Abstract
Introduction:
Instillation of Mitomycin C (MMC) should prevent implantation of cancer cells released during endoscopic treatment and prevent recurrences as seen in carcinoma of the bladder.
Aim:
To develop and evaluate a protocol for a single dose MMC instillation following Holmium: YAG laser ablation of upper urinary tract transitional cell carcinoma (UUT-TCC).
Setting and Design:
A single institute prospective study.
Materials and Methods:
MMC instillations protocol was designed and offered to patients between August 2005 and April 2011. Following tumor ablation, MMC was instilled into upper urinary tract (UUT) over 40 minutes. All the patients were regularly followed up.
Results:
Twenty UUT units (19 patients) were managed for UUT-TCCs using our MMC protocol. Two UUT units had G1pTa tumors, 14 had G2pTa, 2 had G3pTa, and 2 had G3pT1. At a mean follow-up of 24 months (range 1-72 months), 13/20 (65%) of the UUT units remained cancer-free, 3 (15%) UUT units developed stricture and were treated with endoscopic dilatation, only 1 (5%) of these developed long-term complications. None of the patients developed postoperative renal impairment or systemic side-effects.
Conclusions:
Using a set standard protocol, MMC can safely be instilled into the UUT after TCC ablation with minimal complications or side effects, good preservation of renal function, and with a low recurrences rate comparable to the literature.
Keywords: Mitomycin C, transitional cell carcinoma, upper urinary tract, ureteroscopy
INTRODUCTION
Nephroureterectomy has been the gold standard for the management of upper tract transitional cell carcinoma (UUT-TCC).[1] With the development of smaller diameter flexible ureteroscopes in conjunction with flexible laser fibers and improved optics, endoscopic management can be a safe alternative.[2] This minimally invasive approach can reduce the morbidity of treatment whilst preserving renal function. With the expanding role of renal sparing technique, low grade lesions in patients with normal contralateral kidneys can also be treated ureterorenoscopicaly.[1,3] Recent reports suggest that endoscopic management can be an alternative treatment option for low grade superficial tumors even as a first line management.[4,5,6]
However, with a recurrence rate between 30-65% following complete endoscopic treatment, the importance of frequent endoscopic surveillance is emphasized.[7] Not surprisingly, this is higher for high grade lesions with a third of patients proceeding to nephroureterectomy with a long-term follow-up.[8] Contemporary success of adjuvant intravesical treatments for non-muscle invasive bladder transitional cell carcinoma in reducing recurrence and progression rates has encouraged urologists to adopt a similar approach for UUT-TCC using BCG or Mitomycin C (MMC).[9,10,11,12,13,14,15]
The existing methods for MMC instillation either depend on gravity drainage instilled via nephrostomy tube or bladder instillation with an objective to develop a reflux via JJ stents or instilled via retrograde open-ended ureteric catheter. Each method has potential drawbacks, such as tumor seeding from the nephrostomy tract as well as the risk of extravasation and absorption of the topical agent if the percutaneous method is done.[16] Furthermore, various doses and delivery schedules for instillation have been tried with a variable recurrence rate.[9,10,11,12,13,14,15,16,17]
Therefore, we adopted a protocol for endoscopic management and adjuvant MMC installation with an aim to assess the recurrence and complication rates, effect of adjuvant MMC on renal function, and the need for further radical surgery for patients with UUT-TCC.
MATERIALS AND METHODS
Between August 2005 and April 2011, 15 men and 4 women with a mean age of 72 years (range: 57-83 years) underwent ureterorenoscopic UUT-TCC Holmium: YAG laser ablation followed by administration of topical MMC following the adopted algorithm protocol [Figure 1]. One man also developed UUT-TCC in his other UUT, bringing the total UUT units involved to 20. All patients diagnosed with new and recurrent suspected UUT-TCC were selected after an informed consent and institutional audit board approval. The study was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice and the Declaration of Helsinki (September 2004 version). The exclusion criteria were tumor size >1.5 cm, multiple (technically impossible to ablate in one session) tumors, or known high grade G3 tumors prior to surgery.
Figure 1.
Proposed algorithm for management of UT-TCC (CTU: CT Urogram, UT-TCC: Upper tract transitional cell carcinoma, URS: Ureterorenoscopy, MMC: Mitomycin C)
Semi-rigid ureteroscope 7 F Karl Storz or 7.8 F Richard Wolf and 7.5 F Karl Storz Flex X flexible ureterorenoscope were used for the procedure. Laser ablation was done with a curative intent using holmium laser (365 μm or 210 μm fibers) with power levels for ablation ranging from 1.0 J to 1.2 J, and the pulse frequencies from 12 Hz to 15 Hz. For ablation, the laser fiber was directed at and placed in close approximation to the tumor without touching the tissue. Multiple biopsies were taken from the tumor both before and after ablation using a 3 F Karl Storz reusable biopsy forceps for flexible ureteroscope for flat lesions and Nitinol zero tip basket for exophytic lesions. Sterile water was used for irrigation with low pressure gravity flow. Saline was not used for irrigation as diathermy ablation was intended to complete the treatment. As we did not observe any complications related to upper urinary tract irrigation for stone treatment between 2005 and 2009, we decided on water rather than Glycine. Both were reported previously as appropriate and safe for irrigation.[18]
After reviewing all published reports on MMC instillations into upper urinary tract [Table 1], we established a protocol for all patients undergoing endoscopic management of UUT TCC [Figure 1]. Following complete endoscopic ablation of the tumor, 5F open-ended ureteric catheter was left within pelvicalyceal system proximal to ablated area. Forty milligrams of MMC was dissolved in 40 ml of 0.9% normal saline and instilled via an infusion pump over 40 minutes (1 ml/minute). Post installation, the ureteric catheter was clamped for 20 minutes and was only released earlier if the patient complained of pain or discomfort. The ureteric catheter was secured to the 14 F Foley catheter with adhesive tape and was placed to gravity drainage. For tumors in the pelvicalyceal system, the ureteric catheter was removed the following day, whereas for tumors in the ureter, the ureteric catheter was exchanged for a ureteric stent the next day under general anesthetic to prevent stricture formation. Two patients with ureteric TCC had inserted Contour Injection Percuflex stent (Boston Scientific) following laser ablation and did not require coming back to theater following day. Patients were discharged the following day from the hospital with a strict surveillance schedule in place. This involved a 3-monthly check ureterorenoscopy in the first year, a 6-monthly check URS for 2 years followed by an annual URS. This was combined with an annual CT Urography. Follow-up, tumor recurrence, renal function, and need for radical surgery were analyzed retrospectively, and data was kept on a departmental database. Blood MMC levels were not monitored as none of the patients developed any systemic symptoms or deterioration in renal function [Table 2].
Table 1.
A literature review of reported results of topical adjuvant treatment post ablation of UT-TCC
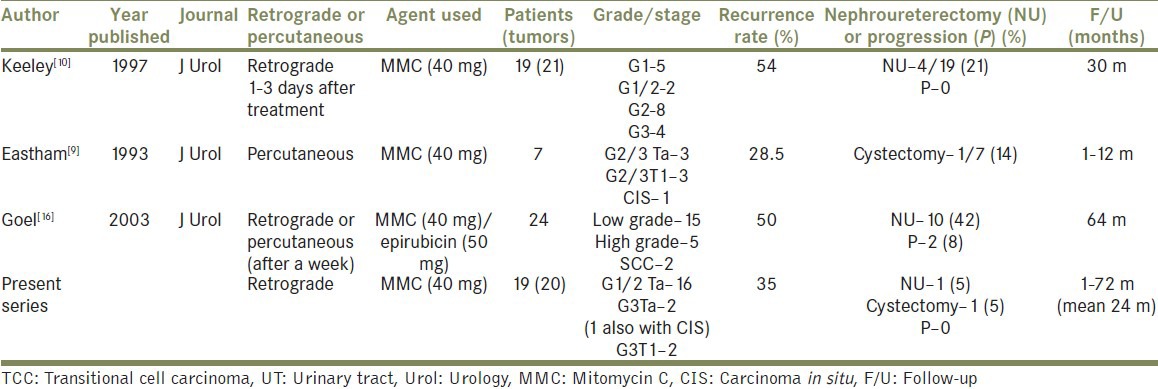
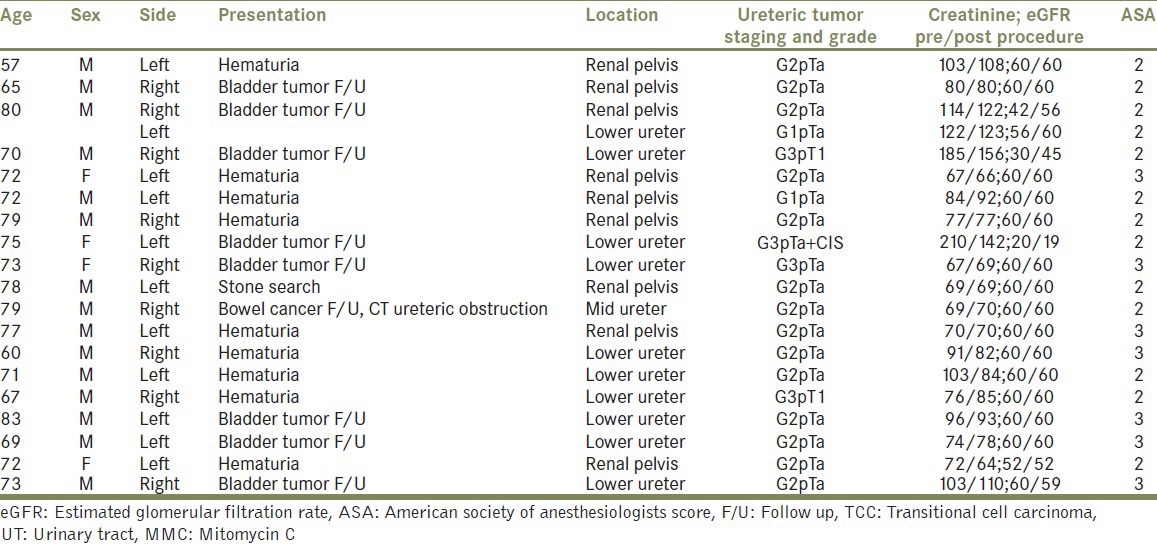
Table 2.
Summary of patients with UT.TCC managed endoscopically with adjuvant MMC instillation
RESULTS
Twenty UUT-TCCs underwent endoscopic ablation followed by instillation of Mitomycin C using the protocol mentioned [Table 2]. All new tumors and recurrences were biopsied prior to complete ablation using Holmium: YAG laser followed by MMC instillation. No significant bleeding was observed during or after the procedures. Seven patients needed a second ablation and MMC re-instillation for recurrences at a mean follow-up of 4 months, all of whom have been tumor-free since their last follow-up.
The mode of presentation was frank hematuria (n = 9), surveillance of bladder tumor (n = 9), of which 2 patients had previous contralateral nephroureterectomy for TCC, with 1 patient being investigated for suspected stones, and another patient was under surveillance check for a bowel cancer, which picked up the ureteric tumor causing obstruction.
The American Society of Anesthesiologists (ASA) score was 2 in 12 patients and 3 in 7 patients. The location of the tumor, side, grade and stage, and renal function are detailed in Table 2.
Nine tumors were in the pelvicalyceal system while 10 in the lower ureter and only 1 in the mid-ureter. Two UUT units had G1pTa tumors, 14 had G2pTa tumors, 2 had G3pTa tumors with one also having a CIS, and 2 had G3pT1 tumors (high grade tumors were pathologically diagnosed after ablation and not known before surgery, otherwise would have been excluded) [Table 2].
The recurrence rate of UUT-TCC was 35% (7/20 urinary tracts). However, at a mean follow-up of 24 months (range: 1-72 months), 13 UUT units remain clear of tumor on the last ureteroscopic assessment. Of those with recurrences, 1 patient had a G3pT1 tumor, but was not keen for a NU and had a recurrent G2pTa tumor, 1 patient with a high grade lower ureteric disease (G3pT1) and a previous contralateral NU and underwent a ureterectomy and ileal substitution, and 1 patient developed muscle invasive TCC around the right ureteric orifice and underwent a cystectomy. The remaining 4 patients with recurrent TCCs underwent ureterorenoscopic ablation followed by MMC instillation and remained tumor-free on check ureterorenoscopies.
COMPLICATIONS
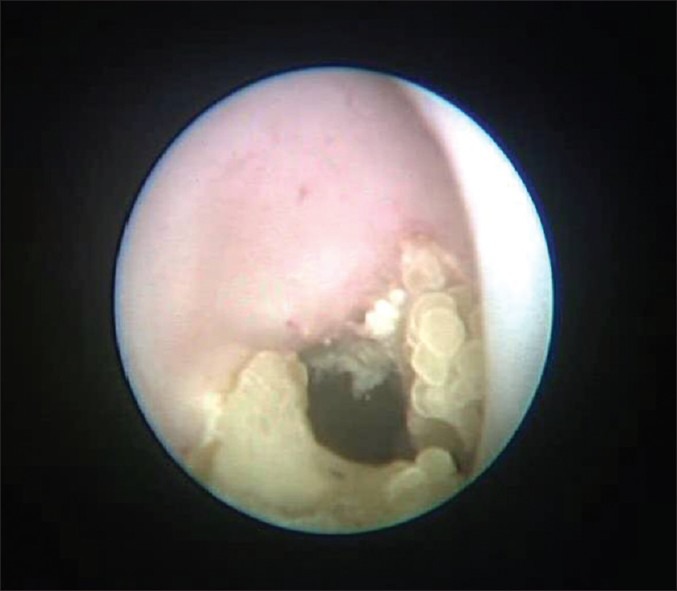
Only 1 patient did not tolerate instillation of MMC, which was stopped after 15 minutes of initiation due to severe loin pain. However, none of the patients developed any clinical systemic side-effects. Initially, in 3/20 cases (15%), we observed local complications, all of which were benign ureteric strictures, which were dilated during their ureteroscopic check and have not recurred since. However, only 5% (n = 1/20 upper urinary tracts) developed a significant long obstructing benign stricture, which lead to a nephroureterectomy due to the kidney being non-functioning on a renogram. Two of the patients that developed strictures were also seen to have benign calcified debris attached on the wall of upper urinary tract. Patient who didn’t tolerate instillation developed a renal stone stuck to the renal pelvis and lower calyx [Figure 1], which was successfully disintegrated with Holmium: YAG laser 6 months after MMC instillation, and he remains recurrence free.
In 1 patient, we successfully dilated stenotic segment of proximal ureter [Figure 2a and b] with Uromax 12 F Balloon Dilator with no evidence of contrast extravasation on retrograde ureteropyelogram after the procedure [Figure 2c], and the ureter was wide for endoscopic inspection 3 months following dilatation [Figure 2d].
Figure 2a.
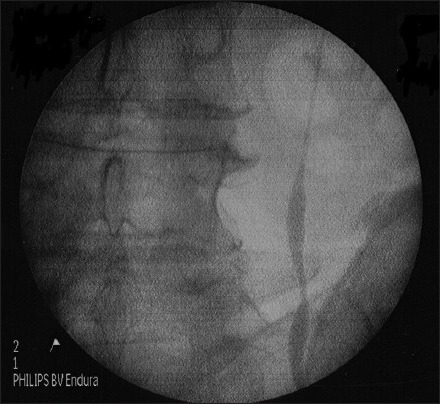
Stenosed ureter pre-dilatation
Figure 2b.
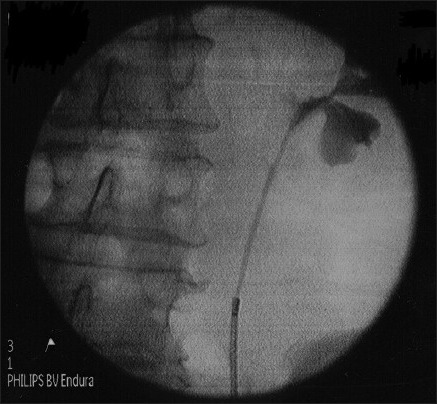
Dilating stenotic segment of proximal ureter with uromax 12 F balloon dilator
Figure 2c.
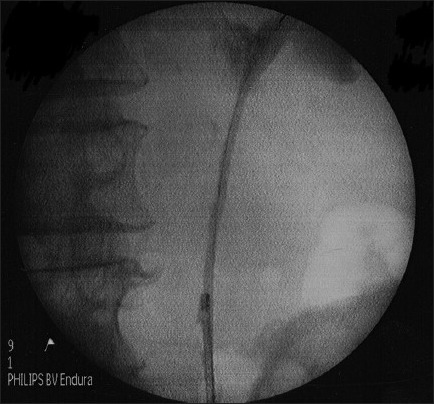
Post procedure, showing no evidence of contrast extravasation on retrograde ureteropyelogram
Figure 2d.
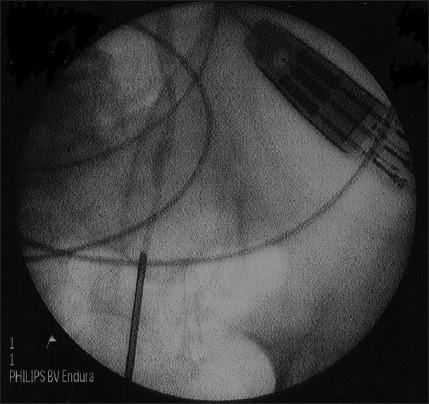
Wide ureter for endoscopic inspection 3 months following dilatation
None of the patients developed worsening renal function; Table 2 details pre- and post-operative renal function. Furthermore, none of the patients had local or distant disease progression, and none of the tumors have been upgraded on subsequent biopsies.
These complications can be classified as Grade IIIb under the Clavien Classification of Surgical Complications.[19]
DISCUSSION
The principle finding of this study was that endoscopic ablation of UUT-TCC followed by adjuvant Mitomycin C delivered using a standardized protocol has minimal complications and tumor recurrences comparable to those reported in the literature.[7] However, it is worth noting that none of the patients with low grade lesions needed nephroureterectomy, and they were all tumor-free on their last follow-up (mean - 24 months). A good cancer control was achieved in 65% of the ureters with preservation of renal function in all patients.
The limitations of this study were that this was a single center small study with significant observations for clinical practice. Also, no formal measurement of the intrarenal pressure as well as the degree of filling of the upper urinary tract was taken whilst instilling the MMC through the infusion pump. With only 19 patients with 20 UUT-TCCs and a mean follow-up of 24 months, some would argue the need for larger numbers, but for a relatively uncommon tumor, our figures are comparable to other published series [Table 1].
The strength of this study compared to other similar studies was the careful selection of UTT-TCC patients for endoscopic laser ablation for effective delivery of adjuvant MMC according to a set standardized protocol meant that all the patients had the same method of treatment.
The adjuvant MMC instillation was started within 6 hours following ablation of tumor, in contrast to previous reports where retrograde instillation of MMC, was done 1-3 days after endoscopic treatment.[10] The proven benefits of immediate adjuvant MMC post bladder tumor resection have changed clinical practice of non-muscle invasive bladder cancer.[20] Based on this, we believe that timing of MMC delivery into upper tract is crucial to its efficacy, hence the basis of the protocol. Unlike previously reported studies, all our patients were discharged 1-2 days postoperatively, and none of the patients developed any treatment related systemic side effect.[11,12,13,14] Sepsis, aplastic anemia, toxic agranulocytosis have been reported with the use of MMC; however, none of the patients in this study experienced any of these.[8]
In a comparison of open nephroureterectomy versus percutaneous resection for management of UTT-TCC, Lee et al. reported a comparable disease-free survival outcome for grade 1 and 2 disease in a 13-year follow-up.[4] In another study over a 9-year period, 19 patients were given percutaneous BCG at 6 weekly installations via a pre-placed nephrostomy tube starting at day 7 after second look nephroscopy, done a week after the percutaneous resection, and found no statistically improvement in survival of those who received BCG when compared to those who did not receive it.[4] Whilst low grade lesions can be treated endoscopically, both studies recommended treating high grade lesions with NU.[4,5] A more recent study also evaluated the cost effectiveness and survival of endoscopic management of UT-TCC with NU, and found the former to be more favorable for low grade superficial tumors.[6]
Possible mechanism and implications for future policy and practices was that endoscopic ablation with adjuvant instillation of Mitomycin C into upper urinary tract can be offered to a carefully selected group of patients, in particular low grade, even with normal contralateral kidneys and good general condition. The high grade and multifocal tumors have a higher risk of recurrence and progression as seen in our study and in other similar studies.[4,5] More aggressive intervention still remains the current recommendation for these cases.[21] As most studies using topical Mitomycin C in UUT-TCC [Table 1]are either small or retrospective, a well-designed prospective multicenter randomized trial is needed to address the issues such as dosage, frequency of installation, and time duration for the adjuvant agent to be in the system.
CONCLUSION
Endoscopic ablation with protocol-based adjuvant MMC for UUT- TCC, in particular low grade lesions seems to be effective in reducing recurrences and tumor progression with good preservation of kidney function and a low rate of MMC related long-term local complications. Though there is a risk of subsequent stricture complication, the majority of which can be easily dilated with no further recurrences.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas. 2011 Update. Eur Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Chen GL, Bagley DH. Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. J Urol. 2000;164:1173–6. [PubMed] [Google Scholar]
- 3.Elliott DS, Blute ML, Patterson DE, Bergstralh EJ, Segura JW. Long-term follow-up of endoscopically treated upper urinary tract transitional cell carcinoma. Urology. 1996;47:819–825. doi: 10.1016/S0090-4295(96)00043-X. [DOI] [PubMed] [Google Scholar]
- 4.Lee BR, Jabbour ME, Marshall FF, Smith AD, Jarrett TW. 13-Year Survival comparison of percutaneous and open nephroureterectomy approaches for management of transitional cell carcinoma of renal collecting system: Equivalent outcomes. J Endourol. 1999;13:289–294. doi: 10.1089/end.1999.13.289. [DOI] [PubMed] [Google Scholar]
- 5.Jarrett TW, Sweetser PM, Weiss GH, Smith AD. Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience. J Urol. 1995;154:1629–35. [PubMed] [Google Scholar]
- 6.Pak RW, Moskowitz EJ, Bagley DH. What is the cost of maintaining a kidney in upper-tract transitional-cell carcinoma? An objective analysis of cost and survival. J Endourol. 2009;23:341–6. doi: 10.1089/end.2008.0251. [DOI] [PubMed] [Google Scholar]
- 7.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol. 2007;4:432–43. doi: 10.1038/ncpuro0875. [DOI] [PubMed] [Google Scholar]
- 8.Lam JS, Gupta M. Ureteroscopic management of upper tract transitional cell carcinoma. Urol Clin North Am. 2004;31:115–128. doi: 10.1016/S0094-0143(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 9.Eastham JA, Huffman JL. Technique of mitomycin C instillation in the treatment of upper urinary tract urothelial tumors. J Urol. 1993;150:324–5. doi: 10.1016/s0022-5347(17)35473-3. [DOI] [PubMed] [Google Scholar]
- 10.Keeley FX, Jr, Bagley DH. Adjuvant mitomycin C following endoscopic treatment of upper tract transitional cell carcinoma. J Urol. 1997;158:2074–7. doi: 10.1016/s0022-5347(01)68157-6. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe JR, Duffy G, Chin JL. Intrarenal bacillus Calmette-Guerin therapy for upper urinary tract carcinoma in situ. J Urol. 1993;149:457–9. doi: 10.1016/s0022-5347(17)36117-7. [DOI] [PubMed] [Google Scholar]
- 12.Vasavada SP, Streem SB, Novick AC. Definitive tumor resection and percutaneous bacille Calmette-Guerin for management of renal pelvic transitional cell carcinoma in solitary kidneys. Urology. 1995;45:381–6. doi: 10.1016/s0090-4295(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Fuchs GJ. New techniques for the administration of topical adjuvant therapy after endoscopic ablation of upper urinary tract transitional cell carcinoma. J Urol. 1998;159:71–5. doi: 10.1016/s0022-5347(01)64015-1. [DOI] [PubMed] [Google Scholar]
- 14.Nonomura N, Ono Y, Nozawa M, Fukui T, Harada Y, Nishimura K. Bacillus Calmette-Guerin perfusion therapy for the treatment of transitional cell carcinoma in situ of the upper urinary tract. Eur Urol. 2000;38:701–4. doi: 10.1159/000020365. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Pineiro JA, Garcia Matres MJ, Martinez-Pineiro L. Endourological treatment of upper tract urothelial carcinomas: Analysis of a series of 59 tumors. J Urol. 1996;156:377–85. doi: 10.1097/00005392-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Goel MC, Mahendra V, Roberts JG. Percutaneous management of renal pelvic urothelial tumors: Long-term followup. J Urol. 2003;169:925–9. doi: 10.1097/01.ju.0000050242.68745.4d. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberg MP, Van Arsdalen KN, Wein AJ. The management of transitional cell carcinoma in solitary renal units. J Urol. 1991;146:700–2. doi: 10.1016/s0022-5347(17)37897-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen GL, Bagley DH. Ureteroscopic surgery for upper tract transitional cell carcinoma; complications and management. J Endourol. 2001;15:399–404. doi: 10.1089/089277901300189420. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 21.Krambeck AE, Thompson RH, Lohse CM, Patterson DE, Elliott DS, Blute ML. Imperative indications for conservative management of upper tract transitional cell carcinoma. J Urol. 2007;178:792–6. doi: 10.1016/j.juro.2007.05.056. [DOI] [PubMed] [Google Scholar]


