not significantly modified by treatments (Figure 3A).
Figure 3.
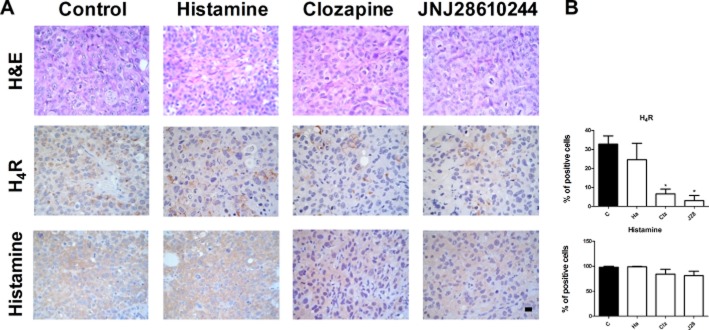
Histological and immunohistochemical analysis of tumour tissues. (A) Representative H&E stained images and immunohistochemical images of H4R and histamine detection in serial paraffin-embedded tumour specimens. x630 original magnification. Scale bar = 20 μm. (B) Positive stained cells were quantified by counting 10 random fields. Data are presented as a percentage per field. Data are shown as means ± SEM. (*P < 0.05 vs. Control. ANOVA and Newman–Keuls Multiple Comparison Test).
In addition, tumours expressed the H4R constitutively, and clozapine and JNJ28610244 significantly decreased the percentage expression levels from 33% in untreated group, to 5% and 3% in clozapine and JNJ28610244, respectively (Figure 3A,B). Tumours also displayed high levels of histamine that were not modified by treatments (Figure 3A,B).
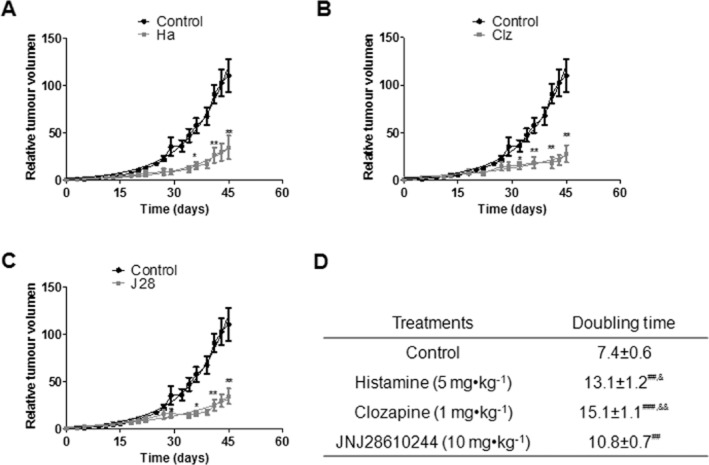
Histamine, clozapine and JNJ28610244 significantly slowed down the tumour growth rate, increasing the exponential doubling time from 7.4 ± 0.6 to 13.1 ± 1.2, 15.1 ± 1.1, and 10.8 ± 0.7 days, respectively (Figure 4A,B,C,D). Tumour volumes of mice subjected to treatments were significantly lower approximately from day 29 of treatment than the tumours of control animals (Figure 4A,B,C).
Figure 4.
Effect of H4R ligands on the triple-negative MDA-MB-231 (human breast cancer cells) xenografted tumour into nude mice. Relative tumour volume of the control group versus (A) histamine (Ha, 5 mg kg−1), (B) clozapine (Clz, 1 mg kg−1), (C) JNJ28610244 (J28, 10 mg kg−1). Data are shown as means ± SEM. Tumour volumes were measured three times a week and a non-linear regression fit was performed to evaluate the exponential growth (*P < 0.05, **P < 0.01, vs. Control; T-test). (D) Median tumour doubling time of each group is depicted numerically (##P < 0.01, ###P < 0.001 vs. Control; T-test; &P < 0.05, &&P < 0.01 vs. JNJ28610244).
In agreement with these results, we observed a decreased in the expression levels of the proliferation marker, PCNA, in tumours of treated animals (Figure 5A,B). Furthermore, we noticed an increased number of apoptotic cells in tumours of histamine-treated animals (Figure 5B).
Figure 5.
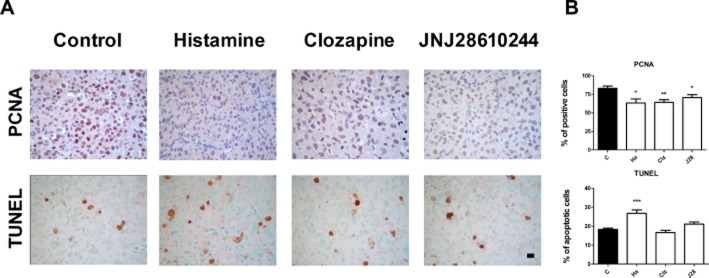
Immunohistochemical analysis of apoptosis and proliferation markers. (A) Representative immunohistochemical images of PCNA, and TUNEL in serial paraffin-embedded tumour specimens; x630 original magnification. Scale bar = 20 μm. (B) Positive stained cells were quantified by counting 10 random fields. Data are presented as a percentage per field. Data are shown as means ± SEM. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control. ANOVA and Newman–Keuls Multiple Comparison Test).
Effect of H4R agonists on tumour angiogenesis
We additionally investigated whether H4R agonist-mediated inhibition of tumour growth could be associated with a reduction in the number of vessels in intratumoural areas.
Results demonstrated a numerous number of medium and big sized congestive vessels in tumours of untreated animals. On the other hand, we observed a reduced number of vessels in tumours of histamine and clozapine treated animals (Figure 6A,B).
Figure 6.
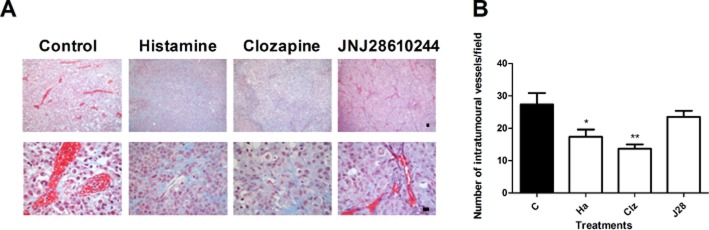
Effect of H4R ligands on tumour vascularization. (A) Representative Masson's trichrome stained images of tumour specimens; x100 and x630 original magnification. Scale bar = 20 μm. (B) The number of intratumoural vessels was quantified in 10 random fields (×100 magnification). Data are shown as means ± SEM. (*P < 0.05, **P < 0.01 vs. Control. ANOVA and Newman–Keuls Multiple Comparison Test).
Role of H4R agonists in survival
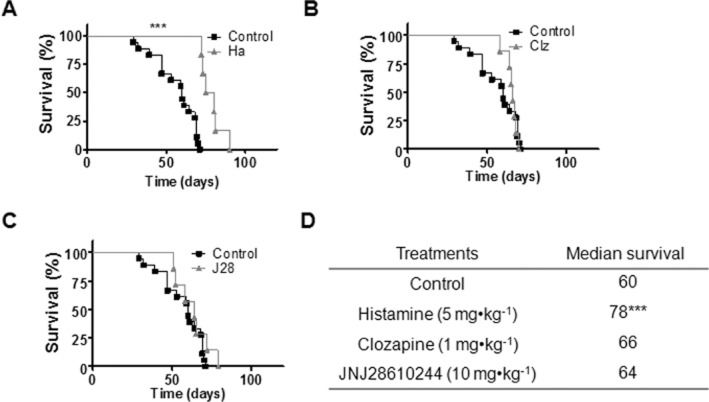
Kaplan–Meier survival curves demonstrated that histamine treatment significantly increased median survival from 60 to 78 days (***P < 0.0005, Log rank test and Gehan-Breslow-Wilcoxon test) (Figure 7A,D). However, no differences were observed in the median survival of clozapine or JNJ28610244-treated groups (Figure 7B,C,D).
Figure 7.
Kaplan–Meier survival of xenografted nude mice against different H4R ligand treatments. Treatments were administered daily by sc injection of saline buffer for the control group, histamine (Ha, 5 mg kg−1), clozapine (Clz, 1 mg kg−1), JNJ28610244 (J28, 10 mg kg−1), 5 days a week until death (***P < 0.0005, Log rank test and Gehan-Breslow-Wilcoxon test).
Effect of H4R agonists on lung micrometastatic disease
MDA-MB-231 xenografted tumours were highly invasive, developing metastasis principally in lungs. Metastases were observed in all groups. No difference in the number of micrometastasis was found after histamine and clozapine treatments. Interestingly, a significant increase in the number of metastasis was evidenced in JNJ28610244-treated group (Figure 8).
Figure 8.
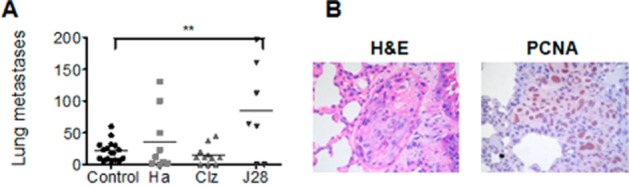
Role of H4R ligands on lung micrometastasis (A) The number of lung micrometastases was evaluated in untreated (Control) and histamine (Ha, 5 mg kg−1), clozapine (Clz, 1 mg kg−1) and JNJ28610244 (J28, 10 mg kg−1) treated mice. Data are shown as means ± SEM. Each dot represents the number of micrometastasis for one mouse. The middle line represents the average number (*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control. ANOVA and Newman–Keuls Multiple Comparison Test). (B) Representative H&E stained images and immunohistochemical images of PCNA in lung metastatic tissue. Pictures were taken at 630× magnification. Scale bar = 20 μm.
Role of H3R and H4R antagonists on tumour growth and survival
We further explored the effect of the H4R antagonist JNJ10191584 and the H3R antagonist JNJ10181457 on tumour growth and survival of tumour-bearing mice. Results show that tumour doubling time was not significantly modified while mean survival was reduced after JNJ10191584 treatment (Figure 9). On the other hand, JNJ10181457 reduced tumour growth without modifying survival (Figure 9). None of these compounds significantly altered lung metastases (Figure 9C).
Figure 9.
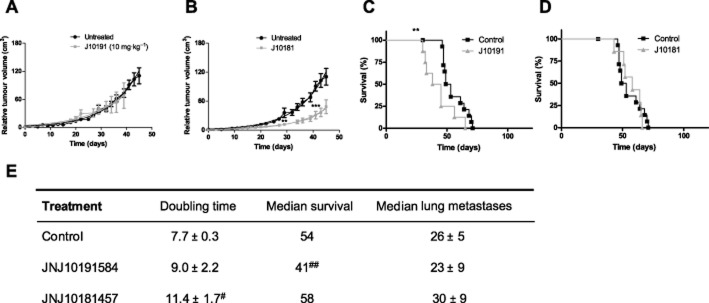
Effect of H4R and H3R antagonists on MDA-MB-231 xenografted tumour into nude mice. Relative tumour volume of the control group versus (A) JNJ10191584 (JNJ1019, 10 mg kg−1), (B) JNJ10181457 (JNJ1018, 10 mg kg−1). Data are shown as means ± SEM. Tumour volumes were measured three times a week and a non-linear regression fit was performed to evaluate the exponential growth (**P < 0.01, ***P < 0.001, vs. Control; T-test). Kaplan–Meier survival of the control group versus (C) JNJ10191584 (JNJ1019, 10 mg kg−1), (D) JNJ10181457 (JNJ1018, 10 mg kg−1). (**P < 0.01, Log rank test and Gehan-Breslow-Wilcoxon test). (E) Median tumour doubling time, median survival and median number of lung metastases of each group are depicted numerically (#P < 0.05, ##P < 0.01 vs. Control).
Discussion
H4R is expressed in human breast tissues and cell lines, exhibiting a key role in histamine-mediated biological processes such as cell proliferation, senescence, and apoptosis in malignant cells (Medina et al., 2008; 2010a; 2011).
In the present study, we aimed at investigating the therapeutic potential of H4R agonist on a triple-negative human breast cancer experimental model.
Histamine binds to the H4R with high affinity, comparable to that of the specific H4R ligands, VUF8430 (agonist) and JNJ7777120 (antagonist) (Lim et al., 2009) and, it has been the most widely used agonist. However, this biogenic amine has cross-reactivity with all four histamine receptors. Clozapine is an atypical antipsychotic, which showed high affinity for the H4R and several works support its use as H4R agonist in vitro and in vivo (Oda et al., 2000; Zhu et al., 2001; Lim et al., 2005; van Rijn et al., 2008; Leurs et al., 2009). The rationality of using clozapine in this study was that this drug has been used for a long time and is clinically available and approved for use in humans. Although not exempt from side effects (e.g. agranulocytosis), they are fully studied and recent epidemiological studies firmly recommend re-evaluating the restrictions for the use of the drug (Vera et al., 2012). In order to evaluate whether clozapine behaves as an H4R agonist, the H4R antagonist JNJ7777120 was employed. It showed more than a thousand fold selectivity over other histamine receptor subtypes and was considered an H4R reference antagonist (Leurs et al., 2009). The inhibitory effect on proliferation induced by clozapine was completely blocked by the combined treatment with the specific H4R antagonist JNJ7777120, confirming that clozapine behaves as an H4R agonist in this model and shows similar effects of other H4R agonists evaluated in these cells (Medina et al., 2011). In addition, in order to confirm the role of H4R in the anti-tumoural effect, a more selective H4R agonist, JNJ28610244 compound, was employed in vitro and also in vivo. This experimental compound has excellent potency and selectivity for the H4R and thus, serves as a useful pharmacological tool for exploring and better understanding H4R function. In addition, JNJ28610244 seems to act as an agonist in vivo as evidenced by its scratching induction in wild-type mice that was not present in H4R-deficient mice (Yu et al., 2010).
JNJ28610244 compound also decreases in vitro cell proliferation dose dependently, an effect that was completely blocked with the H4R antagonist. Moreover, histamine regulates proliferation in a concentration dependent manner, exhibiting an anti-proliferative effect at micromolar concentrations. This effect was partially reversed when cells were treated with the specific antagonist of the H4R, JNJ7777120. These results demonstrate that the activation of H4R is associated to the inhibition of proliferation in MDA-MB-231 cells and are in agreement with previous data (Medina et al., 2008; 2011). Furthermore, the use of siRNA specific for H4R messenger RNA blocked the histamine-induced decreased proliferation in MDA-MB-231 cells. The anti-proliferative effect is associated to an induction of cell apoptosis as it was previously reported (Medina et al., 2011). In this regard, similar responses were reported in human cell lines of pancreatic carcinoma and melanoma, in which histamine, via H4R, inhibits proliferation and modulates cell differentiation (Cricco et al., 2008; Massari et al., 2011). Conversely, H4R was detected in human colon cancer cell lines (HT29, Caco-2, HCT-116), in which histamine exerts both a pro-proliferative and a pro-angiogenic effect via H2R/H4R activation (Cianchi et al., 2005). However, current studies indicate that H4R agonists produce anti-proliferative effects on WiDr colon cancer cells (data not shown). Though these reports make unquestionable the presence of functional H4R in human cancer tissues, the precise role of H4R in cell proliferation seems to be cancer type dependent and must be further investigated.
To test whether H4R agonists modulated the growth of the human breast cancer cell line in vivo, xenograft tumours of MDA-MB-231 were developed into nude mice. In vivo experiments are in agreement with the in vitro studies. Results demonstrate that the employed H4R agonists significantly slowed down the tumour growth rate, evidenced by an increase in the exponential doubling time. Interestingly, in vivo administration of the JNJ10181457, an H3R antagonist, produced a decrease in tumour volume, which could be associated to the increase in MDA-MB-231 cell proliferation exerted through the H3R that was previously reported (Medina et al., 2008).
The developed highly aggressive and very undifferentiated tumours constitutively express the H4R, and exhibit elevated histamine content and high levels of PCNA expression. H4R levels appear to be down regulated in clozapine and JNJ28610244-treated animals.
The reduced tumour growth is associated with a decrease in the expression level of the proliferation marker, PCNA, which was similar in the three H4R agonist groups. Comparable results were observed in M1/15 melanoma cell tumour-bearing nude mice, in which sc daily histamine or clozapine injections suppress tumour growth and decrease PCNA expression (Massari et al., unpubl. data). Furthermore, the H4R agonist clobenpropit inhibited in vivo human cholangiocarcinoma xenograft tumour growth (Meng et al., 2011).
Breast tumour cells are relatively refractory to apoptosis in response to conventional therapies (Jasinski et al., 2008). The promotion of apoptosis is thought to be critical for the effectiveness of anti-cancer therapies. Therefore, apoptosis was investigated in developed tumours. In vitro studies show that H4R agonists enhance MDA-MB-231 cell apoptosis as it was previously reported (Medina et al., 2011), indicating that the activation of H4R produces an apoptotic effect. However, in vivo studies result in more complex picture and only histamine increases significantly the number of tumoural apoptotic cell. Considering that histamine could activate other histamine receptor subtypes, the enhanced histamine-induced apoptosis is in agreement with recent results showing that histamine also enhances apoptosis through the H1R in MDA-MB-231 cells (Martinel Lamas et al., unpublished data). Furthermore, this difference might be associated with the fact that clozapine and JNJ28610244 treatments reduce tumoural expression levels of the H4R. In this line, there are some reports that indicate that the level of expression of the H4R in colon cancer decreases with the advancement of the disease (Fang et al., 2011).
Angiogenesis is a vital process required for solid tumours to expand. In this regard, the diminished tumour growth could also be related with a decrease in the number of vessels, evaluated by Masson trichrome staining, surrounding the tumours of mice treated with histamine and clozapine. No difference in the intratumoural vascularization was observed in JNJ28610244-treated animals compared to untreated ones. Several experimental data demonstrate that histamine is involved in angiogenesis (Ohtsu and Watanabe, 2003). Histamine is reported as an angiogenic factor in different tissues, including the granulation tissue (Ghosh et al., 2001). Also, H2R antagonists (roxatidine and cimetidine) were found to exert suppressive effects on colon cancer implants growth in mice by inhibiting angiogenesis (Tomita et al., 2003). On the other hand, no changes in angiogenesis (evaluated by changes in CD31 immunoreactivity) were detected in a cholangiocarcinoma model after histamine treatment (Francis et al., 2012). In line with present results, it was previously reported that histamine in combination with interleukin-2 inhibited tumour growth and angiogenesis in rat malignant glioma model. Histamine caused an early and pronounced decline in tumour blood flow compared to normal brain (Johansson et al., 2000). Evidence suggests that histamine may exert both pro- or anti-angiogenic effect depending on concentration, presence of cofactors or tumour microenvironment. The mechanisms involved in histamine and clozapine effect remain unknown and deserve further studies. Understanding the role of histamine in cancer angiogenesis could lead to improvement in the development of therapeutic methods targeting this process.
The reduced vascularisation observed upon histamine and clozapine treatment could explain the differences in tumour doubling time, which is similar in clozapine and histamine-treated groups and both higher than doubling time observed in JNJ28610244-treated animals.
Importantly, only histamine induced a significant increase in the survival rate. This improved response observed with histamine on survival could be linked to the pleitropic action of histamine, acting on other histamine receptor subtypes present in breast cancer cells and/or other cell types, including immune cells and endothelial cells (Medina and Rivera, 2010b). In agreement with our results, histamine also enhances survival of M1/15 melanoma bearing mice (Massari et al., unpubl. data). In addition, histamine dihydrochloride (Ceplene) is being safely used in clinical trials as an adjuvant to immunotherapy for the potential treatment of leukaemia and metastatic melanoma, improving efficacy by increasing survival benefit and exhibiting no unexpected or irreversible side effects (Romero et al., 2009).
Metastasis is the most devastating aspect of cancer, accounting for most deaths from neoplasic diseases. Women with metastatic triple-negative breast cancer relapse quickly, and commonly develop visceral metastasis, including lung, liver and brain metastasis (Rakha and Chan, 2011). Therefore, lung metastatic disease was additionally evaluated. Lung micrometastases were not modified upon histamine and clozapine treatments, while JNJ28620144 increased the number of micrometastases. In this line, H4R agonists increased matrix metalloproteinases 2 and 9 that participate in the proteolysis of basement membrane and extracellular matrix proteins, and also enhanced invasiveness of MDA-MB-231 cells in vitro, which are critical steps for cancer metastases (Cricco et al., 2011).
Present results demonstrate the functional expression of H4R in a breast cancer experimental model and show the anti-tumoural properties of H4R agonists, opening new perspectives in histamine pharmacology research aimed at developing a new generation of antihistamines targeting H4R that may contribute for advances in the treatment of cancer. In agreement with presented results, the in vivo treatment with the H4R antagonist JNJ10191584 decreased survival while not significantly modifying tumour growth or lung metastases.
In addition, it is important to highlight side effects were not observed in the most important organs including bone marrow and liver upon H4R agonists' administration (data not shown).
The identification of safe and effective treatments for cancer therapy is vital to improve quality of life. In this regard, H4R ligands offer therapeutic potential as adjuvants for the treatment of breast cancer. Further basic research on anti-cancer properties of these compounds as well as clinical trials to evaluate the therapeutic efficacy in breast cancer is therefore warranted.
Acknowledgments
This work has been supported by grants from the University of Buenos Aires, 20020110200253, 20020100100270, the National Agency of Scientific and Technological Promotion BID PICT-2007-01022, CONICET (PIP 11220110101121), and from the EU-FP7 COST Action BM0806 (Recent advances in histamine receptor H4R research).
We thank the National Cancer Institute of Argentina for financial support through a fellowship to Eliana Carabajal. The technical assistance of Alejandro Paredes is appreciated. We also thank Miss Natalia Rivera for proofreading the manuscript.
We thank Dr. Nicholas Carruthers from Johnson & Johnson Pharmaceutical Research & Development for the JNJ28610244, JNJ7777120, JNJ10181457, 10191584 compounds.
We dedicate this work to the memory of our friend and colleague, Maximo Croci, MD.
Glossary
- BrdU
5-bromo-2′-deoxyuridine
- ER
oestrogen receptor
- H1R
histamine receptor 1
- H2R
histamine receptor 2
- H3R
histamine receptor 3
- H4R
histamine receptor 4
- siRNA
small interfering RNA
Conflict of interest
The authors state no conflict of interest.
References
- Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Schiavone N, Perna F, Magnelli L, Fanti E, et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin Cancer Res. 2005;11:6807–6815. doi: 10.1158/1078-0432.CCR-05-0675. [DOI] [PubMed] [Google Scholar]
- Cricco G, Mohamad N, Sáez MS, Valli E, Rivera ES, Martín G. Histamine and breast cancer: a new role for a well known amine. In: Gunduz M, editor. Breast Cancer – Carcinogenesis, Cell Growth and Signalling Pathways. Rijeka: InTech; 2011. pp. 611–634. [Google Scholar]
- Cricco GP, Mohamad NA, Sambuco LA, Genre F, Croci M, Gutiérrez AS, et al. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm Res. 2008;57:S23–S24. doi: 10.1007/s00011-007-0611-5. [DOI] [PubMed] [Google Scholar]
- Darvas Z, Sakurai E, Schwelberger HG, Hegyesi H, Rivera E, Othsu H, et al. Autonomous histamine metabolism in human melanoma cells. Melanoma Res. 2003;13:239–246. doi: 10.1097/00008390-200306000-00003. [DOI] [PubMed] [Google Scholar]
- Davio CA, Cricco GP, Andrade N, Bergoc RM, Rivera ES. H1 and H2 histamine receptors in human mammary carcinomas. Agents Actions. 1993;38:C172–C174. [Google Scholar]
- De Esch JP, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26:462–469. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Fang Z, Yao W, Xiong Y, Li J, Liu L, Shi L, et al. Attenuated expression of HRH4 in colorectal carcinomas: a potential influence on tumor growth and progression. BMC Cancer. 2011;11:1–11. doi: 10.1186/1471-2407-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Hirasawa N, Ohuchi K. Enhancement by histamine of vascular endothelial growth factor production in granulation tissue via H(2) receptors. Br J Pharmacol. 2001;134:1419–1428. doi: 10.1038/sj.bjp.0704372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–284. [PubMed] [Google Scholar]
- Huang JF, Thurmond RL. The new biology of histamine receptors. Curr Allergy Asthma Rep. 2008;8:21–27. doi: 10.1007/s11882-008-0005-y. [DOI] [PubMed] [Google Scholar]
- Jasinski P, Terai K, Zwolak P, Dudek AZ. Enzastaurin renders MCF-7 breast cancer cells sensitive to radiation through reversal of radiation-induced activation of protein kinase C. Eur J Cancer. 2008;44:1315–1322. doi: 10.1016/j.ejca.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:227–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Johansson M, Henriksson R, Bergenheim AT, Koskinen LO. Interleukin-2 and histamine in combination inhibit tumour growth and angiogenesis in malignant glioma. Br J Cancer. 2000;83:826–832. doi: 10.1054/bjoc.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson G, Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968;48:155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos B, Davio C, Gass H, Gonzalez P, Cricco G, Martin G, et al. Histamine receptors in human mammary gland, different benign lesions and mammary carcinomas. Inflamm Res. 1995;44:S68–S69. doi: 10.1007/BF01674400. [DOI] [PubMed] [Google Scholar]
- Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Lim HD, Adami M, Guaita E, Werfel T, Smits RA, Esch IJ, et al. Pharmacological characterization of the new histamine H4 receptor agonist VUF 8430. Br J Pharmacol. 2009;157:34–43. doi: 10.1111/j.1476-5381.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari NA, Medina VA, Martinel Lamas DJ, Cricco GP, Croci M, Sambuco L, et al. Role of H4 receptor in histamine-mediated responses in human melanoma. Melanoma Res. 2011;21:395–404. doi: 10.1097/CMR.0b013e328347ee53. [DOI] [PubMed] [Google Scholar]
- Medina V, Cricco G, Nuñez M, Martín G, Mohamad N, Correa-Fiz F, et al. Histamine-mediated signaling processes in human malignant mammary cells. Cancer Biol Ther. 2006;5:1462–1471. doi: 10.4161/cbt.5.11.3273. [DOI] [PubMed] [Google Scholar]
- Medina V, Croci M, Crescenti E, Mohamad N, Sanchez-Jiménez F, Massari N, et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010a;61:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina VA, Rivera ES. Histamine as a potential adjuvant to immuno and radiotherapy for cancer treatment: discovering new functions for the oldest biogenic amine. Curr Immunol Rev. 2010b;6:357–370. [Google Scholar]
- Medina VA, Brenzoni PG, Martinel Lamas DJ, Massari N, Mondillo C, Nunez MA, et al. Role of histamine H4 receptor in breast cancer cell proliferation. Front Biosci. 2011;18:1042–1060. doi: 10.2741/e310. [DOI] [PubMed] [Google Scholar]
- Meng F, Han Y, Staloch D, Francis T, Stokes A, Francis H. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology. 2011;54:1718–1728. doi: 10.1002/hep.24573. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Watanabe T. New functions of histamine found in histidine decarboxylase gene knockout mice. Biochem Biophys Res Commun. 2003;305:443–447. doi: 10.1016/s0006-291x(03)00696-x. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pós Z, Hegyesi H, Rivera ES. Histamine and cell proliferation. In: Falus A, editor. Histamine: Biology and Medical Aspects. SpringMed Publishing: Budapest; 2004. pp. 199–217. [Google Scholar]
- Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol) 2011;23:587–600. doi: 10.1016/j.clon.2011.03.013. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, van Marle A, Chazot PL, Langemeijer E, Qin Y, Shenton FC, et al. Cloning and characterization of dominant negative splice variants of the human histamine H4 receptor. Biochem J. 2008;414:121–131. doi: 10.1042/BJ20071583. [DOI] [PubMed] [Google Scholar]
- Rivera ES, Cricco GP, Engel NI, Fitzsimons CP, Martín GA, Bergoc RM. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol. 2000;10:15–23. doi: 10.1006/scbi.2000.0303. [DOI] [PubMed] [Google Scholar]
- Romero AI, Thoren FB, Aurelius J, Askarieh G, Brune M, Hellstrand K. Post-consolidation immunotherapy with histamine dihydrochloride and interleukin-2 in AML. Scand J Immunol. 2009;70:194–205. doi: 10.1111/j.1365-3083.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Neo S, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiligada E, Zampeli E, Sander K, Stark H. Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs. 2009;18:1519–1531. doi: 10.1517/14728220903188438. [DOI] [PubMed] [Google Scholar]
- Tomita K, Izumi K, Okabe S. Roxatidine- and cimetidine-induced angiogenesis inhibition suppresses growth of colon cancer implants in syngeneic mice. J Pharmacol Sci. 2003;93:321–330. doi: 10.1254/jphs.93.321. [DOI] [PubMed] [Google Scholar]
- Vera I, Rezende L, Molina V, Sanz-Fuentenebro J. Clozapine as treatment of first choice in first psychotic episodes. What do we know? Actas Esp Psiquiatr. 2012;40:281–289. [PubMed] [Google Scholar]
- Yu F, Wolin RL, Wei J, Desai PJ, McGovern PM, Dunford PJ, et al. Pharmacological characterization of oxime agonists of the histamine H4 receptor. J Receptor Ligand Channel Res. 2010;3:37–49. [Google Scholar]
- Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, et al. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]