Abstract
Trigonella foenum-graecum is one of the widely used herbs in food and medicine. The seeds of the plants are investigated for antidiabetic potential; however, no efforts have been done to explore the potential of leaves to modify carbohydrate metabolizing enzymes viz. α-amylase and α-glucosidase. The present work was designed to investigate the inhibitory potential of ethyl acetate and water extract of T. foenum-graecum on enzymes α-amylase and α-glucosidase. Different concentrations of extracts were used to study inhibition of enzymatic activity of α-amylase and α-glucosidase. A dose dependent inhibitory effect on enzymes was observed. The current study, for the first time, revealed α-amylase and α-glucosidase inhibitory potential of T. foenum-graecum and the study could be helpful to isolate and characterize compounds responsible for it.
Keywords: α-Amylase, α-glucosidase, Methika, Trigonella foenum-graecum
Introduction
Trigonella foenum-graecum is an herb widely cultivated in India and in some parts of China. In India, seeds are used as condiment. Seeds place an important role in herbal medicine and are recognized for tonic, carminative and aphrodisiac potential. Traditional Chinese medicine finds its use in treatment of weakness.[1,2]
The plant is recognized for medicinal properties like antibacterial,[3] antiinflammatory,[4] hypoglycemic,[5] and hypocholestremic potential.[6] The important phytochemicals found includes saponins, coumarin, scopoletin, and trigonelline. Scientific researches on seeds have proved its ability to reduce blood-glucose and cholesterol in type 1 and type 2 diabetic conditions.[7,8,9,10] The mechanism of action involves stimulation of glucose dependent insulin secretion from beta cells of the pancreas[11] along with inhibition of activity of enzymes like amylase and sucrase.[12]
In current scenario, phytochemicals have received much attention in the treatment of diabetes for various reasons and many researchers have focused on isolation of hypoglycemic agents from medicinal plants.[13] Plant polyphenols and flavonoids are some of the naturally occurring antidiabetic agents,[14] which are known to show an inhibitory effect on carbohydrate hydrolyzing enzyme inhibition, by virtue of their capability to bind with proteins. This phenomenon contributes to lower postprandial hyperglycemia in diabetes.[15]
To the best of our knowledge, there is no scientific evidence on the inhibitory effects of the T. foenum-graecum leaves on carbohydrate hydrolyzing enzymes. The aim of the present study was to evaluate the anti-α-amylase and anti-α-glucosidase inhibitory potential of T. foenum-graecum leaves.
Materials and Methods
Chemicals
Porcine pancreatic amylase (PPA) and α-glucosidase were purchased from Sigma (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO), Tris-HCl buffer, and nitrophenyl glucopyranose were purchased from Central Drug House, India. All the other chemicals used during the work were of analytical grade.
Collection and authentication
The plant was purchased from the local market of Jabalpur (23°N10′, 79°E57′) in the month of November, and was identified at Directorate of Weed Science Research, Madhya Pradesh, India. The leaves were cleaned, shade dried, and coarsely powdered for extraction.
Extraction
T. foenum-graecum leaves were first defatted with petroleum ether and individually extracted (50 g each) with ethyl acetate and water in soxhlet apparatus. Ethyl acetate extract was desaponified using n-butanol. Extracts were filtered through a muslin cloth twice and finally with Whatman filter paper no 4. Finally, the filtrate was lyophilized and stored at 4°C until further use.
Phytochemical screening
Screening of phytochemical constituents of the plant was done using standard procedures.[16,17]
Phytoanalytical Studies
Determination of total phenolic compounds
Total soluble phenolic compounds in the extracts were determined with Folin-Ciocalteu reagent according to the method of using pyrocatechol as a standard phenolic compound.[18] Briefly, 1 ml of extract (1000 μg/ml) in a volumetric flask was diluted with distilled water (46 ml). One millilitre of Folin-Ciocalteu reagent was added and the content of the flask was mixed thoroughly. After 3 min, Na2CO3 (3 ml, 2% w/v) was added and then allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm in a spectrophotometer (Shimadzu-1700). The total concentration of phenolic compounds in the extract determined as microgram of pyrocatechol equivalent by using an equation that was obtained from the standard pyrocatechol graph:
Absorbance = 0.0054 × total phenols (pyrocatechol equivalent) (μg) 0.0058.
Assay for total flavonoid content
Total flavonoid content was determined using the method given elsewhere.[19,20] Briefly, aluminium trichloride (1 ml, 2% w/v) in methanol was mixed with the same volume of the extract (1 ml, 2000 μg/ml). Absorption readings at 415 nm were taken after 10 min against a blank sample consisting of an extract (1 ml, 2000 μg/ml) with methanol (1 ml) and without AlCl3. The concentrations of flavonoid compounds were calculated according to the following equation that was obtained from the standard quercetin graph:
Absorbance = 0.0338 quercetin (μg) - 0.0002; R2= 0.9998.
Enzyme Inhibition Studies
Porcine pancreatic amylase inhibitory
PPA inhibitory assay was performed as per the standard method.[19] 2 mg of starch was suspended in each of the tubes containing 0.2 ml of 0.5 M Tris-Hcl buffer (pH 6.9 and 0.01 M CaCl2). The tubes containing the substrate solution were boiled for 5 min and were then incubated at 37°C for 5 min. 0.2 ml of T. foenum-graecum extract (ethyl acetate and water) was taken in each tube containing different concentrations (10, 20, 40, 60, 80, and 100 μg/ml) of DMSO. PPA was dissolved in Tris-HCl buffer to form a concentration of 2 units/ml and 0.1 ml of this enzyme solution were added to each of the above-mentioned tubes. The reaction was carried out at 37°C for 10 min and was stopped by adding 0.5 ml of 50% acetic acid in each tube. The reaction mixture was centrifuged at 3000 rpm for 5 min at 4°C. The absorbance of the resulting supernatant was measured at 595 nm using a spectrophotometer (Shimadzu-1700). The α-amylase inhibitory activity was calculated as follows:
α-Amylase inhibitory activity = ((Ac+) − (Ac−)) − ((As − Ab))/((Ac+) − (Ac−)) × 100,
where Ac+, Ac−, As, and Ab are defined as the absorbance of 100% enzyme activity (only solvent with enzyme), 0% enzyme activity (only solvent without enzyme activity), a test sample (with enzyme), and a blank (a test sample without enzyme), respectively.
α-Glucosidase inhibitory activity
The α-glucosidase inhibitory activity was determined using the standard method.[20] The enzyme solution was prepared by dissolving 0.5 mg α-glucosidase in 10 ml phosphate buffer (pH 7.0) containing 20 mg bovine serum albumin. It was diluted further to 1:10 with phosphate buffer just before use. Sample solutions were prepared by dissolving 4 mg sample extract in 400 μl DMSO. Five concentrations: 50, 100, 150, 200, and 250 μg/ml were prepared and 5 μl each of the sample solutions or DMSO (sample blank) was then added to 250 μl of 20 mM p-nitrophenyl-α-D -glucopyranoside and 495 μl of 100 mM phosphate buffer (pH 7.0). It was pre-incubated at 37°C for 5 min and the reaction started by addition of 250 μl of the enzyme solution, after which it was incubated at 37°C for exactly 15 min. 250 μl of phosphate buffer was added instead of enzyme for blank. The reaction was then stopped by addition of 1000 μl of 200 mM Na2 CO3 solution and the amount of p-nitrophenol released was measured by reading the absorbance of sample against a sample blank (containing DMSO with no sample) at 400 nm using UV visible spectrophotometer.
Statistical analysis
The results are expressed as mean ± standard error of mean. Experiments were performed in triplicate. Statistical comparison was performed using analysis of variance (ANOVA) followed by Bonferroni's test (*P < 0.05).
Results and Discussion
The aim of current study was to establish the inhibitory activity of T. foenum-graecum against α-amylase and α-glucosidase. The percentage inhibition displayed by each extract is shown in Figure 1 which justifies that only ethyl acetate extract showed prominent α-amylase inhibitory potential (64.55% at concentration 250 μg/ml). The percentage of inhibition ranged from 54.55% to 13.65% in case of ethyl acetate extract and 43.95% to 9.23% in case of water extract. The α-glucosidase inhibitory activity of three extracts of T. foenum-graecum is shown in Figure 2. For all extracts tested, percent α-glucosidase inhibition increased with increasing concentration of extracts. Inhibition in enzyme activity ranged from 52.56% to 10.63% in case of ethyl acetate extract and 33.64% to 7.32% in case of water extract.
Figure 1.
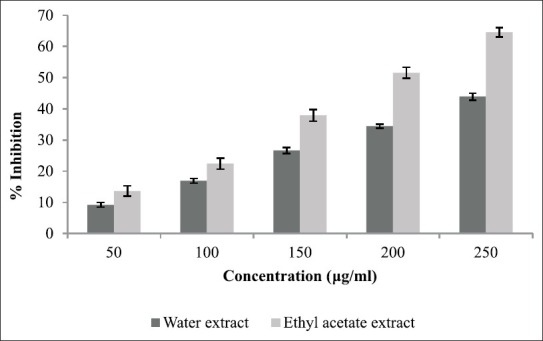
Inhibitory activity of Trigonella foenum-graecum extract against α-amylase
Figure 2.
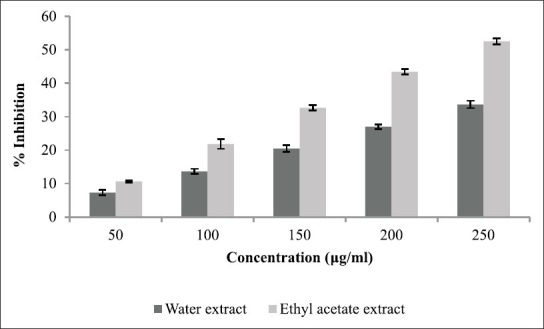
Inhibitory activity of Trigonella foenum-graecum extract against α-glucosidase
Control of sugar in blood by inhibition of carbohydrate metabolizing enzyme is a novel approach. α-Amylase is an enzyme responsible for hydrolysis of complex starch to oligosaccharides, whereas α-glucosidase hydrolyses oligosaccharides, trisaccharides and disaccharides into glucose, and other monosaccharides. Acarbose like drugs inhibits α-glucosidase[21] and are responsible for reducing post-prandial hyperglycemia,[22] such medications are useful to persons who have just diagnosed with type 2 diabetes. Such medications also prove to be useful for individuals taking sulfonylureas and metformin, which help to maintain their blood-glucose levels within a safe limit. In vitro data is also useful, particularly when a large number of compounds are to be tested, or when compounds are synthesized with minor modifications in functional groups or different percentages of extract/fractions, etc.; then a simple in vitro test can be performed to rule out inactive compounds and hence save considerable time and money. The presence of polyphenols[23] and flavonoids (vitexin, tricin, naringenin, quercetin, and tricin-7-O-beta-D-glucopyranoside)[24] in fenugreek might be responsible for such activity. The results of current works are interesting, still sufficient in vivo findings are required to extrapolate its use in humans.
Therefore, plant based α-amylase and α-glucosidase inhibitors are likely to be useful in regulating blood-glucose level. In the current study, T. foenum-graecum demonstrated α-amylase and α-glucosidase inhibitory potential which may serve as a lead for isolation and identification of compounds responsible for it.
References
- 1.Morcos SR, Elhawary Z, Gabrial GN. Protein-rich food mixtures for feeding the young in Egypt. 1. formulation. Z Ernahrungswiss. 1981;20:275–82. doi: 10.1007/BF02021639. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa M, Murakami T, Komatsu H, Murakami N, Yamahara J, Matsuda H, et al. Medicinal foodstuffs. IV. Fenugreek seed.: Structures of trigoneosides Ia, Ib, IIa, IIb, IIIa, and IIIb, new furostanol saponins from the seeds of Indian Trigonella foenum-graecum L. Chem Pharm Bull (Tokyo) 1997;45:81–7. doi: 10.1248/cpb.45.81. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti M, Khan M, Ahmed B, Jamshaid M, Ahmad W. Antibacterial activity of Trigonella foenum-graecum seeds. Fetoterapia. 1996;67:372–4. [Google Scholar]
- 4.Ahmadiani A, Javan M, Semnanian S, Barat E, Kamalinejad M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J Ethnopharmacol. 2001;75:283–6. doi: 10.1016/s0378-8741(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 5.Khosla P, Gupta DD, Nagpal RK. Effect of Trigonella foenum graecum (Fenugreek) on blood glucose in normal and diabetic rats. Indian J Physiol Pharmacol. 1995;39:173–4. [PubMed] [Google Scholar]
- 6.Puri D, Prabhu KM, Murthy PS. Hypocholesterolemic effect of the hypoglycemic principle of fenugreek (Trigonella foenum graecum) seeds. Indian J Clin Biochem. 1995;9:13–6. [Google Scholar]
- 7.Kumar GS, Shetty AK, Sambaiah K, Salimath PV. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr Res. 2005;25:1021–8. doi: 10.1007/s11130-005-5104-5. [DOI] [PubMed] [Google Scholar]
- 8.Puri D, Prabhu KM, Murthy PS. Mechanism of action of a hypoglycemic principle isolated from fenugreek seeds. Indian J Physiol Pharmacol. 2002;46:457–62. [PubMed] [Google Scholar]
- 9.Ajabnoor MA, Tilmisany AK. Effect of Trigonella foenum graceum on blood glucose levels in normal and alloxan-diabetic mice. J Ethnopharmacol. 1988;22:45–9. doi: 10.1016/0378-8741(88)90229-2. [DOI] [PubMed] [Google Scholar]
- 10.Amin R, Abdul-Ghani AS, Suleiman MS. Effect of Trigonella feonum graecum on intestinal absorption. Proc. of the 47th Annual Meeting of the American Diabetes Association (Indianapolis U.S.A.) Diabetes. 1987;36:211a. [Google Scholar]
- 11.Youn JY, Park HY, Cho KH. Anti-hyperglycemic activity of Commelina communis L.: Inhibition of alpha-glucosidase. Diabetes Res Clin Pract. 2004;66:S149–55. doi: 10.1016/j.diabres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Andrade-Cetto A, Becerra-Jiménez J, Cárdenas-Vázquez R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol. 2008;116:27–32. doi: 10.1016/j.jep.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Mai TT, Thu NN, Tien PG, Van Chuyen N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol (Tokyo) 2007;53:267–76. doi: 10.3177/jnsv.53.267. [DOI] [PubMed] [Google Scholar]
- 14.Harborne JB. Phytochemical Methods. London: Chapman and Hall; 1984. Methods in Plant Analysis; pp. 1–32. [Google Scholar]
- 15.Kokate CK. Practical Pharmacognosy. New Delhi: Vallabh Prakashan; 2003. Extraction and Phytochemical Screening; p. 157. [Google Scholar]
- 16.Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 17.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–4. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 18.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 19.Hansawasdi C, Kawabata J, Kasai T. Alpha-amylase inhibitors from Roselle (Hibiscus sabdariffa Linn.) tea. Biosci Biotechnol Biochem. 2000;64:1041–3. doi: 10.1271/bbb.64.1041. [DOI] [PubMed] [Google Scholar]
- 20.Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and funga-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Inn Food Sci Emerg Technol. 2007;8:46–54. [Google Scholar]
- 21.Sima AA, Chakrabarti S. Long-term suppression of postprandial hyperglycaemia with acarbose retards the development of neuropathies in the BB/W-rat. Diabetologia. 1992;35:325–30. doi: 10.1007/BF00401199. [DOI] [PubMed] [Google Scholar]
- 22.Carrascosa JM, Molero JC, Fermín Y, Martínez C, Andrés A, Satrústegui J. Effects of chronic treatment with acarbose on glucose and lipid metabolism in obese diabetic Wistar rats. Diabetes Obes Metab. 2001;3:240–8. doi: 10.1046/j.1463-1326.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaviarasan S, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed polyphenols protect liver from alcohol toxicity: A role on hepatic detoxification system and apoptosis. Pharmazie. 2007;62:299–304. [PubMed] [Google Scholar]
- 24.Shang M, Cai S, Han J, Li J, Zhao Y, Zheng J, et al. Studies on flavonoids from Fenugreek (Trigonella foenumgraecum L.) Zhongguo Zhong Yao Za Zhi. 1998;23:614–6. 639. [PubMed] [Google Scholar]


