Abstract
Trivanga Bhasma, a metallic preparation containing Bhasmas of Naga (lead), Vanga (tin) and Yashada (zinc), was studied for repeated dose toxicity in Swiss albino mice to estimate No Observed Effect Level (NOEL) or No Observed Adverse Effect Level (NOAEL). A total of 80 Swiss albino mice of either sex with an average body weight of 28-30 g were equally divided into four groups (Group I, II, III, and IV). Group I served as control and was given vehicle (honey: water in 2:3 ratio) Group II, III, and IV received Trivanga Bhasma @ 7.8, 39.5,and 78 mg/kg body weight for 90 consecutive days. The effect of drug was assessed on body weight, feed and water consumption changes, hematological, and histopathological parameters. At the end of the study, all animals were sacrificed and examined for gross pathological changes. Histopathological evaluation was performed for control and high dose group. Trivanga Bhasma was found to be safe. No significant clinical signs were noted in all groups studied. No major alterations were observed during histopathological evaluation. Hence, dose rate of 78 mg/kg body weight was established as NOAEL. It is suggested to carry out a toxicity study at possible higher doses and in a different species so as to establish target organ of toxicity.
Keywords: Mice, Naga, toxicity study, Trivanga Bhasma, Vanga, Yashada
Introduction
Bhasmas are Ayurvedic drugs prepared by using various minerals and metals along with specified herbs. The minerals and metals used in these preparations may be toxic in nature at higher dose or at cumulative dose. The purpose of toxicity study is two-fold, that is, to find out the effect caused and the level of exposure at which the effect is observed. Long-term toxicity studies are designed to detect effects on organs or body systems and the dose range over which the effect develops due to repeated administration for long period. The data collected from toxicity studies will help to understand dose-toxicity response and to establish a safe dose for use in clinical trials.[1]
Trivanga Bhasma is a classical Ayurvedic preparation containing Bhasmas of three metals, namely, lead, zinc, and tin indicated in diabetes mellitus and urinary disorders.[2] These metals undergo various processes described in classical literature to yield desired product. Finally, these Bhasmas are tested following Ayurvedic specifications before being used as a drug for treatment. As these preparations contain substantial amount of heavy metals, it is obvious to be concerned about safety of these drugs.[3] However, simple measurement of heavy metal content of the drug does not explain how it may be acting in-vivo.[4] Hence, it is essential to study its effects in biological system, which will give some idea about target organ of the toxicity. Meticulous perusal of available literature indicated that there is paucity of data on toxicity studies of Trivanga Bhasma, hence, present study was undertaken with an objective to evaluate the toxicological profile, identify the target organs of toxicity and to establish No Observed Effect Level (NOEL) or No Observed Adverse Effect Level (NOAEL) after administration of Trivanga Bhasma in mice for 90 consecutive days.
Materials and Methods
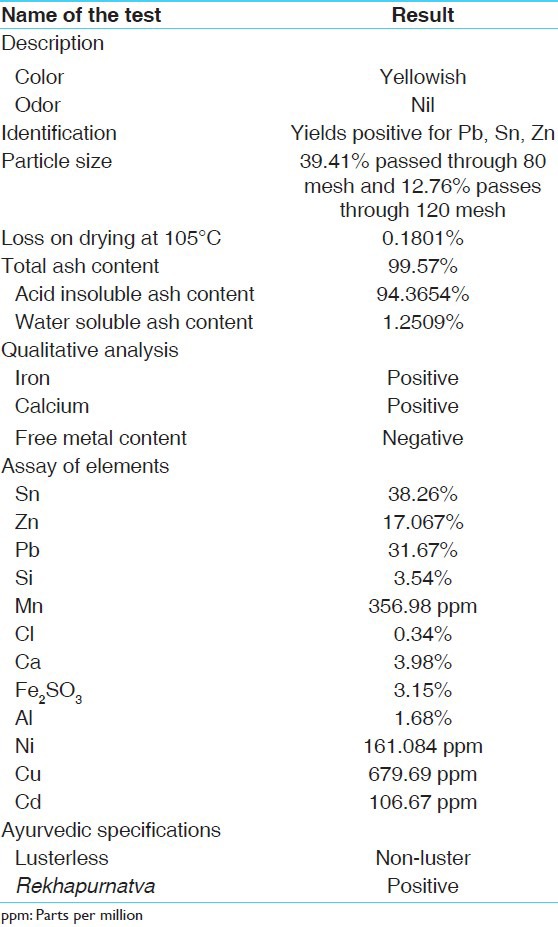
Trivanga Bhasma was provided by the Central Council for Research in Ayurvedic Sciences, New Delhi along with chemical analysis as described in Table 1.
Table 1.
Chemical analysis of Trivanga Bhasma
An approval for the study was obtained from Institutional Animal Ethics Committee (IAEC) (No 6-17/2003-CRI/Tech/777 dated February 2, 2009). A total of 80 Swiss albino mice of either sex with body weight ranging from 28 to 30 g were obtained from Institutional Animal Breeding Facility. Mice were acclimatized for 7 days. Temperature and relative humidity were maintained at 25 ± 1°C and 40-70%, respectively and illumination were controlled to give approximately a sequence of 12 hours light and 12 hours dark.[5] Mice were individually housed in polypropylene cages (27 × 19 × 14 cm) with lids and rice husk bedding. Pelleted rodent diet obtained from National Institute of Nutrition, Hyderabad, was provided along with deionized water using plastic nozzle bottles ad libitum.
The animals were weighed and randomly divided into four groups of 10 animals/sex each in such a way that mean body weights are equal and total weight variation should not exceed ±20% of the mean. Chronic toxicity study was conducted by single daily administration of test drug @ 7.8 mg/kg i.e. Therapeutically Equivalent Dose (TED), 39 mg/kg (TED × 5), and 78 mg/kg (TED × 10) body weight along with vehicle control for 90 consecutive days. The dose suggested for Trivanga Bhasma is 60 mg/day, which was converted to mice therapeutic dose using standard dose extrapolation method.[6] The test drug was given as suspension in vehicle [honey mixed with water (2:3)] by gavage, and control receiving vehicle.
All animals were observed for morbidity and mortality twice daily. General clinical observations were made twice a day at the same time throughout study. The animals were observed for changes in skin, fur, eyes, mucus membrane, occurrence of secretions, and excretions. For neurological examination, the animals were taken outside the cage in a standard arena. The behavior of the animals was recorded. Body weights and feed consumption of each animal were recorded at the start of study and thereafter at weekly intervals. Differential Leukocyte Count (DLC) was done manually. Animals were sacrificed on 91st day of the study, and were subjected to a detailed gross pathological examination. The tissues were weighed and collected in 10% neutral buffered formalin. Testes were collected in modified Davidson's fluid and subsequently transferred to 10% neutral buffered formalin after 24 hours. Organs from Control group and High Dose group were processed as per Registry of Industrial Toxicology Animal-data (RITA) guidelines[7] and subjected to histopathological evaluation. In-life phase observations were recorded and analyzed statistically by Student's t test followed by analysis of variance (ANOVA).[8]
Results
No abnormality in clinical signs was detected across all the groups throughout the study and during neurological examination except excitation and subsequent death on next day of one male in therapeutic dose group in 8th week. Another male from therapeutic dose group was found dead in 11th week of the study. However, gross pathological examination revealed no abnormal changes. Except these two, no mortality was found in any of the study groups. No treatment related effect on body weights [Tables 2 and 3], feed consumption, water consumption, fecal consistency, organ weights [Tables 4 and 5], and hematological parameters [Tables 6 and 7] were observed on comparison to control group. No treatment-related adverse effect was recorded upon detailed histopathological evaluation [Figures 1-6].
Table 2.
Weekly body weight record of males (in gm)
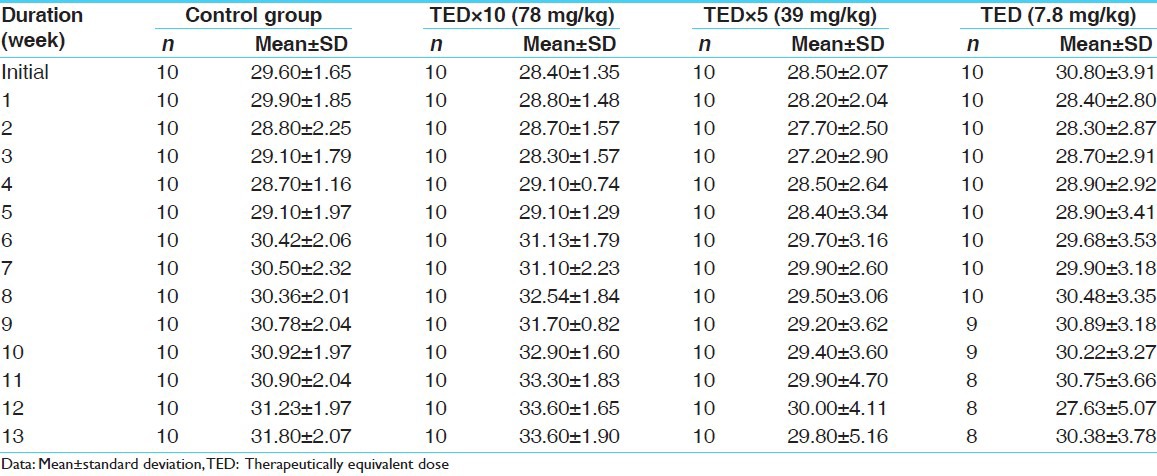
Table 3.
Weekly body weight record of females (in gm)
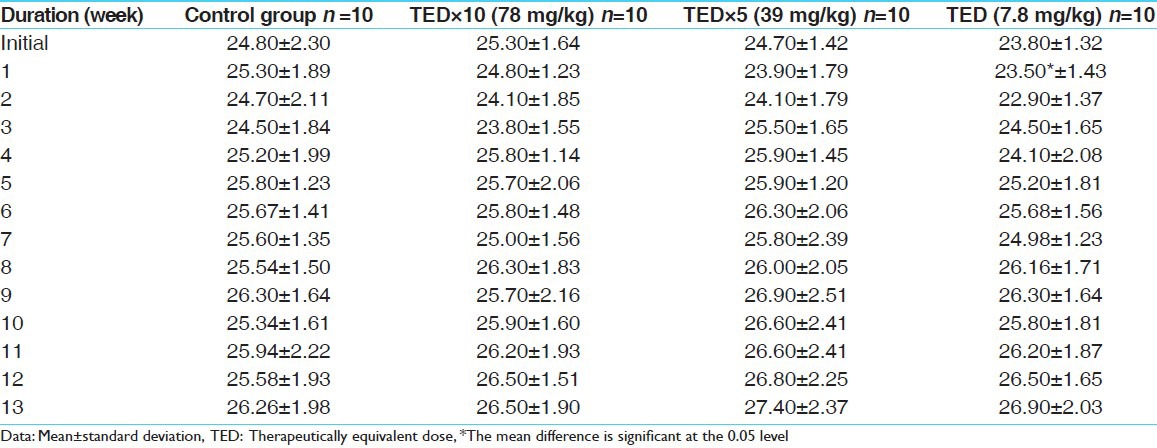
Table 4.
Organ weight record of males (in gm)
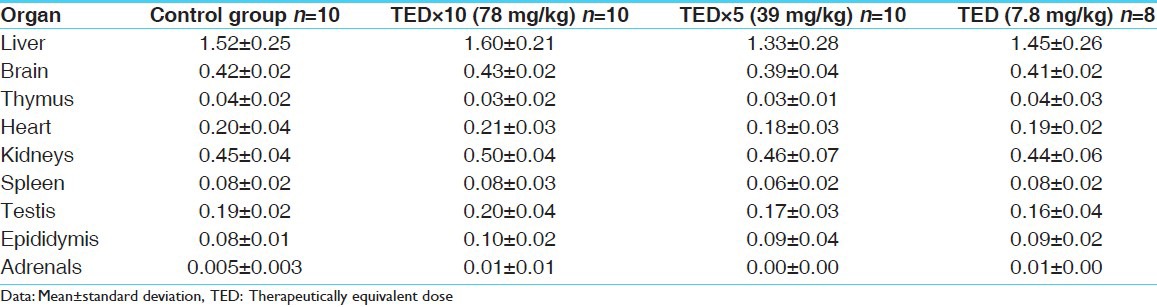
Table 5.
Organ weight record of females (in gm)
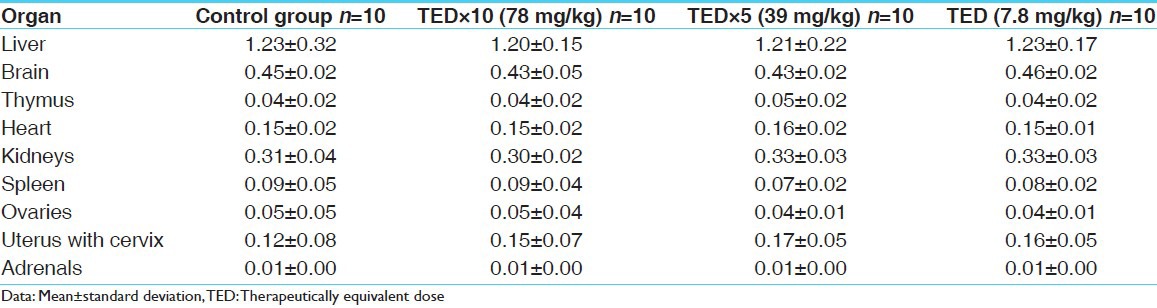
Table 6.
Hematological parameters of males
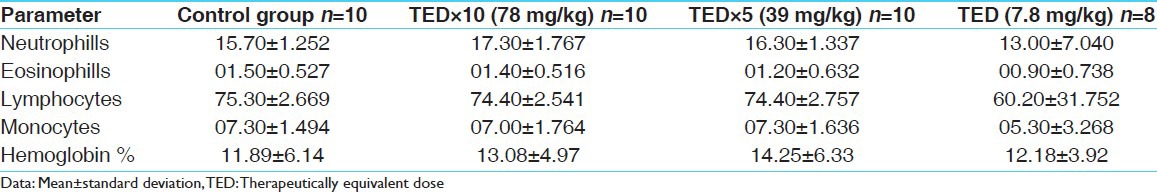
Table 7.
Hematological parameters of females
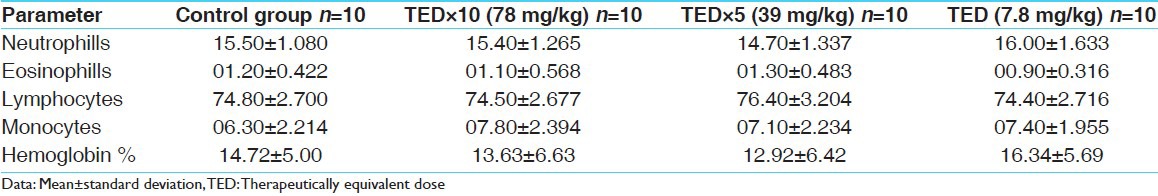
Figure 1.
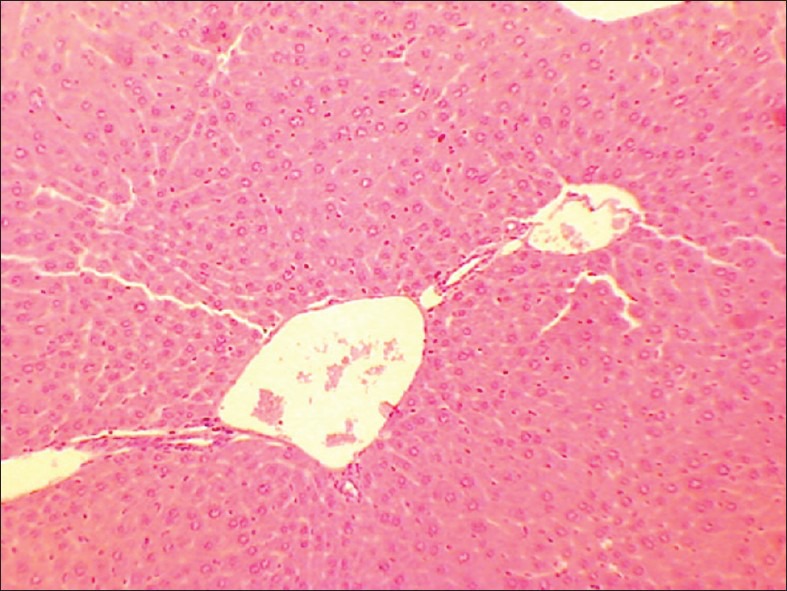
Control liver
Figure 6.
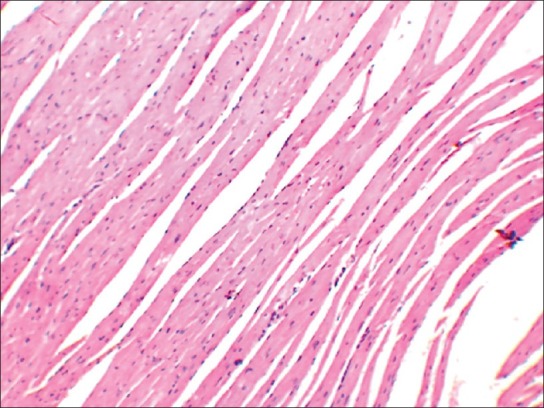
High dose (TED ×10) heart
Figure 2.
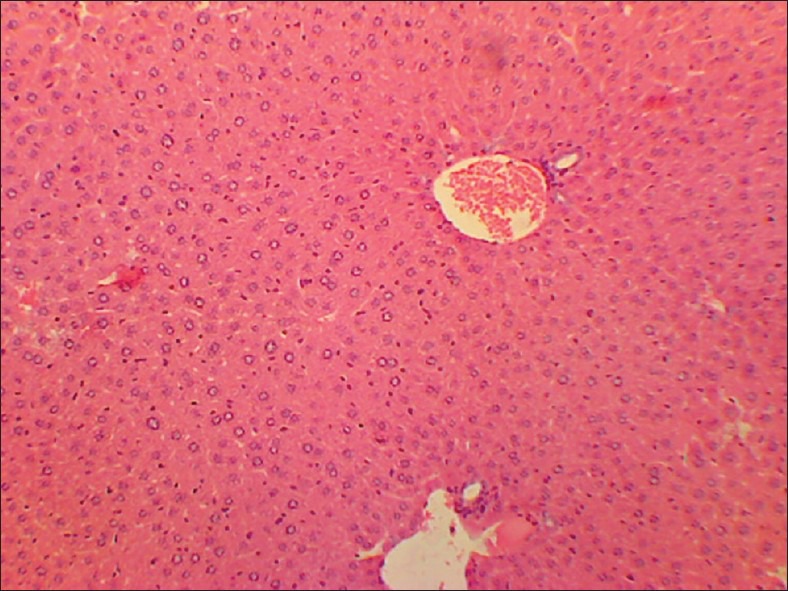
High dose (TED ×10) liver
Figure 3.
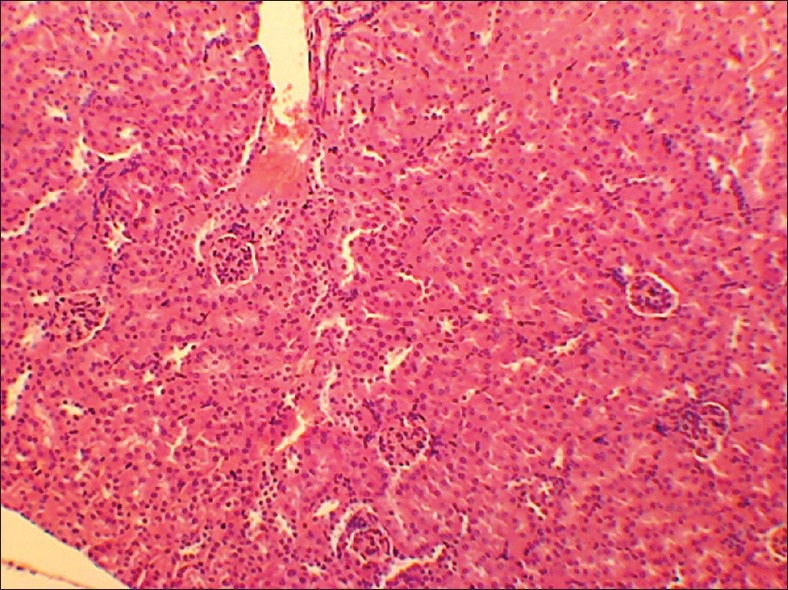
Control kidney
Figure 4.
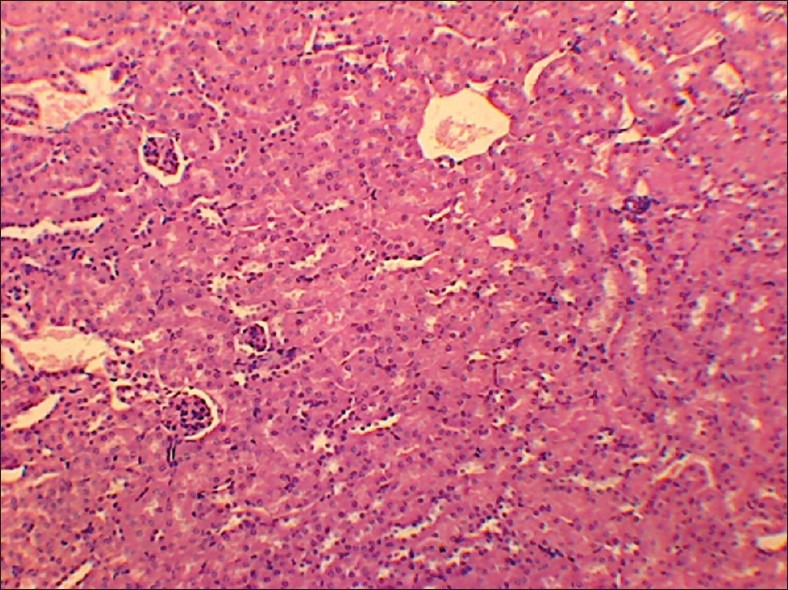
High dose (TED ×10) kidney
Figure 5.
Control heart
Discussion
Trivanga Bhasma contains heavy metals lead (31.67%), tin (38.36%), and zinc (17.1%) and is prepared in compliance with Ayurvedic classical literature.[2] However, no treatment-related effect was observed up to 10 times therapeutic dose levels. Present study indicates 78 mg/kg body weight as NOEL for Trivanga Bhasma in Swiss albino mice. Body weight change is an important index of assessment of toxicity.[4] In this study; the test drug even at the highest dose level studied showed normal weight gain. This indicates the drug has not caused any adverse effect on normal physiological functions of the body. Histopathological evaluation also indicated normal cyto-architecture and hence, it is concluded that Trivanga Bhasma is safe at dose rate of 78 mg/kg.
The dose selected in this study was extrapolation of the human therapeutic dose, which was found to be safe. The dose conversion factor was based on surface area. It is suggested to carry out a toxicity study at higher doses so as to establish target organ of toxicity. Mice were selected as biological system because of its easy availability, small size, and large background data. However, it is difficult to study all desired parameters in Mice owing to its small size and less blood volume. It is suggested to carry out study in different biological system for example, rats.
Conclusion
It is concluded that Trivanga Bhasma is generally well tolerated at dose rate of 78 mg/kg (TED × 10) in mice.
References
- 1.Anonymous. Schedule “Y”. Drugs and Cosmetics (II Amendment) Rules. Ministry of Health and Family Welfare, Government of India. 2005 [Google Scholar]
- 2.Anonymous. 2nd revised ed. New Delhi: Under Ministry of Health and Family Welfare, Govt. of India; 2004. The Ayurvedic Formulary of India, Part-1, Controller of Publication. [Google Scholar]
- 3.Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of Ayurvedic herbal medicine products. JAMA. 2004;292:2868–73. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- 4.Lavekar GS, Ravishankar B, Gaidhani SN, Shukla VJ, Ashok BK, Padhi MM. Mahayograj Guggulu heavy metal estimation and safety studies. Int J Ayurveda Res. 2010;1:150–8. doi: 10.4103/0974-7788.72486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breeding of and Experiments on Animals (Control and Supervision) Rules, 1998. Sub section (1) of Section 17 of, the Prevention of Cruelty to Animals Act, 1960 (59 of 1960) under the notification of the Government of India, Ministry of environment and Forests number S.O. 789 (E) dated, the September, 1998 in the Gazette of India [Google Scholar]
- 6.Paget GE, Barnes JM. Evaluation of drug activities, pharmacometrics. In: Lawrence DR, Bacharach AL, editors. Vol. 1. New York: Academic press; 1969. p. 161. [Google Scholar]
- 7.Morawietz G, Ruehl-Fehlert C, Kittel B, Bube A, Keane K, Halm S, et al. Revised guides for organ sampling and trimming in rats and mice – Part 3. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol. 2004;55:433–49. doi: 10.1078/0940-2993-00350. [DOI] [PubMed] [Google Scholar]
- 8.Snedecor GW, Cochran WG. 6th ed. Ames, Iowa: Iowa State University Press; 1967. Statistical Methods; pp. 258–96. [Google Scholar]


