Abstract
Background
Alcohol is the most widely abused substance and its chronic consumption causes neurobehavioral disorders. It has been shown that alcohol affects the function of immune cells. Dendritic cells (DC) serve as the first line of defense against infections and are known to accumulate neurotransmitters such as 5-hydroxytryptamine (5-HT). The enzyme monoamine oxidase-A (MAO-A) degrades 5-HT that is associated with clinical depression and other neurological disorders. 5-HT is selectively transported into neurons through the serotonin transporter (SERT), which is a member of the sodium- and chloride-dependent neurotransmitter transporter (SLC6) family. SERT also serves as a receptor for psychostimulant recreational drugs. It has been demonstrated that several drugs of abuse such as amphetamine and cocaine inhibit the SERT expression; however, the role of alcohol is yet to be elucidated. We hypothesize that alcohol can modulate SERT and MAO-A expression in DC, leading to reciprocal downregulation of 5-HT in extracellular medium.
Methods
Dendritic cells were treated with different concentrations (0.05% to 0.2%v/v) of alcohol for 24–72 hours and processed for SERT and MAO-A expression using Q-PCR and Western blots analysis. In addition, SERT function in DC treated with alcohol both in the presence and absence of imipramine, a SERT inhibitor was measured using 4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide uptake assay. 5-HT levels in culture supernatant and intracellular 5-hydroxy indole acetic acid (5-HIAA) and cyclic AMP were also quantitated using ELISA.
Results
Dendritic cells treated with 0.1% alcohol for 24 hours showed significant upregulation of SERT and MAO-A expression compared with untreated DC. We also observed that 0.1% alcohol enhanced the function of SERT and decreased extracellular 5-HT levels compared with untreated DC cultures, and this was associated with the elevation of intracellular 5-HIAA and cyclic AMP levels.
Conclusions
Our study suggests that alcohol upregulates SERT and MAO-A by elevating cyclic AMP, which may lead to decreased concentration of 5-HT in the extracellular medium. As 5-HT is a major neurotransmitter and an inflammatory mediator, its alcohol-mediated depletion may cause both neurological and immunological deregulation.
Keywords: Serotonin Transporter, Alcohol, Dendritic Cells, Serotonin, Monoamine Oxidase A
Alcohol is the most commonly used and abused substance worldwide (Oak et al., 2006). It is a potent immunosuppressive agent and it has been recognized for many centuries as an important risk factor for the development of infections (Song et al., 2005). Alcoholic individuals frequently show an immunodeficiency state, which is associated with a higher risk of cancer (Laso et al., 2007; Nelson and Kolls, 2002). In addition, alcohol abuse has been demonstrated to deplete serotonin or 5-hydroxytryptamine (5-HT) contributing to neuropsychiatric conditions such as depression and anxiety disorders and alcohol dependence (Tollefson, 1989, 1991).
5-Hydroxytryptamine is a classical neurotransmitter and vasoactive aminewhich regulates a variety of physiologic states and behaviors, including pain, appetite, mood, and sleep (Mossner and Lesch, 1998). It is an important inflammatory mediator with significant immunomodulatory effects (Leon-Ponte et al., 2007; Meredith et al., 2005). Extracellular 5-HT is cleared by the serotonin transporter (SERT), which leads to its inactivation in the brain and periphery by the enzyme called monoamine oxidase-A (MAO-A) (Henry et al., 2003). In addition, SERT dysfunction has been found in individuals suffering fromboth depression and anxiety disorders (Hariri and Holmes, 2006). Substitution of Val425 for Ile425 in SERT gene was observed in patients with obsessive compulsive disorder(OCD)(Ozaki et al., 2003). Prasad and colleagues (2009) documented that variants in SERT gene, Ile425Leu, Phe465-Leu, and Leu550Val are associated with OCD and autism.
Serotonin transporter is a member of sodium- and chloride- dependent neurotransmitter transporter (SLC6) family (Saier, 1999). It is a 630-amino-acid protein and has 12 transmembrane domains and its C and N terminal regions lie in the cytoplasm (Qian et al., 1995; Tate and Blakely, 1994). It selectively transports serotonin together with Na+ and Cl− into cells and in the same reaction transports K+ out of the cell and modulates serotonergic signaling and neurotransmission (Blakely et al., 1994). It has been shown that SERT is also a receptor for antidepressant drugs and psychostimulant drugs of abuse such as 3,4-methylenedioxymethamphetamine, amphetamine, and cocaine, suggesting that these drugs are able to modulate the transport of serotonin into the cell (Tatsumi et al., 1997). However, the effect of alcohol on SERT expression and function is not clear.
Alcohol may contribute to neuropsychiatric conditions by affecting several cells in the brain. Dendritic cells (DC) are responsible for processing and presentation of antigen to T cells (Bell et al., 1999; Lanzavecchia and Sallusto, 2001) and serve as the first line of defense against infection (Nair et al., 2005). These cells play a key role in the initiation of adaptive immune responses and their functions are negatively affected by both acute and chronic alcohol exposure in humans (Dolganiuc et al., 2003; Laso et al., 2007).
It has been reported that DC express SERT and can accumulate serotonin then playing an important role in the transport of serotonin in the brain (Axelsson et al., 1978; O’Connell et al., 2006). However, the mechanisms by which alcohol affects the expression and function of SERT in DC are still unknown. In the present study, we demonstrated that alcohol upregulates the expression and function of SERT in DC leading to the depletion of extracellular 5-HT.
MATERIAL AND METHODS
Isolation of Monocytes
Human leukopacks were obtained from Community Blood Centers of South Florida, Inc., Miami, Florida. Monocytes were isolated using RosetteSep human monocyte enrichment cocktail (StemCell Technologies, Vancouver, Canada). The cocktail contained a combination of mouse and rat monoclonal antibodies directed against antigens on CD2, CD3, CD8, CD19, CD56, CD66b, and glycophorin A. It crosslinks unwanted cells in human whole blood to multiple RBCs, forming immunorosettes that pellet along with the free RBCs when centrifuged over Ficoll-Hypaque (Sigma Aldrich, St Louis, MO). Monocytes were collected at the interface between the plasma and Ficoll-Paque.
Generation of DC From Monocytes
Monocytes were cultured at a concentration of 1 × 106/ml in complete RPMI medium containing 20 ng of recombinant human GM-CSF/ml and 20 ng of recombinant IL-4/ml (R&D Systems, Inc., Minneapolis, MN) for 6 days (Cao et al., 2005; Nair et al., 2005). On alternate days, 1-ml medium was replaced with fresh medium containing GM-CSF and IL-4 at a concentration of 20 ng/ml. On day 6, the cells were positive for the expression of DC markers, CD40, CD80, and CD86 (Nair et al., 2005).
Treatment of DC With Alcohol
The DC were treated with different concentrations of alcohol (0.05, 0.1, and 0.2%) for 24 to 72 hours. At these concentrations, alcohol did not affect the viability of the cells. After 24 to 72 hours, the cells were harvested and RNA was extracted.
RNA Extraction and Quantitative Real-Time PCR
The cytoplasmic RNA from the cell pellet was extracted using RNA purification kit (Qiagen, Valencia, CA). The cell pellet was lysed and homogenized in the presence of guanidine-thiocyanate-containing buffer, which inactivates RNases. Ethanol was added to provide appropriate binding conditions and the sample was then applied to an RNeasy mini spin column (Qiagen), where the total RNA binds to the membrane and contaminants were washed away. The RNA was eluted and quantitated and was stored at −80°C. Equal quantities of RNA from all the samples were reverse transcribed using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). The cDNA was stored at −20°C until the analysis.
The expression of SERT and MAO-A genes was quantitated using Brilliant QPCR master mix (Stratagene, Cedar Creek, TX) and the primer sequences depicted in Table 1. TaqMan probes labeled with a reporter at 51 end and a quencher at 31 end were used to monitor the amplification of gene. The RT–PCR was performed as described (Cunningham, 2001).
Table 1.
Primer Sequences for Real-Time PCR
| Primer | Primer sequence |
|---|---|
| GAPDH | Hs99999905 (Applied Biosystems) |
| Serotonin transporter | Forward—51 CACAACCTCCTCCTCCAGTTC Reverse—51 CCCTTCCATCATCAGGCTTAGC |
| Monoamine oxidase A | Hs00165140 (Applied Biosystems) |
Western Blot Analysis
After 24 hours exposure to alcohol, the DC were harvested and the cell lysates were prepared in protein extraction reagent (Pierce Biotechnology, Rockford, IL) containing protease inhibitor (Pierce Biotechnology), vortexed and kept on ice for 10 minutes, and centrifuged at 13,306 g for 10 minutes at 4°C. The supernatants were collected and the protein levels were quantified using the protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Equal quantities of protein were subjected to SDS–PAGE and transferred to nitrocellulose membrane (Bio-Rad Laboratories), blocked with 10% nonfat dry milk, washed with tris-buffered saline–tween 20 (TBST), and incubated overnight with mouse monoclonal antibody against human SERT (Abcam, Cambridge, MA) and rabbit polyclonal antibody against MAO-A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The blots were washed with TBST, incubated with goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Inc.) for SERT and goat antirabbit IgG-HRP (Santa Cruz Biotechnology, Inc.) for MAO-A, and developed using the super signal west pico chemiluminescent substrate (Pierce Biotechnology).
Flow Cytometric Analysis
The expression of SERT in DC treated with 0.1% alcohol for 24 hours was also analyzed by indirect flow cytometry. The cell pellet was washed with PBS/EDTA, resuspended in 500 ml of 1 × PBS and incubated with mouse monoclonal antibody to SERT (Advanced Targeting Systems, San Diego, CA) for 30 min at room temperature. Then, the cells were washed with PBS/EDTA and incubated with phycoerythrin (PE)-conjugated donkey anti-mouse IgG monoclonal antibody (eBioscience, San Diego, CA) for 30 min in dark at room temperature. PE-conjugated control antibody was isotype- matched IgG. After washing with PBS/EDTA, the cell pellet was resuspended in 2% paraformaldehyde and analyzed using FACSCalibur flow cytometer through CellQuest software (BD Biosciences, San Jose, CA). The results are expressed as the mean fluorescence intensity (MFI).
Measurement of SERT Activity Using 4-[4-(Dimethylamino)-Styryl]-1-Methylpyridinium Iodide (ASP) Uptake Assay
Dendritic cells were treated with 0.1% alcohol both in the presence and absence of SERT inhibitor, imipramine at a concentration of 10 μM (Fowler et al., 2006) for 24 hours. Cell culture supernatant was collected to measure the concentration of extracellular 5-HT. The cells were treated with 1 μM of 4-[4-(dimethylamino)-styryl]-1-methylpyridinium iodide (ASP)—a fluorescent substrate for organic cation transporter, incubated in a CO2 incubator for 1 hour and washed thrice with Krebs Ringers bicarbonate buffer containing 1 mM of imipramine. The cells were lysed by 1% SDS, and the ASP transported into the cells was quantified by measuring its fluorescence intensity (λex = 485 nm, λem = 620 nm).
Quantification of Serotonin (5-HT) Concentration in Culture Medium by ELISA
The concentration of 5-HT in the culture medium was measured using 5-HT competitive ELISA kit (GenWay Biotech Inc., San Diego, CA). The 5-HT in the samples and controls were acylated with acetic anhydride and acetone and were applied to microtiter plate coated with anti-rabbit antiserum (goat). Standards were also applied to the microtiter plate. Biotinylated 5-HT and serotonin antiserum were added to each of the wells and incubated overnight at 4°C. The wells were washed and anti-biotin antibody (goat) conjugated to alkaline phosphatase was added followed by the application of p-nitrophenylphosphate substrate solution. The optical density was read at 405 nm using a microplate reader from Molecular Devices (Sunnyvale, CA).
Quantification of Intracellular 5-Hydroxy Indole Acetic Acid by ELISA
After exposing the DC to 0.1% alcohol for 24 hours, the cells were harvested, and the cell lysates were prepared in protein extraction reagent (Pierce Biotechnology) and centrifuged at 12,000 rpm for 10 minutes at 4°C. The supernatant was collected to measure intracellular 5-hydroxy indole acetic acid (5-HIAA) by using 5-HIAA competitive ELISA kit (IBL Transatlantic Corp., Toronto, CA). The samples were diluted and then methylated with methylation reagent. The methylated samples and standards were applied to microtiter plate coated with anti-rabbit IgG followed by the addition of 5-HIAA biotin and 5-HIAA antiserum and incubated overnight at 2–8°C. The wells were washed, and anti-biotin antibodies conjugated to alkaline phosphatase and p-nitrophenylphosphate solution were added. The optical density was measured at 405 nm.
Quantification of Intracellular Cyclic AMP Concentration by ELISA
Intracellular cyclic AMP concentration of DC treated with 0.1% alcohol was measured using the cyclic AMP direct immunoassay kit (EMD Chemicals, Gibbstown, NJ). The DC treated with alcohol for 24 hours as well as control culture cells were harvested and treated with 0.1 N hydrochloric acid, incubated for 10 minutes, and centrifuged at 600 g at room temperature. The supernatant was collected to measure the cyclic AMP. Cyclic AMP standards and the supernatant were applied to microtiter plate coated with goat anti-rabbit IgG. Alkaline phosphatase conjugated to cyclic AMP and anti-cyclic AMP were added to each of the wells, and the plate was incubated at room temperature for 2 hours on plate shaker. The wells were washed, p-nitrophenylphosphate substrate solution was added to every well, and the optical density was measured at 405 nm.
Statistical Analysis
Mean ± SD of 3 individual experiments was calculated, analyzed by Student’s t-test, and the values were considered to be significant when p < 0.05.
RESULTS
Alcohol Upregulates the Expression of SERT Gene in DC
Data presented in Fig. 1 show the dose- and time-dependent effect of alcohol on SERT gene expression in DC. DC were treated with alcohol at different concentrations (0.05, 0.1, and 0.2%) for 24, 48, and 72 hours, respectively. RNA was extracted and reverse transcribed, and SERT gene expression was quantitated by real-time PCR using GAPDH as a constitutively expressed gene. DC treated with 0.1% alcohol for 24 hours showed significant upregulation of SERT gene [Transcript Accumulation Index (TAI) = 2.77, p < 0.035] when compared with control culture (TAI = 1). However, no significant difference in SERT gene expression was observed at alcohol concentrations, 0.05% (TAI = 0.97) and 0.2% (TAI = 1.12). The effect of alcohol on SERT gene expression was higher at 24 hours but not at 48 and 72 hours. Therefore, a 24-hour incubation period and an alcohol concentration of 0.1% were selected for subsequent experiments.
Fig. 1.
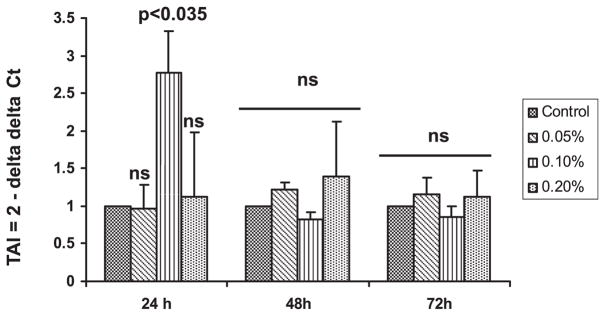
Alcohol upregulates serotonin transporter (SERT) gene expression. Dendritic cells (DC) were treated with different concentrations (0.05% to 0.2%) of alcohol for 24–72 hours. The DC were harvested and cytoplasmic RNA from the cell pellet was extracted, reverse transcribed, and the cDNA was used to analyze SERT gene expression by real-time PCR. Alcohol at a concentration of 0.1% upregulated SERT gene expression in DC treated for 24 hours. Values representmean ± SD from 3 individual experiments.
Alcohol Upregulates SERT Protein Expression in DC
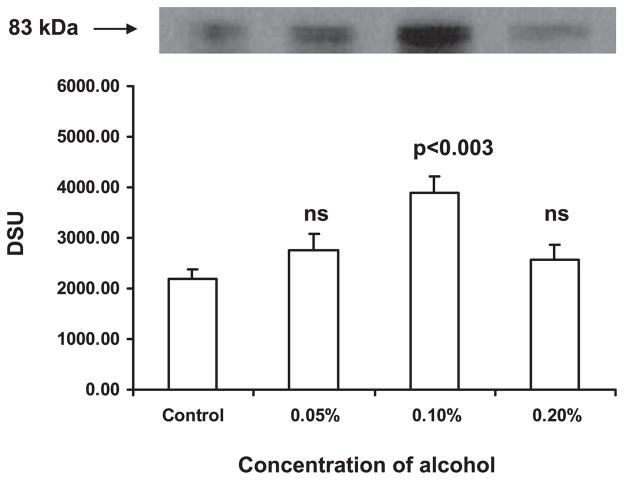
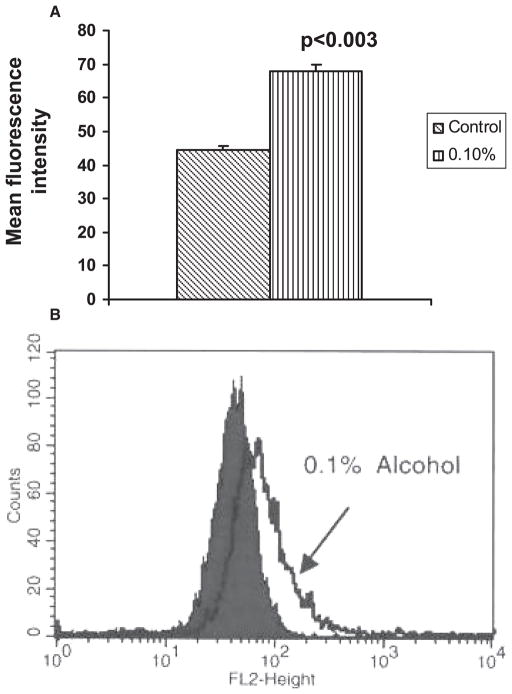
To study whether alcohol affects SERT protein expression in DC, the protein extracted from the cells treated with alcohol (0.05 to 0.2%) for 24 hours was analyzed by Western blotting (Fig. 2). We observed that the alcohol at 0.1% caused the upregulation of SERT protein expression when compared with control DC. The expression of SERT protein in DC after exposure to 0.1% alcohol for 24 hours was also analyzed by flow cytometry (Fig. 3A,B). The MFI was higher (68; p < 0.003) in DC treated with alcohol when compared with control DC (42) indicating that 0.1% alcohol caused the upregulation of SERT protein.
Fig. 2.
Dose-dependent effect of alcohol on serotonin transporter (SERT) protein expression in dendritic cells (DC). DC were treated with alcohol at concentrations 0.05 to 0.2% for 24 hours. The DC were harvested, cell lysates were prepared and centrifuged, supernatant was collected, and protein concentration in supernatant was estimated. Equal quantities of protein from all the samples were subjected to SDS–PAGE, transferred to nitrocellulose membrane, and incubated with mouse monoclonal SERT antibody and then with goat anti-mouse IgG-HRP. The blot was developed. About 0.1% alcohol caused the upregulation of SERT protein (83 kDa).
Fig. 3.
(A) Flow cytometric analysis of SERT protein expression. Dendritic cells (DC) treated with 0.1% alcohol for 24 hours were harvested, washed with PBS/EDTA, incubated with mouse monoclonal antibody to SERT and stained with donkey anti-mouse IgG conjugated to PE, and analyzed by FACS Calibur flow cytometer with appropriate control antibodies. (B) Overlay plot of a representative experiment shows the upregulation of SERT in cells treated with 0.1% alcohol when compared with control cells. Isotype controls were calibrated but were not shown.
Alcohol Enhances the Function of SERT and Depletes 5-HT Level in Cell Culture Supernatant
As alcohol upregulated SERT both at gene and protein levels, we determined whether alcohol could increase the function of SERT. Therefore, we treated the DC with 0.1% alcohol both in the presence and absence of imipramine, an inhibitor of SERT for 24 hours, and the culture supernatant was collected. The cells were incubated with 4-[4-(dimethylamino)-styryl]-1-methylpyridinium iodide (ASP) and measured the uptake of ASP by SERT. The MFI of intracellular ASP was higher in the cells treated with alcohol (206; p < 0.02) when compared with control DC (132) indicating that alcohol enhances the transport of ASP through SERT. The uptake of ASP into the cells treated with imipramine (85.67; p < 0.05) and 0.1% alcohol + imipramine (75.33; p < 0.035) was lower when compared with control culture as well as 0.1% alcohol treated cells (Fig. 4). To study whether alcohol increases the transport of 5-HT into the DC and depletes extracelluar 5-HT, we quantitated the levels of 5-HT in the DC culture medium using ELISA kit and observed that 0.1% alcohol decreased 5-HT levels (12.48 ng/ml; p < 0.03) compared with control culture (16.76 ng/ml). However, the 5-HT concentration in the culture medium of DC treated with imipramine (20.69 ng/ml; p < 0.002) and 0.1% alcohol + imipramine (21.04 ng/ml; p < 0.035) was higher than that observed in control culture medium as well as 0.1% alcohol treated DC culture medium (Fig. 5).
Fig. 4.
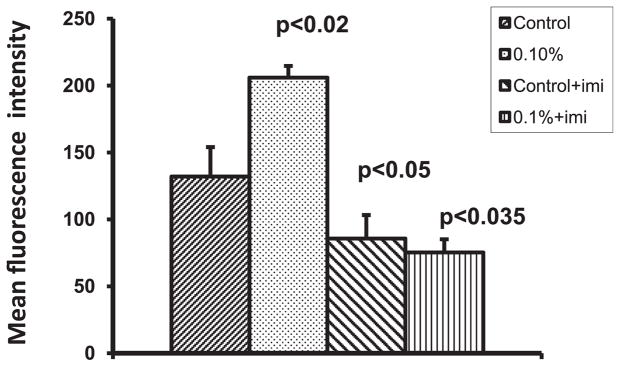
Alcohol enhances SERT function. Dendritic cells (DC) were treated with 0.1% alcohol both in the presence and absence of imipramine for 24 hours. The cells were incubated with 4-[4-(dimethylamino)-styryl]-1-methylpyridinium iodide (ASP) and the uptake of ASP by DC was measured. The ASP fluorescence intensity was higher in alcohol-treated DC. Imipramine treatment lowered the uptake of ASP by DC.
Fig. 5.
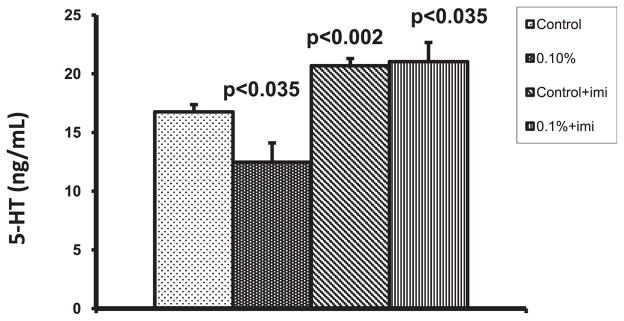
Effect of alcohol on serotonin (5-hydroxytryptamine, 5-HT) levels in dendritic cells (DC) culture supernatant. The supernatant from DC culture treated with alcohol both in the presence and absence of imipramine for 24 hours was collected and analyzed for 5-HT levels using 5-HT competitive ELISA kit. 0.1% alcohol treatment downregulated the 5-HT level. Imipramine treated cells showed higher levels of 5-HT.
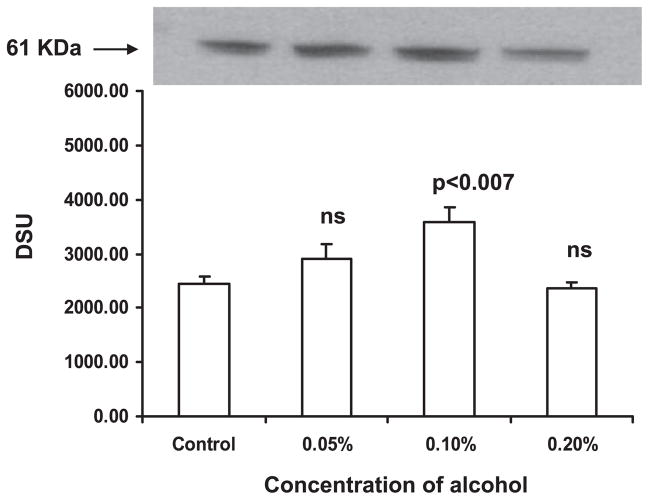
Alcohol Upregulates MAO-A Expression in DC
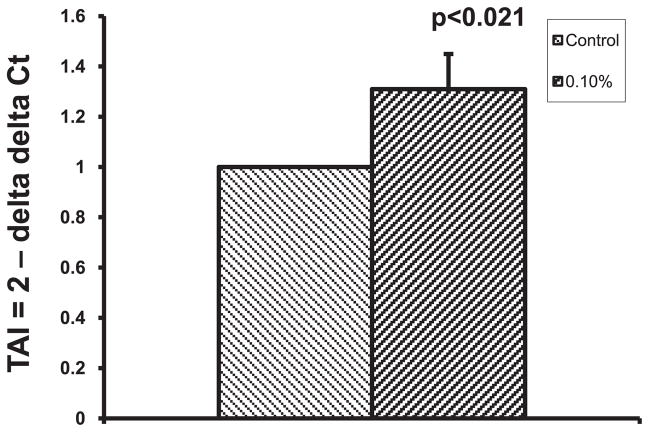
Monoamine oxidase-A is an enzyme involved in the degradation of 5-HT. As we observed decreased levels of 5-HT in DC culture medium with an increase in SERT expression, we analyzed the expression of 5-HT metabolizing enzyme, MAO-A, by Western blotting and observed that alcohol at 0.1% caused the upregulation of MAO-A compared with control culture (Fig. 6). In addition, we analyzed whether the increase in MAO-A protein expression is due to the upregulation of MAO-A gene, by real-time PCR, and found that MAO-A gene is upregulated in 0.1% alcohol-treated DC (TAI = 1.31; p < 0.021; Fig. 7).
Fig. 6.
Western blot illustrating monoamine oxidase-A (MAO-A, 61 kDa) expression in dendritic cells (DC). Equal quantities of protein from DC treated with 0.05 to 0.2% alcohol for 24 hours were run on SDS–PAGE; transferred to nitrocellulose membrane; the membrane was incubated with rabbit polyclonal antibody against MAO-A and goat anti-rabbit IgG-HRP; and the blot was developed. MAO-A was upregulated in DC treated with 0.1% alcohol.
Fig. 7.
Monoamine oxidase-A (MAO-A) gene is upregulated by alcohol. After 24 hours treatment with 0.1% alcohol, the dendritic cells were harvested, cytoplasmic RNA from the cell pellet was extracted, reverse transcribed to cDNA, and the cDNA was used for the MAO-A gene expression analysis by real-time PCR. Alcohol caused the upregulation of MAO-A gene expression.
Alcohol Enhances Intracellular 5-HIAA Concentration
5-Hydroxy indole acetic acid is the end product of 5-HT catabolism. The alcohol-mediated upregulation of MAO-A may lead to increased intracellular degradation of 5-HT to 5-HIAA. Therefore, we measured the concentration of intracellular 5-HIAA by ELISA and observed that 5-HIAA concentration is higher in alcohol-treated DC (0.97 μg/ml; p < 0.037) than in control DC (0.67 μg/ml) (Fig. 8).
Fig. 8.
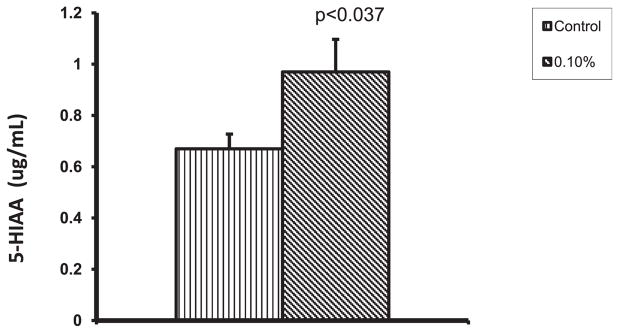
Alcohol increases the concentration of intracellular 5-hydroxy indole acetic acid (5-HIAA). The dendritic cells (DC) treated with 0.1% alcohol for 24 hours were lysed and the intracellular 5-HIAA concentration was measured by using 5-HIAA competitive ELISA kit. The concentration of 5-HIAA was found to be higher in alcohol-treated DC.
Alcohol Increases Intracellular Cyclic AMP
Previous studies have indicated that cyclic AMP upregulates SERT mRNA. Therefore, we studied whether alcohol-induced upregulation of SERT is associated with an increase in the cyclic AMP level and we observed that cyclic AMP concentration is higher in alcohol-treated DC (5.50 pmol/ml; p < 0.047) than in control DC (1.73 pmol/ml) (Fig. 9).
Fig. 9.
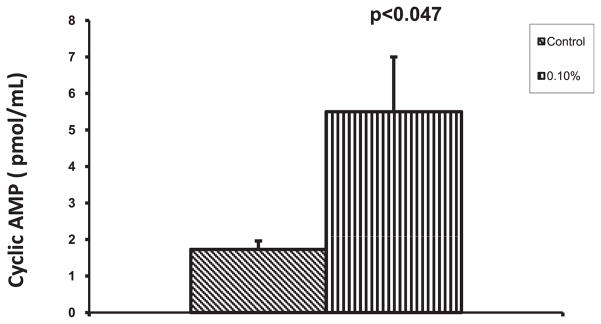
Alcohol elevates cyclic AMP level. The dendritic cells (DC) were incubated with 0.1% alcohol for 24 hours and lysed. The intracellular cyclic AMP concentration was measured by using cyclic AMP direct immunoassay kit. Alcohol caused elevation of cyclic AMP concentration in DC.
DISCUSSION
The SERT, in addition to its role in transporting serotonin (5-HT) into the cells, serves as a molecular target for several antidepressants and different drugs of abuse (Magnani et al., 2004). In the present study, we examined whether alcohol affects the SERT expression and function in DC that lead to immunederegulation. Earlier studies have shown that hematopoietic cells express neurotransmitter transporters (O’Connell et al., 2006; Pacheco et al., 2006). DC express SERT, take up 5-HT and store in intracellular vesicles, and subsequently release via Ca2+-mediated exocytosis (O’Connell et al., 2006). Previous studies have also shown that T and B lymphocytes are functionally responsive to 5-HT, indicating a role of 5-HT in adaptive immune responses. Inhibition of 5-HT synthesis inhibits IL-2-stimulated human T-cell proliferation (Aune et al., 1994).
Our dose–response and kinetic studies show that 0.1% ethanol caused the upregulation of SERT in DC as evidenced by real-time PCR, Western blots, and flow cytometry. This increase in the expression of SERT was detected in DC exposed to ethanol for 24 hours. However, the increase in the concentration of ethanol to 0.2% and exposure time to 48 and 72 hours did not increase the SERT expression. Therefore, in our subsequent experiments, we used 0.1% alcohol treatment for 24 hours. Szabo and colleagues (1992) reported that ethanol at as low concentration as 25 mM (0.1%) was effective in inducing TGFβ production in monocytes (precursors of DC) and suggested that continued presence of alcohol was not necessary to modulate monocyte functions (Szabo et al., 2004).
As SERT was upregulated, we hypothesized that it may enhance the function of SERT. 4-[4-(Dimethylamino)styryl]-1-methylpyridinium iodide (ASP) is a fluorescent molecule. It is a substrate for the organic cation transporter (Jørgensen et al., 2008; Stachon et al., 1996). Fowler and colleagues (2006) reported that the function of SERT can be measured by measuring the uptake of ASP by SERT. Therefore, we treated the DC with alcohol both in the presence and absence of imipramine, an inhibitor of SERT to study whether alcohol-mediated upregulation of SERT leads to an increase in the uptake of ASP by DC. We observed that the MFI of ASP is higher in DC treated with alcohol than that observed in the control cells indicating that alcohol-mediated upregulation of SERT enhances the function of SERT. In addition, we found that the ASP fluorescence intensity is lower in the cells treated with imipramine when compared with the cells that were not treated with imipramine. This observation implies that SERT is a major path of ASP transport in DC. Our results also show that alcohol caused depletion of 5-HT in the culture supernatant. This may be because of an increase in the transporter function as a consequence of its upregulation by alcohol. However, the 5-HT concentration in culture supernatant of DC treated with imipramine was higher, which may be due to the obstruction of 5-HT entry into DC by imipramine leading to the retention of 5-HT in extracellular medium. In consistent with our findings, other studies have shown that the upregulation of SERT causes the downregulation of 5-HT in extracellular fluid (ECF; Brenner et al., 2007). In SERT knockout mice, extracellular 5-HT clearance was retarded with an increase in ECF levels of 5-HT (Boyce-Rustay et al., 2006; Mathews et al., 2004). All these studies suggest that transport of 5-HT into the cells depends on the extent of SERT expression. Once 5-HT enters the cells, it can be stored in intracellular vesicles for subsequent exocytosis or can be catabolized by MAO-A.
Monoamine oxidase is an enzyme of the outer mitochondrial membrane, and it exists in 2 forms: MAO-A and MAO-B (Bach et al., 1988; Ou et al., 2006). MAO-A degrades 5-HT to 5-HIAA, which is eliminated as an excretory product. In the present study, we observed an increase in the expression of MAO-A accompanied by an increase in the intracellular 5-HIAA concentration, which may be attributable to enhanced intracellular 5-HT concentration and its subsequent degradation to 5-HIAA by MAO-A. The catalytic activity of monoamine oxidases generates reactive oxygen species, which may cause oxidative damage to mtDNA and lead to apoptosis, aging, and neurodegenerative processes (Hauptmann et al., 1996). In addition, the expression of MAO-A has been reported to be related to alcohol dependence and neuropsychiatric conditions (Huang et al., 2007; Schmidt et al., 2000; Vanyukov et al., 1995). Earlier reports have shown that alcohol enhances intracellular cyclic AMP concentration (Atkinson et al., 1977; Rabe et al., 1990; Uhlemann et al., 1979). Therefore, we studied whether alcohol-induced SERT and MAO-A upregulation is mediated by increased cyclic AMP levels, because cyclic AMP has been reported to cause the upregulation of SERT mRNA and increase SERT-specific ligand binding in human placental choriocarcinoma cells (Ramamoorthy et al., 1993). Alcohol significantly enhanced the cyclic AMP level in DC. From our results, it can be suggested that alcohol upregulates SERT and MAO-A by elevating intracellular cyclic AMP. Alcohol-mediated upregulation of SERT would lead to decreased concentration of 5-HT in the extracellular medium. As 5-HT is a major neurotransmitter and an inflammatory mediator, its depletion may cause neurological and immunological deregulation.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH): 7 R01 DA 012366-09, 7R01 DA 014218-06, 7 R01 DA 015628-06, 7 R01 DA 021537-02, 5 R01 AA 017405-02, 1 R37 DA 025576-01. Dr. Aruna Kode is gratefully acknowledged for her technical assistance in ASP uptake assay.
References
- Atkinson JP, Sullivan TJ, Kelly JP, Parker CW. Stimulation by alcohols of cyclic AMP metabolism in human leukocytes. Possible role of cyclic AMP in the anti-inflammatory effects of ethanol. J Clin Invest. 1977;60:284–294. doi: 10.1172/JCI108776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune TM, Golden HW, McGrath KM. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J Immunol. 1994;153:489–498. [PubMed] [Google Scholar]
- Axelsson S, Elofsson R, Falck B, Sjoborg S. In vitro uptake of L-dopa and catecholamines into the epidermal Langerhans cell. Acta Derm Venereol Suppl (Stockh) 1978;58:31–35. [PubMed] [Google Scholar]
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Young JW, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daw LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, Kilic F. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–215. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Lee SH, Lu J. CD83 is preformed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem J. 2005;385:85–93. doi: 10.1042/BJ20040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. Assessing differential gene expression. The Scientist. 2001;15:27. [Google Scholar]
- Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003;27:1023–1031. doi: 10.1097/01.ALC.0000071745.63433.32. [DOI] [PubMed] [Google Scholar]
- Fowler A, Seifert N, Acker V, Woehrle T, Kilpert C, de Saizieu A. A nonradioactive high-throughput/high-content assay for measurement of the human serotonin reuptake transporter function in vitro. J Biomol Screen. 2006;11:1027–1034. doi: 10.1177/1087057106294698. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of serotonin transporter in neural function. Trends Cogn Sci. 2006;10:187–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hauptmann N, Grimsby J, Shih JC, Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch Biochem Biophys. 1996;335:295–304. doi: 10.1006/abbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- Henry LK, Adkins EM, Han Q, Blakely RD. Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J Biol Chem. 2003;278:37052–37063. doi: 10.1074/jbc.M305514200. [DOI] [PubMed] [Google Scholar]
- Huang SY, Lin WW, Wan FJ, Chang AJ, Ko HC, Wang TJ, Wu PL, Lu RB. Monoamine oxidase-A polymorphisms might modify the association between the dopamine D2 receptor gene and alcohol dependence. J Psychiatry Neurosci. 2007;32:185–192. [PMC free article] [PubMed] [Google Scholar]
- Jørgensen S, Nielsen Eø, Peters D, Dyhring T. Validation of a fluorescence-based high-throughput assay for the measurement of neurotransmitter transporter uptake activity. J Neurosci Methods. 2008;169:168–176. doi: 10.1016/j.jneumeth.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Leon-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell signaling through the 5-HT7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Hasse J. Partitioning of serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J NeurosciMethods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J. Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology. 2005;115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan SD, Schwartz SA, Reynolds J, Whitney R, Bernstein Z, Chawda RP, Sykes D, Hewitt R, Hsiao CB. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule grabbing non-integrin expression by dendritic cells in HIV-1 patients. J Immunol. 2005;174:6617–6626. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defense and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- O’Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4- mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006;103:10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pacheco R, Oliva H, Martinez-Navio JM, Climent N, Ciruela F, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177:6695–6704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe CS, Giri PR, Hoffman PL, Tabakoff B. Effect of ethanol on cyclic AMP levels in intact PC12 cells. Biochem Pharmacol. 1990;40:565–571. doi: 10.1016/0006-2952(90)90557-2. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Cool DR, Mahesh VB, Leibach FH, Melikian HE, Blakely RD, Ganapathy V. Regulation of the human serotonin transporter. Cholera toxin-induced stimulation of serotonin uptake in human placental choriocarcinoma cells is accompanied by increased serotonin transporter mRNA levels and serotonin transporter-specific ligand bining. J Biol Chem. 1993;268:21626–21631. [PubMed] [Google Scholar]
- Saier MH., Jr A functional – phylogenetic system for the classification of transport proteins. J Cell Biochem. 1999;32–33:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Sander T, Kuhn S, Smolka M, Rommelspacher H, Samochowiec J, Lesch KP. Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious – depressive alcoholics. J Neural Transm. 2000;107:681–689. doi: 10.1007/s007020070069. [DOI] [PubMed] [Google Scholar]
- Song K, Zhao XJ, Marrero L, Oliver P, Nelson S, Kolls JK. Alcohol reversibly disrupts TNF-alpha/TACE interactions in the cell membrane. Respir Res. 2005;6:123–130. doi: 10.1186/1465-9921-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachon A, Schlatter E, Hohage H. Dynamic monitoring of organic cation transport processes by fluorescence measurements in LLC-PK1 cells. Cell Physiol Biochem. 1996;6:72–81. [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alchol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Verma BK, Fogarasi M, Catalano DE. Induction of transforming growth factor-beta and prostaglandin E2 production by ethanol in human monocytes. J Leukoc Biol. 1992;52:602–610. doi: 10.1002/jlb.52.6.602. [DOI] [PubMed] [Google Scholar]
- Tate CG, Blakely RD. The effect of N-linked glycosylation on activity of the Na(+) - and Cl(−) – dependent serotonin transporter expressed using recombinant baculovirus in insect cells. J Biol Chem. 1994;269:26303–26310. [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Tollefson GD. Serotonin and alcohol: interrelationships. Psychopathology. 1989;22:37–48. doi: 10.1159/000284625. [DOI] [PubMed] [Google Scholar]
- Tollefson GD. Anxiety and alcoholism: a serotonin link. Br J Psychiatry Suppl. 1991;159:34–39. [PubMed] [Google Scholar]
- Uhlemann ER, Robberecht P, Gardner JD. Effect of alcohols on the actions of VIP and secretin on acinar cells from guinea pig pancreas. Gastroenterology. 1979;76:917–925. [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Yu LM, Tarter RE, Deka R. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset alcoholism/substance abuse. Am J Med Genet. 1995;60:122–126. doi: 10.1002/ajmg.1320600207. [DOI] [PubMed] [Google Scholar]