Abstract
Importance
Growth hormone–releasing hormone (GHRH) has been previously shown to have cognition-enhancing effects. The role of neurotransmitter changes, measured by proton magnetic resonance spectroscopy, may inform the mechanisms for this response.
Objective
To examine the neurochemical effects of GHRH in a subset of participants from the parent trial.
Design
Randomized, double-blind, placebo-controlled substudy of a larger trial.
Setting
Clinical research unit at the University of Washington School of Medicine.
Participants
Thirty adults (17 with mild cognitive impairment [MCI]), ranging in age from 55 to 87 years, were enrolled and successfully completed the study.
Interventions
Participants self-administered daily subcutaneous injections of tesamorelin (Theratechnologies Inc), a stabilized analogue of human GHRH (1 mg/d), or placebo 30 minutes before bedtime for 20 weeks. At baseline and weeks 10 and 20, participants underwent brain magnetic resonance imaging and spectroscopy protocols and cognitive testing and provided blood samples after fasting. Participants also underwent glucose tolerance tests before and after intervention.
Main Outcomes and Measures
Brain levels of glutamate, inhibitory transmitters γ-aminobutyric acid (GABA) and N-acetylaspartylglutamate (NAAG), and myo-inositol (MI), an osmolyte linked to Alzheimer disease in humans, were measured in three 2×2×2-cm3 left-sided brain regions (dorsolateral frontal, posterior cingulate, and posterior parietal). Glutamate, GABA, and MI levels were expressed as ratios to creatine plus phospho-creatine, and NAAG was expressed as a ratio to N-acetylaspartate.
Results
After 20 weeks of GHRH administration, GABA levels were increased in all brain regions (P<.04), NAAG levels were increased (P=.03) in the dorsolateral frontal cortex, and MI levels were decreased in the posterior cingulate (P=.002). These effects were similar in adults with MCI and older adults with normal cognitive function. No changes in the brain levels of glutamate were observed. In the posterior cingulate, treatment-related changes in serum insulin-like growth factor 1 were positively correlated with changes in GABA (r=0.47; P=.001) and tended to be negatively correlated with MI (r=−0.34; P=.06). Consistent with the results of the parent trial, a favorable treatment effect on cognition was observed in substudy participants (P=.03). No significant associations were observed between treatment-related changes in neurochemical and cognitive outcomes. Glucose homeostasis in the periphery was not reliably affected by GHRH administration and did not account for treatment neurochemical effects.
Conclusions
Twenty weeks of GHRH administration increased GABA levels in all 3 brain regions, increased NAAG levels in the frontal cortex, and decreased MI levels in the posterior cingulate. To our knowledge, this is the first evidence that 20 weeks of somatotropic supplementation modulates inhibitory neurotransmitter and brain metabolite levels in a clinical trial, and it provides preliminary support for one possible mechanism to explain favorable GHRH effects on cognition in adults with MCI and in healthy older adults.
Trial Registration
clinicaltrials.gov Identifier: NCT00257712
Circulating levels of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) decline across the life span, a condition referred to as somatopause.1 Most of the GH measured in plasma is produced in the pituitary,2 with some central nervous system tissues, such as the hippocampus, also producing GH.3 Several different peptides act as regulators for the GH system: 1 that is inhibitory, somatostatin, and 2 that are excitatory, ghrelin and GH-releasing hormone (GHRH).4 As with GH, GHRH levels decrease with age.4,5 When given as an injectable supplement, GHRH acts on its own receptors and directly stimulates GH release2 and IGF-1 synthesis by the liver.4 Unlike extrinsic GH supplementation, GHRH administration modulates intrinsic regulatory systems and evokes a normal pulsatile pattern of GH release.4
The behavioral effects of somatotropic supplementation have been examined in numerous animal studies and in 6 double-blind placebo-controlled clinical studies to date.6–13 Supplementation with IGF-1 improves spatial and reference memory in aged rats,6–8 and, if given to younger animals for 21 months, halts the behavioral decline in spatial memory that occurs with aging.9 In humans, 3 of 4 GH supplementation studies demonstrated improved cognitive function for 6 to 24 months,10–13 and 1 did not.14 We have conducted 2 randomized clinical trials of GHRH administration.15,16 In the first study, 6 months of treatment with GHRH (1-29 NH2) (sermorelin acetate; Serono Laboratories) improved performance on tests of fluid intelligence (working memory, planning and organization, selective attention, and processing speed) in healthy older adults.16 Our subsequent trial replicated and extended this work to demonstrate positive cognitive effects not only in healthy older adults but also in adults with amnestic mild cognitive impairment (MCI) who are at increased risk of Alzheimer disease (AD) dementia.15
Although the favorable effects of somatotropic supplementation on neuronal activity, including synaptic transmission and receptor density, are robust in animal studies (reviewed by Deak and Sonntag17), research on the neurobiological effects of these hormones in humans is quite limited. In 1 study, the cerebral glucose metabolic rate in the frontal cortex, as measured with positron emission tomography, was positively correlated with serum IGF-1 levels in older adults.18 In another imaging study, serum IGF-1 levels were positively correlated with brain levels of N-acetylaspartate (NAA), a putative marker of neuronal activity, in adults with persistent childhood GH deficiency.19 Imaging studies using magnetic resonance spectroscopy (MRS) survey the common brain metabolites of choline-containing compounds, creatine plus phosphocreatine (Cr), myo-inositol (MI), NAA, and the sum of glutamate plusglutamine, spectra that often include contributions from γ-aminobutyric acid (GABA). New techniques are increasingly used, such as J-resolved point-resolved spectroscopy (J-PRESS),20 which permits separation of spectral composites into the constituent neurotransmitters (glutamate, glutamine, N-acetylaspartylglutamate [NAAG], and GABA), along with established approaches (eg, Mescher-Garwood [MEGA] PRESS) to target single neurotransmitters with high specificity.21,22 Herein, we describe the results of a brain imaging study using MRS to examine the effects of GHRH on inhibitory and excitatory neurotransmitters in a subgroup of participants enrolled in a large randomized clinical 20-week trial.15 In light of results from studies demonstrating brain areas vulnerable to aging- or AD-related processes,23–27 we evaluated dorsolateral frontal, posterior cingulate, and posterior parietal regions for changes.
METHODS
The 20-week parent study was registered on ClinicalTrials.gov (NCT00257712) and was approved by the University of Washington and Veterans Affairs Puget Sound Health Care System institutional review boards and by the Veterans Affairs Research and Development Committee. The Consolidated Standards of Reporting Trials (CONSORT) flowchart describing participant flow through the parent trial is published elsewhere.15 At baseline and at weeks 10 and 20, participants completed magnetic resonance imaging (MRI) and MRS imaging protocols and cognitive testing. At baseline and other specified time points during the study, blood samples were collected for measurements of IGF-1, insulin, and glucose after participants had been fasting. Participants also completed oral glucose tolerance tests before and after treatment to further assess GHRH effects on glucose homeostasis.
PARTICIPANTS
Written informed consent was obtained for all MRS substudy participants. From 152 older adults with normal cognitive function (86 adults) or amnestic MCI28 (66 adults) enrolled in the parent trial, 30 participants (18 men) were enrolled in the MRS substudy (9 with MCI and 7 with normal cognitive function received placebo; 8 and 6, respectively, received GHRH). All participants successfully completed the imaging protocols.
INTERVENTION
In the parent study, participants were randomized in a 1:1 ratio in blocks of 4 to receive either placebo or 1.0 mg/d of tesamorelin (henceforth, GHRH), a human GHRH analogue (acetate salt of N-[trans-3-hexenoyl]-human GHRH [1-44] amide; Theratechnologies Inc), which results in a pulsatile GH response and elevated serum IGF-1 to young adult normal levels,29 injected subcutaneously 30 minutes before bedtime for 20 weeks. The GHRH dose was reduced because of an adverse event (eg, arthralgia and fluid retention) within the first 4 weeks of treatment in 4 substudy participants (2 women with normal cognitive function and 1 woman and 1 man with MCI), and the GHRH dose was increased for 1 substudy participant (a man with MCI) when his serum concentration of IGF-1 failed to increase by at least 15% over baseline. Each unblinded dose adjustment was yoked with a similar adjustment for a placebo-treated participant. Other details regarding blinding, compliance, safety monitoring, and dose adjustments in the parent trial are published elsewhere.15
COGNITION
Four parallel versions of the cognitive protocol were administered at baseline, at weeks 10 and 20, and after a 10-week wash-out (no MRS data were collected at week 30) in counterbalanced order. The protocol included tests of executive function (Stroop Color-Word Interference, Task Switching, Self-ordered Pointing Test, word fluency) and episodic memory (story recall, Hopkins Verbal Learning Test, Visual-Spatial Learning Test, delayed match-to-sample task). Composite scores were constructed by summing change z scores within cognitive domain before analysis. Additional details regarding cognitive testing and outcomes are published elsewhere.15
ASSAYS
Serum levels of IGF-1 were initially measured with a 2-site immunoradiometric assay, which included an extraction step to remove IGF-1–binding proteins (DSL/Beckman Coulter; 2.6% intra-assay and 4.5% interassay coefficients of variation). When the manufacturer discontinued this assay, IGF-1 was measured using radioimmunoassay (Mediagnost/IBL America; 3.5% intra-assay and 5.2% interassay coefficients of variation). The 2 assays correlated linearly and all radioimmunoassay values were normalized to the immunoradiometric assay standard with a linear correction algorithm.
Insulin and glucose were measured under fasting and challenge conditions in light of well-established GH effects on energy metabolism. For the oral glucose tolerance test, blood was first collected after a 12-hour fast for glucose and insulin measurements; participants then consumed a 75-g dextrose solution, and blood was sampled 1 and 2 hours later. At screening, participants meeting American Diabetes Association standard glycemic criteria for diabetes, indicated by fasting plasma glucose levels above 125 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or 2-hour oral glucose tolerance test levels above 199 pg/mL, were excluded from enrollment.
IMAGING ACQUISITION AND PROCESSING
Before MRI, participants were given instructions to hydrate for 24 hours by drinking eight 8-oz glasses of water and to avoid caffeine for 2 hours before the procedure. They were positioned comfortably on the 3T Philips scanner bed. Supplemental padding was used to minimize head movement in an 8-channel head coil. Localizers in all 3 planes were obtained with a 3-dimensional fast low-angle shot sequence, followed by a high-resolution magnetization-prepared rapid gradient echo (MPRAGE) volume acquisition in the sagittal plane (field of view, 24 cm; 256×256 matrix; isotropic 1×1×1-mm3 voxels; 10 minutes). The MPRAGE sequence was then reformatted to generate coronal and axial image volumes.
Next, 2×2×2-cm3 voxels were localized in the left-sided dorsolateral frontal, posterior cingulate, and posterior parietal regions (Figure 1). Three-plane voxel coordinates and anatomic landmarks were tagged and screen saved to ensure accurate voxel relocalization for follow-up MRI at weeks 10 and 20. At each voxel location, higher-order shimming was performed, followed by a 24-echo water-suppressed PRESS acquisition (echo time [TE], 35–380 milliseconds in 15-millisecond steps; repetition time, 2 seconds; number of signals acquired, 16; 2048 points; bandwidth, 2000; approximately 14 minutes with shimming and setup), with water-unsuppressed acquisitions used to evaluate for eddy current evaluation. Data were saved to a Linux workstation and processed by using custom MATLAB software (MathWorks) with C-scripts for TE-averaged (sum of 24 echoes) and J-PRESS data sets (spectra reformatted as described elsewhere).20 Metabolites measured included GABA, NAAG, glutamate, glutamine, and MI, for J-PRESS data sets and choline-containing compounds, Cr, and NAA for TE-averaged data sets. Metabolites were referenced to Cr and NAAG to NAA by convention.30 Gray-white (G/W) matter voxel fractions were obtained by using SPM software (http://www.fil.ion.ucl.ac.uk) with custom MATLAB scripts for voxel extraction (modified from the original source code by Mikael Montelius, MS).
Figure 1.
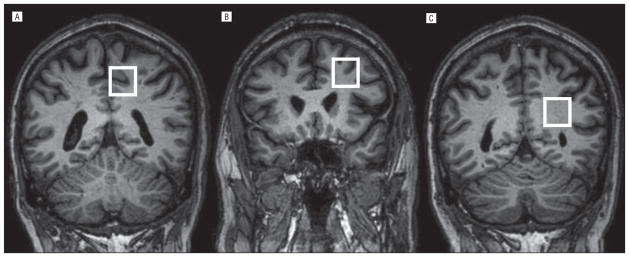
Voxel locations (white boxes) in the posterior cingulate (A), dorsolateral frontal (B), and parietal (C) regions.
STATISTICAL ANALYSIS
The analytic plan was similar to that used in the parent trial.15 The primary outcomes for the MRS substudy included the inhibitory transmitters GABA and NAAG and the excitatory transmitter glutamate. Although GHRH-related changes from baseline to week 20 were predicted in light of the behavioral results, changes at week 10 were examined in post hoc exploratory analyses. Before analysis, multiple regression and correlation procedures were used to create residualized change scores at weeks 10 and 20 (ie, residuals from the regression of longitudinal data on the baseline value). Residuals that serve as change scores are adjusted for baseline differences and are inherently more stable than delta values.31 Age, sex, and G/W matter voxel fractions were used as covariates in all models.
The GABA/Cr, NAAG/NAA, and glutamate/Cr ratios were subjected to a single multivariate analysis of variance (MANOVA), with treatment group (GHRH vs placebo), diagnosis (MCI vs normal cognitive function), and brain region (dorsolateral frontal, posterior cingulate, or posterior parietal) as independent variables. When the MANOVA results proved significant, subsequent univariate analyses of variance (ANOVAs) were conducted on the constituent outcomes. When appropriate, pairwise comparisons by group, diagnosis, or brain region were performed with use of ANOVAs rather than t tests to permit covariates in the model. Similarly structured exploratory ANOVAs were performed on secondary neurochemical outcomes (all referenced relative to Cr) including MI, choline-containing compounds, NAA, and glutamine. The effects of GHRH on cognition and glucose homeostasis in MRS sub-study participants were examined in post hoc analyses. Correlational analyses explored relationships between baseline and treatment-related changes in neurochemical outcomes, serum IGF-1 levels, glucose homeostasis, and cognitive composites of executive function and verbal episodic memory scores (composites that were sensitive to GHRH effects in the parent trial). Baseline neurochemical differences by diagnosis (MCI vs normal cognitive function) were also examined by using ANOVA, with adjustment for age, sex, and G/W matter voxel fraction. All analyses were performed with Stata software (StataCorp).32
RESULTS
At baseline, characteristics of the participants were comparable across treatment groups for diagnosis, cognitive status, age, and educational level; body composition; and serum IGF-1, fasting insulin, and glucose levels (Table 1); no differences in metabolites were observed by treatment group assignment or diagnosis (data not shown).
Table 1.
Baseline Characteristics by Treatment Group and Diagnosis
| Characteristic | Mean (SD)a
|
|||
|---|---|---|---|---|
| GHRH Group
|
Placebo Group (n = 16)
|
|||
| Normal Cognitive Function | MCI | Normal Cognitive Function | MCI | |
| Participants, No. (No. female) | 6 (3) | 8 (2) | 7 (3) | 9 (4) |
| Age, y | 66.8 (9.0) | 69.4 (8.3) | 64.4 (8.7) | 70.1 (7.2) |
| Educational level, y | 17.5 (2.6) | 17.8 (2.4) | 16.7 (2.5) | 16.8 (2.0) |
| MMSE | 29.3 (1.0) | 29.4 (1.4) | 29.4 (0.5) | 28.8 (1.5) |
| Story recall scoreb | 60.3 (14.3) | 40.1 (16.4) | 60.0 (11.6) | 44.7 (12.8) |
| BMI | 25.7 (3.2) | 28.1 (3.9) | 25.3 (5.0) | 27.4 (3.4) |
| IGF-1, ng/mL | 158 (92) | 231 (78) | 206 (66) | 161 (46) |
| Insulin, μIU/mL | 4.2 (0.8) | 8.0 (5.6) | 5.5 (3.7) | 6.4 (3.8) |
| Glucose, mg/dL | 97 (7.2) | 104 (11.0) | 93 (6.5) | 97 (12.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GHRH, growth hormone–releasing hormone; glucose, fasting plasma glucose concentration; IGF-1, fasting serum concentration of insulinlike growth factor 1; insulin, fasting plasma insulin concentration; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination score (maximum score, 30).
SI conversion factors: To convert IGF-1 to nanomoles per liter, multiply by 0.131; insulin to picomoles per liter, by 6.945; and glucose to millimoles per liter, by 0.0555.
Data represent mean (SD) values unless otherwise noted.
Significant baseline difference by diagnosis (P <.05).
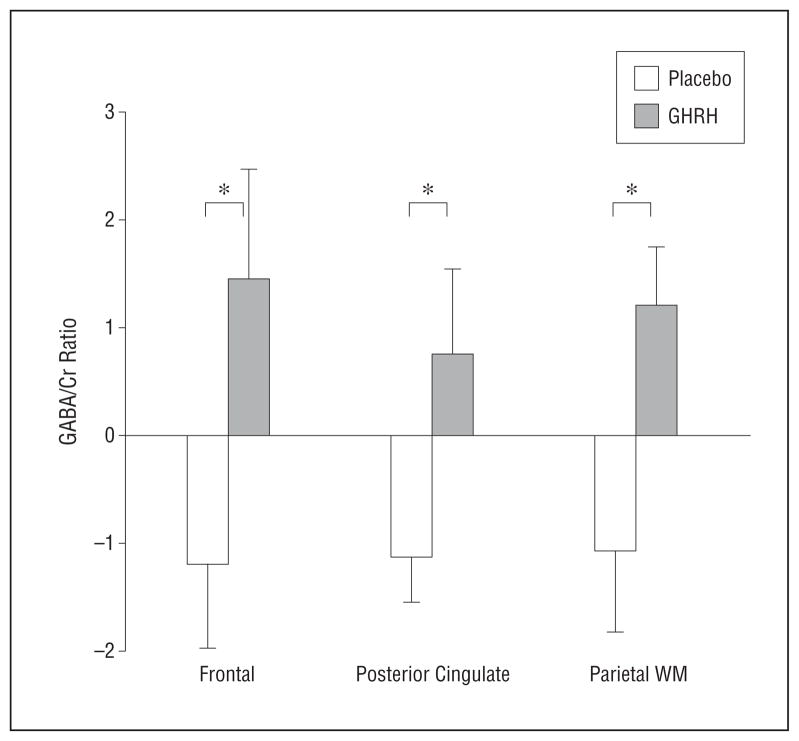
The omnibus MANOVA on GABA/Cr, NAAG/NAA, and glutamate/Cr indicated a significant effect of GHRH (main effect of treatment, F3,22 = 6.17; P = .003), which was comparable in adults with MCI and in healthy older adults (no treatment × diagnosis interaction; P = .48). The results of subsequent ANOVAs on constituent outcomes indicated that for GABA/Cr, ratios increased with 20 weeks of GHRH administration (main effect of treatment, F24 = 13.62; P = .001; Figure 2) in all brain regions sampled (no treatment × region interaction; P = .98) for adults with or without MCI (no treatment × diagnosis interaction; P = .90) (eFigure, A; http://www.jamaneuro.com).
Figure 2.
Mean (SE) baseline to week 20 change in the ratio of γ-aminobutyric acid (GABA) to creatine plus phosphocreatine (Cr) by treatment group (placebo vs growth hormone–releasing hormone [GHRH]), expressed as residualized change scores. WM, white matter. *P <.05.
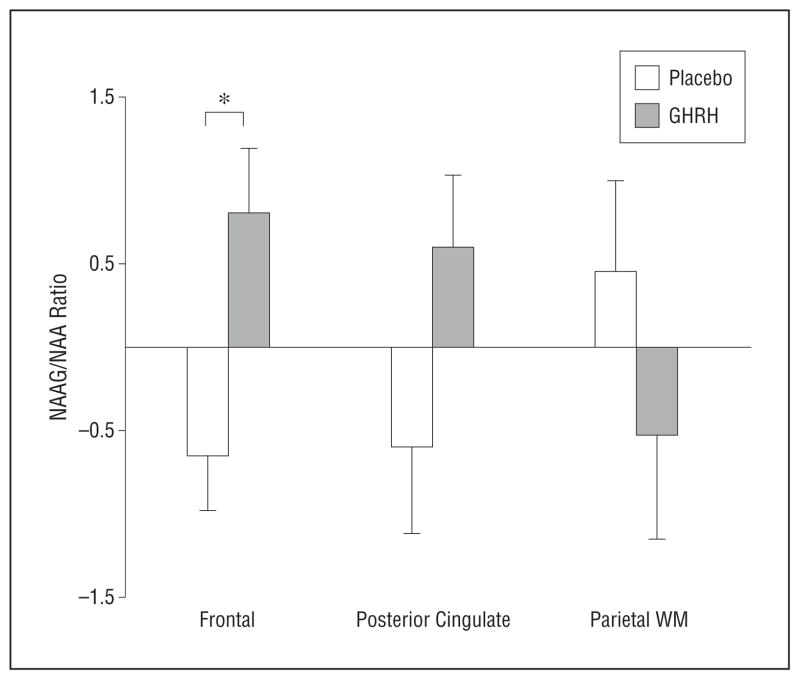
Separate exploratory analyses by brain region confirmed widespread effects of GHRH on GABA/Cr ratios (dorsolateral frontal region, F22 = 4.57 [P = .04]; posterior cingulate, F23 = 7.37 [P = .01]; posterior parietal, F23 = 5.58 [P = .03]). (The posterior parietal region sampled included relatively more white matter, and J-PRESS tends to overestimate GABA in white matter [J.E.J., written communication, July 2, 2012].) There were regional differences in NAAG/NAA ratios in response to GHRH administration (treatment × region interaction, F2,50 = 4.06; P = .02) (Figure 3); the results of post hoc analyses indicated treatment-related increases in regions with relatively more gray (frontal or cingulate region) than white matter (parietal region) for both diagnostic groups (no treatment × diagnosis interaction; P = .77) (eFigure, B), most notably in the frontal cortex (P = .03). The univariate analysis of glutamate/Cr ratios indicated no significant main or interaction effects involving treatment (P >.35).
Figure 3.
Mean (SE) baseline to week 20 change in the ratio of N-acetylaspartylglutamate (NAAG) to N-acetylaspartate (NAA) by treatment group (placebo vs growth hormone–releasing hormone [GHRH]), expressed as residualized change scores. WM, white matter. *P <.05.
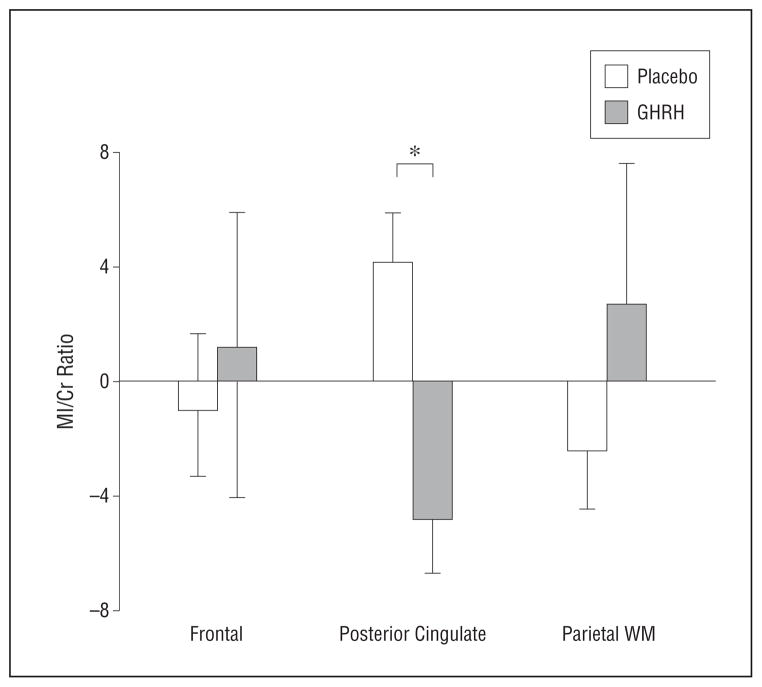
The result of secondary analyses indicated that MI/Cr ratios decreased with 20 weeks of GHRH administration in the posterior cingulate (F23 = 11.57; P = .002) in adults with or without MCI (no treatment × diagnosis interaction; P = .21) (eFigure, C), but not in the dorsolateral frontal (P = .61) or parietal (P = .53) regions. This difference was supported by a treatment × region interaction in the omnibus analysis (ANOVA, F2,50 = 3.88; P = .03) (Figure 4). There were no other treatment effects on neurochemical measurements, and GABA, NAAG, and MI levels were unchanged from baseline to 10 weeks of treatment (all P >.14).
Figure 4.
Mean (SE) baseline to week 20 change in the ratio of myo-inositol (MI) to creatine plus phosphocreatine (Cr) by treatment group (placebo vs growth hormone–releasing hormone [GHRH]), expressed as residualized change scores. WM indicates white matter. *P <.05.
Post hoc analysis of GHRH effects on executive function and verbal memory composite scores indicated that the benefits observed for the larger group in the parent trial were also observed in the smaller group of MRS participants (MANOVA for main effect of treatment, with adjustment for age, educational level, and Mini-Mental State Examination score, F2,20 = 4.02; P = .03) (Table 2). Cognitive response to GHRH did not differ between participants who opted in and those who opted out of the MRS substudy (P = .53). There were no reliable effects of treatment on peripheral measures of glucose homeostasis. Although fasting insulin levels increased for GHRH-treated adults with MCI in the parent trial (P = .005), this effect failed to reach significance in the substudy (P = .12).
Table 2.
Changes in Cognitive Performance by Treatment Group and Diagnosisa
| Test | z Score, Mean (SE)
|
|||
|---|---|---|---|---|
| GHRH Group
|
Placebo Group
|
|||
| Normal Cognitive Function | MCI | Normal Cognitive Function | MCI | |
| Executive function composite | 0.39 (0.24) | −0.07 (0.19) | −0.09 (0.11) | −0.42 (0.13) |
| Task-switching accuracy | 0.61 (0.18) | 0.11 (0.16) | −0.18 (0.24) | −0.75 (0.37) |
| Stroop interference RT | 0.30 (0.18) | −0.23 (0.47) | −0.18 (0.18) | −0.56 (0.29) |
| SOPT accuracy | 0.51 (0.32) | 0.11 (0.28) | 0.27 (0.39) | −0.17 (0.36) |
| Word fluency | 0.22 (0.43) | −0.29 (0.41) | −0.29 (0.31) | −0.22 (0.27) |
| Verbal memory composite | 0.39 (0.45) | 0.21 (0.25) | 0.16 (0.20) | −0.48 (0.17) |
| Story total recall | 0.40 (0.44) | 0.44 (0.42) | 0.33 (0.26) | −0.25 (0.23) |
| HVLT total recall | 0.37 (0.48) | −0.02 (0.45) | −0.004 (0.32) | −0.71 (0.27) |
Abbreviations: GHRH, growth hormone–releasing hormone; HVLT, Hopkins Verbal Learning Test; MCI, mild cognitive impairment; RT, reaction time measured using voice onset latency; SOPT, Self-ordered Pointing Test.
Values represent mean changes in performance, expressed as z scores of residualized change scores, by treatment assignment and diagnosis at week 20 relative to baseline. The z scores are unadjusted for covariates that were included in the model (age, educational level, and baseline Mini-Mental State Examination score).
Exploratory correlational analyses indicated that in the posterior cingulate region, the baseline GABA/Cr ratio was positively correlated with the serum IGF-1 concentration (r = 0.40; P = .03), as was the change from baseline to week 20 in these measures for the entire MRS sub-study cohort (r = 0.47; P = .001). Although changes in GABA/Cr ratios and fasting insulin concentrations were associated for the substudy cohort (r = 0.41; P = .03), when the fasting insulin concentration was included as an additional covariate in the structured ANOVA for GABA/Cr, the initial findings remained unchanged. Treatment-related changes in MI/Cr ratios tended to be negatively correlated with IGF-1 (r = −0.34; P = .06). In the dorsolateral frontal cortex, although the serum IGF-1 concentration and NAAG/NAA ratio were positively correlated at baseline (r = 0.41; P = .02), treatment-related changes in these measures from baseline to week 20 were not (r = −0.07; P = .71). There were no other associations involving neurochemical outcomes, serum IGF-1 and fasting insulin levels, and cognitive composite scores (all P > .05). A summary of the correlational analyses is provided online (eTable).
DISCUSSION
In adults with MCI and healthy older adults, 20 weeks of GHRH administration increased GABA/Cr ratios in the left dorsolateral frontal, left posterior cingulate, and left posterior parietal regions and NAAG/NAA ratios in the left dorsolateral frontal region. Treatment with GHRH did not affect glutamate, the predominant excitatory neurotransmitter in the brain. Myo-inositol, an osmolyte and precursor of phospholipids that has been found to be elevated with increasing neuropathological severity associated with AD,33–35 was decreased in the left posterior cingulate region after treatment. Although the implications of MI changes have been the source of much speculation, this and our other neurochemical findings suggest that GHRH may have beneficial effects on neurotransmitter and osmotic changes linked to aging and AD.
The role of GABA as the major inhibitory neurotransmitter, ubiquitous in the central nervous system, is well characterized (reviewed by Rossignol36). Although the animal literature suggests that GABA receptor changes may underlie the beneficial memory effects of somatotropic supplemention,8 to our knowledge, our findings provide the first evidence that GHRH administration increases inhibitory neurotransmitter levels in the brain. The diverse functional role of NAAG as a peptide neurotransmitter has only recently been characterized (reviewed by Neale et al37). In a few postmortem studies of AD, NAAG levels are reduced (levels in the occipital lobe are less than levels in the temporal lobe, which are less than levels in the frontal lobe).38 Unlike neurotransmitters that act on ionotropic receptors, NAAG acts on metabotropic glutamate receptor 3, present on both neurons and glia.37 A secondary form of NAAG, NAAG2, was recently identified, and its function and distribution are still undetermined.39 In the absence of clear evidence to indicate that NAAG has effects other than inhibiting neurotransmission, perhaps the most parsimonious conclusion from our data at this point is that 20 weeks of GHRH treatment enhances inhibitory features of brain network activity (GABA and NAAG).
A range of studies demonstrates aging-related changes in GABA and an important role of efficient inhibitory processing for successful cognition. In the auditory cortex, the synthesis enzyme for GABA, glutamic acid decarboxylase, is reduced with aging within most cortical layers (II–V).40 Although this effect is not as widespread in the parietal cortex, enzyme reductions in the hippocampus support focused involvement in that region.40 Aging- or disease-related changes in the balance of inhibitory to excitatory activity in the brain may represent an important marker of brain function efficiency. Majdi and colleagues41 reported that the expression of proteins associated with excitatory and inhibitory postsynaptic sites in the prefrontal and parietal cortical regions were altered in older cognitively impaired mice compared with older unimpaired or young mice, implicating a potential important role of excitatory-inhibitory tone in aging-related cognitive impairment.
In a chemically induced model of behavioral deficit using the noncompetitive N-methyl-D-aspartate receptor antagonist phencyclidine,42 transplantation of embryonic medial ganglionic eminence into the frontal lobe either before or after phencyclidine treatment selectively causes growth of GABAergic interneurons and a reversal of cognitive impairment.42 In theoretical models, it has been suggested that deficits in GABA result in poorer performance because of an increase in background noise relative to stable stimulus strength, whereas supplementation overcomes this condition.43 Consistent with this interpretation, reduced levels of dorsolateral prefrontal GABA in humans are related to greater impulsivity44 and increased perseveration in schizophrenia.45
Our study has several limitations. The sample size was small and only 3 brain regions were analyzed with MRS. Although the method used (J-PRESS) has been shown to be sufficient and reproducible for measuring GABA,46 further studies using more sensitive editing approaches (eg, MEGA-PRESS) would be helpful to improve sensitivity. The anatomic coordinates selected for examination in the brain may have precluded our ability to detect associations between GHRH effects on neurochemical outcomes and cognition in our study. For example, in studies in which the anatomic target for MRS is highly localized to the behavior being surveyed, close concordance between GABA and function is observed; a high correlation was demonstrated between visual discrimination tasks and magnetoencephalographic measures of gamma band activity (probably mediated by GABA23), functional MRI findings, and GABA in the visual cortex.21,47 Similar findings are reported for motor control and GABA in the supplementary motor area48 and for tactile discrimination and GABA in the sensorimotor cortex49; control regions selected to assess cortical GABA levels remote from the site of interest demonstrated no concordance with performance.49,50
Future work evaluating metabolite-behavior changes induced by GHRH in targeted task-specific regions will be useful to evaluate whether GHRH-related effects on inhibitory neurochemistry tone might account for GHRH-related improvements in cognitive function. Our data do not permit us to resolve whether brain neurochemical treatment effects were due to indirect GHRH effects in the periphery or direct GHRH effects in the brain. Although the role of GH on glucose homeostasis is well established, we did not observe consistent treatment effects on glucose or insulin under fasting or challenge conditions, and treatment-related change in neurochemical outcomes were not correlated with these measures. The effects of GHRH on brain glucose metabolism have yet to be explored in clinical trials.
In summary, 20 weeks of GHRH administration increased brain levels of the inhibitory transmitters GABA and NAAG and decreased MI levels both in adults with MCI and in healthy older adults. These treatment-related changes are consistent with amelioration of aging- or disease-related biochemical processes in the brain. Although larger and longer-duration treatment trials are needed to confirm these preliminary findings, our pilot study provides additional evidence to support positive GHRH effects on brain function in normal and pathological aging.
Acknowledgments
Funding/Support: This project was supported by the National Institute on Aging, National Institutes of Health (grants R01 AG030484-01 [principal investigator, Dr Friedman] and AG025515 [principal investigator, Dr Vitiello]). A portion of this work was conducted through the Clinical Research Center Facility at the University of Washington Medical Center, which is supported by the National Center for Research Resources, National Institutes of Health (grant ULRR025014).
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: Presented in part at the Alzheimer’s Association International Conference; July 14–19, 2012; Vancouver, British Columbia, Canada.
Online-Only Material: The eFigure and eTable are available at http://www.jamaneuro.com.
Additional Contributions: Tesamorelin and placebo were donated for the study by Theratechnologies Inc. Jane Corkery-Hahn, MS, M. Ashley Heald, MA, and Monica Kletke, BS, assisted in conducting this research.
Author Contributions: Study concept and design: Friedman, Baker, Borson, and Vitiello. Acquisition of data: Friedman, Baker, Barsness, and Merriam. Analysis and interpretation of data: Friedman, Baker, Jensen, Craft, Merriam, Otto, Novotny, and Vitiello. Drafting of the manuscript: Friedman, Baker, Jensen, Barsness, and Vitiello. Critical revision of the manuscript for important intellectual content: Friedman, Baker, Borson, Jensen, Craft, Merriam, Otto, Novotny, and Vitiello. Statistical analysis: Friedman and Baker. Obtained funding: Friedman, Baker, and Vitiello. Administrative, technical, and material support: Friedman, Baker, Jensen, Barsness, Craft, Merriam, and Vitiello. Study supervision: Friedman, Baker, and Vitiello.
References
- 1.Lieberman SA, Hoffman AR. The somatopause: should growth hormone deficiency in older people be treated? Clin Geriatr Med. 1997;13(4):671–684. [PubMed] [Google Scholar]
- 2.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14(1):20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 3.Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci U S A. 2006;103(15):6031–6036. doi: 10.1073/pnas.0507776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merriam GR, Schwartz RS, Vitiello MV. Growth hormone-releasing hormone and growth hormone secretagogues in normal aging. Endocrine. 2003;22(1):41–48. doi: 10.1385/ENDO:22:1:41. [DOI] [PubMed] [Google Scholar]
- 5.Merriam GR, Buchner DM, Prinz PN, Schwartz RS, Vitiello MV. Potential applications of GH secretagogs in the evaluation and treatment of the age-related decline in growth hormone secretion. Endocrine. 1997;7(1):49–52. doi: 10.1007/BF02778062. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107 (4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 7.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129(1):119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55(2):B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- 10.Baum HB, Katznelson L, Sherman JC, et al. Effects of physiological growth hormone (GH) therapy on cognition and quality of life in patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1998;83(9):3184–3189. doi: 10.1210/jcem.83.9.5112. [DOI] [PubMed] [Google Scholar]
- 11.Deijen JB, de Boer H, Blok GJ, van der Veen EA. Cognitive impairments and mood disturbances in growth hormone deficient men. Psychoneuroendocrinology. 1996;21(3):313–322. doi: 10.1016/0306-4530(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 12.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Soares CN, Musolino NR, Cunha Neto M, et al. Impact of recombinant human growth hormone (RH-GH) treatment on psychiatric, neuropsychological and clinical profiles of GH deficient adults: a placebo-controlled trial. Arq Neuropsiquiatr. 1999;57(2A):182–189. doi: 10.1590/s0004-282x1999000200003. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86(4):1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 15.Baker LD, Barsness SM, Borson S, et al. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69(11):1420–1429. doi: 10.1001/archneurol.2012.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006;27(2):318–323. doi: 10.1016/j.neurobiolaging.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67(6):611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arwert LI, Veltman DJ, Deijen JB, Lammertsma AA, Jonker C, Drent ML. Memory performance and the growth hormone/insulin-like growth factor axis in elderly: a positron emission tomography study. Neuroendocrinology. 2005;81(1):31–40. doi: 10.1159/000084872. [DOI] [PubMed] [Google Scholar]
- 19.van Dam PS, de Winter CF, de Vries R, et al. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology. 2005;30(4):357–363. doi: 10.1016/j.psyneuen.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JE, Licata SC, Ongür D, et al. Quantification of J-resolved proton spectra in two-dimensions with LC Model using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22(7):762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- 21.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29(50):15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novotny EJ, Jr, Hyder F, Shevell M, Rothman DL. GABA changes with vigabatrin in the developing human brain. Epilepsia. 1999;40(4):462–466. doi: 10.1111/j.1528-1157.1999.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 24.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(8):1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 26.Ishiwata A, Sakayori O, Minoshima S, Mizumura S, Kitamura S, Katayama Y. Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta Neurol Scand. 2006;114(2):91–96. doi: 10.1111/j.1600-0404.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 27.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 29.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96(1):150–158. doi: 10.1210/jc.2010-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57(6):977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1983. [Google Scholar]
- 32.Stata. College Station, TX: StataCorp; 2009. Stata Statistical Software: Release 11 [computer program] [Google Scholar]
- 33.Huang W, Alexander GE, Chang L, et al. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a 1H MRS study. Neurology. 2001;57 (4):626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- 34.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarci K, Lowe V, Przybelski SA, et al. Magnetic resonance spectroscopy, β-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77(10):951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2012;2011:649325. doi: 10.1155/2011/649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neale JH, Olszewski RT, Zuo D, et al. Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem. 2011;118(4):490–498. doi: 10.1111/j.1471-4159.2011.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passani LA, Vonsattel JP, Carter RE, Coyle JT. N-acetylaspartylglutamate, N-acetylaspartate, and N-acetylated alpha-linked acidic dipeptidase in human brain and their alterations in Huntington and Alzheimer’s diseases. Mol Chem Neuropathol. 1997;31(2):97–118. doi: 10.1007/BF02815236. [DOI] [PubMed] [Google Scholar]
- 39.Lodder-Gadaczek J, Becker I, Gieselmann V, Wang-Eckhardt L, Eckhardt M. N-acetyl aspartylglutamatesynthetase II synthesizes N-acetylaspartylglutamylglutamate. J Biol Chem. 2011;286(19):16693–16706. doi: 10.1074/jbc.M111.230136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132(4):1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 41.Majdi M, Ribeiro-da-Silva A, Cuello AC. Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience. 2009;159(2):896–907. doi: 10.1016/j.neuroscience.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka DH, Toriumi K, Kubo K, Nabeshima T, Nakajima K. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci. 2011;31(40):14116–14125. doi: 10.1523/JNEUROSCI.2786-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujiwara H, Zheng M, Miyamoto A, Hoshino O. Insufficient augmentation of ambient GABA responsible for age-related cognitive deficit. Cogn Process. 2011;12(2):151–159. doi: 10.1007/s10339-010-0375-7. [DOI] [PubMed] [Google Scholar]
- 44.Boy F, Evans CJ, Edden RA, et al. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70(9):866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goto N, Yoshimura R, Kakeda S, et al. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol. 2009;24(8):639–645. doi: 10.1002/hup.1070. [DOI] [PubMed] [Google Scholar]
- 46.Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. J Magn Reson. 2011;208 (2):210–218. doi: 10.1016/j.jmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31(46):16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]