Abstract
Background and Purpose
Here, we have investigated whether learning and/or short-term memory was associated with release of ACh and glutamate in the rat nucleus accumbens (NAc). Additionally, neurotransmitter release in the NAc was assessed during facilitation of cognitive processes by antagonists of inhibitory histamine autoreceptors.
Experimental Approach
The olfactory, social memory test was used in combination with push–pull superfusion of the NAc. A male, juvenile rat was exposed twice to an adult male rat at intervals of 60 or 90 min, and release of ACh and glutamate was determined in the NAc of the conscious adult rat. Histamine receptor antagonists were applied i.c.v.
Key Results
First exposure of a juvenile rat to an adult rat increased ACh and glutamate release in the NAc of the adult rat. Repetition of exposure after 60 min did not change release of ACh and glutamate, while contact time to recognition (CTR) was shortened. Repetition of exposure after an interval of 90 min prolonged CTR and enhanced accumbal ACh and glutamate release rates. Injection (i.c.v.) of thioperamide (histamine H3 receptor antagonist) together with famotidine (H2 receptor antagonist), 80 min prior to second exposure, diminished CTR and abolished ACh and glutamate release when second exposure was carried out 90 min after the first one.
Conclusions and Implications
Histaminergic neurons per se facilitated short-term memory, without activation of cholinergic and/or glutamatergic neurons in the NAc of rats. Cholinergic and glutamatergic neurons within the NAc contributed to learning but not to recall of memory.
Linked Articles
This article is part of a themed issue on Histamine Pharmacology Update. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2013.170.issue-1
Keywords: learning, short-term memory, neurotransmitter release, histaminergic neurons, nucleus accumbens (NAc), push-pull superfusion
Introduction
Histaminergic transmission in the CNS modulates cognitive processes and endogenous histamine facilitates short-term memory in the olfactory, social memory test (Prast et al., 1996). In the CNS, release of neuronal histamine is repressed by constitutively active, inhibitory H3 autoreceptors (see Philippu and Prast, 2001a) and post-trial administration of the H3 receptor antagonist thioperamide improves memory consolidation in the open-field test (Molinengo et al., 1999; receptor nomenclature follows Alexander et al., 2011). The H3 receptor antagonist, FUB 181 [3-(4-chlorophenyl)propyl-3-(1H-imidazol-4-yl)propyl ether], ameliorates scopolamine-induced learning deficits in mice exposed to an elevated-plus maze test (Muir et al., 1994).
ACh released from central cholinergic neurons plays an essential role in cognitive processes, such as learning, recall of acquired information (Hasselmo and Bower, 1993; Nakazato et al., 2000; De Jaeger et al., 2013) and attentional processes (Muir et al., 1994; Torres et al., 1994; Sarter and Paolone, 2011). While cholinomimetic compounds facilitate learning and memory (Dawson and Iversen, 1993; Iliev et al., 1999), cholinergic receptor antagonists, such as scopolamine, diminish cognitive performance (Ingles et al., 1993; Rush, 1998).
Studies of the cholinergic involvement in learning and memory are mainly focused on the cerebral cortex (Blandina et al., 1996; Barkai and Hasselmo, 1997), although striatal areas also participate in cognitive processes (Setlow, 1997; Laplante et al., 2012). For instance, spatial and non-spatial novelty elicits responses in the limbic afferents of the nucleus accumbens (NAc) (Schacter et al., 1989; Sargolini et al., 1999). Nevertheless, little is known about the function of the NAc, the main part of the ventral striatum, on learning and memory.
The present study was carried out to investigate the release of the neurotransmitters ACh and glutamate in the NAc during acquisition and short-term memory, and to evaluate the influence of histaminergic neurons on recognition and neurotransmitter release within the NAc. For this purpose, the olfactory, social memory test was carried out on adult, male rats. The olfactory, social memory test is a well-accepted model evaluating mnemonic processes (Carr et al., 1976; Thor and Holloway, 1982; Schacter et al., 1989; Lemaire et al., 1994; Noack et al., 2010). Locomotor activity of the adult rat was also monitored. The release of ACh and glutamate in the NAc was assessed using the push–pull superfusion technique, while the H3 receptor antagonist thioperamide and the H2 receptor antagonist famotidine were applied i.c.v.
Methods
Animals
All animal care and experimental protocols were approved by the Kommission für Tierversuchsangelegenheiten, Bundesministerium für Wissenschaft, Forschung und Kunst, Austria. All procedures used were as humane as possible. The ARRIVE guidelines for reporting experiments involving animals have been followed (Kilkenny et al., 2010; McGrath et al., 2010). Thirty-two adult, 5- to 6-month-old male, sexually experienced rats (Sprague Dawley, 450–500 g) were housed in pairs, under conditions of constant temperature (23 ± 2°C) and a 12 h light/dark cycle (light period: 07:00–19:00 h). Water and food were freely available. Two weeks before experiments were carried out, the rats were separated. For the olfactory, social memory test, juvenile, 4-week-old male rats, housed four to six per cage under the above conditions, were used as social stimuli. On the day of the experiment, a juvenile rat was placed in a separate cage with water and food ad libitum.
Push–pull superfusion technique
Adult rats were anaesthetized with sodium pentobarbital (40 mg·kg−1, i.p.) and ketamine (50 mg·kg−1, i.p.), and the head was fixed in a stereotaxic frame. A guide cannula (outer diameter 1.25 mm, inner diameter 0.90 mm) was inserted stereotaxically (Paxinos and Watson, 1998) into the ventral striatum until its tip was 2 mm above the target area (the NAc; coordinates, AP +1.3 mm, ML −2.5 mm from bregma, DV −7.5 mm from dura). The guide cannula with its stylet was fixed at the skull with dental screws and cement. For i.c.v. injection of drugs or vehicle [artificial CSF (aCSF); composition, (mmol·L−1) NaCl 140.0, KCl 3.0, CaCl2 1.2, MgCl2 1.0, Na2HPO4 1.0, NaH2PO4 0.3 and glucose 3.0, pH 7.2.], a guide cannula (outer diameter 0.65 mm, inner diameter 0.4 mm) with its stylet was stereotaxically inserted contralaterally to the push–pull guide cannula (coordinates, AP –1.3 mm, ML +1.7 mm from bregma, DV –3.8 mm from dura). This guide cannula (for the i.c.v injections) was also fixed with cement. Two days after surgery, the stylet of the guide cannula to the push–pull cannula was removed and replaced by a push–pull cannula (outer cannula: outer diameter 0.80 mm, inner diameter 0.50 mm; inner cannula: outer diameter 0.20 mm, inner diameter 0.10 mm). The push–pull cannula was 2 mm longer than its guide cannula, thus reaching the NAc. The NAc of the freely moving rat was superfused with aCSF, containing 1 μmol·L−1 neostigmine, at a flow rate of 20 μL·min−1. The superfusate was continuously collected in time periods of 10 min at −20°C. Drugs were dissolved in aCSF and 10 μL was injected over a period of 30 s through a stainless steel cannula (outer diameter 0.38 mm, inner diameter 0.2 mm) which was 1.5 mm longer than its guide cannula, thus reaching the lateral ventricle (AP −1.3 mm, ML +1.7 mm from bregma, DV −3.8 mm from dura) (Paxinos and Watson, 1998). In control rats, 10 μL of aCSF was injected in the lateral ventricle. Localizations of the push–pull cannulae and i.c.v. cannulae were verified on histological brain slices (50 μm) stained with cresyl violet.
Determination of ACh and glutamate
Neurotransmitters were determined in the superfusate after separation by HPLC. ACh was electrochemically detected as previously described (Prast et al., 1995). Briefly, mobile phase, which consisted of 100 mmol·L−1 K2HPO4, 5 mmol·L−1 KCl, 1 mmol·L−1 tetramethylammonium hydroxide, 1 mmol·L−1 Na-EDTA and 0.5 mL·L−1 of the microbiocide kathon GC (pH 7.9) was pumped at a flow rate of 0.4 mL·min−1. ACh and choline were separated on an analytical column (80 × 3 mm, ChromSpher C18) loaded with lauryl sulfate (100 mg/20 mL). At the post-column enzyme reactor (20 × 1 mm nucleosil-NH2) to which ACh esterase and choline oxidase were covalently bound, ACh was hydrolysed to acetate and choline. Subsequently, choline was oxidized to betaine and hydrogen peroxide. The peroxide was electrochemically detected by a platinum electrode at +500 mV with an amperometric detector (BAS LC-4B, Bioanalytical Systems Inc., West Lafayette, IN, USA). ACh (injection volume 100 μL) was quantified using calibration curves from external standards injected at the beginning and the end of the sample analyses. The detection limit for ACh (signal to noise ratio = 3) was 5 fmol per sample collected.
Glutamate was fluorimetrically detected after pre-column derivatization with ortho-phthaldialdehyde (OPA) as previously described (Kraus and Prast, 2002). The HPLC system consisted of a solvent gradient delivery pump (JASCO PU-1580, Tokyo, Japan), an auto sampler (CMA 200, refrigerated microsampler, CMA Microdialysis, Stockholm, Sweden) and an analytical column (Nucleosil 100-5 C18, 5 μm). The mobile phase consisted of 0.1 mol·L−1 sodium acetate buffer (adjusted with acetic acid to pH 6.95) : methanol : tetrahydrofurane = 92.5:5: 2.5, vol% (eluent A). This solution was mixed in a continuous gradient with eluent B (methanol : tetrahydrofurane = 97.5:2.5, vol%); the initial concentration of eluent B was 10%. The gradient was as follows: eluent B 10–25% (0–0.5 min), isocratic run (0.5–14 min), 25–40% (14–18 min), 40–100% (18–24 min), 100–0% (24–26 min), isocratic run (26–36 min). Over the next 5 min, the mobile phase returned to its initial composition. For the pre-derivatization, 50 μL of the superfusate was mixed automatically within the auto sampler, with 10 μL of OPA and after a reaction time of 60 s, 50 μL was injected. The fluorescence detector (JASCO FP-920) was set at 365 and 450 nm excitation and emission wavelengths respectively. The retention time for glutamate was 8 min. Glutamate release was quantified using calibration curves of external standards injected at the beginning and the end of the sample analyses. The detection limit was 20 fmol per sample collected. ACh and glutamate were determined in samples obtained from the same animal.
Recordings of contact time to recognition (CTR) and locomotor activity
A video camera, placed 1 m above the superfusion cage, recorded the 10 min of exposure of the juvenile rat to the adult rat. Recordings were evaluated optically. Social investigatory behaviour of the adult rat, expressed as the CTR, was defined as the time elapsed while the adult rat was close to or in direct contact with the juvenile rat by sniffling or inspecting his body. Locomotion was recorded by the video camera 10 min before and during the 10 min of exposure of the juvenile rat to the adult rat. Locomotor activity was expressed in locomotor activity units. One unit was defined as the movement of the adult rat from one quadrant of the cage to another. Each adult rat was exposed to the same juvenile in the first and the second contact. Each adult rat was exposed to a different juvenile.
Materials
Thioperamide maleate [N-cyclohexyl-4-(imidazol-4-yl)-1-piperidine-carbothioamide; Tocris Cookson, Bristol, UK] and famotidine (N′-[amino-sulfonyl]-3-[(2-[diaminomethyleneamino]-4-thiazolyl)methylthio]-propanamidine; Sigma, Deisenhofen, Germany) were used in this study. Sodium pentobarbital and ketamine were also supplied by Sigma, Deisenhofen, Germany.
Data analysis
Data are expressed as mean values ± SEM. Values of CTR were compared by the Mann–Whitney U-test. Locomotor activity was compared by Wilcoxon's rank test for paired data, and the Mann–Whitney U-test was performed for comparison of groups during different time periods between the two exposures of the juvenile to the adult rat. Neurotransmitter release rates were analysed by Friedman's test followed by Wilcoxon's rank test for paired data, using as controls the means of the three values prior to exposure of the juvenile to the adult rat. Data were computed by applying CSS3.
Results
Mean basal outputs of neurotransmitters in the NAc of the conscious adult rat were (fmol·min−1; mean values ± SEM) as follows: ACh 58.0 ± 7.7 (n = 30), glutamate 871.6 ± 113.0 (n = 29).
Effect of thioperamide applied together with famotidine on learning and memory during performance of the olfactory, social memory test
The time taken to recognize the juvenile rat by the adult rat (CTR) during first exposure was 124 ± 29 s (mean value ± SEM; n = 9). During the second exposure which took place 60 min after the first exposure, CTR was decreased. When the second exposure took place 90 min after the first one, CTR was similar to that during the first exposure. Injection (i.c.v.) of 5 μg thioperamide (H3 receptor antagonist) together with 20 μg famotidine (H2 receptor antagonist) immediately after the first exposure diminished CTR during the second exposure 90 min later. Similar injection of the vehicle (10 μL of aCSF) was ineffective (Figure 1).
Figure 1.
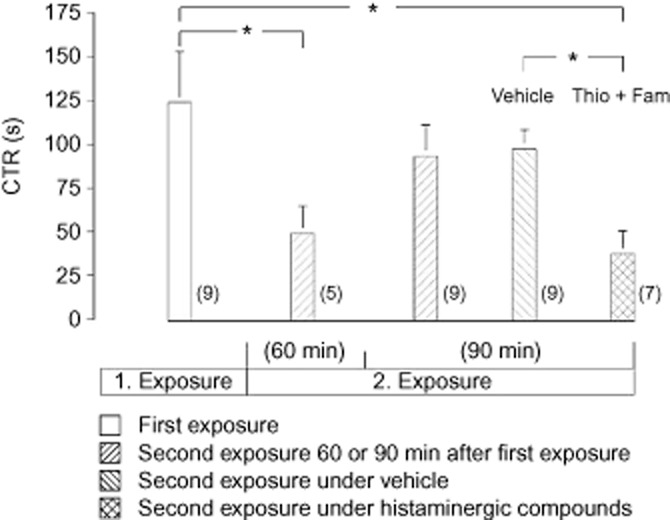
Social interactions of a juvenile rat with an adult rat, assessed by the CTR. Effect of i.c.v. injection of thioperamide (Thio; 5 μg) together with famotidine (Fam; 20 μg) on CTR. Histamine receptor antagonists or aCSF (vehicle) was injected immediately after the first exposure. Each exposure lasted 10 min. Mean values ± SEM. Number of experiments are indicated in parentheses. *P < 0.05, significantly different as shown.
Effect of thioperamide applied together with famotidine on locomotor activity during performance of the olfactory, social memory test
The first exposure of the juvenile rat markedly elevated locomotor activity of the adult rat compared to locomotor activity 10 min prior to the exposure (Table 1). The second exposure after 60 min also raised locomotor activity but to a lesser degree than during the first exposure. A 90 min interval between the two exposures resulted in an increase in locomotor activity similar to that during the first contact. Injection (i.c.v.) of thioperamide (5 μg) together with famotidine (20 μg) immediately after the first exposure did not affect the locomotor activity of the adult rat during the second exposure that was carried out 90 min after the first one. Injection (i.c.v.) of the vehicle was ineffective (Table 1).
Table 1.
Locomotor activity of an adult rat during exposure of a juvenile rat; effect of i.c.v. injection of thioperamide together with famotidine during the second exposure
| Control | Exposure | |
|---|---|---|
| 1. Exposure (n = 9) | 2.88 ± 1.60 | 49.00 ± 13.85** |
| 2. Second exposure after 60 min (n = 5) | 1.80 ± 1.11 | 9.20 ± 4.09*,++ |
| 3. Second exposure after 90 min (n = 9) | 0.40 ± 0.20 | 38.30 ± 13.37**,¶ |
| 4. Second exposure after 90 min + vehicle (n = 9) | 0.62 ± 0.32 | 39.40 ± 14.41**,¶ |
| 5. Second exposure after 90 min + Thio + Fam (n = 7) | 1.00 ± 0.68 | 28.00 ± 13.95**,¶ |
Control, locomotor activity 10 min prior to exposure; Thio, thioperamide (i.c.v., 5 μg); Fam, famotidine (i.c.v., 20 μg); vehicle (aCSF). Intracerebroventricular injection of the compounds was carried out immediately after the first exposure. Each exposure lasted 10 min. Mean values ± SEM. Number of experiments is shown in parentheses. *P < 0.05, **P < 0.01, significantly different from control (Wilcoxon's rank test). ++P < 0.01, 2 significantly different from 1, 3, 4 and 5. ¶ values for conditions 3, 4 and 5 were not significantly different from each other. (Mann–Whitney U-test).
Effect of thioperamide applied together with famotidine on ACh and glutamate release in the NAc
Injection of 5 μg thioperamide together with 20 μg famotidine into the lateral ventricle did not influence basal release rates of ACh (Figure 2A) and glutamate (Figure 2B) in the NAc. Injection of vehicle was also ineffective (Figure 2A,B).
Figure 2.
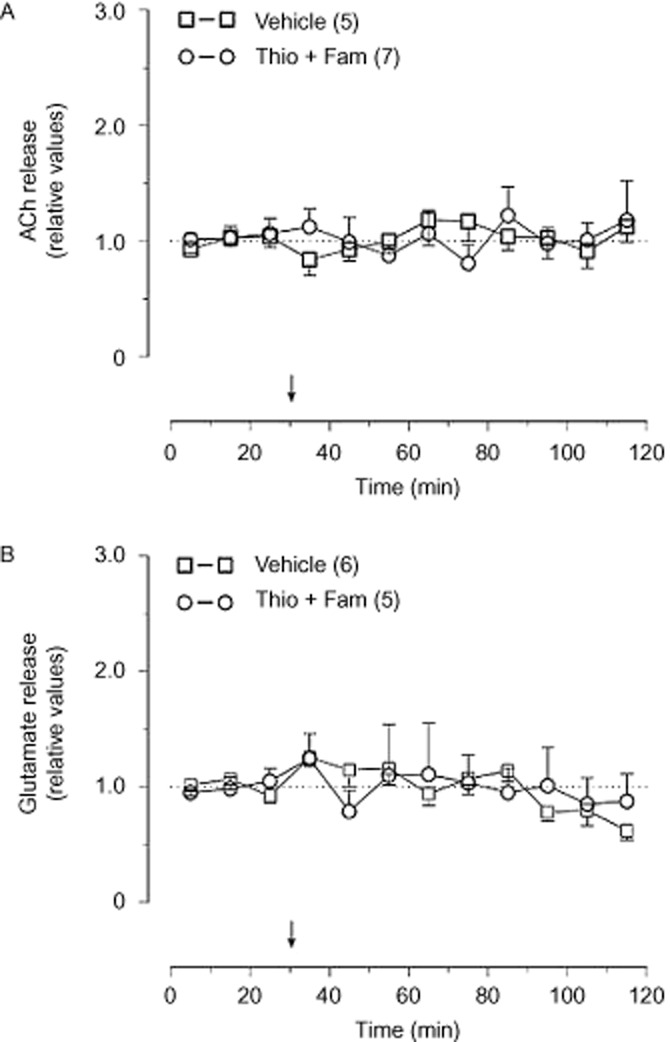
Effect of thioperamide (Thio; 5 μg, i.c.v.) together with famotidine (Fam; 20 μg, i.c.v.) on basal release of ACh (A) and glutamate (B) in the NAc. The basal release rates in the three samples preceding i.c.v. injection were taken as 1. Arrows indicate drug injection. Mean values ± SEM. Number of rats is indicated in parentheses.
Exposure of a juvenile rat to the adult rat led to a long-lasting increase in ACh release. A second exposure, performed 60 min after the first exposure, did not influence ACh outflow, but a second exposure after an interval of 90 min, did enhance ACh release. Thioperamide (5 μg) injected together with famotidine (20 μg) immediately after the first exposure abolished the enhanced release of ACh during the second exposure (Figure 3A). Injection of vehicle did not affect the increased ACh release during this second exposure (n = 8; not shown).
Figure 3.
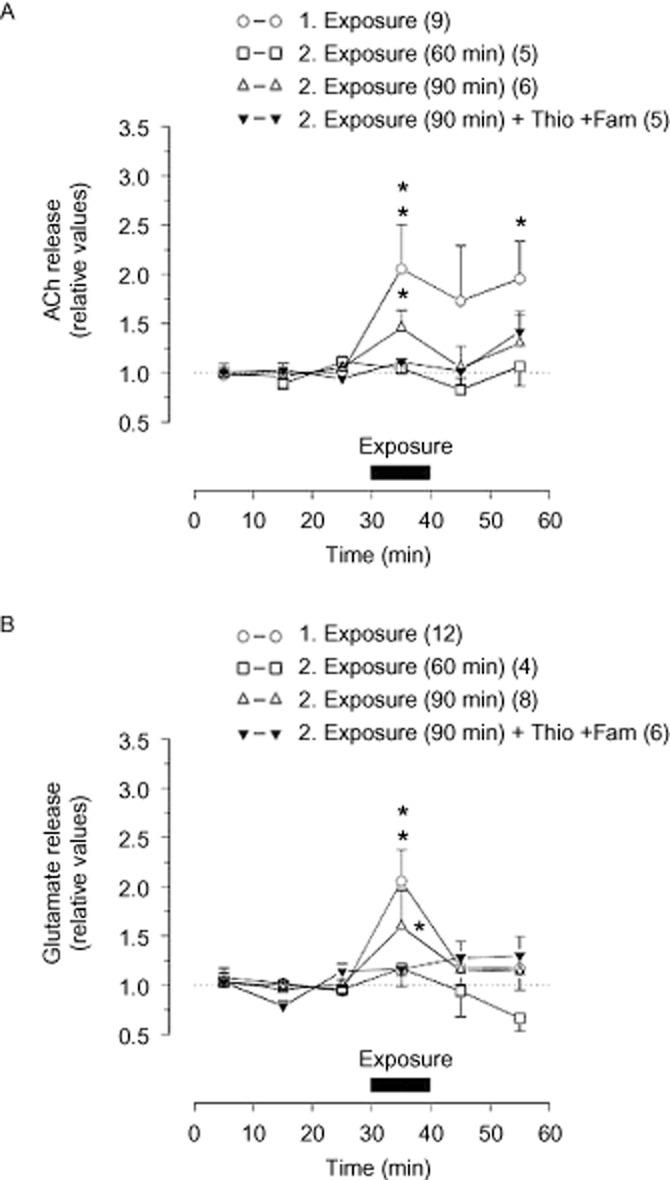
Release of ACh (A) and glutamate (B) in the NAc during performance of the olfactory, social memory task. Effect of i.c.v. injection of 5 μg thioperamide (Thio) together with 20 μg famotidine (Fam) on transmitter release. Histamine receptor antagonists or aCSF (vehicle) was injected immediately after the first exposure. Each exposure lasted 10 min and second exposure took place 60 or 90 min after the first exposure. Horizontal bars indicate exposure of the juvenile rat to the adult rat. Basal release rate in the three samples preceding the exposure was taken as 1. Mean values ± SEM. Number of rats is indicated in parentheses. *P < 0.05; **P < 0.01, significantly different from basal release rate.
Release of glutamate was also increased during the first exposure. A second exposure 60 min after the first exposure did not affect glutamate release, but when the second exposure was carried out after an interval of 90 min, the outflow of glutamate was enhanced. Glutamate release during this later second exposure was abolished by injection of the histamine receptor antagonists, thioperamide and famotidine (Figure 3B), while i.c.v. injection of the vehicle was ineffective (n = 8; not shown).
Discussion and conclusions
The olfactory, social memory test (Carr et al., 1976; Thor and Holloway, 1982) is mainly a chemosensory-mediated memory of a social conspecific (Schacter et al., 1989). It is based on the time needed by an adult, sexually experienced rat to become acquainted with an unfamiliar juvenile rat; the time required being an index of short-term memory. When re-exposure is carried out in less than 1 h, the adult rat recognizes the juvenile rat and contact time is short (Thor and Holloway, 1982). An interval of 2 h between the two exposures requires a complete re-investigation of the juvenile rat by the adult rat so that the CTR during first and second exposures are similar (Dantzer et al., 1987). Combining assays of transmitter release by the push–pull superfusion technique, with the olfactory, social memory test makes it possible to correlate neuronal activity in a distinct brain area with memory processes.
During the first exposure, release of ACh and glutamate increased in the NAc of the adult rat. Participation of the NAc during learning is supported by the finding that bilateral damage to the medial NAc impaired acquisition in the Morris water task (Sutherland and Rodriguez, 1989). Novel visual stimuli also evoked neuronal responses within the ventral striatum (Williams et al., 1993) and neuronal activity within the NAc was elevated during conditioning to social interaction (El Rawas et al., 2012).
A 60 min interval between first and second exposure markedly decreased CTR, pointing to immediate recognition of the juvenile rat by the adult rat. Outflow of ACh and glutamate in the NAc was not affected during the second exposure. Obviously, release of these neurotransmitters in the NAc is not necessary for recognition. This notion is consistent with many other observations. For example, damage to the NAc only slightly affects retention of acquired place navigation in the Morris water task (Sutherland and Rodriguez, 1989). Furthermore, decreased cerebral cholinergic neurotransmission impairs acquisition of passive-avoidance learning, rather than maintenance of memory (Ikarashi et al., 2000; see Robinson et al., 2011).
Prolongation of the time interval between the two exposures from 60 to 90 min led to a CTR that was similar to that during the first exposure. Moreover, ACh and glutamate release was now markedly increased in the NAc. Hence, the 90 min interval between the two exposures seems to initiate processes of learning that are associated with activation of cholinergic and glutamatergic transmission.
The significance of histaminergic projections within neuronal circuits on mnemonic processing is now well established (Philippu and Prast, 2001; Alvarez, 2009; Zlomuzica et al., 2009; Köhler et al., 2011). Intracerebroventricular injection of histamine, histidine and of the H3 receptor antagonist thioperamide facilitates short-term memory (Prast et al., 1996). On the contrary, central administration of the H3 receptor agonist immepip, or inhibition of neuronal histamine synthesis by α-fluoromethylhistidine, diminishes memory (Prast et al., 1996). H3 receptor antagonists have also been proposed for reversing addictive drug-induced cognitive deficits (Alleva et al., 2012). When the H2 receptor antagonist famotidine or thioperamide is applied to the NAc by local superfusion, outflow of ACh in this nucleus is enhanced (Prast et al., 1999a,b). Combined i.p. injection of thioperamide with an H2 receptor antagonist significantly enhanced the improving effect of thioperamide on scopolamine-induced learning deficit in the elevated-plus maze test (Miyazaki et al., 1995). Moreover, activation of histamine H2 receptors decreased performance in a passive avoidance task (Onodera et al., 1998), while an analogue of the H2 receptor antagonist ranitidine (JWS-USC-75-IX) improved memory (Terry et al., 2011).
It should be mentioned, however, that the facilitating effect of histaminergic neurons on mnemonic processing is not consistently observed under all experimental conditions. Eidi et al. (2003) showed that post-training i.c.v. injection of histamine reduced memory retention in a passive avoidance task and histamine applied into the ventral hippocampus impaired learning processes (Alvarez et al., 2001).
Based on our former study and on the findings of Miyazaki et al. (1995), here we have assayed release of ACh and glutamate during histaminergic facilitation of short-term memory, induced by injecting thioperamide together with famotidine i.c.v. These histamine receptor antagonists were applied together because it has been shown that in this way the facilitating effect on mnemonic processes is most obvious. We found that, when the second exposure was carried out 90 min after the first one, i.c.v. injection of thioperamide and famotidine markedly shortened CTR and abolished the enhanced release of ACh and glutamate, which occurred under control conditions. Consequently, facilitation of memory by increasing neuronal histamine release was not due to activation of cholinergic and/or glutamatergic neurons within the NAc. Note that thioperamide given i.c.v. together with famotidine did not affect basal release of glutamate and ACh in the NAc, while superfusion of the NAc with these compounds modulated basal release of ACh (Prast et al., 1999a,b). This discrepancy might be due to the more selective and direct action of these compounds when applied by superfusion of the NAc than by i.c.v. injection.
It has been reported that the selective H3 receptor antagonist thioperamide also inhibits H4 receptors. However, comparison of Ki values reveals that the antagonistic action of this compound on H4 receptors is only weak (Leurs et al., 2009; see also Lovenberg et al., 2000; Hough, 2001). Moreover, Connelly et al. (2009) demonstrated that H4 receptors do not act as heteroreceptors in the brain and they do not influence either histamine release, or glutamatergic transmission. Hence, it is unlikely that histamine H4 receptors participate in these thioperamide-elicited effects.
The NAc is known to participate in motor activity (Mogenson and Yang, 1991). Thus, it might be claimed that activation of cholinergic and/or glutamatergic transmission within the NAc reflects cerebral processes controlling locomotor activity. However, thioperamide and famotidine did not markedly influence the enhanced locomotion elicited during the second exposure. Another indication that neurotransmitter release during this memory task was not due to locomotor activity is the finding that injection of NMDA and AMPA receptor antagonists into the NAc enhances locomotor activity (Mogenson et al., 1980; Ouagazzal and Amalric, 1995). However, in our experiments, exposure of a juvenile rat to the adult rat elicited glutamate release and increased locomotor activity. Thus, neurotransmitter release, induced during exposure of a social conspecific, seems to be mainly due to learning of a new, social event.
It is worthwhile mentioning that, when in vivo techniques are used, the involvement of more than one neurotransmitter in a certain function is probable. Hence, under in vivo conditions, the agonists and/or antagonists of histamine receptors that are applied to the CNS should be those compounds with very low affinity for non-histamine receptors.
In conclusion, acquisition of information by rats induced ACh and glutamate release, whereas short-term memory did not affect release of these neurotransmitters, in the NAc. Hence, within the NAc, histaminergic neurons facilitated, per se, the short-term memory without influencing cholinergic and glutamatergic transmission. Further studies are necessary to clarify which histamine receptors are responsible for the memory-improving effect of histamine in various brain regions.
Acknowledgments
This work was supported by the Fonds zur Förderung der wissenschaftlichen Forschung (FWF). The authors are grateful to Gospava Gajić for the excellent technical assistance.
Glossary
- CTR
contact time to recognition
- FUB 181
[3-(4-chlorophenyl)propyl-3-(1H-imidazol-4-yl)propyl ether]
- NAc
nucleus accumbens
- OPA
ortho-phthaldialdehyde
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva L, Tirelli E, Brabant C. Therapeutic potential of histaminergic compounds in the treatment of addiction and drug-related cognitive disorders. Behav Brain Res. 2012;237C:357–368. doi: 10.1016/j.bbr.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Alvarez EO. The role of histamine on cognition. Behav Brain Res. 2009;199:183–189. doi: 10.1016/j.bbr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Alvarez EO, Ruarte MB, Banzan AM. Histaminergic systems of the limbic complex on learning and motivation. Behav Brain Res. 2001;124:195–202. doi: 10.1016/s0166-4328(01)00213-3. [DOI] [PubMed] [Google Scholar]
- Barkai E, Hasselmo MH. Acetylcholine and associative memory in the piriform cortex. Mol Neurobiol. 1997;15:17–29. doi: 10.1007/BF02740613. [DOI] [PubMed] [Google Scholar]
- Blandina P, Giorgetti M, Bartolini L, Cecchi M, Timmerman H, Leurs R, et al. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br J Pharmacol. 1996;119:1656–1664. doi: 10.1111/j.1476-5381.1996.tb16086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr WJ, Yee L, Gable D, Marasco E. Olfactory recognition of conspecifics by domestic Norway rats. J Comp Physiol Psychol. 1976;90:821–828. doi: 10.1037/h0077266. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RLM, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009;157:55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Iversen SD. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behav Brain Res. 1993;57:143–153. doi: 10.1016/0166-4328(93)90130-i. [DOI] [PubMed] [Google Scholar]
- De Jaeger X, Cammarota M, Prado MA, Izquierdo I, Prado VF, Pereira GS. Decreased acetylcholine release delays the consolidation of object recognition memory. Behav Brain Res. 2013;238:62–68. doi: 10.1016/j.bbr.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Eidi M, Zarrindast MR, Eidi A, Oryan S, Parivar K. Effects of histamine and cholinergic systems on memory retention of passive avoidance learning in rats. Eur J Pharmacol. 2003;465:91–96. doi: 10.1016/s0014-2999(03)01440-7. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, et al. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front Behav Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001;59:415–419. [PubMed] [Google Scholar]
- Ikarashi Y, Kuribara H, Shiobara T, Takahashi A, Ishimaru H, Maruyama Y. Learning and memory in mice treated with choline oxidase, a hydrolytic enzyme for choline. Pharmacol Biochem Behav. 2000;65:519–522. doi: 10.1016/s0091-3057(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Iliev A, Traykov V, Prodanov D, Mantchev G, Yakimova K, Krushkov I, et al. Effect of the acetylcholinesterase inhibitor galanthamine on learning and memory in prolonged alcohol intake rat model of acetylcholine deficit. Methods Find Exp Clin Pharmacol. 1999;21:297–301. doi: 10.1358/mf.1999.21.4.538182. [DOI] [PubMed] [Google Scholar]
- Ingles JL, Beninger RJ, Jhamandas K, Boegman RJ. Scopolamine injected into the rat amygdala impairs working memory in the double Y-maze. Brain Res Bull. 1993;32:339–344. doi: 10.1016/0361-9230(93)90197-j. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler CA, da Silva WC, Benetti F. Histaminergic mechanisms for modulation of memory systems. Neural Plast. 2011;2011:328602. doi: 10.1155/2011/328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MM, Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbria. Neuroscience. 2002;112:331–343. doi: 10.1016/s0306-4522(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Laplante F, Zhang ZW, Huppé-Gourgues F, Dufresne MM, Vaucher E, Sullivan RM. Cholinergic depletion in nucleus accumbens impairs mesocortical dopamine activation and cognitive function in rats. Neuropharmacology. 2012;63:1075–1084. doi: 10.1016/j.neuropharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Böhme GA, Piot O, Roques BP, Blanchard JC. CCK-A and CCK-B selective receptor agonists and antagonists modulate olfactory recognition in male rats. Psychopharmacology (Berl) 1994;115:435–440. doi: 10.1007/BF02245565. [DOI] [PubMed] [Google Scholar]
- Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJP. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Pyati J, Chang H, Wilson SJ, Erlander MG. Cloning of rat histamine H3 receptor reveals distinct species pharmacological profiles. J Pharmacol Exp Ther. 2000;293:771–778. [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Imaizumi M, Onodera K. Effects of thioperamide, a histamine H3-receptor antagonist, on a scopolamine-induced learning deficit using an elevated plus-maze test in mice. Life Sci. 1995;57:2137–2144. doi: 10.1016/0024-3205(95)02206-x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Molinengo L, Di Carlo G, Ghi P. Combined action of thioperamide plus scopolamine, diphenhydramine, or methysergide on memory in mice. Pharmacol Biochem Behav. 1999;63:221–227. doi: 10.1016/s0091-3057(98)00229-9. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato E, Yamamoto T, Ohno M, Watanabe S. Cholinergic and glutamatergic activation reverses working memory failure by hippocampal histamine H1 receptor blockade in rats. Life Sci. 2000;67:1139–1147. doi: 10.1016/s0024-3205(00)00713-x. [DOI] [PubMed] [Google Scholar]
- Noack J, Richter K, Laube G, Haghgoo HA, Veh RW, Engelmann M. Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol Learn Mem. 2010;94:568–575. doi: 10.1016/j.nlm.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Onodera K, Miyazaki S, Imaizumi M. Cognitive involvement by negative modulation of histamine H2 receptors in passive avoidance task in mice. Methods Find Exp Clin Pharmacol. 1998;20:307–310. doi: 10.1358/mf.1998.20.4.485684. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Amalric M. Competitive NMDA receptor antagonists do not produce locomotor hyperactivity by a dopamine-dependent mechanism. Eur J Pharmacol. 1995;294:137–146. doi: 10.1016/0014-2999(95)00518-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. Sydney: Academic Press; 1998. [Google Scholar]
- Philippu A, Prast H. Role of histaminergic and cholinergic transmission in cognitive processes. Drug News Perspect. 2001a;14:523–529. doi: 10.1358/dnp.2001.14.9.858409. [DOI] [PubMed] [Google Scholar]
- Philippu A, Prast H. Importance of histamine in modulatory processes, locomotion and memory. Behav Brain Res. 2001;124:151–159. doi: 10.1016/s0166-4328(01)00226-1. [DOI] [PubMed] [Google Scholar]
- Prast H, Fischer H, Werner E, Werner-Felmayer G, Philippu A. Nitric oxide modulates the release of acetylcholine in the ventral striatum of the freely moving rat. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:67–73. doi: 10.1007/BF00169191. [DOI] [PubMed] [Google Scholar]
- Prast H, Argyriou A, Philippu A. Histaminergic neurons facilitate social memory in rats. Brain Res. 1996;734:316–318. [PubMed] [Google Scholar]
- Prast H, Tran MH, Fischer H, Kraus M, Lamberti C, Grass K, et al. Histaminergic neurons modulate acetylcholine release in the ventral striatum: role of H3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999a;360:558–564. doi: 10.1007/s002109900097. [DOI] [PubMed] [Google Scholar]
- Prast H, Tran MH, Lamberti C, Fischer H, Kraus M, Grass K, et al. Histaminergic neurons modulate acetylcholine release in the ventral striatum: role of H1 and H2 histamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999b;360:552–557. doi: 10.1007/s002109900098. [DOI] [PubMed] [Google Scholar]
- Robinson L, Platt B, Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav Brain Res. 2011;221:443–465. doi: 10.1016/j.bbr.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Rush DK. Scopolamine amnesia of passive avoidance: a deficit of information acquisition. Behav Neural Biol. 1998;50:255–274. doi: 10.1016/s0163-1047(88)90938-7. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Roullet P, Oliverio A, Mele A. Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience. 1999;93:855–867. doi: 10.1016/s0306-4522(99)00259-6. [DOI] [PubMed] [Google Scholar]
- Sarter M, Paolone G. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci. 2011;125:825–835. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter GB, Yang CR, Innis NK, Mogenson GJ. The role of the hippocampal-nucleus accumbens pathway in radial-arm maze performance. Brain Res. 1989;494:339–349. doi: 10.1016/0006-8993(89)90602-1. [DOI] [PubMed] [Google Scholar]
- Setlow B. The nucleus accumbens and learning and memory. J Neurosci Res. 1997;49:515–521. doi: 10.1002/(SICI)1097-4547(19970901)49:5<515::AID-JNR1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rodriguez AJ. The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behav Brain Res. 1989;32:265–277. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Herman EJ, Callahan PM, Beck WD, Warner S, et al. The prototypical ranitidine analog JWS-USC-75-IX improves information processing and cognitive function in animal models. J Pharmacol Exp Ther. 2011;336:751–766. doi: 10.1124/jpet.110.175422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–1006. [Google Scholar]
- Torres EM, Perry TA, Blockland A, Wilkinson LS, Wiley RG, Lappi DA, et al. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rolls ET, Leonard CM, Stern C. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Ruocco LA, Sadile AG, Huston JP, Dere E. Histamine H1 receptor knockout mice exhibit impaired spatial memory in the eight-arm radial maze. Br J Pharmacol. 2009;157:86–91. doi: 10.1111/j.1476-5381.2009.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


