Abstract
Background and Purpose
Among the pathogenic mechanisms of asthma, a role for oxidative/nitrosative stress has been well documented. Recent evidence suggests that histamine H4 receptors play a modulatory role in allergic inflammation. Here we report the effects of compound JNJ 7777120 (JNJ), a selective H4 receptor antagonist, on antigen-induced airway inflammation, paying special attention to its effects on lipocortin-1 (LC-1/annexin-A1), a 37 kDA anti-inflammatory protein that plays a key role in the production of inflammatory mediators.
Experimental Approach
Ovalbumin (OA)-sensitized guinea pigs placed in a respiratory chamber were challenged with antigen. JNJ (5, 7.5 and 10 mg·kg−1) was given i.p. for 4 days before antigen challenge. Respiratory parameters were recorded. Bronchoalveolar lavage (BAL) fluid was collected and lung specimens taken for further analyses 1 h after antigen challenge. In BAL fluid, levels of LC-1, PGD2, LTB4 and TNF-α were measured. In lung tissue samples, myeloperoxidase, caspase-3 and Mn-superoxide dismutase activities and 8-hydroxy-2-deoxyguanosine levels were measured.
Key Results
OA challenge decreased LC-1 levels in BAL fluid, induced cough, dyspnoea and bronchoconstriction and increased PGD2, LTB4 and TNF-α levels in lung tissue. Treatment with JNJ dose-dependently increased levels of LC-1, reduced respiratory abnormalities and lowered levels of PGD2, LTB4 and TNF-α in BAL fluid.
Conclusions and Implications
Antigen-induced asthma-like reactions in guinea pigs decreased levels of LC-1 and increased TNF-α and eicosanoid production. JNJ pretreatment reduced allergic asthmatic responses and airway inflammation, an effect associated with LC-1 up-regulation.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: histamine H4 receptors, JNJ 7777120, asthma-like reaction, airway inflammation, lipocortin-1, cytokines, prostaglandins, oxidative stress, apoptosis
Introduction
Bronchial hyper-responsiveness and inflammation are characteristic of acute asthma and serve to trigger events of adverse airway remodelling, which include loss of airway epithelium, goblet cell and smooth muscle cell hyperplasia, and fibrosis (Rennard, 1996). Within the complex framework of mechanisms involved in the pathogenesis of asthma, a key role for oxidative/nitrosative stress, epithelial cell apoptosis and airway inflammation has been well documented (Andreadis et al., 2003). Besides the classical allergen-dependent pathways, reactive oxygen and nitrogen species produced during airway inflammation activate neutrophils, eosinophils and mast cells to release histamine, prostanoids and cytokines, which exacerbate airway hyperresponsiveness and favour the transition to subacute/chronic disease (Comhair et al., 2005).
The inflammatory responses resulting from the release of histamine have, for many years, been thought to be mediated by histamine H1 receptors (receptor nomenclature follows Alexander et al., 2011). Consequently, H1 receptor antagonists have been used as major components of the treatment of allergies. Recent evidence suggests that histamine can play diverse roles in inflammation and immune responses through the activation of another histamine-activated GPCR, the H4 receptor, expressed on dendritic and inflammatory cells (Thurmond et al., 2008).
The discovery of the histamine H4 receptors has given investigators new tools to further address the function of histamine and its receptors in allergic and inflammatory processes. Thus, histamine, acting through H4 receptors, induces chemotaxis of murine mast cells in vitro (Hofstra et al., 2003), and leads to changes of their tissue localization in vivo (Thurmond et al., 2004). Both effects are consistent with the previously reported distribution of mast cells in the epithelial lining of nasal mucosa in patients with rhinitis induced by allergen (Slater et al., 1996). Activation of histamine H4 receptors also induces chemotaxis of human eosinophils, enhances the effect of chemotactic agents and stimulates up-regulation of adhesion molecules (Ling et al., 2004). Indeed, H4 receptor expression and function on mast cells, eosinophils, basophils, dendritic and T-cells suggests that this receptor may play a role in allergen-induced asthmatic responses.
In a murine model of ovalbumin (OA)-induced asthma-like reaction, H4 receptors were involved in the activation of CD4+ cells by dendritic cells (Dunford et al., 2006). The administration of JNJ7777120 (JNJ), a novel selective H4 receptor antagonist, showed significant anti-inflammatory effects during both the sensitization and effector phases. These findings indicate that H4 receptors are also involved in the initial priming of the immune system after allergen challenge (Thurmond et al., 2008).
Indeed, the poor clinical efficacy of H1 receptor blockers in asthma would suggest the possible involvement of other histamine receptors in this disease and H4 receptor-deficient mice show reduced lung inflammation and Th2-derived cytokine production upon allergen challenge (Dunford et al., 2006). Moreover, the recent finding that resolution of an inflammatory reaction is an active phenomenon brought out by endogenous anti-inflammatory mediators, such as lipocortin-1 (LC-1), also known as annexin-A1, has opened new pathways to understand the mechanism of action of anti-inflammatory drugs (D'Acquisto et al., 2008). LC-1, a glucocorticoid-modulated protein, was initially characterized by its ability to inhibit prostanoid release (Cirino et al., 1987). Further studies indicated that LC-1 also inhibited leukocyte migration, reduced fluid extravasation and exhibited anti-nociceptive activity (Cirino et al., 1989; Perretti and Flower, 1993; Ayoub et al., 2008). The allergic guinea pig model is an old model, still largely used to reproduce the different syndromes presented by human asthma. The animals are sensitized to OA and the bronchial responsiveness, as well as the cough reflex response, is measured after antigen inhalation and this model has been widely used to evaluate the anti-asthmatic effect of class of compounds (Masini et al., 2005; 2007; Giannini et al., 2008; Cinci et al., 2010; Evans et al., 2012).
In this study we investigate the effects of a selective histamine H4 receptor antagonist in reducing airway inflammation and hyper-responsiveness, oxidative stress and epithelial cell apoptosis and the possible interactions with LC-1.
Methods
Animals
All animal care and experimental protocols complied with the Italian and the European Community regulations on animal experimentation for scientific purposes (D.M. 116192; O.J. of E.C. L358/1 12/18/1986) and in agreement with the Good Laboratory Practice. The protocols were approved by the animal care committee of the University of Florence (Florence, Italy). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Sixty male Hartley albino guinea pigs were used. They were purchased from a commercial dealer (Rodentia, Bergamo, Italy) and housed in a controlled environment for 7 days at 22°C with a 12-h light/12-h dark cycle before use. During the experimental time, the animals were maintained under the same conditions and provided with standard chow and water ad libitum. At the end of the treatments, the animals weighed 350 to 400 g.
Animal sensitization and treatment
The guinea pigs were randomly divided into six experimental groups, with 10 animals in each group.
Group 1
Animals were injected with phosphate-buffered saline (PBS, 5 mL·kg−1, i.p., plus 5 mL·kg−1 s.c.) and, 18–21 days later, received an aerosol of OA (Fluka, Buchs, Switzerland) dissolved in saline (5 mg·mL−1). They are referred to as naïve OA-challenged animals and used as negative controls.
Other guinea pigs were sensitized with 100 mg·kg−1, i.p., plus 100 mg·kg−1 s.c. OA dissolved in saline (20 mg·mL−1). After 18–21 days, they were challenged with an OA aerosol (5 mg·mL−1 saline) to check for sensitization by evaluation of allergen-elicited respiratory changes, as described later. The animals were withdrawn from antigen exposure at the first signs of respiratory abnormalities. The guinea pigs reactive to the inhaled antigen were used for the further experiments. Three days after the challenge, the sensitized guinea pigs were randomly divided in the following groups, 10 animals each.
Group 2
Animals were treated with s.c. injections (0.5 mL) of PBS for 4 days before challenge with an aerosol of saline alone. These are referred to as sensitized, unchallenged controls.
Group 3
Animals were treated with s.c. injections (0.5 mL) of PBS for 4 days before challenge with aerosolized OA (5 mg·mL−1 saline). These are referred to as the sensitized, OA-challenged group.
Groups 4–6
Animals were treated with s.c. injections (0.5 mL) of the selective H4 receptor antagonist JNJ7777120 (JNJ; kindly provided by Johnson & Johnson, San Diego, CA, USA; Hill et al., 1997). JNJ was dissolved in PBS as vehicle and tested over three daily doses (5, 7.5 and 10 mg·kg−1) for 4 days before challenge with aerosolized OA. These are referred to as sensitized, JNJ-treated OA-challenged group.
Challenge with inhaled OA and evaluation of respiratory activity
Five guinea pigs from all groups were individually placed in an airtight transparent whole-body plethysmographic chamber, as described previously (Suzuki et al., 2004; Masini et al., 2005). The changes in inner pressure in the respiratory chamber induced by breathing were monitored with a high-sensitivity pressure transducer (P75 type 379, HSE, Heidelberg, Germany) connected to a polygraph (Universal oscillograph, Harvard, Edenbridge, UK). Animals from the naïve group were included in the aerosol challenge to reveal possible breath alterations due to non-specific stimulation of the airways by the aerosol droplets. The respiratory activity of the animals subjected to the different treatments was monitored for 5 min after the onset of aerosol administration and classified according to the previously reported criteria (Suzuki et al., 2004; Masini et al., 2005). In particular, cough was assumed as a transient change in the pressure (a rapid inspiration followed by a rapid expiration), whereas dyspnoea was assumed as a series of irregular breaths of abnormally elevated frequency (tachypnoea) and amplitude or as repeated gasping.
The following parameters were evaluated: (i) latency time for the first cough stroke (s); (ii) cough severity, the product of cough frequency (cough strokes per min) and mean cough amplitude (excess pressure over the normal breath); and (iii) latency time for the onset of dyspnoea (s).
Measurement of airway bronchoconstriction
Anaesthesized guinea pigs from each group (n = 4) were mechanically ventilated by a constant volume method, as reported previously (Masini et al., 2005). Animals were injected with 100 mg·kg−1 b. wt. sodium pentobarbital (Abbott, Latina, Italy) to induce anaesthesia and abolish natural respiration. Body temperature was maintained constant at 37°C. The trachea was cannulated with polyethylene tube (inner diameter, 2 mm), and the animals ventilated with a small-animal respirator (Harvard), adjusted to deliver a tidal volume of 10 mL at a rate of 45 strokes per min. Changes in lung resistance to inflation (as the pressure at the airway opening, PAO) were evaluated as previously reported (Masini et al., 2005). Changes in inflation pressure, which are directly related to airway resistance, were recorded for 5 min after the beginning of OA aerosol and expressed as percentage changes over the basal values. These animals were also used for collection of bronchoalveolar lavage (BAL) fluid, as detailed later.
Post mortem analysis
At the end of the breath recording period, the animals were killed 1 h after aerosol administration with a lethal dose of sodium pentobarbital. No macroscopic alterations in liver or kidney, which could be related to a toxic effect of JNJ treatment, was observed. Lung tissue samples from each animal from the middle and the lower lobes were taken for biochemical and morphological analyses, as described below. BAL fluid was collected by insertion of a cannula into the trachea and instillation of 3 mL of PBS, pH 7.4, with three flushes, into the bronchial tree. BAL fluid was centrifuged at 1100× g for 30 min, the cell-free supernatant was collected, its volume measured and frozen at-70°C until needed.
Histological and morphometrical analysis
Three or four samples of lung tissue from each animal (about 150 mg each) were fixed by immersion in Mota fluid, dehydrated in graded ethanol and embedded in paraffin. Tissue sections, 5 μm thick, were stained with hematoxylin and eosin and used to evaluate the surface area of alveolar air spaces, as described (Masini et al., 2005). Four randomly-chosen microscopical fields per animal (2 fields per section) were analysed. At the chosen magnification, each field corresponds to a tissue area of 570 224 μm2 that includes an average of 300 alveolar profiles. The same tissue sections were used to evaluate the surface area of bronchial lumina, selected by: (i) histological appearance of small-sized, muscular bronchi; and (ii) transverse or slightly oblique cross-section. In each guinea pig, measurements were carried out on four to six randomly chosen bronchi from the tissue sections cut from the two different lung samples, examined with a ×20 objective (test area: 125 400 μm2). For both alveolar and bronchial lumenal areas, digital micrographs of the microscopical fields to be analysed were taken and surface area measurements were carried out using the ImageJ 1.33 free-share image analysis software (http://rsb.info.nih.gov/ij) upon appropriate threshold to include only blank, tissue-free air spaces. The mean values (±SEM) of alveolar and bronchial luminal areas were then calculated for each experimental group.
Immunohistochemistry for eosinophilic major basic protein (eMBP)
Immunohistochemistry for eosinophil identification was carried out on histological sections, 5 μm thick, of Mota-fixed, paraffin-embedded lung tissue fragments. Sections were rehydrated, treated with 0.1% trypsin for 10 min to retrieve antigen, then with 0.3% (v/v) H2O2 in 60% (v/v) methanol to quench endogenous peroxidase, and, finally, incubated overnight with mouse monoclonal anti-human eMBP antibodies (clone BMK13, Chemicon, Temecula, CA, USA; 1:50 working dilution in PBS). Immune reaction was revealed by indirect immunoperoxidase method (Vectastain Elite kit, Vector, Burlingame, CA, USA), using 3,3′-diaminobenzidine as chromogen. As negative controls, sections incubated with only the primary or the secondary antisera were used.
Observations were carried out with a Reichert–Jung Microstar IV light microscope (Cambridge Instruments, Buffalo, NY, USA). In each guinea pig, the number of MBP-positive eosinophils was counted in 10 randomly chosen microscopical fields at a ×200 final magnification (test area: 72 346 μm2). Values obtained from two different observers were averaged.
Evaluation of mast cell granule release
This assay was carried out on histological lung tissue sections, 5 μm thick, stained with Astra blue (Fluka, Buchs, Switzerland). This cationic dye binds specifically to heparin containing mast cell granules and can be used as a probe to evaluate the cells’ content of secretion granules by optical density measurements. Digital images of individual mast cells, randomly taken from the two different lung samples using a ×100 oil immersion objective, were analysed with the ImageJ 1.33 software. In each animal, 30 different mast cells were analysed and the mean optical density value (±SEM) was then calculated for the entire experimental group.
Determination of myeloperoxidase (MPO) activity
MPO, a tissue indicator of leukocyte accumulation into the pulmonary tissue was determined as previously described (Mullane et al., 1985). Frozen lung tissue samples of about 100 mg were homogenized in a solution containing 0.5% hexadecyltrimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and then centrifuged for 30 min at 20 000 g at 4°C. An aliquot (100 μL) of the supernatant was used to determine MPO activity using an Immunoassay kit following the instructions of the manufacturer (PrognostiX Inc., Cleveland, OH, USA). The rate of change in absorbance was measured spectrophotometrically at 450 nm. MPO activity was expressed in mU·mg−1 wet tissue.
Determination of malondialdehyde (MDA)
MDA an end-product of cell membrane lipid peroxidation by reactive oxygen species (ROS), was determined by measurement of the chromogen obtained from the reaction of MDA with 2-thiobarbituric acid (TBA; Aruoma et al., 1989). About 100 mg of lung tissue were homogenized with 1 mL of 50 mM Tris-HCl buffer containing 180 mM KCl and 10 mM EDTA, final pH 7.4. Then, 0.5 mL of TBA (1% w/v) in 50 mM NaOH and 0.5 mL of HCl (25% w/v in water) were added to 0.5 mL of sample. The mixture was placed in test tubes and heated in boiling water for 10 min. After cooling, the chromogen was extracted in 3 mL of 1-butanol, and the organic phase was separated by centrifugation at 2000× g for 10 min. The absorbance of the organic phase was read spectrophotometrically at 532 nm wavelength. Values are expressed as nmol of TBA reactive substances (MDA equivalents) mg−1 of protein, using a standard curve of 1,1,3,3- tetramethoxypropane. Protein concentrations of the homogenates were determined with the Bradford method (Bradford, 1976).
Determination of 8-hydroxy-2′-deoxyguanosine (8-OHdG)
Lung tissue DNA isolation was performed according to Masini et al. (2005). Lung samples were homogenized in 1 mL of 10 mM PBS, pH 7.4, sonicated on ice for 1 min, added with 1 mL of 10 mM Tris-HCl buffer, pH 8, containing 10 mM EDTA, 10 mM NaCl and 0.5% SDS, incubated for 1 h at 37°C with 20 μg·mL−1 RNase 1 (Sigma-Aldrich, Milano, Italy) and overnight at 37°C under argon in the presence of 100 μg·mL−1 proteinase K (Sigma-Aldrich). The mixture was extracted with chloroform/isoamyl alcohol (10/2 v/v). DNA was precipitated from the aqueous phase with 0.2 volumes of 10 M ammonium acetate, solubilized in 200 μL of 20 mM acetate buffer, pH 5.3, and denatured at 90°C for 3 min. The extract was then supplemented with 10 IU of P1 nuclease (Sigma-Aldrich) in 10 μL and incubated for 1 h at 37°C with 5 IU of alkaline phosphatase (Sigma-Aldrich) in 0.4 M phosphate buffer, pH 8.8. All of the procedures were performed in the dark under argon. The mixture was filtered by an Amicon Micropure-EZ filter (Millipore Corporation, Billerica, MA, USA), and 50 μL of each sample was used for 8-OHdG determination using a Bioxytech enzyme immunoassay kit (Oxis, Portland, OR, USA), following the instructions provided by the manufacturer. The values are expressed as ng 8-OHdG mg−1 protein. Protein concentrations of the homogenates were determined with the Bradford method (Bradford, 1976).
Measurement of Mn-superoxide dismutase (Mn-SOD) activity
The frozen lung samples were homogenized with 10 mM phosphate buffered saline, pH 7.4, sonicated on ice for 1 min, and centrifuged at 100× g for 10 min. Supernatants were used for SOD measurement. The assay of Mn-SOD activity was carried out based on SOD-induced inhibition of the conversion of nitro blue tetrazolium (NBT) into formazan mediated by O2•– generated by xanthine–xanthine oxidase mixtures (Nishida et al., 2002). The reaction was performed in sodium carbonate buffer, 50 mM, pH 10.1, containing 0.1 mM EDTA, 25 μM NBT (Sigma), 0.1 mM xanthine and 2 nM xanthine oxidase (Sigma). The rate of reduction of NBT was monitored with a spectrophotometer (Lambda 5, Perkin Elmer, Monza, Italy) set at 560 nm wavelength. The amount required to inhibit the rate of reduction of NBT to formazan by 50% was defined as 1 unit of SOD activity. Specific Mn-SOD activity was determined by preincubating the sample for 30 min with 2 mM NaCN to inhibit total SOD activity. Values are expressed as mU·mg−1 proteins. Protein concentrations of the homogenates were determined with the Bradford method (Bradford, 1976).
Determination of caspase-3 activity
The enzymatic activity of caspase-3 was determined using the Ac-Asp-Glu-Val-Asp-AMC (Ac-DEVD-AMC, Bachem, Bubendorf, Switzerland) fluorescent substrate (Stennicke and Salvesen, 1997). Lung tissue samples were homogenized with 10 mM HEPES (pH 7.4), containing 0.5% 3-[ (3-cholamido-propyl)dimethylammonio]-1-propane-sulfonate (CHAPS), 42 mM KCl, 5 mM MgCl2, 1 mM DTT, 1 mM PMSF, 2 μg·mL−1 leupeptin and 1 μg·mL−1 pepstatin A. The homogenate was then centrifuged at 10 000× g for 10 min. The supernatants (containing 250 μg total protein) were incubated with 40 μM of Ac-DEVD-AMC for 60 min at 37°C. Substrate cleavage was monitored fluorometrically (Spectrofluo JY3 D, Jobin Yvon, Paris, France) at 380 nm excitation and 460 nm emission wavelengths. Data are expressed as arbitrary units mg−1 protein. One unit of enzyme activity is defined as the amount of enzyme required to liberate 40 μmol of Ac-DEVD-AMC upon 60 min at 37°C. Protein concentrations of the homogenates were determined with the Bradford method (Bradford, 1976).
Reverse transcriptase-PCR (RT-PCR)
Total RNA was isolated according to the manufacturer's protocol (Trireagent, Sigma, Milano, Italy) and reverse transcribed (Omniscript, Qiagen, Milano, Italy) by using random primers. The RNA purity was validated by PCR and gel electrophoresis by using primers for the GAPDH gene. A typical PCR reaction (HotStarTaq, Qiagen) was prepared for amplification of LC-1, TNFα and IL-4 mRNA, and calibration was performed by amplification of the same cDNA sample with primers for GAPDH mRNA. Primer sequences were as follows: LC-1 5-AAGCCGGAGAAAGGAGAAAG-3 (sense) and 5-TCTCCCTTGGTTTCATCCAG-3 (antisense), product size 379 pb; TNF-α 5-CTCATGTTGTGGCAAACCAG-3 (sense) and 5-CTCCCAGGTAGATGG TTCA-3 (antisense) product size 321 pb; IL-4 5-CATCGGCATTTTGAACGAGNG TCA-3′ (sense) 5′-CTTATCGATGAATCCAGGCATCG-3′ (antisense) GAPDH, 5-GTCGGTTGTGGATCTGACCT-3 (sense) and 5-TGCTGTAGCCGAACTCATTG-3 (antisense) product size 245 pb (Public Software Primer3; Baguet and Bix, 2004). Amplification was performed as follows: 30 s denaturation at 94°C, 30 s annealing at 56°C, 1 min extension at 72°C for 30 cycles for LC-1, TNF-α and GAPDH genes; 30 s annealing at 60°C, and 1 min extension at 72°C for 45 cycles for IL-4 gene. Amplification products were highlighted with ethidium bromide on 1.5% agarose gel. The intensities of the bands were quantified by densitometric analysis using the ImageJ 1.33 software.
Western blot analysis for LC-1 and TNF-α protein
Total proteins from the lung samples were obtained as described previously (Cianchi et al., 2006). Lung tissues were placed in lysis buffer (0.9% NaCl, 20 mmol·L−1 Tris-HCl pH 7.6, 0.1% Triton X-100, 1 mmol·L−1 PMSF, and 0.01% leupeptin) and homogenized. The total proteins (70 μg, as measured by the bicinchoninic acid assay) were subjected to Western blotting analysis as previously described (Cianchi et al., 2004). The primary antibodies used were rabbit polyclonal anti-LC-1 (Alexis Biochemicals; 1:250 working dilution), mouse monoclonal anti–TNFα (Santa Cruz Biotechnology; 1:500 working dilution), and goat polyclonal anti-β actin (Santa Cruz Biotechnology; 1:1000 working dilution). Binding of each primary antibody was determined by the addition of suitable peroxidase-conjugated secondary antibodies (Amersham; anti-mouse and anti-rabbit antibodies, 1:5000 working dilution; anti-goat antibody, 1:10 000 working dilution). Densitometric analysis was carried out with the ImageJ software.
Determination of LC-1, TNF-α, IL-4 PGD2, LTB4 in BAL fluid
The levels of LC-1, PGD2, LTB4 and the Th1/Th2 prototypic cytokines TNF-α and IL-4 were measured in BAL fluid of four animals per group using commercial elisa kits (Cayman Chemical, Ann Arbor, MI, USA), following the protocol provided by the manufacturer. Results are expressed as ng substance mL−1 BAL fluid.
Data analysis
Data are expressed as mean ± SEM. Statistical analysis was performed by one-way ANOVA, followed by Student's-Newman–Keuls multiple comparison post hoc test; P < 0.05 was considered significant. Calculations were done using a Graph-Pad Prism 2.0 statistical program (GraphPad Software, San Diego, CA, USA).
Results
Evaluation of respiratory activity
The results of the plethysmographic assay are shown in Figure 1A–C. The non-sensitized guinea pigs (group 1) after inhalation of OA aerosol showed no abnormal breathing. Similar results were obtained with the sensitized guinea pigs exposed to aerosolized saline (group 2). The sensitized, vehicle-treated animals (group 3) showed significant alterations of breathing pattern after exposure to OA aerosol, characterized by severe cough episodes and early onset of dyspnoea. On the contrary, the sensitized animals treated with increasing doses of JNJ before exposure to OA showed a significant reduction in respiratory alterations compared with the vehicle-treated animals. An increase in the latency for dyspnea and a decrease in cough severity was observed.
Figure 1.

Evaluation of respiratory activity in animals from different experimental groups. Latency for the onset of bronchospasm (A); cough severity (B); latency for the onset of dyspnoea (C). Treatment with increasing doses of JNJ reduces the alterations found in sensitized guinea pigs challenged with OA. Bronchoconstriction, evaluated as PAO (D), was significantly reduced by treatment with increasing doses of JNJ. Values are the mean ± SEM per group, n = 10. *P < 0.05, **P ≤ 0.01 and ***P ≤ 0.001, significantly different from OA.
Bronchoconstriction, assessed as airflow resistance to passive ventilation (as PAO) in the anaesthetized animals, was a prominent feature in the sensitized, vehicle-treated animals (group 3), and was significantly and dose-dependently decreased by treatment with JNJ (Figure 1D).
Histological and morphometric analysis
Microscopic evaluation of lung tissue in non-sensitized guinea pigs (Figure 2A) and in those sensitized, but not exposed to OA (Figure 2B) showed normal histological features, while the sensitized, vehicle-treated animals exposed to OA (group 3) showed a reduction of the bronchiolar lumen and dilatation of the alveolar air spaces (Figure 2C). These changes were not present in histological sections from the sensitized animals treated with increasing doses of JNJ (Figure 2D–F). The morphometric analysis of the bronchial and alveolar area confirmed the visual observations: JNJ treatment significantly inhibited the bronchial lumen constriction and the alveolar inflation observed in the sensitized, vehicle-treated animals (Figure 2G,H). The morphometric analysis of lung mast cells revealed a significant reduction in optical density – related to granule discharge – in the sensitized, vehicle-treated, OA-challenged animals (group 3) compared with the controls of groups 1 and 2 (Figure 3A–G). In the animals treated with increasing JNJ doses, the optical density of lung mast cells was significantly increased compared with the animals of group 3, clearly indicating that JNJ significantly reduced mast cell granule secretion.
Figure 2.
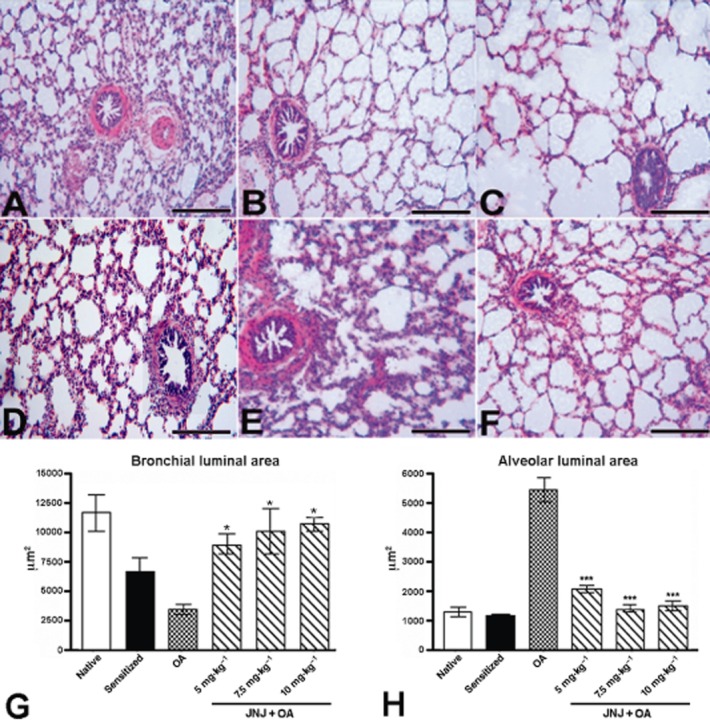
Sections of lung tissue from different experimental groups examined by light microscopy. Non-sensitized guinea pigs (A) and sensitized animals not exposed to OA (B) showed normal histological features. Conversely, in the sensitized guinea pigs challenged with OA aerosol (C), the lumen of bronchioles is considerably reduced and the alveolar spaces greatly increased. In the guinea pigs treated with JNJ at doses of 5, 7.5 and 10 mg·kg−1 (D–F), the intrapulmonary bronchioles and most of the alveolar spaces appear regular. Haematoxylin-eosin staining, bars = 100 μm. Morphometric analysis of bronchial (G) and alveolar (H) luminal areas in lung tissue sections. The samples from sensitized guinea pigs challenged with OA show a significant increase of decrease in mean luminal bronchial and alveolar areas. These changes were significantly reduced in the animals treated with increasing doses of JNJ. Values are the mean ± SEM per group, n = 6. *P ≤ 0.05 and ***P ≤ 0.001, significantly different from OA.
Figure 3.
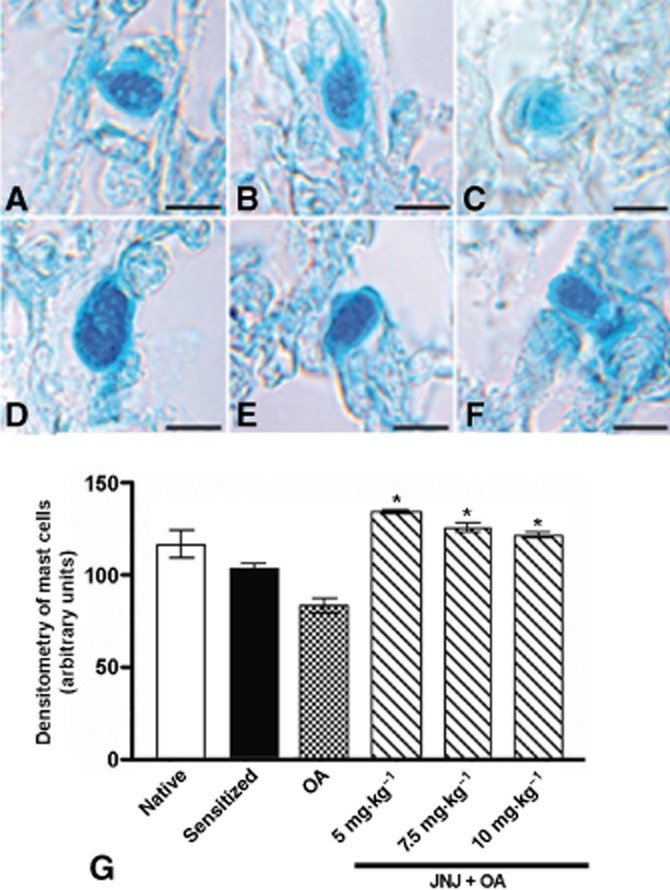
Astra-blue staining (A–F) and densitometric analysis (G) of lung mast cells. Compared with the controls (A,B), a prominent reduction of mast cells staining, directly related to granule discharge, is clearly visible in sections from the sensitized guinea pigs challenged with OA (C). These changes were significantly reduced in animals treated with JNJ at doses of 5, 7.5 and 10 mg·kg−1 (D–G). Values are the mean ± SEM per group, n = 6. *P ≤ 0.05, significantly different from OA. Bars = 10 μm.
Immunohistochemistry of eMBP and evaluation of MPO activity
As shown in Figure 4, the number of eMBP-positive eosinophils and the activity of MPO, a marker of leukocyte infiltration in lung tissue were significantly increased in the lungs from sensitized, vehicle-treated, OA-challenged animals (group 3) compared with the control groups 1 and 2. Treatment of the animals with JNJ decreased the inflammatory response, as indicated by the significant reduction in the number of immunoreactive eosinophils and the values of the MPO.
Figure 4.
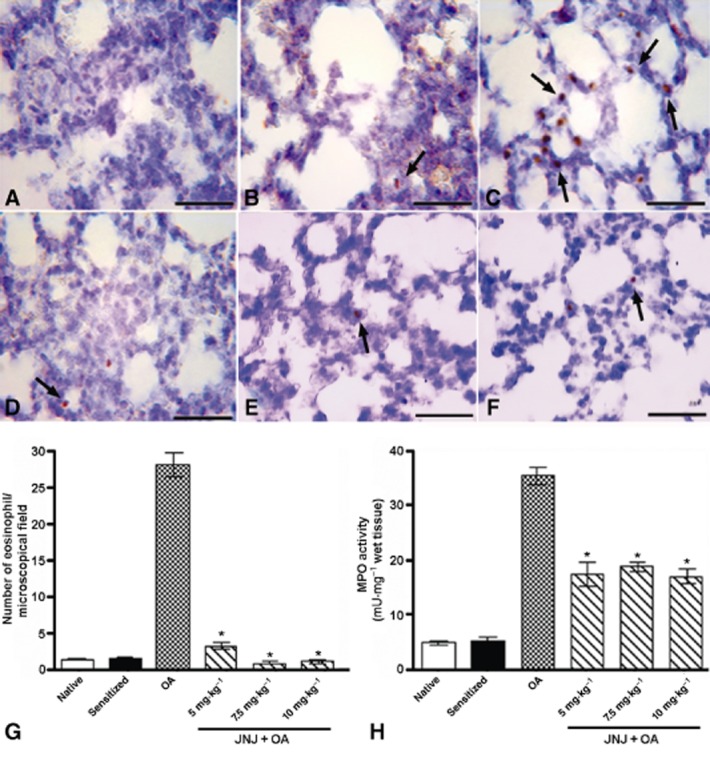
eMBP immunostaining (A–F) and relative densitometric analysis (G); MPO activity quantification (H). The treatment with JNJ at doses of 5, 7.5 and 10 mg·kg−1 body weight (D–F), attenuated the inflammatory response induced by OA challenge (C) in sensitized guinea pigs, reducing the number of eosinophils positive for eMBP (indicated by arrows) (G) and the activity of neutrophil marker MPO (H). Values are the mean ± SEM per group, n = 6. *P ≤ 0.001, significantly different from OA. Bars = 100 μm.
Determination of MDA production, 8-OHdG levels and Mn-SOD activity
The production of MDA (Figure 5A) evaluated as TBA equivalent, a marker of ROS-induced lipoperoxidation damage, was markedly increased in the lung tissue of sensitized, vehicle-treated guinea pigs exposed to OA (Group 3) compared with the control groups 1 and 2. In the animals treated with JNJ, the production of MDA was significantly decreased compared with the animals of group 3. Similar results were also observed by evaluation of the tissue levels of 8-OHdG (Figure 5B), a marker of oxidative DNA damage, while Mn-SOD activity (Figure 5C), which is inversely related to ROS-mediated tissue injury, was significantly increased by JNJ treatment.
Figure 5.
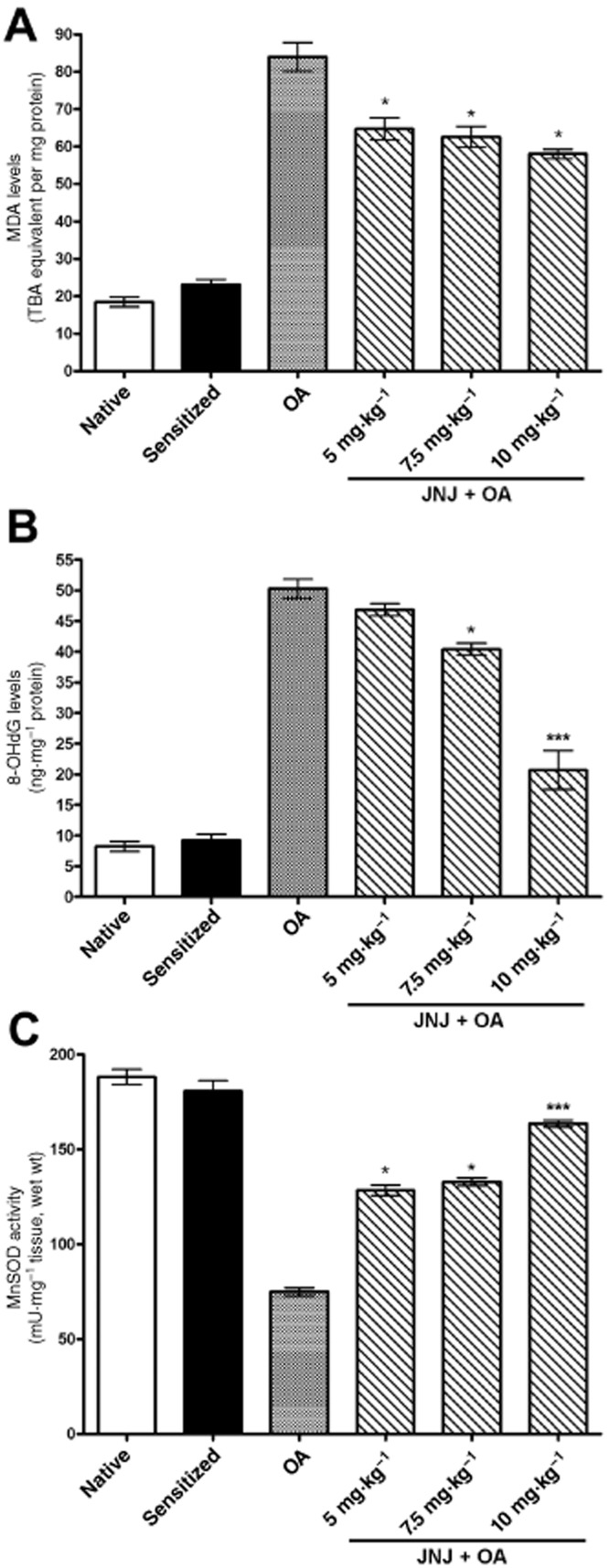
Lung tissue levels of MDA (A) and 8-OHdG (B), key markers of oxidative injury, are significantly increased, while the activity of Mn-SOD (C) is significantly decreased in the sensitized guinea pigs challenged with OA, compared with the controls. Treatment with increasing doses of JNJ caused a significant, dose-related reversal of the noted parameters. Values are the mean ± SEM per group, n = 10. *P ≤ 0.01, ***P ≤ 0.001, significantly different from OA.
Determination of caspase-3 activity
The activity of caspase-3 (Figure 6), a marker of apoptosis, was significantly increased in the lung tissue of sensitized, vehicle treated guinea pigs exposed to OA (Group 3) compared with the control groups 1 and 2. In the samples from guinea pigs treated with JNJ, caspase-3 activity was significantly decreased compared with group 3.
Figure 6.
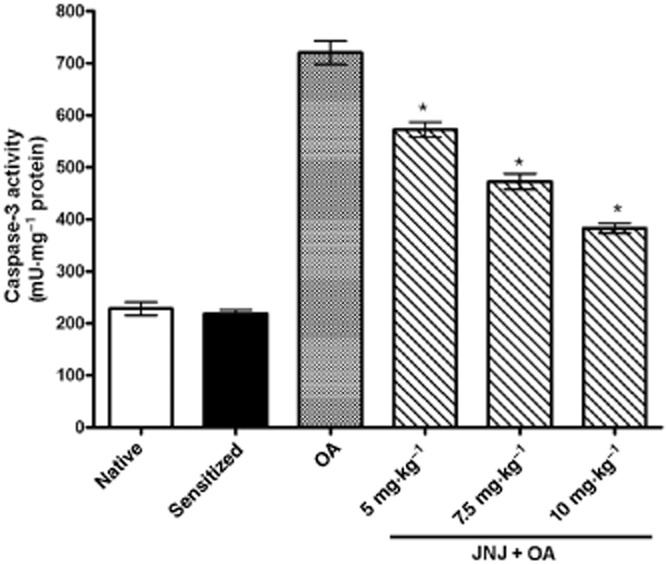
Lung tissue caspase-3 activity, a marker of apoptosis, is significantly increased in the sensitized guinea pigs challenged with OA as compared with the controls. Treatment with increasing doses of JNJ caused a significant, dose-related reversal of the noted parameters. Values are the mean ± SEM per group, n = 6. *P ≤ 0.01, significantly different from OA.
Evaluation of LC-1 expression
The levels of LC-1 transcript, evaluated by RT-PCR, were significantly decreased in lung tissue of sensitized, vehicle-treated guinea pigs exposed to OA (group 3) compared with the control groups 1 and 2. In the samples from guinea pigs treated with JNJ (10 mg·kg−1) the expression of LC-1 mRNA and protein was significantly increased compared with group 3 (Figure 7A). Consistently, the levels of LC-1 in BAL fluid were significantly and in a dose-related way increased in the samples taken from animals treated with JNJ (Figure 7B).
Figure 7.
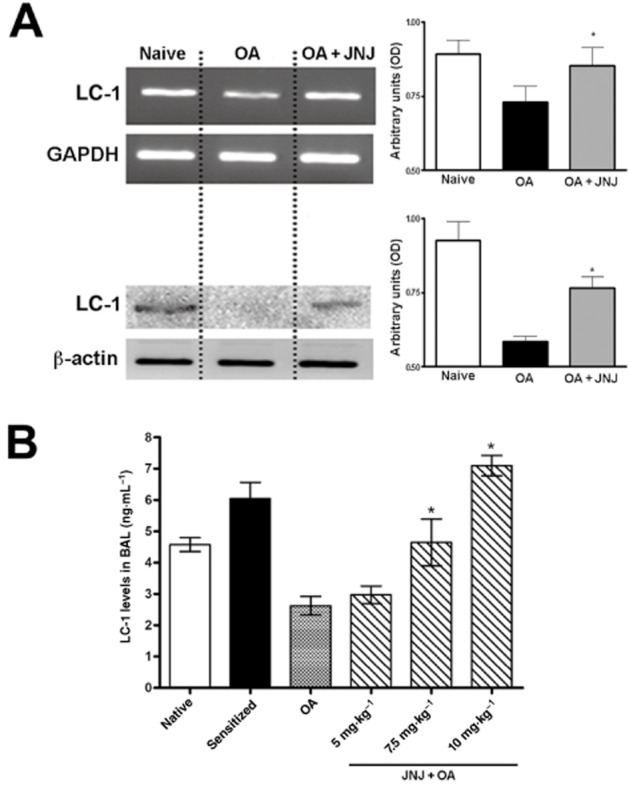
mRNA for lipocortin-1 and protein levels in lung tissue samples (A), assessed by RT-PCR and Western blotting, respectively, are significantly decreased in the sensitized guinea pigs challenged with OA, but not in those treated with 10 mg·kg−1 JNJ. Similarly, lipocortin-1 levels immunodetected in BAL fluid (B) are markedly decreased in the sensitized guinea pigs challenged with OA, while JNJ at the doses of 7.5 and 10 mg·kg−1 reversed such decreases. Values are the mean ± SEM per group, n = 6. *P ≤ 0.01, significantly different from OA.
Concentration of TNF-α, PGD2, LTB4 and IL-4 in BAL
As shown in Figure 8A–D, the levels of the inflammatory cytokines TNF-α and IL-4, the chemotactic agent LTB4 and the mast cell-derived PGD2 were elevated in BAL fluid taken from sensitized, vehicle treated guinea pigs exposed to OA (group 3) compared with the control groups 1 and 2. In the animals treated with JNJ the BAL levels of TNFα, IL-4, PGD2 and LTB4 were significantly and dose-dependently decreased, compared with the vehicle-treated counterparts.
Figure 8.
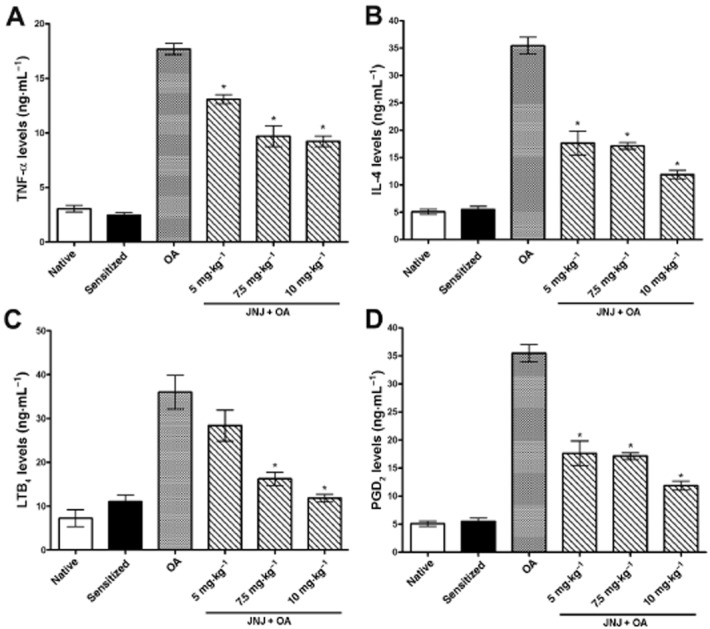
BAL levels of the pro-inflammatory cytokines TNF-α (A) and IL-4 (B), the chemoattractant LTB4 (C), and the mast cell-derived PGD2 (D) are significantly increased in the sensitized guinea pigs challenged with OA as compared with the controls. Treatment with increasing doses of JNJ caused a significant, dose-related reversal of the noted variables. Values are the mean ± SEM per group, n = 5. *P ≤ 0.001, significantly different from OA.
The anti-inflammatory effect of JNJ was confirmed by RT-PCR for TNF-α and IL-4 performed in BAL fluid, showing a decrease in mRNAs for these pro-inflammatory cytokines in the guinea pigs treated with JNJ (10 mg·kg−1) compared with the vehicle-treated counterparts (Figure 9).
Figure 9.

RT-PCR analysis for IL-4 and TNF-α mRNA performed in BAL fluid of guinea pigs from different experimental groups. The assayed pro-inflammatory cytokines are significantly up-regulated in the sensitized guinea pigs e challenged with OA, but not in those treated with 10 mg·kg−1 JNJ. Values are the mean ± SEM per group, n = 6. *P ≤ 0.01, significantly different from OA.
Discussion
The results of this study show that JNJ, a selective antagonist of the histamine H4 receptor, blocked the functional, histopathological and biochemical changes induced by OA challenge in an in vivo model of allergic asthma in guinea pigs and suggest that these effects are mediated by down-regulation of key pro-inflammatory cytokines, such as TNF-α and IL-4 and, at least in part, up-regulation of LC-1. This last protein, also known as annexin A1, was originally identified as a corticosteroid-regulated protein and involved in the anti-inflammatory effects of the corticosteroids (D'Acquisto et al., 2008). However, more recent observations have shown that its anti-inflammatory actions are much broader. In fact, LC-1 inhibits the activity of cytoplasmic phospholipase A2, thus playing a key role in down-regulation of inflammatory lipid mediators such as PGs and LTs, and inhibits the extravasation of leukocytes and release of inflammatory mediators (D'Acquisto et al., 2008). Further studies are required to unveil the exact mechanisms linking blockade of H4 receptors by JNJ and LC-1 up-regulation, but it is possible that JNJ could reduce proteolytic cleavage of LC-1 induced by allergen challenge (Chung et al., 2004), thereby extending LC-1 bioactivity. Mast cells contains abundant LC-1 located in, or on, the α-granules and these cells are an active site of LC-1 synthesis (Oliani et al., 2000). Moreover, mast cells in the LC-1 null animal exhibit histological signs of constitutive activation being partially degranulated and there is evidence that LC-1 inhibits mast cell granule release in vitro or in models of allergic inflammation (Bandeira-Melo et al., 2005). Given that histamine H4 receptors are expressed by various immune and inflammatory cells, such as dendritic cells, CD4+ and CD8+ T-cells (Zampeli and Tiligada, 2009), it seemed logical to assume that JNJ could reduce the complex cytokine interplay involved in the pathogenesis of allergic asthma. Asthma is characterized by infiltration of leukocytes, especially eosinophils, in the lung tissue, which produce large amounts of superoxide anion and other harmful mediators, which are responsible for bronchoconstriction and airway remodelling (Jarjour and Calhoun, 1994). In fact, the forced expiratory volume is inversely correlated with the increased production of superoxide by leukocytes (Jarjour and Calhoun, 1994). In addition, superoxide and peroxynitrite can promote the expression of genes encoding pro-inflammatory cytokines, IL-1, TNF-α and IL-6, which can spark endothelial cell damage (Ndengele et al., 2005). We have previously shown that superoxide and peroxynitrite promote mast cell degranulation and histamine release (Masini et al., 2005), as well as activation of COX and PG production (Mollace et al., 2005).
The present results show that treatment with JNJ significantly reduced overall leukocyte infiltration in the lung tissue, measured as MPO activity and particularly eosinophils, measured as eMBP-positive cells. It also reduced the release of proinflammatory cytokines and prostanoids, for example TNF-α, IL-4, PGD2 and LTB4, in the BAL fluid. These results are in agreement with previous data showing marked anti-inflammatory properties of JNJ in vitro and in vivo models (Thurmond et al., 2004; 2008). As mentioned earlier, eosinophils also play a key role in the pathogenesis, symptoms and severity of allergic asthma (Fujimoto et al., 1997; Bousquet et al., 2000). Eosinophils are involved in adverse airway remodelling through the release of TGF-β, which shifts stromal cells towards the myofibroblastic pro-fibrotic phenotype (Kisseleva and Brenner, 2008). Eosinophils, together with mast cells, also play a key role in angiogenesis (Nissim Ben Efraim and Levi-Schaffer, 2008). In particular, stimulation of H4 receptors on eosinophils triggers cellular changes required for chemotaxis, actin polymerization, shape changes and expression of adhesion molecules involved in cell migration such as CD11b and ICAM-1 (Ling et al., 2004). Treatment with JNJ effectively reduced eosinophil chemotaxis, confirming the notion mentioned earlier that that this process involves histamine H4 receptors (Ling et al., 2004; Zampeli and Tiligada, 2009).
It is well known that mast cells play a crucial role in the pathogenesis of allergic asthma in humans. Mast cell-released mediators are implicated in the mechanisms of bronchoconstriction, smooth muscle cell proliferation and inflammatory cell recruitment, which in turn maintain and amplify the inflammatory signalling (Wardlaw et al., 1988). Of note, treatment with JNJ prevented activation of lung mast cells, as demonstrated by the significant reduction of anaphylactic granule discharge.
Our results show that JNJ was able to counteract the allergic inflammatory process through the up-regulation of LC-1. In turn, the reduction of the lung inflammatory response was associated with diminished oxidative damage, as assessed by measurements of the tissue levels and activity of MDA, 8-OHdG and Mn-SOD. Moreover, the decreased lung inflammatory response and oxidative injury induced by JNJ treatment resulted in a decreased activity of caspase-3, the key enzyme of cell apoptosis, thus decreasing the conditions for airway remodelling.
It is currently believed that allergic asthma is a multifactorial process in which multiple inflammatory and immune cells cooperate through reciprocal interactions. The expression of H4 receptors by many of these cells indicates its involvement in the modulation of allergic inflammatory lung response and suggests that targeting this receptor could be a novel causative therapeutic strategy for asthmatic and allergic diseases (Zhang et al., 2006).
In conclusion, this study provides further support to the concept that histamine H4 receptors can play a major role in the modulation of immune and inflammatory response in allergic asthma and suggests that H4 receptor antagonists can be viewed as novel drugs to down-regulate eosinophil and mast cell activation and the downstream events leading to airway dysfunction and remodelling.
Acknowledgments
This research was supported by a COST action BM 0806 and by a grant from Ente Cassa di Risparmio di Firenze (E. Masini).
The authors are grateful to Dr. Maria Cristina Vinci for skilful technical assistance in RT-PCR.
Glossary
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- BAL
bronchoalveolar lavage
- eMBP
eosinophilic major basic protein
- JNJ
JNJ7777120, 1-[ (5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine
- LC-1
lipocortin-1
- MDA
malondialdehyde
- Mn-SOD
Mn-superoxide dismutase
- MPO
myeloperoxidase
- NBT
nitro blue tetrazolium
- OA
ovalbumin
- PAO
pressure at the airway opening
- TBA
2-thiobarbituric acid
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Laughton MJ, Quinlan GJ, Gutteridge JMC. The mechanism of initiation of lipid peroxidation: evidence against a requirement for an iron (II)- iron (III) complex. Biochem J. 1989;258:617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub SS, Yazid S, Flower RJ. Increased susceptibility of annexin-A1 null mice to nociceptive pain is indicative of a spinal antinociceptive action of annexin-A1. Br J Pharmacol. 2008;154:1135–1142. doi: 10.1038/bjp.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet A, Bix M. Chromatin landscape dynamics of the IL4-IL13 locus during T helper 1 and 2 development. Proc Natl Acad Sci USA. 2004;101:11410–11415. doi: 10.1073/pnas.0403334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Bonavita AG, Diaz BL, Silva PM, Carvalhpo VF, Jose PJ, et al. A novel effect for annexin 1-derived peptide ac2-26: reduction of allergic inflammation in the rat. J Pharmacol Exp Ther. 2005;313:1416–1422. doi: 10.1124/jpet.104.080473. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chung YW, Oh HY, Kim JY, Kim JH, Kim IY. Allergen-induced proteolytic cleavage of annexin-1 and activation of cytosolic phospholipase A2 in the lungs of a mouse model of asthma. Proteomics. 2004;4:3328–3334. doi: 10.1002/pmic.200400895. [DOI] [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Fantappiè O, Messerini L, Sardi I, Lasagna N, et al. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin Cancer Res. 2004;10:2694–2704. doi: 10.1158/1078-0432.ccr-03-0192. [DOI] [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, et al. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther. 2006;5:2716–2726. doi: 10.1158/1535-7163.MCT-06-0318. [DOI] [PubMed] [Google Scholar]
- Cinci L, Masini E, Bencini A, Valtancoli B, Mastroianni R, Calosi L, et al. Suppression of allergen-induced respiratory dysfunction and airway inflammation in sensitized guinea pigs by Mn(II)(Me(2)DO2A), a novel superoxide scavenger compound. Free Radic Biol Med. 2010;48:1525–1534. doi: 10.1016/j.freeradbiomed.2010.02.041. [DOI] [PubMed] [Google Scholar]
- Cirino G, Flower RJ, Browning JL, Sinclair LK, Pepinsky RB. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature. 1987;328:270–272. doi: 10.1038/328270a0. [DOI] [PubMed] [Google Scholar]
- Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA. 1989;86:3428–3432. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA, Xu W, Ghosh S, Thunnissen FB, Almasan A, Calhoun WJ, et al. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166:663–674. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acquisto F, Perretti M, Flower RJ. Annexin-A1: a pivotal regulator of the innate and adaptive immune systems. Br J Pharmacol. 2008;155:152–169. doi: 10.1038/bjp.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- Evans RL, Nials AT, Knowles RG, Kidd EJ, Ford WR, Broadley KJ. A comparison of antiasthma drugs between acute and chronic ovalbumin-challenged guinea-pig models of asthma. Pulm Pharmacol Ther. 2012;25:453–464. doi: 10.1016/j.pupt.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kubo K, Matsuzawa Y, Sekiguchi M. Eosinophil cationic protein levels in induced sputum correlate with the severity of bronchial asthma. Chest. 1997;112:1241–1247. doi: 10.1378/chest.112.5.1241. [DOI] [PubMed] [Google Scholar]
- Giannini L, Nistri S, Mastroianni R, Cinci L, Vannacci A, Mariottini C, et al. Activation of cannabinoid receptors prevents antigen-induced asthma-like reaction in guinea pigs. J Cell Mol Med. 2008;12:2381–2394. doi: 10.1111/j.1582-4934.2008.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–136. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule up-regulation. Br J Pharmacol. 2004;142:161–171. doi: 10.1038/sj.bjp.0705729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E, Bani D, Vannacci A, Pierpaoli S, Mannaioni PF, Comhair SAA, et al. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitised guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med. 2005;39:520–531. doi: 10.1016/j.freeradbiomed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, et al. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J Pharmacol Exp Ther. 2007;324:548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–193. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- Nishida S, Teramoto K, Kimoto-Kinoshita S, Tohda Y, Nakajima S, Tomura T, et al. Changes of CuZn-superoxide dismutase activity of guinea pig lung in experimental asthma. Free Radic Res. 2002;36:601–606. doi: 10.1080/10715760210872. [DOI] [PubMed] [Google Scholar]
- Nissim Ben Efraim AH, Levi-Schaffer F. Tissue remodeling and angiogenesis in asthma: the role of the eosinophil. Ther Adv Respir Dis. 2008;2:163–171. doi: 10.1177/1753465808092281. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Christian HC, Manston J, Flower RJ, Perretti M. An immunocytochemical and in situ hybridization analysis of annexin 1 expression in rat mast cells: modulation by inflammation and dexamethasone. Lab Invest. 2000;80:1429–1438. doi: 10.1038/labinvest.3780150. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin-1. J Immunol. 1993;150:992–999. [PubMed] [Google Scholar]
- Rennard SI. Repair mechanisms in asthma. J Allergy Clin Immunol. 1996;98:278–286. doi: 10.1016/s0091-6749(96)70076-3. [DOI] [PubMed] [Google Scholar]
- Slater A, Smallman LA, Drake-Lee AB. Increase in epithelial mast cell numbers in the nasal mucosa of patients with perennial allergic rhinitis. J Laryngol Otol. 1996;110:929–933. doi: 10.1017/s0022215100135388. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3 -6 -7 and -8. J Biol Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Masini E, Mazzocca C, Cuzzocrea S, Ciampa A, Suzuki H, et al. Inhibition of poly(ADP-ribose) polymerase prevents allergen-induced asthma-like reaction in sensitised guinea pigs. J Pharmacol Exp Ther. 2004;311:1241–1248. doi: 10.1124/jpet.104.072546. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay A. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Venable JD, Thurmond RL. The histamine H4 receptor in autoimmune disease. Expert Opin Investig Drugs. 2006;15:1443–1452. doi: 10.1517/13543784.15.11.1443. [DOI] [PubMed] [Google Scholar]


