Abstract
Background
Growth hormone–releasing hormone (GHRH), growth hormone, and insulinlike growth factor 1 have potent effects on brain function, their levels decrease with advancing age, and they likely play a role in the pathogenesis of Alzheimer disease. Previously, we reported favorable cognitive effects of short-term GHRH administration in healthy older adults and provided preliminary evidence to suggest a similar benefit in adults with mild cognitive impairment (MCI).
Objective
To examine the effects of GHRH on cognitive function in healthy older adults and in adults with MCI.
Design
Randomized, double-blind, placebo-controlled trial.
Setting
Clinical Research Center, University of Washington School of Medicine in Seattle.
Participants
A total of 152 adults (66 with MCI) ranging in age from 55 to 87 years (mean age, 68 years); 137 adults (76 healthy participants and 61 participants with MCI) successfully completed the study.
Intervention
Participants self-administered daily subcutaneous injections of tesamorelin (Theratechnologies Inc), a stabilized analog of human GHRH (1 mg/d), or placebo 30 minutes before bedtime for 20 weeks. At baseline, at weeks 10 and 20 of treatment, and after a 10-week washout (week 30), blood samples were collected, and parallel versions of a cognitive battery were administered. Before and after the 20-week intervention, participants completed an oral glucose tolerance test and a dual-energy x-ray absorptiometry scan to measure body composition.
Main Outcome Measures
Primary cognitive outcomes were analyzed using analysis of variance and included 3 composites reflecting executive function, verbal memory, and visual memory. Executive function was assessed with Stroop Color-Word Interference, Task Switching, the Self-Ordered Pointing Test, and Word Fluency, verbal memory was assessed with Story Recall and the Hopkins Verbal Learning Test, and visual memory was assessed with the Visual-Spatial Learning Test and Delayed Match-to-Sample.
Results
The intent-to-treat analysis indicated a favorable effect of GHRH on cognition (P=.03), which was comparable in adults with MCI and healthy older adults. The completer analysis showed a similar pattern, with a more robust GHRH effect (P=.002). Subsequent analyses indicated a positive GHRH effect on executive function (P=.005) and a trend showing a similar treatment-related benefit in verbal memory (P=.08). Treatment with GHRH increased insulinlike growth factor 1 levels by 117% (P<.001), which remained within the physiological range, and reduced percent body fat by 7.4% (P<.001). Treatment with GHRH increased fasting insulin levels within the normal range by 35% in adults with MCI (P<.001) but not in healthy adults. Adverse events were mild and were reported by 68% of GHRH-treated adults and 36% of those who received placebo.
Conclusions
Twenty weeks of GHRH administration had favorable effects on cognition in both adults with MCI and healthy older adults. Longer-duration treatment trials are needed to further examine the therapeutic potential of GHRH administration on brain health during normal aging and “pathological aging.”
Trial Registration
clinicaltrials.gov Identifier: NCT00257712
Somatotrophic hormones such as growth hormone–releasing hormone (GHRH), growth hormone (GH), and insulinlike growth factor 1 (IGF-1) play an important role in brain function.1 In a healthy system, GHRH, also known as growth hormone–releasing factor, increases circulating levels of IGF-1 by way of direct effects on GH pulsatile release from the pituitary, which then stimulates an increase in production and release of IGF-1 from the liver. An increase in circulating levels of IGF-1 provides important negative feedback to control and limit pituitary GH release. In 2 clinical trials,2,3 the direct manipulation of IGF-1 and GH levels to restore age- or disease-related deficits was associated with inconsistent treatment effects on cognition, mood, and body composition. The indirect manipulation of IGF-1 and GH levels via GHRH administration is gaining popularity and has been approved by the US Food and Drug Administration to treat hypothalamic GH deficiency and human immunodeficiency virus–related lipodystrophy.4 Treatment with GHRH stimulates a cascade of somatotrophic endocrine activities that results in a natural pulsatile GH release instead of the prolonged increase in GH levels that occurs with GH supplementation. Treatment with GHRH also preserves the normal negative feedback regulation of GH by IGF-1, minimizing risk of drug overdose and adverse events.
Somatotrophic hormone levels decrease with advancing age,5,6 and such decreases have adverse effects on cognition in animals5–7; in humans, these decreases in somatotrophic hormone levels are often are associated with poorer executive function and short-term memory8–10 and increased Alzheimer disease (AD) pathology in the brain.11–13 The GHRH-induced GH secretory response has been relatively well studied in aging and early AD, with reports of delayed response but normal peak GH concentration and area under the curve,14,15 and postmortem studies comparing patients with AD with age-matched controls suggest that IGF-1 binding is preserved in the hippocampus and frontal cortex.16 Together, these studies suggest that, if GH and IGF-1 levels can be sufficiently elevated in older at-risk adults, the mechanisms for normal function related to GH and IGF-1 will likely be intact.
Insulinlike growth factor 1 deficiency is hypothesized to play a role in the pathogenesis of many neurodegenerative disorders, including AD. Insulinlike growth factor 1 readily crosses the blood-brain barrier17 and binds to receptors throughout the brain, with the highest receptor densities in the superficial and deep cortical layers, olfactory bulb, amygdala, thalamic nuclei, and hippocampus.5 Insulinlike growth factor 1 has numerous favorable effects on neurobiological processes compromised by aging and AD,5,18–20 with potent neurotrophic and neuroprotective actions including the stimulation of neurite outgrowth, the promotion of neuronal survival in the hippocampus and the entorhinal cortex (a key site of pathology in mild cognitive impairment [MCI] and AD),21 the promotion of vascular growth and blood flow, the regulation of tau phosphorylation,22 and protection from the neurotoxic effects of Aβ.23 In transgenic AD mouse models, circulating levels of IGF-1 are low, and when IGF-1 levels are restored in these animals, the Aβ burden is reduced.24,25 In patients with moderate to severe AD, IGF-1 levels are reduced relative to age-matched cognitively normal adults.13 In AD brains, increasing pathology is associated with greater deficits in IGF-1 and insulin signaling in the temporal cortex indicating possible brain IGF-1/insulin resistance,26,27 an association that has been well characterized by others.28
In humans, most of the work linking age-related IGF-1 reductions to cognitive decline is provided by cross-sectional studies.29,30 Previously, we reported favorable cognitive effects of GHRH in a controlled trial of healthy older adults,31 particularly on tasks of executive function including selective attention, problem solving, working memory, and planning/organization (episodic memory was not assessed). In exploratory analyses, the GHRH effects were equally robust in participants who obtained lower baseline scores (≤27) on the Mini-Mental State Examination (MMSE), suggesting a potential therapeutic benefit for adults with MCI.
Herein, we present the results of our 5-month randomized controlled trial of GHRH administration, building on our previous work and on the reports by others linking lower IGF-1 levels with poorer cognitive performance.11,13,32–34 We expanded the sample to include adults with amnestic MCI presumably in the early stage of AD pathogenesis with characteristic mild executive dysfunction and episodic memory deficits,35 refined the cognitive battery to include domain-specific tests targeting episodic memory and executive function, and administered a degradation-protected human GHRH analog (tesamorelin, acetate salt of N-[trans-3-hexenoyl]-human GHRH (1-44) amide; Theratechnologies Inc) that results in a natural pulsatile GH response and effectively increases serum IGF-1 to young adult normal levels.36 In light of our previous findings, we hypothesized that GHRH administration would improve cognitive performance in healthy adults, particularly executive function. In MCI, we hypothesized that treatment with GHRH would attenuate or stabilize the cognitive decline expected to occur within a 6-month period for adults with the amnestic subtype.37 We also examined the effects of GHRH on IGF-1, adiposity, and measures of glucoregulation to explore putative mechanisms linking GHRH with improved cognitive function.
METHODS
PARTICIPANTS
Our study was registered on clinicaltrials.gov (NCT00257712) and approved by the institutional review boards of the University of Washington and the Veterans Affairs Puget Sound Health Care System and by the Veterans Affairs Research and Development Committee. Written informed consent was obtained for all participants, and medical procedures were conducted through the University of Washington Clinical Research Center (supported by the National Institutes of Health/National Center for Research Resources). A total of 152 older adults were enrolled, including 86 participants (37 men and 49 women) with normal cognitive status and 66 participants (26 men and 40 women) who met the published diagnostic guidelines for amnestic MCI (single or multiple domain).38 During screening, a standardized battery of cognitive tests was administered, and scores were compared with an age- and education-adjusted estimate of premorbid ability (Shipley Vocabulary test). When candidates obtained delayed memory scores at least 1.5 SDs below this estimate, a diagnosis of amnestic MCI was considered by expert consensus, also taking into consideration medical and social history, and the results of the physical examination and clinical laboratory screening. Exclusion criteria included diabetes and other significant medical conditions, neurologic disease that might affect cognition other than MCI, and a baseline serum IGF-1 concentration greater than the midrange for healthy young adults (300 ng/mL [to convert to nanomoles per liter, multiply by 0.131]; 1 participant was excluded because of this criterion). Use of estrogen therapy, antihypertensives, or antidepressants was permitted, whereas use of diabetic medications or androgens (testosterone or dehydroepiandrosterone) was not.
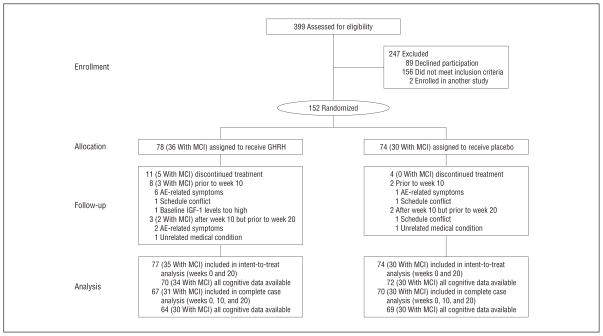
The baseline characteristics for those who completed our study were comparable across treatment groups for diagnosis, cognitive status, age, education, body composition, and circulating levels of IGF-1, insulin, and glucose (Table 1). Estrogen use was balanced across treatment groups (9 participants from the placebo group and 8 participants from the GHRH group). As expected, participants with MCI were older (P=.02) and obtained lower scores at baseline on the MMSE (P=.01) and total Story Recall (P=.001). Consequently, age and baseline MMSE score were included as covariates in analyses. A Consolidated Standards of Reporting Trials flow diagram is provided in Figure 1.
Table 1.
Baseline Characteristics of Participants Who Completed the Study
| Characteristic | Mean (SD)
|
|||
|---|---|---|---|---|
| Participants Who Received GHRH (n = 67)
|
Participants Who Received Placebo (n = 70)
|
|||
| Normal Controls (n = 36) | Adults With MCI (n = 31) | Normal Controls (n = 40) | Adults With MCI (n = 30) | |
| Sex, No. | ||||
| Female | 20 | 19 | 21 | 18 |
| Male | 16 | 12 | 19 | 12 |
| Age,a y | 67.2 (7.9) | 70.2 (8.3) | 65.9 (6.9) | 69.2 (8.2) |
| Education, y | 16.1 (2.1) | 16.5 (2.6) | 16.4 (2.6) | 16.6 (2.3) |
| MMSE scorea | 28.9 (1.3) | 28.2 (1.9) | 29.1 (1.0) | 28.6 (1.2) |
| Story Recall scorea,b | 55.6 (12.2) | 42.8 (15.7) | 52.7 (14.2) | 44.1 (16.4) |
| Body fat,c % | 35.1 (10.1) | 38.2 (8.6) | 34.3 (9.7) | 34.3 (9.3) |
| Lean muscle,d kg | 47.9 (11.2) | 43.5 (10.0) | 48.1 (10.3) | 46.3 (12.4) |
| Fasting serum IGF-1 level, ng/mL | 153 (71) | 164 (89) | 178 (80) | 164 (77) |
| Fasting plasma insulin level, μIU/mL | 7.0 (4.2) | 6.7 (4.1) | 6.6 (5.6) | 8.0 (8.1) |
| Fasting plasma glucose level, mg/dL | 102 (9.1) | 102 (11.4) | 100 (10.3) | 99 (10.0) |
Abbreviations: GHRH, growth hormone–releasing hormone; IGF-1, insulinlike growth factor 1; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Examination (maximum score, 30).
SI conversion factors: To convert IGF-1 to nanomoles per liter, multiply by 0.131; to convert insulin to picomoles per liter, multiply by 6.945; and to convert glucose to millimoles per liter, multiply by 0.0555.
P < .05 (baseline difference by diagnosis).
Immediate + delayed memory score on Story Recall, a test of verbal memory for thematic information (maximum score, 80).
Dual-energy x-ray absorptiometry measurement of total body fat, expressed as percentage of total body mass.
Dual-energy x-ray absorptiometry measurement of lean muscle mass.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram of healthy older adults and adults with mild cognitive impairment (MCI). AE indicates adverse event; GHRH, growth hormone–releasing hormone; and IGF-1, insulinlike growth factor 1.
PROCEDURE
Participants were randomly assigned to either group using a 1:1 ratio in blocks of 4 to receive 1.0 mg/d of tesamorelin (hereafter referred to as GHRH) or placebo as a subcutaneous injection 30 minutes before bedtime for 20 weeks. Treatment allocation was stratified by diagnosis, sex, and estrogen use. Outcomes were assessed between 8 and 10 AM at baseline, at weeks 10 and 20, and again 10 weeks after treatment was discontinued (week 30). Before and after treatment, participants received an oral glucose tolerance test (OGTT) and a dual-energy x-ray absorptiometry scan. Participants and study personnel involved in data collection were blinded to treatment assignment.
COGNITION
Four parallel versions of the cognitive protocol were administered at baseline and at weeks 10, 20, and 30 (washout) in counterbalanced order, and they included tests of executive function and verbal and visual episodic memory with documented sensitivity to aging and early AD,39–44 or to changes in metabolic function affecting glucose and insulin activity.45–48
Executive Function
Tests of executive function included a computer-administered version of Stroop Color-Word Interference,49,50 Task Switching,51 the Self-Ordered Pointing Test,52 and Word Fluency. For the Stroop Color-Word Interference, a test of selective attention and response inhibition, color names were presented on a computer screen in concordant or discordant font colors (eg, the word “red” presented in red or green font). For each of 4 alternating trial blocks, participants either read the word or named the color as quickly as possible, and response latency (voice onset) and content were recorded. Each trial was preceded by a displayed reminder of task instruction to minimize memory load. For Task Switching, a test of cognitive flexibility, pairs of stimuli that included a letter and a number were presented clockwise around a 2 × 2 matrix displayed on a computer screen. Every 2 trials, the participant made an odd-even decision or a consonant-vowel decision. Each trial was triggered by the previous response, and reaction time and accuracy were recorded. For the Self-Ordered Pointing Test, a test of visual working memory, participants were instructed to touch each design of a 10-design array. After each touch, the designs were rearranged within the array. This procedure was repeated a total of 3 times using the same 10-design array, and the number of errors (ie, the participant touched the same design more than once) was recorded. For Word Fluency, the total number of words generated across four 60-second trials was recorded, including 2 trials of “letter” fluency (eg, words beginning with the letter “s”) and 2 trials of “category” fluency (eg, animals).
Episodic Memory
Story Recall and the Hopkins Verbal Learning Test were administered to assess verbal memory, and the Visual-Spatial Learning Test53,54 and Delayed Match-to-Sample55 were administered to assess visual memory. For Story Recall, participants heard a narrative containing 40 informational bits and then re-called as much as possible immediately and after a 25-minute delay. Credit was awarded for verbatim recall and accurate paraphrases. For the Hopkins Verbal Learning Test, participants heard 16 words and recalled as many items as possible across 3 learning trials and then again after a 25-minute delay. For both verbal memory tests, total recall (immediate + delayed) was analyzed. For the Visual-Spatial Learning Test, a visual analog of the Hopkins Verbal Learning Test, participants viewed 8 designs that were placed on a 5 × 6 array for 10 seconds. The designs were then removed and mixed with 8 distractors, and participants selected the 8 studied designs and placed them in their correct positions on the array; this procedure was repeated 2 additional times. After a 25-minute delay, participants again selected the 8 designs from the distractors and placed them in their remembered locations on the array, and total recall of correct design paired with correct location was recorded. For Delayed Match-to-Sample, 20 abstract geometric designs were presented in series for 10 seconds on a touch-screen monitor. Following a 25-minute delay, 20 design triplets were displayed in series, and participants touched the single design per set that was previously studied.
MOOD AND SLEEP
At baseline and at weeks 10 and 20, GHRH effects on mood and sleep were assessed using the Geriatric Depression Scale56 and the Pittsburgh Sleep Quality Index.57
INSULIN SENSITIVITY, GLUCOSE TOLERANCE, AND BODY COMPOSITION
Insulin sensitivity, glucose tolerance, and adiposity were measured in light of well-established GH effects on energy metabolism. Participants completed an OGTT during screening and at week 20. Following a 12-hour fast, blood samples were collected for glucose and insulin measurements; participants then consumed a 75-g dextrose solution, and blood samples were obtained 60 and 120 minutes later. At screening, participants meeting the American Diabetes Association standard glycemic criterion for diabetes, which is indicated by a fasting plasma glucose level greater than 125 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or a 2-hour OGTT glucose level exceeding 199 pg/mL, were excluded from enrollment. At baseline and week 20, dual-energy x-ray absorptiometry scans were performed to quantify body fat and lean muscle mass.
INSULINLIKE GROWTH FACTOR 1
Serum levels of IGF-1 were initially measured using a 2-site immunoradiometric assay, which included an extraction step to remove IGF-1 binding proteins (Diagnostic Systems Laboratories/Beckman Coulter; intra-assay coefficient of variation, 2.6%; interassay coefficient of variation, 4.5%). Following Beckman’s acquisition of Diagnostic Systems Laboratories, this assay was discontinued, and for the remaining 18% of participants, IGF-1 was measured using a radioimmunoassay with coated tubes (Mediagnost/IBL-America; intra-assay coefficient of variation, 3.5%; interassay coefficient of variation, 5.2%). The 2 assays correlated linearly (r = 0.91), and all radioimmunoassay values were normalized to the immunoradiometric assay standard using a linear correction algorithm.
SAFETY AND COMPLIANCE
Circulating levels of IGF-1 were measured before study entry at screening and baseline. At weeks 2, 4, 8, 10, 16, and 20, IGF-1 was measured to ensure levels were within the physiologic range, adverse events were assessed, a brief physical examination was performed, and changes in medications, exercise regime, or health status were recorded. When an adverse event became problematic for a participant, the dose was reduced by 0.25 mg/d by a physician investigator blinded to treatment group assignment. A separate, unblinded physician investigator (G.R.M.) with no direct contact with participants or psychometrists adjusted the dose either when achieved IGF-1 exceeded physiologic levels or when IGF-1 failed to increase by at least 15% over baseline for participants in the active group. Each of these GHRH dose adjustments was yoked with a similar adjustment for a placebo-treated participant to maintain the blind for participants, staff, and other investigators. Compliance was monitored during study visits via the number of returned vials and the number of entries in a self-reported log.
STATISTICAL ANALYSES
The principal analysis was based on intent to treat. Participants who discontinued treatment were asked to return to the clinic for cognitive testing at week 20. Completer analyses were performed on cognitive outcomes obtained at weeks 10 and 20. Exploratory analyses of week-30 data were also performed to examine effects of treatment discontinuation. Multiple regression and correlation procedures were used to create residualized change scores for data collected at weeks 10, 20, and 30 relative to baseline, which are inherently more stable than arithmetic difference scores. The primary cognitive outcomes included 3 composites reflecting executive function, verbal memory, and visual memory. The composites were derived from summed z scores per cognitive domain, adjusted for number of tests administered. For the intent-to-treat analysis, an omnibus multivariate analysis of variance was performed on the 3 composite scores, with treatment group and diagnosis (for participants with MCI and normal controls) as independent variables. For the completer analysis, week (ie, weeks 10 and 20) was also included as an independent variable in the model. When the omnibus multivariate analysis of variance proved significant, univariate analyses of variance were performed on the constituent-dependent measures. When appropriate, pairwise comparisons were performed using t tests. Similarly structured analyses of variance were performed on serum IGF-1, body fat and lean muscle mass, fasting plasma insulin and glucose, and insulin response to the OGTT. Age and MMSE score were included as covariates in all analyses. Sex and education were statistically considered as covariates, but they were dropped if non-contributory. Exploratory analyses examined associations between treatment-related changes in cognition, mood, sleep, IGF-1 level, and body composition. For completer analyses, the a priori plan was to use standard multiple imputation procedures to handle missing data when missing data exceeded 5% and casewise deletion otherwise. All analyses were performed using STATA.58
RESULTS
COGNITION
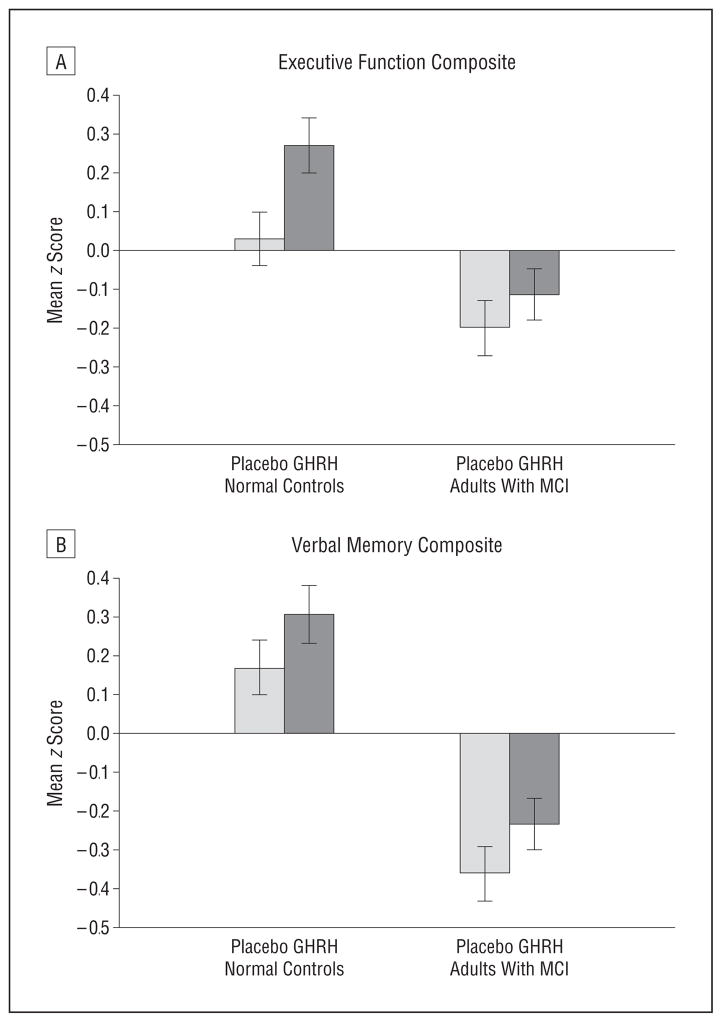
For the intent-to-treat analysis, the omnibus multivariate analysis of variance on the 3 composite scores indicated favorable effects on cognitive function at week 20 over baseline for adults allocated to receive GHRH vs placebo (F3,133 = 3.11, P = .03). Although overall cognitive performance differed by diagnosis, as expected (F3,133 = 5.97, P < .001), GHRH had comparable beneficial effects both in healthy older adults and in adults with MCI (no treatment × diagnosis interaction; P = .22), consistent with predictions based on our earlier work (ie, comparable GHRH effects observed for participants with higher and lower MMSE scores).31 For the completer analysis, missing data did not exceed 5%, and the omnibus multivariate analysis of variance produced a similar but more robust pattern of results. Treatment with GHRH had a favorable effect on cognition (F3,125 = 5.26, P = .002), and even though the healthy adults outperformed those with MCI overall (F3,125 = 11.15, P < .001), the cognitive benefit relative to placebo was comparable for both groups (no treatment × diagnosis interaction; P = .57). The results of the completer analysis indicated similar GHRH benefits at weeks 10 and 20 (no interaction involving week on treatment). Education was included as a covariate in the intent-to-treat and completer analyses (together with age and baseline MMSE score), whereas sex was not. Separate univariate analyses on the constituent composites scores indicated a GHRH-related improvement in normal controls and attenuation of decline in adults with MCI for executive function (effect size: f = 0.37, F1,127 = 8.34, P = .005; Figure 2A) and a trend showing the same pattern of results for verbal memory (effect size: f = 0.24, F1,130 = 3.09, P = .08; Figure 2B). For both analyses, favorable effects were comparable in adults with MCI and in normal controls (no treatment × diagnosis interaction; P > .27). Visual memory was not reliably affected by treatment with GHRH in our study (P > .15), likely in part owing to the high variability in scores and the near-chance performances on the Delayed Match-to-Sample. For the executive function and verbal memory composites, further inspection of the mean change z scores for the constituent tests indicated a positive influence of GHRH on all but Word Fluency (Table 2). Exploratory analyses of week-30 washout data (change scores relative to baseline) using analysis of variance suggested a lingering GHRH-related benefit for executive function (P = .08) but not verbal memory (P = .32).
Figure 2.
Cognitive response to growth hormone–releasing hormone (GHRH). Mean z scores representing change from baseline in composites of executive function (A) and verbal memory (B), expressed as residualized change scores. Treatment with GHRH had favorable effects on executive function (P = .005) as measured by Task Switching accuracy, Stroop Color-Word Interference reaction time (voice onset latency) on “interference” trials, Self-Ordered Pointing Test accuracy, and Word Fluency. A similar trend was observed for verbal memory (P = .08) as measured by total recall (immediate + delayed) on the Hopkins Verbal Learning Test and total Story Recall. Pairwise comparisons between subgroups defined by diagnosis are not presented because the main finding indicates a treatment effect for the groups combined and no treatment × diagnosis interaction. Visual memory did not benefit from GHRH administration. Error bars indicate standard error of the mean. MCI indicates mild cognitive impairment.
Table 2.
Performance on Constituent Tests of the Executive Function and Verbal Memory Composites by Treatment Groupa
| Test | Mean z Score (SE)
|
|
|---|---|---|
| GHRH Group | Placebo Group | |
| Executive function | ||
| Task Switching accuracy | 0.29 (0.13) | −0.13 (0.12) |
| Stroop Color-Word Interference RT | 0.27 (0.13) | −0.14 (0.13) |
| SOPT accuracy | 0.32 (0.13) | 0.06 (0.12) |
| Word Fluency | 0.16 (0.13) | 0.15 (0.13) |
| Verbal memory | ||
| Total Story Recall | 0.32 (0.13) | 0.10 (0.12) |
| HVLT total recall | 0.28 (0.14) | 0.16 (0.13) |
Abbreviations: GHRH, growth hormone–releasing hormone; HVLT, Hopkins Verbal Learning Test; RT, reaction time measured using voice onset latency; SOPT, Self-Ordered Pointing Test.
Values represent mean change in performance due to treatment (averaged across weeks 10 and 20) relative to baseline, adjusted for age, baseline MMSE score, and educational level.
MOOD AND SLEEP
Treatment with GHRH did not affect mood (P = .31) or sleep quality (P = .98) in adults with MCI or in healthy older adults (no treatment × diagnosis interaction).
IGF-1, INSULIN SENSITIVITY, GLUCOSE TOLERANCE, AND BODY COMPOSITION
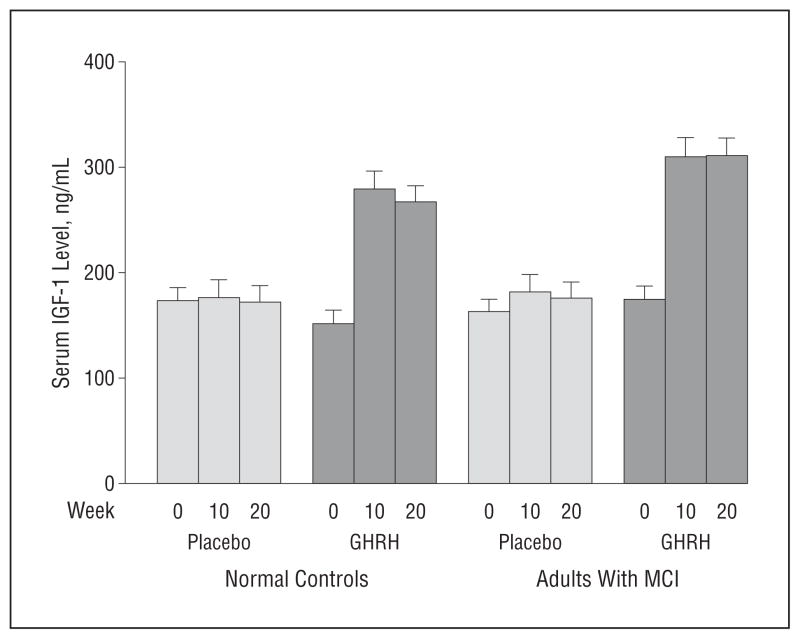
Serum levels of IGF-1 increased by 117% with GHRH administration (F1,131 = 109.73, P < .001), with no interaction involving week on treatment or diagnosis (Figure 3) indicating comparable effects at weeks 10 and 20 in adults with MCI and normal controls. The IGF-1 response to GHRH did not differ by sex (P = .69). Ten weeks after termination of treatment (week 30), IGF-1 concentration returned to basal levels. For the entire sample, treatment-related increases in serum IGF-I were associated with higher executive function composite change scores (r = 0.2, P = .03), but there were no other associations involving cognition and IGF-1.
Figure 3.
Mean serum insulinlike growth factor 1 (IGF-1) levels at baseline (week 0) and at weeks 10 and 20 (to convert to nanomoles per liter, multiply by 0.131). The IGF-1 levels increased with the administration of growth hormone–releasing hormone (GHRH) (P < .001), with no differences observed as a function of time (week 10 vs week 20) or diagnosis (healthy vs mild cognitive impairment [MCI]). Error bars indicate standard error of the mean.
Treatment with GHRH increased fasting plasma insulin levels in adults with MCI but not in normal controls (treatment × diagnosis interaction, F1,128 = 6.94, P = .005; the mean [SD] values were −0.20 [1.2] ng/mL for normal controls who received placebo and −0.02 [0.9] ng/mL for normal controls who received GHRH [P = .95], and the mean [SD] values were −1.91 [10.5] ng/mL for adults with MCI who received placebo and 2.32 [12.5] ng/mL for adults with MCI who received GHRH [P < .001]). Fasting plasma glucose, glycosylated hemoglobin A1c, and 2-hour OGTT glucose and insulin responses were not affected by treatment. Treatment with GHRH reduced body fat by 7.4% (F1,129 = 41.30, P < .001) and increased lean muscle mass by 3.7% (F1,129 = 27.60, P < .001), and these effects were similar in adults with MCI and normal controls, and in both men and women. The GHRH-related changes in fasting plasma insulin level and body composition were not correlated with the treatment-related change in cognition for the entire group or within subgroups defined by diagnosis. To examine potential moderators of cognitive response, analyses of composites were rerun, including treatment-related changes in fasting insulin level, body composition, and IGF-1 level as covariates. When the omnibus models were adjusted for IGF-1 concentration, the GHRH effect on cognition was no longer apparent; treatment effects on fasting insulin level or body composition were not contributory. In the executive function analysis of variance, a similar pattern of results was observed, whereas, for verbal memory, the treatment effect was equally attenuated when the analysis of variance was adjusted for IGF-1 level or for insulin level.
SAFETY AND COMPLIANCE
The compliance rate for self-administered injections was 98.7%. Adverse events primarily consisted of local skin reactions (redness, itching, or stinging) and increased arthralgias. Other adverse events reported, although less frequently, included gastrointestinal upset, numbness or tingling in the hands, weight gain, and fluid retention. Although increased fluid retention can potentially precipitate or exacerbate symptoms of congestive heart failure and hypertension, coronary adverse events were not observed in our study. Objective measures of body weight indicated no difference across treatment groups (P = .95). Adverse events were reported by 68% of the participants in the GHRH group and by 36% of the participants in the placebo group, and more frequently by women than by men (eTable, http://www.archneurol.com). Adverse event–related dose adjustments occurred for 13 of 78 GHRH-treated participants (17%) (11 women [6 with MCI] and 2 men [1 with MCI] and for 2 of 74 participants in the placebo group (3%) (2 women [1 with MCI]). Dose adjustments at week 4 were made for 1 healthy male control with MCI in the GHRH group when the serum IGF-1 concentration exceeded the physiologic range for age (dose reduced to 0.50 mg/d) and for 1 male adult with MCI in the GHRH group when the serum IGF-I concentration failed to increase by at least 15% over baseline (dose increased to 1.5 mg/d). Dose adjustments not related to adverse events were always yoked with a similar dose change in the placebo group.
COMMENT
We examined the effects of treatment with GHRH on cognition and serum IGF-1 level in healthy older adults and adults with amnestic MCI in light of our previous findings showing treatment benefits for those with lower baseline MMSE scores and reports by others linking lower IGF-1 levels with poorer cognitive performance.11,13,32–34 Five months of daily subcutaneous injections of GHRH increased circulating IGF-1 to young adult normal levels and had a favorable effect on executive function in adults with MCI and healthy older adults. A trend indicated a positive GHRH effect on verbal memory. Although treatment with GHRH reduced body fat and increased lean muscle mass, these effects did not modulate cognitive response.
To our knowledge, there are only 2 other randomized controlled studies examining the cognitive effects of an intervention that promotes natural stimulation of GH secretion in older adults: our first 5-month trial of GHRH administration in cognitively normal adults31 and a large, multisite 12-month trial of a ghrelin mimetic in patients with mild to moderate AD.59 In the present study, we used a different GHRH analog than that administered in our previous trial31 in light of its preferred safety profile and robust IGF-1–elevating effect, we refined the cognitive battery to better assess executive function and episodic memory, and we expanded the sample to include older adults with MCI. Although we cannot make head-to-head comparisons of GHRH effects on specific cognitive tests across our 2 trials, the therapeutic promise of GHRH is strengthened by the consistency of overall findings.
In the AD trial,59 the hormone administered was an orally active GH secretagogue (MK-677) and biologically similar to ghrelin, which is produced by the stomach. MK-677 increased serum IGF-1 concentrations by 73% at 12 months, but the results of the primary analyses indicated no treatment effects on disease progression as measured by standard outcomes in AD clinical trials. The results of the clinician-based assessment at 12 months, however, indicated a trend for improvement (P = .06) in the MK-677 group, particularly for noncarriers of the apolipoprotein E (APOE) ε4 allele (ie, ε4-negative adults). Although APOE genotyping was not performed in our study, it is possible that a high proportion of our participants were noncarriers of the ε4 allele, given the inclusion of a sizeable group of well-characterized cognitively normal participants who are less likely to be ε4 carriers.60 Other differences between our study and the MK-677 study relate to the specific hormone administered (GHRH vs ghrelin mimetic), with different physiological effects (MK-677 increased blood glucose levels and hemoglobin A1c levels, and GHRH did not), treatment efficacy on serum IGF-1 level (117% with GHRH vs 73% with MK-677), and sensitivity of cognitive outcomes to treatment effects on executive function and episodic memory. Despite methodological dissimilarities in the 2 trials, it may be that the key difference is the timing of the intervention relative to stage of disease, such that stimulation and restoration of GH and IGF-1 activities result in beneficial effects but only when pathologic processes that cause decline in cognitive function can still be prevented, delayed, or reversed.
In our study, treatment with GHRH increased fasting plasma insulin levels in adults with MCI but not in normal controls. Insulinlike growth factor 1 and insulin have similar metabolic effects,61 and IGF-1 is a potent insulin sensitizer.62 Early AD pathology is linked to subclinical changes in insulin activity in the periphery and in the central nervous system63,64 such that adults with amnestic MCI may be particularly sensitive to metabolic changes affecting insulin availability.28,65 Our data suggest that IGF-1 plays a role in treatment response on executive function, whereas, for verbal memory, both IGF-1 and insulin are likely to contribute. Although speculative, GHRH-stimulated increases in circulating insulin may help to override the early negative effects of AD pathology to boost performance on abilities (such as declarative memory) that are particularly sensitive to insulin-related dysfunction.47
Although somatotrophic hormone levels decrease with advancing age,5,6 with a potential adverse effect on cognition,5,6,11–13 lower GH and IGF-1 levels are not always associated with negative consequences. In animal models of GH and IGF-1 deficiency and in studies of caloric restriction, reduced GH and IGF-1 levels and activity are associated with a longer life span.66,67 Moreover, transgenic mice that produce abnormally high levels of GH and IGF-1 have a shorter life span and early age-related cognitive changes.68 The relationship between lower levels of IGF-1 and “delayed aging,” attributed to improved insulin signaling and glucose metabolism,66 contrasts with reports in human studies indicating no effect of caloric restriction on GH and IGF-169 and no effect of reduced GH and IGF-1 levels on life span in GH-deficient adults70 who paradoxically present with glucose intolerance and insulin resistance.71 In addition, increasing GH and IGF-1 levels in GH-deficient adults does not affect glucose and insulin response to a glucose challenge.72 Nonetheless, the favorable effects of increased GH and IGF-1 levels on cardiovascular health,73 adiposity, lean muscle mass, and potentially cognition may tip the scales in favor of restoration of somatotrophic hormone activity in older adults, particularly in those at increased risk of decline in cognitive function.
The generalizability of our findings is limited by the small sample size, particularly for subgroup analyses by diagnosis, and by sample demographics, including educational level (mean level, 16.4 years) and minority representation (90% of participants were white), that do not accurately represent the larger US population. The MCI group was older than the normal control group; however, age was included as a covariate in all analyses, and significant differences were observed between treatment groups that were comparable with regard to age. Although more women than men reported adverse events (eTable) with potential effects on unblinding and treatment efficacy (adverse event–related dose adjustments were made for 11 women and 2 men), the achieved serum IGF-1 concentration and cognitive response to GHRH administration did not differ by sex. Growth hormone–releasing hormone was administered for only 5 months, which limits our ability to assess longer-term cognitive efficacy and safety of the hormone for older adults, particularly in the absence of important mechanistic studies to account for cognition-enhancing effects of GHRH analogs in the brain. Although we achieved statistical significance in our omnibus completer analysis showing medium to large effect sizes for executive function and verbal memory, improvement on the individual tests was small (~0.25 SDs), and without information about functional status (data not collected), the clinical significance of our findings remains unclear. Finally, our findings describe GHRH effects for a group of older adults that includes those with and without MCI. It can be argued that the inclusion of healthy adults in our study weakens the therapeutic relevance of GHRH for cognitively impaired populations. However, we observed comparable benefits of GHRH on cognition in adults with MCI and in normal controls. Alternatively, the added value of including healthy adults may relate to notable GHRH-related improvements in cognitive abilities characteristically compromised with advancing age that can precede or mask early neurodegenerative disease, a finding with potential implications for the role of GHRH in secondary and primary prevention.
The potential to preserve, or even enhance, cognitive function in normal aging and in populations where cognitive functions are failing rapidly owing to neurodegenerative disease clearly has important implications not only for the affected individual but also for the support system that bears the social and financial burdens of long-term caregiving. Studies attempting to prevent or arrest cognitive decline in MCI using a variety of different approaches such as cholinesterase inhibitors, vitamins B and E, anti-inflammatories, antihypertensives, and statins have been largely negative.74–78 In normal aging, similar drug intervention trials have also been unsuccessful. The common goal of promising nonpharmacological approaches, such as those that target diet or exercise,79,80 is to augment or restore health to optimum levels. Restoration of age-related changes in somatotrophic axis activity, with consequences for GH and IGF-1 availability, may represent an analogous strategy that optimizes the health of the neuroendocrine environment with multiple protective and restorative effects on brain function, including cognition. Our results replicate and expand our earlier positive findings,31 demonstrating that GHRH administration has favorable effects on cognitive function not only in healthy older adults but also in adults at increased risk of cognitive decline and dementia. Larger and longer-duration treatment trials are needed to firmly establish the therapeutic potential of GHRH administration to promote brain health in normal aging and “pathological aging.”
Acknowledgments
Funding/Support: This research was supported by National Institutes of Health/National Institute of Aging grants AG025515 (to Dr Vitiello) and AG030484 (to Dr Friedman) and by the US Department of Veterans Affairs. A portion of this work was conducted through the Clinical Research Center Facility at the University of Washington Medical Center, which is supported by the National Institutes of Health/National Center for Research Resources grant ULRR025014.
Footnotes
Financial Disclosure: Tesamorelin and placebo were provided at no cost to the study by Theratechnologies Inc (Montreal, Quebec, Canada).
Online-Only Material: The eTable is available at http://www.archneurol.com.
Additional Contributions: We thank Jane Corkery-Hahn, BA, M. Ashley Heald, and Monica Kletke, BS, for their expert assistance in conducting this research.
Author Contributions: Dr Baker had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis, which was conducted without input from the supporting institutions. Study concept and design: Baker, Borson, Merriam, and Vitiello. Acquisition of data: Baker, Barsness, Borson, Merriam, and Vitiello. Analysis and interpretation of data: Baker, Borson, Merriam, Friedman, Craft, and Vitiello. Drafting of the manuscript: Baker, Barsness, Borson, and Vitiello. Critical revision of the manuscript for important intellectual content: Baker, Borson, Merriam, Friedman, Craft, and Vitiello. Statistical analysis: Baker and Craft. Obtained funding: Baker, Friedman, Craft, and Vitiello. Administrative, technical, and material support: Baker, Barsness, Borson, Merriam, and Vitiello. Study supervision: Baker and Vitiello.
References
- 1.Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55(2):B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86(4):1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 3.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon S. Tesamorelin: a review of its use in the management of HIV-associated lipodystrophy. Drugs. 2011;71(8):1071–1091. doi: 10.2165/11202240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Trejo JL, Carro E, Lopez-Lopez C, Torres-Aleman I. Role of serum insulin-like growth factor I in mammalian brain aging. Growth Horm IGF Res. 2004;14(suppl A):S39–S43. doi: 10.1016/j.ghir.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74(4):512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- 8.Bellar D, Glickman EL, Juvancic-Heltzel J, Gunstad J. Serum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience. 2011;178:133–137. doi: 10.1016/j.neuroscience.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Aleman A, de Vries WR, de Haan EH, Verhaar HJ, Samson MM, Koppeschaar HP. Age-sensitive cognitive function, growth hormone and insulin-like growth factor 1 plasma levels in healthy older men. Neuropsychobiology. 2000;41(2):73–78. doi: 10.1159/000026636. [DOI] [PubMed] [Google Scholar]
- 10.Deijen JB, Arwert LI, Drent ML. The GH/IGF-I Axis and cognitive changes across a 4-year period in healthy adults. ISRN Endocrinol. 2011;2011:249421. doi: 10.5402/2011/249421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez A, Cacabelos R, Sanpedro C, García-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;28(4):533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc. 2005;53(10):1748–1753. doi: 10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- 13.Luppi C, Fioravanti M, Bertolini B, et al. Growth factors decrease in subjects with mild to moderate Alzheimer’s disease (AD): potential correction with dehydroepiandrosterone-sulphate (DHEAS) Arch Gerontol Geriatr. 2009;49(suppl 1):173–184. doi: 10.1016/j.archger.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Heuser IJ, Baronti F, Marin CA, et al. Growth hormone secretion in Alzheimer’s disease: 24-hour profile of basal levels and response to stimulation and suppression studies. Neurobiol Aging. 1992;13(2):255–260. doi: 10.1016/0197-4580(92)90037-x. [DOI] [PubMed] [Google Scholar]
- 15.Pavlov EP, Harman SM, Merriam GR, Gelato MC, Blackman MR. Responses of growth hormone (GH) and somatomedin-C to GH-releasing hormone in healthy aging men. J Clin Endocrinol Metab. 1986;62(3):595–600. doi: 10.1210/jcem-62-3-595. [DOI] [PubMed] [Google Scholar]
- 16.Jafferali S, Dumont Y, Sotty F, Robitaille Y, Quirion R, Kar S. Insulin-like growth factor-I and its receptor in the frontal cortex, hippocampus, and cerebellum of normal human and Alzheimer disease brains. Synapse. 2000;38(4):450–459. doi: 10.1002/1098-2396(20001215)38:4<450::AID-SYN10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135(5):1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 18.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7(1):45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 19.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70(5):384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Alzheimer’s Disease Neuroimaging Initiative. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272(31):19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 23.Doré S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94(9):4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8(12):1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 25.Carro E, Trejo JL, Gerber A, et al. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol Aging. 2006;27(9):1250–1257. doi: 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholerton B, Baker LD, Craft S. Insulin resistance and pathological brain ageing. Diabet Med. 2011;28(12):1463–1475. doi: 10.1111/j.1464-5491.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 29.Aleman A, de Vries WR, Koppeschaar HP, et al. Relationship between circulating levels of sex hormones and insulin-like growth factor-1 and fluid intelligence in older men. Exp Aging Res. 2001;27(3):283–291. doi: 10.1080/036107301300208718. [DOI] [PubMed] [Google Scholar]
- 30.Aleman A, Verhaar HJ, De Haan EH, et al. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999;84(2):471–475. doi: 10.1210/jcem.84.2.5455. [DOI] [PubMed] [Google Scholar]
- 31.Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006;27(2):318–323. doi: 10.1016/j.neurobiolaging.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Aleman A, Torres-Alemán I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89(3):256–265. doi: 10.1016/j.pneurobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 33.van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol. 2004;490(1–3):87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 34.Rollero A, Murialdo G, Fonzi S, et al. Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology. 1998;38(2):73–79. doi: 10.1159/000026520. [DOI] [PubMed] [Google Scholar]
- 35.Saunders NL, Summers MJ. Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol. 2010;32(4):350–357. doi: 10.1080/13803390903042379. [DOI] [PubMed] [Google Scholar]
- 36.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96(1):150–158. doi: 10.1210/jc.2010-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovacevic S, Rafii MS, Brewer JB Alzheimer’s Disease Neuroimaging Initiative. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2009;23 (2):139–145. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 39.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64 (6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 40.Daigneault S, Braun CM. Working memory and the Self-Ordered Pointing Task: further evidence of early prefrontal decline in normal aging. J Clin Exp Neuropsychol. 1993;15(6):881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- 41.Kramer AF, Hahn S, McAuley E, et al. Exercise, aging and cognition: healthy body, healthy mind? In: Rogers WA, Fisk AD, editors. Human Factors Interventions for the Health Care of Older Adults. Mahwah, NJ: Lawrence Erlbaum Associates Inc; 2001. pp. 91–120. [Google Scholar]
- 42.Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol. 2007;20(4):219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- 43.Lonie JA, Herrmann LL, Tierney KM, et al. Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer’s disease. J Neuropsychol. 2009;3(pt 1):79–92. doi: 10.1348/174866408X289935. [DOI] [PubMed] [Google Scholar]
- 44.Nutter-Upham KE, Saykin AJ, Rabin LA, et al. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23(3):229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 48.Bayer-Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68(6):743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golden CJ. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 50.Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. J Exp Psychol Hum Percept Perform. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- 51.Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121(pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 52.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 53.Malec J, Ivnik R, Smith G, Tangalos E. Visual Spatial Learning Test: normative data and further validation. Psychol Assess. 1992;4(4):433–441. doi: 10.1037/1040-3590.4.4.433. [DOI] [Google Scholar]
- 54.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ, Malec JF. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Auditory Verbal Learning Test and the Visual Spatial Learning Test. Clin Neuropsychol. 2005;19(3–4):464–523. doi: 10.1080/13854040590945193. [DOI] [PubMed] [Google Scholar]
- 55.Buccafusco JJ, Terry AV, Jr, Murdoch PB. A computer-assisted cognitive test battery for aged monkeys. J Mol Neurosci. 2002;19(1–2):179–185. doi: 10.1007/s12031-002-0030-6. [DOI] [PubMed] [Google Scholar]
- 56.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.STATA Statistical Software [computer program]. Release 11. College Station, TX: StataCorp; 2009. [Google Scholar]
- 59.Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML MK-677 Protocol 30 Study Group. Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 2008;71(21):1702–1708. doi: 10.1212/01.wnl.0000335163.88054.e7. [DOI] [PubMed] [Google Scholar]
- 60.Saunders AM. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J Neuropathol Exp Neurol. 2000;59(9):751–758. doi: 10.1093/jnen/59.9.751. [DOI] [PubMed] [Google Scholar]
- 61.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 62.Laager R, Ninnis R, Keller U. Comparison of the effects of recombinant human insulin-like growth factor-I and insulin on glucose and leucine kinetics in humans. J Clin Invest. 1993;92(4):1903–1909. doi: 10.1172/JCI116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20(3):723–736. doi: 10.3233/JAD-2010-091687. [DOI] [PubMed] [Google Scholar]
- 64.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil-Bea FJ, Solas M, Solomon A, et al. Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):405–413. doi: 10.3233/JAD-2010-100795. [DOI] [PubMed] [Google Scholar]
- 66.Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7(3):285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 67.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18(6):455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartke A. Growth hormone and aging: a challenging controversy. Clin Interv Aging. 2008;3(4):659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Redman LM, Veldhuis JD, Rood J, Smith SR, Williamson D, Ravussin E Pennington CALERIE Team. The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell. 2010;9(1):32–39. doi: 10.1111/j.1474-9726.2009.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aguiar-Oliveira MH, Oliveira FT, Pereira RM, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. 2010;95(2):714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simpson H, Savine R, Sönksen P, et al. GRS Council. Growth hormone replacement therapy for adults: into the new millennium. Growth Horm IGF Res. 2002;12(1):1–33. doi: 10.1054/ghir.2001.0263. [DOI] [PubMed] [Google Scholar]
- 72.Roemmler J, Gockel A, Otto B, Bidlingmaier M, Schopohl J. Effects on metabolic variables after 12-month treatment with a new once-a-week sustained-release recombinant growth hormone (GH: LB03002) in patients with GH deficiency. Clin Endocrinol (Oxf ) 2012;76(1):88–95. doi: 10.1111/j.1365-2265.2011.04146.x. [DOI] [PubMed] [Google Scholar]
- 73.Gatenby VK, Kearney MT. The role of IGF-1 resistance in obesity and type 2 diabetes-mellitus-related insulin resistance and vascular disease. Expert Opin Ther Targets. 2010;14(12):1333–1342. doi: 10.1517/14728222.2010.528930. [DOI] [PubMed] [Google Scholar]
- 74.Isaac MG, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD002854. doi: 10.1002/14651858.CD002854.pub2. [DOI] [PubMed] [Google Scholar]
- 75.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 76.Thal LJ, Ferris SH, Kirby L, et al. Rofecoxib Protocol 078 study group. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 77.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry. 2006;77(4):429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? a randomised controlled trial. Br J Sports Med. 2008;42(5):344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 79.Jak AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273–291. doi: 10.1007/7854_2011_141. [DOI] [PubMed] [Google Scholar]
- 80.Gu Y, Scarmeas N. Dietary patterns in Alzheimer’s disease and cognitive aging. Curr Alzheimer Res. 2011;8(5):510–519. doi: 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]