Abstract
Developmental signals that control growth and differentiation are regulated by environmental factors that generate reactive oxygen species (ROS) and alter steady state redox environments in tissues and fluids. Protein thiols are selectively oxidized and reduced in distinct spatial and temporal patterns in conjunction with changes in glutathione/glutathione disulfide (GSH/GSSG) and cysteine/cystine (Cys/CySS) redox potentials (E0) to regulate developmental signaling. The purpose of this study was to measure compartment specific thiol redox status in cultured organogenesis-stage rat conceptuses and to evaluate the impact of thiol oxidation on the redox proteome. The visceral yolk sac (VYS) has the highest initial (0 hr) total intracellular GSH (GSH + 2GSSG) concentrations (5.5 mM) and the lowest Eh (−223 mV) as determined by HPLC analysis. Total embryo (EMB) GSH concentrations ranged lower (3.2 mM) and were only slightly more oxidized than the VYS. Total GSH concentrations in yolk sac fluid (YSF) and amniotic fluid (AF) are >500-fold lower than in tissues and are highly oxidized (YSF Eh = −121 mV and AF Eh = −49 mV). Steady state total Cys concentrations (Cys + 2CySS) were significantly lower than GSH in tissues but were otherwise equal in VYS and EMB near 0.5 mM. On gestational day 11, total GSH and Cys concentrations in EMB and VYS increase significantly over the 6 hr time course while Eh remains relatively constant. The Eh (GSH/GSSG) in YSF and AF become more reduced over time while Eh (Cys/CySS) become more oxidized. Addition of L-buthionine-S,R-sulfoximine (BS0) to selectively inhibit GSH synthesis and mimic the effects of some GSH-depleting environmental chemicals, significantly decreased VYS and EMB GSH and cys concentrations and increased Eh over the 6 hr exposure period, showing a greater overall oxidation. In the YSF, BSO caused a significant increase in total Cys concentrations to 1.7 mM but did not significantly change the Eh for Cys/CySS. A significant net oxidation was seen in the BSO-treated AF compartment after 6 hr. Biotinylated iodoacetamide (BIAM) labeling of proteins revealed the significant thiol-oxidation of many EMB proteins following BSO treatment. Quantitative changes in the thiol proteome, associated with developmentally-relevant pathways, were detected using isotope coded affinity tag (ICAT) labeling and mass spectroscopy. Adaptive pathways were selectively enriched with increased concentrations of proteins involved in mRNA processing (splicesome) and mRNA stabilization (glycolysis, GAPDH), as well as, protein synthesis (aminoacyl-tRNA) and protein folding (antigen processing, Hsp70, protein disulfide isomerase). These results show the ability of chemical and environmental modulators to selectively alter compartmental intracellular and extracellular GSH and Cys concentrations and change their corresponding Eh within the intact viable conceptus. The altered Eh were also of sufficient magnitude to alter the redox proteome and change relative protein concentrations suggesting that the mechanistic links through which environmental factors inform and regulate developmental signaling pathways may be discovered using systems developmental biology techniques.
Introduction
The establishment of anatomical structure and function in the embryo (EMB) during early post-implantation development is the result of programmed cell growth and differentiation which are controlled through complex, tightly regulated, and highly integrated cell signaling activities. The master control of signaling required to create a viable EMB ultimately resides in the genetic code but is also reliant on a myriad of environmental instructions that are recognized in distinct spatial and temporal patterns throughout embryogenesis. The mechanisms through which the environment exerts its influences on developmental signaling are not well understood and are too often overlooked with respect to their roles as critical regulators of embryogenesis. The physical factors, xenobiotics, nutrients, and other environmental cues that relay their instructions to the appropriate developmental signaling pathways must do so within the context of dynamically changing, yet coordinated, microenvironments located within multiple conceptal compartments. The visceral yolk sac (VYS), amnion, and EMB proper represent tissue barriers within the conceptus that separate fluid spaces into discrete physiological compartments. Environmental stimuli may communicate with the conceptus through the brush border-VYS-maternal interface or between any of the many fluid-tissue interfaces found within the conceptus to regulate growth and differentiation. A wide variety of environmental sensors have been proposed to help explain how extracellular signals elicit changes in intracellular pathways and serve to regulate cell functions [1–3]. In addition to these important sensor functions, it must also be recognized that reactive oxygen species (ROS) and their associated redox signaling pathways contribute significantly to developmental regulation [4–6]. An enormous volume of recent research has implicated redox signaling as a critical pathway for regulation of growth and differentiation in many cell types, including those associated with acute and chronic diseases such as cancer [7, 8], neurodegenerative disorders [9, 10], diabetes [11], atherosclerosis [12], heart disease [13–15], and birth defects [6, 16]. The identification and characterization of redox-related signal pathways and the elucidation of how they function in complex networks have led to a vastly improved understanding of how cells respond to normal environmental cues as well as those which are altered by exogenous chemicals or disease.
In the relative hypoxia of early development, increased concentrations of ROS are most often thought of to produce only oxidation, toxicity, and cell damage, but even at low concentrations they also serve as signal molecules and second messengers in regulating endogenous cell processes [17]. The weight of evidence to support a role for reactive oxygen species (ROS) as essential signaling molecules in the control and regulation of critical cell processes such as proliferation, differentiation, migration, metabolism, and apoptosis is significant and continues to accrue [18–20]. The functional roles of ROS as regulatory and signaling molecules and redox signaling during development, in general, have been widely understudied. Several compelling lines of evidence now exist to implicate ROS in developmental control and programming, which is further supported by suggestions that many known teratogens elicit oxidative stress as part of their mode of teratogenic action [16, 21].
Conserved regulatory pathways in virtually all cell types have evolved proteins that contain cysteine-based regulatory nodes or switches that are capable of sensing physiological changes and responding to environmental and chemical insults. Increased oxidation within the intracellular redox environment, results in a more positive redox potential (Eh) and altered cellular function by virtue of the oxidation of specific redox-sensitive cysteine nodes located within critical active or regulatory sites. Soluble thiol redox couples, which are represented predominantly by cysteine (Cys)/cystine (CySS) in the extracellular milieu and glutathione (GSH)/glutathione disulfide (GSSG) in intracellular compartments, establish the steady state Eh required for cells to perform their designated functions. Several developmentally-relevant signaling molecules, transcription factors, and regulatory enzymes are known to be compartmentally regulated by Eh including TNFα, EGF, NFκB, AP-1, Nrf2, and thioredoxin, among others [5, 6, 22–25].
The effects that environmental stimuli and chemical toxins have on redox-regulated developmental pathways are believed to be compartment and context specific. Based on this premise, the objectives of this study were: (1) to determine the steady state concentrations of GSH, GSSG, Cys, and CySS and their respective Eh in the major tissue and fluid compartments of the gestational day (GD) 11, early organogenesis-stage rat conceptus in whole embryo culture (WEC), (2) to show how compartmental thiol concentrations and Eh change independently in response to inhibition of GSH synthesis produced by L-buthionine-S,R-sulfoximine (BSO) treatment, and (3) to determine whether observed redox potential shifts and related signaling changes were sufficient to oxidize protein thiols and change selected protein concentrations within the embryonic proteome.
It is anticipated that patterns of cys accumulation and flux will change as GSH synthesis is compromised. Exactly how the conceptus adapts to these changes is unknown and a major interest in these studies. In the developing conceptus, cells, tissues and rudimentary organs receive signals from their immediate environments, which are then transmitted directly to other cells and tissues or carried through fluid filled compartments in the form of diffusible molecules or changes in the biochemical steady state environment. Each of these spatial entities are likely to exist as very different redox microenvironments, which could explain the responses to context-specific environmental cues that promote changes in proliferation, differentiation and migration, as well as the changes induced by environmental insults. The assessment of these differences forms the basis for the current report.
MATERIALS AND METHODS
Chemicals and Reagents: L-Buthionine–S,R-sulfoximine, glutathione, glutathione disulfide, cysteine, cystine, γ-glutamyl-glutamate, iodoacetic acid, iodoacetamide, bicinchoninic acid, and penicillin/streptomycin (10,000 units/ml penicillin, 10,000 μg/ml streptomycin sulfate) were purchased from Sigma/Aldrich (St. Louis, MO). Hanks balanced salt solution (HBSS) was purchased from GIBCO/Life Technologies (Grand Island, NY). Dansyl chloride was purchased from Fluka Chemie/Sigma-Aldrich (St. Louis, MO); The biotinylated iodoacetamide reagent (BIAM) was supplied by Invitrogen/Molecular Probes (Eugene, OR); streptavidin beads for pull-down from GE Healthcare/Amersham (Pittsburgh, PA); 2D gel electrophoresis supplies from BIORAD (Hercules, CA); and streptavidin-conjugated AlexaFluor 680 from Invitrogen/Molecular Probes (Eugene, OR) provided fluorescence detection of BIAM-bound proteins. Cleavable Isotope Coded Affinity Tag (ICAT) reagent Kits for Protein Labeling were purchased from AB Sciex/Applied Biosystems (Framingham, MA).
Animals
All experiments were conducted in rat WEC using viable GD10-11 conceptuses obtained from primagravids time-mated (specific pathogen free; SPF) Sprague-Dawley rats (Charles River, Portage, MI). A sperm-positive vaginal smear on the morning following mating was used to confirm pregnancy, and this time was designated GD 0. Pregnant dams were shipped 4–5 days prior to use, were maintained on a 12 hr light −12 hr dark cycle, and were allowed access to standard commercial rodent feed and water ad libitum. Anesthesia, exsanguination, and uteri removal were conducted as previously described and according to an approved institutional animal use and care protocols. [26, 27].
Culture Conditions, Exposure, and Sampling
Gravid uteri were placed in HBSS (pH 7.4 without indicator dyes) and each implantation site was removed using forceps and irridectomy scissors. Decidual masses were opened using fine watchmakers forceps and intact conceptuses were removed. Reichert’s membranes were torn away using fine forceps and the conceptus, consisting of an intact inverted VYS, ectoplacental cone (EPC), and up to 10 intact viable conceptuses were transferred into 10 ml of warmed culture medium, not to exceed 1 conceptus per ml of medium. Culture medium was prepared from heat inactivated rat serum (50%), HBSS (50%), and penicillin/streptomycin [28]. Prior to the addition of conceptuses, culture medium and culture bottle headspace were saturated with 20% O2, 5% CO2, 75% N2 and medium was warmed to 37 °C. Gestational day 10 embryos at the onset of culture had open anterior neuropores, complete dorsal flexion, and 8–12 somite pairs. Culture occurred in sealed sterile glass bottles on a roller apparatus in a constant 37 °C incubator. After 20 hr of culture, the culture medium and headspace were re-saturated with 95% O2 and 5% CO2. All test agents were added directly to the culture medium in the appropriate vehicle.
Determination of Thiol Concentrations and Redox Potential
Two tissues sources; VYS and EMB, and two fluid compartments; yolk sac fluid (YSF – the fluid compartment that separates the VYS and the amnion) and amniotic fluid (AF – the fluid compartment found within the amniotic sac and which directly bathes the EMB) were sampled for each GD 11 conceptus. Tissue and fluid samples were collected using a series of micromanipulations performed under a stereo dissecting microscope. One or two intact conceptuses were placed in a 150 μl drop of cold HBSS on a plastic Petri dish. The VYSs were torn away, taking care not to rupture the amnion, and the conceptus gently agitated to disperse the YSF contents into the drop. In our sampling protocol, conceptal blood from ruptured vitelline vessels combines with the YSF (we have not yet been able to completely separate these two fluid compartments). The VYS is lifted out using fine forceps and placed in 300 μl of 1X HPLC preservation buffer (5% perchloric acid, 0.2 M boric acid, and 10 μM γ-glutamylglutamate [γ-EE]). Without disturbing the tissue, the drop is then collected using a fine tip pipette and combined with an equal volume of 2X HPLC preservation buffer containing 10% perchloric acid, 0.4 M boric acid, and 20 μM γ-EE. The conceptus is then resuspended in a 300 μl drop of 1X HPLC preservation buffer and the amnion torn open to release its contents into the drop. The fluid is collected as before and added to an equal volume of 2X HPLC preservation buffer. The remaining EMB is resuspended a third time in a 300 μl drop containing 1X HPLC preservation buffer and EMB and fluid are collected into a microcentrifuge tube. The result of this process is the collection of the two tissue and two fluid samples saved in equal volumes of preservation buffer that contains the internal standard (γ-EE) at a final concentration of 5 μM. These samples are snap-frozen in liquid nitrogen and are stored at −74 °C for up to three months prior to analysis.
Samples are thawed on ice and prepared for HPLC derivitization using dansyl chloride as described previously according to the methods of Jones [29] and as modified by Harris et al [30]. Glutathione, GSSG, Cys, and CySS were resolved and quantified using reverse-phase HPLC analysis on a Waters 2695 Alliance Separations Module fitted with a Supelcosil LC-NH2 column (Sigma-Aldrich, St. Louis, MO). Mobile phases consisted of A) 80% HPLC grade methanol and 20% ddiH2O, and B) 62.5% methanol, 12.5% glacial acetic acid, 214 mg/ml CH3COONa-3H2O in ddiH2O with gradient flow at a rate of 1.0 ml/min. Peaks were visualized by fluorescence detection using a Waters 2474 fluorescence detector (excitation 335 nm and emission at 518 nm) and processed with Waters Empower software (Waters, Milford, MA).
Calculation of Cys/CySS and GSH/GSSG Eh are accomplished using the Nernst equation for pH 7.4: GSH/GSSG, Eh = − 264+30 log ([GSSG]/[GSH]2), Cys/CySS, Eh = − 250+30 log ([CySS]/[Cys]2) and require absolute concentrations for each of the thiol species of interest. Because cell volumes (tissue) and conceptal fluid compartment volumes could not be measured directly, estimations were calculated using two methods. Tissues concentrations for thiols in the VYS and EMB were estimated from total tissue protein using the bicinchoninic acid (BCA) method adapted to the microplate reader [31]. Fluid volumes in the YSF and AF compartments were estimated from average digital measurements of a typical GD 11 conceptus as determined using NIH Image software. Spherical volumes were calculated for the VYS, amnion, and EMB using the equation, 4πr3, and sequentially subtracted to approximate the volume of each compartment.
Analysis of the Redox Proteome using Biotinylated Iodoacetamide (BIAM) Derivitization
Viable rat conceptuses in WEC were exposed to 1 mM BSO for 6 hrs on GD11 as described previously. At the conclusion of exposure, intact treated and control conceptuses were washed in two changes of cold HBSS (pH 7.4). Ectoplacental cones were removed and VYS dissected away from the EMB proper. Tissues were placed immediately in lysis buffer (RIPA) containing 20 μM BIAM and sonicated briefly to disperse cells. All samples were then incubated in the dark for 10 min at 37°C. Reactions were stopped with the addition of 1 μl of un-biotinylated iodoacetamide (IAM) at a final concentration of 5 mM. Samples were incubated once again for 10 min at 37°C and stored frozen at −74°C until prepared for final analysis.
BIAM-labeled samples were processed into an enriched fraction prior to resolution on 2D gels and transfer to nitrocellulose using a streptavidin pull-down technique as described previously [32]. The streptavidin, 680 AlexaFluor (Invitrogen) conjugate is diluted 1:5000 from the 2mg/ml stock solution. Nitrocellulose membranes to which the proteins have been transferred were immersed in the diluted streptavidin probe in the dark and with constant rocking for 45 min. Membranes were removed, washed in PBS and imaged on the Odyssey Infrared Imager (LICOR).
Sulfhydryl-Modification and Analysis of Proteins with Cleavable ICAT Reagents
Samples were obtained from conceptuses cultured from GD10 and treated as above with BSO on GD11 in WEC for 6 hr. Conceptuses were harvested from culture and processed for ICAT analysis as described elsewhere [33, 34] and according to manufacturer’s instructions (AB Sciex/Applied Biosystems). In brief, 100 μg of untreated control EMB and 100 μg of BSO-treated EMB tissues were labeled with the maleimide ICAT reagents (Light 12C isotope for untreated controls and 13C Heavy isotope for the BSO-treated test samples, thus, providing a direct comparison of relative protein abundance between treated and control samples.
Protein samples were prepared for mass spectrometry analysis per instructions provided by the manufacturer (Applied Biosystems), allowing for fragmentation analysis of labeled proteins. The labeled and digested samples were subjected to strong cation exchange chromatography (SCX). A Dionex U-3000 capillary HPLC equipped with a capillary pump and a microbore pump were used for the analysis. The microbore pump was used to generate a linear gradient for the SCX separation. The samples were reconstituted in the starting buffer of 5 mM KH2PO4 (pH 3)/5% acetonitrile (ACN). An aliquot was injected onto a SCX column (Polysulfoethyl-Asp, 1 × 15 cm, Dionex). The peptides were eluted off the SCX column using a linear gradient of 0 – 100% 1 M NaCl/5% ACN/5 mM KH2PO4 over 20 minutes at a flow rate of 50 ul/min. The eluent was collected by time into 10–14 fractions. An aliquot of each fraction was then analyzed by RP-HPLC on the maXis LC-MS/MS system as described below.
Electrospray ionization (ESI) mass spectrometric analysis was performed using a Bruker model maXis ESI-Q-TOF instrument interfaced with an on-line nanospray source (Bruker Daltonics) to perform LC-MS/MS using a U3000 RSLCnano HPLC configured for nanoliter per minute flows. The Dionex U-3000 RSLCnano nanobore HPLC was configured with a binary nanoflow ultra-high pressure pump and a ternary high pressure microbore pump. The system used a pulled-loop autosampler configured with a 20 ul sample loop. A desalting trap column (0.3 × 5 mm, 5 um C18 PepMap 120 A, Dionex) was used and the analytical column used was a C18 PepMap (0.075 × 250 mm, 2 um, 120A, Dionex). The solvents used were 0.1% formic acid in water (A) and 80% ACN/0.1% formic acid (B). The gradient was 2–55% B in 90 min. The eluent from the analytical column was introduced into the maXis using the Bruker on-line nanospray source. The source was operated at a spray voltage of 900 V with a drying gas of nitrogen flowing at 6 L/min. The capillary temperature was set to 160°C. The mass spectrometer was set to acquire spectra of m/z 50 to 1900. MS/MS data was acquired in an automated fashion using the 3 most intense ions from the MS scan with precursor active exclusion for 90 s after 3 spectra were acquired for each parent ion. MS data was acquired at a scan speed of 3 Hz and MS/MS data was acquired at a scan speed of 1–1.5 Hz depending on the intensity of the parent ion. MS internal calibration was achieved by the use of a lock mass (HP-1222, Agilent Technologies).
The collected data was processed by DataAnalysis (Bruker Daltonics) to produce deconvoluted and internally calibrated data and saved as an xml peak list which was uploaded to our Proteinscape database (Bruker Daltonics). Proteinscape automatically submitted the peak list to the in-house MASCOT server based upon preset search conditions. For ICAT quantitation, the data was submitted from Proteinscape to WARP-LC (Bruker Daltonics), which analyzed the peak information and performed the quantitation based upon preset parameters.
The principle output from an ICAT analysis following trypsin digestion and resolution by mass spectroscopy was the H/L ratio (Heavy/Light). Only H/L ratios that deviated from unity by more than 25% were considered significantly different and included in subsequent analysis. Proteins meeting these criteria were interrogated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (http://www.genome.jp/kegg) to predict functional pathway associations and links among proteins. Only proteins for which KEGG had available pathway data were included for the purposes of this study. We did not provide a detailed summary of all protein changes identified by ICAT quantitation, but will rather show a summary of selective changes in the VYS and EMB proteomes due to BSO treatment. Multiple proteins mapping to functional pathways in the KEGG database were ranked from most to least according to the pathways having the most proteins affected.
Statistical Analysis
All values presented in this report are expressed as the mean ± standard error of the mean (SEM). A confidence level of 95% (α=0.05) was used as threshold for statistical significance. Statistical outliers were removed after meeting the criteria of being 1.5 times the interquartile range from each measure. Student’s t-tests and ANOVA using the Tukey’s post-hoc test were used for statistical comparison.
RESULTS
The measured changes in concentrations of soluble thiols and the Eh in the conceptus can be viewed separately from spatial and temporal perspectives. Results are organized to first show the temporal and spatial changes in total thiols, which is a sum of all the reduced species of a given couple plus 2X the oxidized concentration, because each disulfide form contains two copies of the reduced form. This analysis is followed by a description of the individual oxidized and reduced forms and their respective Eh values.
Control Total Glutathione Concentrations
Time 0 measurements refer to samples taken on GD 11 approximately 30 min after saturation of culture media/culture bottle headspace with 95% O2/5% CO2, (standard WEC protocol) and represent the beginning of a period during which the conceptus is responding and adapting to the increased oxygen tension. In the VYS total glutathione (GSH + 2GSSG) concentrations increased by 63% from time 0 hr to 6 hr, beginning with an average total glutathione concentration of 5.49 ± 0.49 mM and ending at 8.95 ± 1.31 mM (Table 1). At time 0 the control EMB had a 42% lower total glutathione concentration (3.19 ± 0.36 mM) than the VYS. Total glutathione concentrations increase by 35% in the embryo over the 6hr incubation to 4.29 ± 0.48 mM. The YSF and AF contain much lower initial (0 hr) concentrations of total glutathione at 0.02 ± 0.00 mM and 0.01 ± 0.00 mM, respectively, but increased significantly only in the YSF to 0.07 ± 0.01 mM over the 6 hr time course.
TABLE 1.
Total glutathione and cysteine concentrations found in major tissue and fluid compartments of the rat GD 11 conceptus grown in whole embryo culture. Concentrations were determined using HPLC as described in Methods
| Tissue or Fluid Compartment | Time (hr) | Total Glutathione GSH + 2GSSG (μM) | Total Cysteine cys + 2cySS (μM) | ||
|---|---|---|---|---|---|
| CONTROL | BSO | CONTROL | BSO | ||
| Visceral Yolk Sac | 0 | 5487 ± 493 (12) | 470 ± 57 (12) | ||
| I | 4830 ± 706 (5) | 3219 ± 347a,b (10) | 376 ± 148 (5) | 488 ± 67b (10) | |
| 3 | 6801 ± 1070 (8) | 3111 ± 364a,b (8) | 480 ± 107 (8) | 342 ± 40b (8) | |
| 6 | 8946 ± 1313b (7) | 1073 ± 52a,b (7) | 753 ± 181b (7) | 156 ± 55a,b (8) | |
| Yolk Sac Fluid | 0 | 24 ± 2 (9) | 556 ± 36 (7) | ||
| I | 33 ± 9 (5) | 25 ± 5 (9) | 559 ± 76 (4) | 707 ± 124 (8) | |
| 3 | 63 ± 13b (6) | 19 ± 4a (6) | 1237 ± 343 (6) | 1217 ± 469 (8) | |
| 6 | 65 ± 13b (5) | 25 ± 6a (7) | 454 ± 86 (4) | 1716 ± 559 (7) | |
| Amniotic Fluid | 0 | 11 ± 2 (9) | 42 ± 7 (8) | ||
| I | 13 ± 4 (5) | 10 ± 2 (11) | 44 ± 6 (4) | 120 ± 33a (11) | |
| 3 | 24 ± 6 (6) | 13 ± 2 (8) | 95 ± 16 (5) | 173 ± 70 (8) | |
| 6 | 16 ± 3 (4) | 7 ± 1a (8) | 78 ± 23 (4) | 224 ± 79 (8) | |
| Embryo | 0 | 3186 ± 357 (12) | 491 ± 70 (12) | ||
| I | 2563 ± 383 (5) | 2058 ± 197b) | 391 ± 146 (5) | 606 ± 128 (10) | |
| 3 | 3132 ± 363 (8) | 2099 ± 248a,b (8) | 635 ± 155 (7) | 624 ± 132 (8) | |
| 6 | 4293 ± 481 (7) | 1038 ± 59a,b (8) | 1403 ± 423b (7) | 635 ± 116 (8) | |
Control Total Cysteine Concentrations
Total cysteine (Cys + 2CySS) concentrations in VYS and EMB were nearly identical at 0.47 ± 0.06 mM and 0.49 ± 0.07 mM, respectively (Table 1). Over the 6 hr time course, total concentrations of Cys increased to 0.75 ± 0.12 mM in the VYS and to 1.4 ± 0.42 mM or 185% of the 0 hr levels in the EMB. Control total cysteine concentrations in the YSF increased from 0.56 ± 0.04 mM at 0 hr to 0.76 ± 0.09 mM at 6 hr. Total cysteine in the AF increased from 0.04 ± 0.01 mM at 0 hr to 0.08 ± 0.00 mM at 6 hr.
BSO (1 mM) Treatment
Inhibition of de novo GSH biosynthesis by the addition of BSO resulted in 88% and 76% reductions of total glutathione, relative to concurrent controls, in the VYS and the EMB at 6 hr, respectively. Similar reductions in concentration were seen for total glutathione in YSF and AF. When total Cys concentration changes were considered following BSO addition, the values in VYS and EMB decreased by 63% and 77%, respectively, relative to concurrent controls. In contrast, total cysteine concentrations were significantly increased by 127% in the YSF and 190% in the AF, indicating that the excess Cys normally used for new GSH synthesis in the VYS may have been shuttled to the YSF for storage. The preceding values provide the framework within which oxidized and reduced thiols and their corresponding Eh values can be evaluated (Table 1).
Comparison of reduced and oxidized GSH and Cys
GSH concentrations increase with time in untreated, control conceptuses across the 0, 1, 3, and 6 hr time points in VYS, EMB, and YSF (Figure 2). As expected, GSH was uniformly decreased as a consequence of BSO exposure. AF concentrations trended slightly higher at 1 hr but, subsequently, returned to previous levels. These changes were not significant. Control GSH/GSSG ratios at 0 hr were 16 (VYS), 13(EMB), 1.5(YSF) and 0.1 (AF) indicating a clear trend toward higher relative concentrations of oxidized species in the fluid compartments. After 6 hr of BSO treatment, GSH/GSSG ratios decreased to 5 and 9 in the VYS and EMB, respectively, but increase to 4 for YSF and 0.7 for AF. GSSG concentrations followed a spatial and temporal distribution pattern similar to GSH but represented less than 5% of the total glutathione. This result suggests that relatively little GSH is maintained or sequestered in the major fluid compartments of the conceptus at this stage of development and that most of the remaining GSH, even following BSO treatment, remains in the reduced form. Control Cys/CySS ratios were much lower compared to GSH/GSSG because the bulk of free Cys usually exists in the oxidized (CySS) form in tissues and especially in fluid compartments such as YSF and AF (Figure 3). Cys/CySS concentration ratios of 1.9, 0.6, 0.1, and 0.4 were seen in controls at 0 hr for VYS, EMB, YSF, and AF, indicating that the reduced form predominates only in the VYS, BSO (1 mM) treatment resulted in a further significant reduction in Cys/CySS ratios in these tissues and compartments by virtue of a larger relative increase in CySS concentrations.
Figure 2.
Changes in reduced glutathione (GSH) and the oxidized glutathione disulfide (GSSG) measured in VYS, YSF, AF, and E in GD 11 rat conceptuses for controls (solid circles) and following 1mM BSO (open circles) over the 6 hr sampling period. In accord with normal WEC protocols, the time 0 designated on the figure represents the time when cultures are saturated with 95% O2 to ensure continued optimal growth. Control and treated conceptuses are responding to the additional oxygenation over the 6 hr sampling period. The † symbol indicates that control or BSO-treated values have significantly (p< 0.05) increased or decreased at a designated time point relative to the 0 hr media control. The * symbol indicated that BSO treatment produced a significant (p< 0.05) change relative to the media control value at any given time point.
Figure 3.
Changes in reduced cysteine (cys) and the oxidized cystine (cySS) measured in VYS, YSF, AF, and E in GD 11 rat conceptuses for controls (solid circles) and following 1mM BSO (open circles) over the 6 hr sampling period. In accord with normal WEC protocols, the time 0 designated on the figure represents the time when cultures are saturated with 95% O2 to ensure continued optimal growth. Control and treated conceptuses are responding to the additional oxygenation over the 6 hr sampling period. The † symbol indicates that control or BSO-treated values have significantly (p< 0.05) increased or decreased at a designated time point relative to the 0 hr media control. The * symbol indicated that BSO treatment produced a significant (p< 0.05) change relative to the media control value at any given time point.
Reduced Cys concentrations in the VYS were 0.23 ± 0.06 mM at 0 hr. Also at 6 hr, concentrations in the YSF, AF and EMB ranged between 0.10 ± 0.02 mM and 0.03 ± 0.01mM and exhibited a much lower ratio of reduced and oxidized forms when compared to total GSH. Oxidized CySS concentrations at 0 hr were 0.12 ± 0.02 mM and 0.16 ± 0.03 mM in the VYS and EMB, respectively. In the YSF CySS concentrations ranged from 0.27 ± 0.02 mM at 0 hr and increased to 0.31 ± 0.04 mM at 6 hr. CySS concentrations in the AF ranged from 0.02 ± 0.03 mM to 0.07 ± 0.02 mM over the same time frame. When both oxidized and reduced forms were considered together, total cysteine concentrations more than doubled over the 6 hr time period in the VYS while total amounts in the EMB increased nearly 3-fold. Steady state levels of Cys and CySS are regulated to remain relatively low in cells and tissues due to inherent toxicity. As with cysteine concentrations in other extracellular biological fluid compartments such as plasma, the majority of total cysteine was found in the oxidized state with concentrations of CySS at 0.27 ± 0.02 mM in the YSF and 0.02 ± 0.00 mM in the AF. The temporal pattern of change occurring over the 6 hr culture period was also of interest. In the YSF, the fluid compartment separating the amnion and VYS, Cys decreased over time by 78% and 93% for YSF and AF, respectively, while CySS doubles in the YSF and decreased by 36% in the AF.
As expected, due to its selective inhibition of GSH biosynthesis, BSO produces a significant 87% and 68% reduction in GSH concentrations in the VYS and EMB, respectively, over the 6 hr culture period. The overall rate of GSH depletion is largely dependent on the intrinsic rate of GSH turnover within the cells and tissues. Total Cys/CySS showed an initial 22% increase 1 hr after BSO addition but decreased thereafter in the VYS and EMB to approximately half of its concurrent control value. The Cys/CySS was not being utilized for GSH synthesis and appeared to be sequestered in fluid compartments (YSF and AF) based on the 50% increase in Cys and CySS in these compartments during the 6 hr culture period. This suggests that concentrations of Cys/CySS not being utilized for GSH biosynthesis were released by the VYS and EMB into extracellular spaces rather than being allowed to accumulate at toxic levels in cells and tissues. The mechanisms of this regulation are unknown, although the ability of YSF fluid to sequester amino acids in relatively high concentrations has been known for some time [35]. The already low amounts of GSH in the YSF and AF were further and significantly reduced by BSO treatment when compared to concurrent controls (Figure 3).
Redox Potentials
The characterization of changes in Cys/CySS and GSH/GSSG steady states based on absolute concentrations of the major soluble thiols is instructive for understanding the flux of these molecules and their adaptive biochemistry but this level of investigation contributes little to an understanding of how their changes could affect biochemical and metabolic functions, as well as, conceptal signaling and regulation. For this purpose, the determination of soluble thiol concentrations, followed by computation of half-cell redox potentials (Eh), provides a more meaningful picture of their roles in regulating intracellular environments conducive to signaling and control of critical cellular functions.
As seen in Figure 4, control tissue Eh values for the GSSG/GSH redox couple in the EMB are found to be, on average, about 10–15 mV more oxidized when compared to the corresponding VYS. GSSG/GSH Eh values range between −223 ± 0.01 mV to −234 ± 0.01 mV in the VYS and −219 ± 0.01 mV to −224 ± 0.01 mV in the EMB proper on GD 11. In the fluid compartments, GSH/GSSG Eh is, as expected, much more oxidized ranging between −122 ± 0.01 mV to −143 ± 0.01 in the YSF and from −50 ± 0.00 mV to −88 ± 0.00 mV in the AF.
Figure 4.
Redox potentials (Eh) in sampled tissues (VYS, EMB) and major fluid compartments (YSF, AF) of the GD 11 rat conceptus, grown in whole embryo culture (WEC). Bubble diagrams (as described in Figure 1) show changes in redox potential for GSH/GSSG and cys/cySS redox couples occurring at 1, 3, and 6 hr in whole embryo culture following media saturation with 95% O2 and the addition of 1mMBSO to inhibit GSH biosynthesis. Redox potentials are measured in mV. Heat maps show direction of changes with deeper shades of blue indicating more reducing conditions and a move toward red as tissues and fluids become more oxidized. The symbol * indicates that treated values are significantly different from concurrent unexposed controls (p <0.05). The symbol ** indicates that treated values are significantly different from concurrent unexposed controls (p <0.01).
Control Eh values for the Cys/CySS redox couple remained relatively constant in the VYS, between −142 ± 0.02 mV and −150 ± 0.01 mV, over the 6 hr culture period while the EMB was more oxidized with Eh in the −119 ± 0.01 mV to −137 ± 0.01 mV range. As with the GSH/GSSG redox couple, Cys/CySS was even more oxidized in the fluid compartments at Eh values of −63 ± 0.01 ± mV to −95 ± 0.01 mV in the YSF and −57 ± 0.01 mV to −95 ± 0.00 ± mV in the AF.
Inhibition of GSH synthesis with BSO caused a net oxidation in VYS, YSF, and EMB relative to concurrent controls over the 6 hr culture period. Increases in GSH/GSSG Eh were significant (p<0.05) in the VYS at 3 hr (Δ 18 mV) and 6 hr (Δ 34 mM); in the YSF at 3 hr (Δ 15 mV); and in the EMB at 6 hr (Delta; 18 mV). Significant trends toward greater oxidation following BSO treatment were seen in VYS, AF and EMB across the culture period. Significant oxidation (p< 0.05) in the Cys/CySS redox couple, based on increased Eh, was seen in the VYS (Δ 46 mV), AF (Δ 2 mV), and EMB (Δ 22mV) at 6 hr following BSO treatment.
Alterations of the Thiol Proteome
Alterations in the embryonic proteome as a result of BSO exposure were assessed using two different methodological approaches. Each method targeted the redox proteome as the respective labeling reagents react specifically with reduced protein sulfhydryls (Cys). The first analysis, using the BIAM reagent, separates embryonic proteins on 2-dimensional gels and provided a qualitative assessment of the proteins that may have been redox-regulated through thiol oxidation at accessible protein Cys –SH groups (Figure 5). The net oxidation caused by treatment is akin to other more indirect methods that purport to show “oxidative stress”. The second analysis, using ICAT reagents, also labeled proteins at accessible –SH groups but provided a means to quantify the direct relative gain or loss of specific proteins in embryonic tissues (Table 2). Control proteins (light isotope label) and treated proteins (heavy isotope label) were mixed, digested with trypsin, producing tryptic peptides which were then fractionated and subsequently analyzed using LC-MS (liquid chromatography-mass spectrometry). The recorded magnitude of heavy/light isotope (H/L) ratios showed the extent to which total protein concentrations changed due to a specific treatment.
Figure 5.
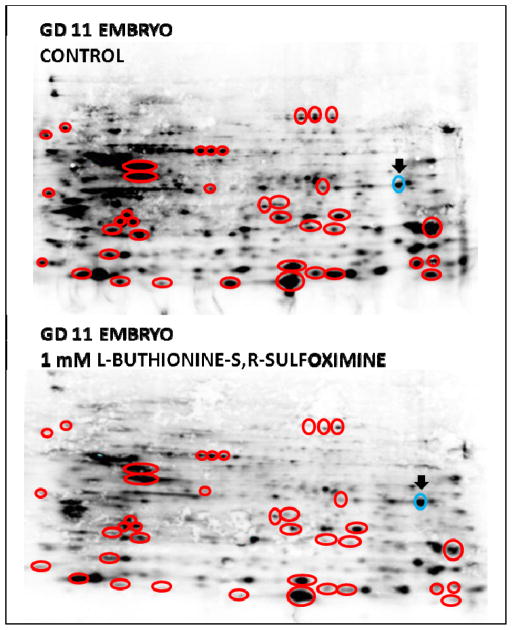
Gestational day 11 (GD 11) embryonic proteins covalently labeled at reduced cysteines with biotinylated iodoacetamide reagent (BIAM), separated on 2- dimensional SDS-PAGE gels and transferred to nitrocellulose membranes from embryos exposed to 1 mM BSO in WEC for 6 hr. Streptavidin tagged with AlexaFluor 680 conjugate was used to detect labeled proteins. BIAM reagents selectively bind only to accessible reduced thiols on embryonic proteins. Control embryonic homogenates (upper panel) show significant numbers of proteins containing reduced thiols that bound BIAM reagent. Identical embryos exposed to 1 mM BSO show a significant reduction in the number and intensity of tagged spots. Corresponding spots (circles) picked on the basis of showing at least a 25% decrease in intensity (digital desitometry) indicate proteins oxidized as a result of BSO exposure.
Table 2.
A summary of protein concentrations altered in the GD 11 EMB after a 6 hr exposure to 1 mM BSO and as determined by isotope-coded affinity tag (ICAT)-labeling and resolution using mass spectroscopy. Relative changes in protein concentration are quantified based on heavy (treatment)/light (control) isotope ratios. Only proteins showing changes in excess of 15% are shown. The table is also restricted to an analysis of the most abundant EMB proteins and the 10 KEGG pathways with the highest number of altered proteins mapping to that pathway
| Pathway (Mapped from KEGG Database) | % Enrichment | p-value |
|---|---|---|
| A Spliceosome | 2.2 | 0.020 |
| B Glycolysis/Gluconeogenesis | 1.8 | 0.035 |
| C Antigen processing and presentation | 1.8 | 0.039 |
| D Aminoacyl-tRNA biosynthesis | 1.3 | 0.046 |
| E Base excision repair | NS | |
| F Tight junction | NS | |
| G Ribosome | NS | |
| H Purine metabolism | NS | |
| I Cysteine and methionine metabolism | NS | |
| J Selenoamino acid metabolism | NS | |
|
| ||
| Proteins in Significantly Enriched Pathways | GI Accession | H/L |
|
| ||
| Spliceosome | ||
| Acin1 protein [Rattus norvegicus] | 60688451 | 5.17 |
| calcium homeostasis endoplasmic reticulum protein [Rattus norvegicus] | 157823443 | 4.69 |
| PRP19/PSO4 pre-mRNA processing factor 19 homolog (S. cerevisiae), isoform CRA_d [Rattus norvegicus] | 149062429 | 3.56 |
| U2af2 protein [Rattus norvegicus] | 59808930 | 3.55 |
| heterogeneous nuclear ribonucleoprotein M isoform b [Rattus norvegicus] | 158186696 | 3.17 |
| Glycolysis/Gluconeogenesis | ||
| glyceraldehyde-3-phosphate dehydrogenase [Rattus norvegicus] [PREDICTED: similar to] | 62653546 | 3.87 |
| dihydrolipoamide dehydrogenase, isoform CRA_a [Rattus norvegicus] | 149051076 | 3.28 |
| Tpi1 protein [Rattus norvegicus] | 38512111 | 2.91 |
| aldolase A, isoform CRA_g [Rattus norvegicus] | 149067838 | 2.59 |
| Antigen Processing and Presentation | ||
| heat shock 70 kDa protein 4 [Rattus norvegicus] | 24025637 | 3.75 |
| protein disulfide isomerase associated 3, isoform CRA_a [Rattus norvegicus] | 149023097 | 2.50 |
| calreticulin, isoform CRA_b [Rattus norvegicus] | 149037838 | 1.97 |
| heat shock protein HSP 90-alpha [Rattus norvegicus] | 28467005 | 1.52 |
| Aminoacyl-tRNA Biosynthesis | ||
| Asparaginyl-tRNA synthetase [Rattus norvegicus] | 68534368 | 5.12 |
| methionyl-tRNA synthetase [Rattus norvegicus] | 189083764 | 3.96 |
| aspartyl-tRNA synthetase, cytoplasmic [Rattus norvegicus] | 16758642 | 3.32 |
|
| ||
| Proteins Most Affected by Treatment from All Pathways | ||
|
| ||
| Protein | GI Accession | H/L |
| peptidyl-prolyl cis-trans isomerase FKBP5 [Rattus norvegicus] [E] | 58865920 | 15.58 |
| heat shock protein 75 kDa, mitochondrial precursor [Rattus norvegicus] | 84781723 | 5.38 |
| Acin1 protein [Rattus norvegicus] [A] | 60688451 | 5.17 |
| Asparaginyl-tRNA synthetase [Rattus norvegicus] [D] | 68534368 | 5.12 |
| APEX [Rattus norvegicus] | 12247526 | 4.76 |
| mitochondrial carrier homolog 2 (C. elegans) (predicted), isoform CRA_b [Rattus norvegicus] | 149022590 | 4.73 |
| calcium homeostasis endoplasmic reticulum protein [Rattus norvegicus] [A] | 157823443 | 4.69 |
| lactate dehydrogenase A-like [Rattus norvegicus] [PREDICTED] | 293344081 | 4.64 |
| Epb4.1l2 protein [Mus musculus] [F] | 27371130 | 4.62 |
| karyopherin (importin) beta 1 [Rattus norvegicus] | 149054034 | 4.48 |
| ribosomal protein S11 [Marmota monax] | 92111517 | 4.45 |
| 60S ribosomal protein L4 [Mus musculus] [G] | 30794450 | 4.42 |
| importin 5 [Rattus norvegicus] [PREDICTED] | 293354006 | 4.41 |
| Ipo4 protein [Mus musculus] | 13097471 | 4.41 |
| phosphoribosylformylglycinamidine synthase [Rattus norvegicus] [H] | 157786806 | 4.40 |
| poly(rC) binding protein 2, isoform CRA_d [Mus musculus] | 148672030 | 4.40 |
| exportin-2 [Rattus norvegicus] | 157820325 | 4.37 |
| b4 integrin interactor [Mus musculus] | 2910995 | 4.36 |
| leucine-rich PPR motif-containing protein, mitochondrial precursor [Rattus norvegicus] | 56605990 | 4.35 |
| eukaryotic translation initiation factor 3, subunit M [Mus musculus] | 123241991 | 4.34 |
| eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked [Mus musculus] | 123270417 | 4.33 |
| Mat2a protein [Mus musculus] [I],[J] | 34849522 | 4.32 |
| T-complex protein 1 subunit eta [Mus musculus] | 238814391 | 4.31 |
| exportin 1, CRM1 homolog (yeast) [Mus musculus] | 123857927 | 4.31 |
| mCG11470 [Mus musculus] | 148686738 | 4.24 |
BIAM Labeling of Reduced Protein Sulfhydryls
Two-dimensional gels from control and BSO-treated EMB were labeled with BIAM reagent, resolved on a 2D-SDS-PAGE gel, blotted to a nitrocellulose membrane, and probed with fluorophore-labeled streptavidin. Results are shown in Figure 5. A global qualitative comparison following streptavidin binding, visualization, and densitometry showed reduced intensity of signal in EMB tissues that had been treated with BSO. Selected spots were compared between treated and control gels for label intensity using computer assisted densitometry. All circled spots were determined to have changed from control by at least 25%, based on densitometry measurements. The BIAM reagent only reacts with reduced –SH (-S−) groups so as the Cys thiols become oxidized, less BIAM binds and the attenuated signal from proteins visualized using the streptavidin tag in the treated sample indicates a net oxidation has occurred. Note the significant decrease in staining in the upper left quadrant due to BSO treatment. Only one spot was found to increase in intensity (blue circle).
ICAT Labeling and Analysis of the Conceptal Sulfhydryl-Modified Proteome
BSO treatment also produced significant changes in the relative abundance of individual proteins in the EMB. The principal effect of BSO in the EMB was to increase the relative amounts of thiol-labeled proteins (tagged with ICAT reagent) that were grouped into functional pathways and analyzed using the KEGG (http://www.genome.jp/kegg) database. Table 2 shows results from 10 pathways mapped in KEGG, four of which were determined to be highly enriched and statistically significant (enrichment refers to the magnitude of net increase of a particular protein), including the number of proteins within a pathway and the relative magnitude/direction of change based on H/L ratios. The analysis, as performed, identified only a subset of the most abundant proteins that were altered by BSO treatment. Only the proteins showing a H/L ratio shift in excess of 25% are used in the data presented. The specific proteins found to be associated with each of the pathways that are significantly affected by BSO can be found in Table 2. Proteins in affected pathways selective for EMB and found to be significantly enriched include the Splicesome, Glycolysis/Gluconeogenesis, Antigen Processing and Presentation, and Aminoacyl-tRNA Biosynthesis pathways. Of the proteins found to have changed in the various pathways, several were common to multiple pathways, limiting the number of proteins that are exclusive to a given pathway. Several specific proteins identified from fragment analysis that are not associated with a significantly enriched pathway are also listed in Table 2.
DISCUSSION
Excessive oxidation and depletion of GSH has often been associated with the generic condition of attenuated antioxidant and protective function known as “oxidative stress”. Recent compelling evidence from studies of cancer and other chronic diseases now also suggests that variations in the redox states of GSH, Cys, Trx and other abundant redox-couples play direct regulatory and signaling roles in the same pathways and receptors that control embryonic growth and development [7, 15, 36]. Fluctuations in the redox state of the pyridine nucleotides such as NADPH and NADH could also impact cellular signaling, especially under conditions of hypoxia and physiologic stress. The most important role of pyridine nucleotides in redox signaling, however, will be through the transfer of electrons for reduction of GSH, cys, and Trx [37]. Sensitive regulatory targets may be located in intracellular or extracellular compartments and may have differential sensitivities to environmental signals, which can change gene expression as well as to regulate the quality and quantity of the conceptal proteome. Intracellular steady state concentrations of GSH are higher in VYS and EMB by approximately 250-fold when compared to their respective, adjacent, fluid compartments of the YSF and AF. This is similar to mature organisms in which extracellular fluids, including plasma, maintain GSH in μM concentrations while adjacent intracellular tissue values fall within the mM range [38–40]. An inverse relationship exists for the GSH precursor Cys in the YSF where extracellular fluid concentrations exceed intracellular VYS (Figure 2,3; Table 1). In the AF, which directly bathes the EMB, Cys concentrations remain 12-fold lower than in the adjacent EMB tissues.
Glutathione biosynthesis and turnover in tissues of the organogenesis-stage rat conceptus have been previously characterized [26, 30, 41–43], but the adjacent fluid compartments, their respective redox potentials, and redox proteomic responses to environmental cues and GSH modulators have not been evaluated. This is an important consideration for redox regulation during development because the regulatory domains of many growth factor receptors and transporters are located on the extracellular leaf of the plasma membrane [40, 44]. The VYS has been shown to be the most metabolically active tissue in the conceptus with regards to GSH biosynthesis [15, 42, 43]. This is likely due, in part, to its role as the maternal-embryonic interface where, during organogenesis, the tissue’s outward-facing brush border captures maternal proteins along with their specific cargoes and processes them through lysosomes to provide all of the amino acids and other components required for conceptal biosynthesis [45–47]. Previous experiments have shown that specific activities of γ-glutamate cysteine ligase (GCLc), the rate-limiting enzyme in GSH biosynthesis, are 2–3 times higher in the VYS compared to the corresponding EMB proper [42, 43]. Glutathione modulating agents that form covalent adducts (diethyl maleate; DEM) or inhibit GSH synthesis (BSO) similarly deplete GSH in both major tissue compartments, but the recovery profiles for these tissues are very different. DEM exposure in rat WEC, for example, produces a rapid depletion of GSH in both VYS and EMB. Following removal of the DEM, GSH recovery begins immediately in the VYS producing GSH concentrations after 3 hr that exceed those observed prior to exposure. Recovery of GSH in the EMB, however, is delayed showing no increases in the EMB until VYS levels are replete [43]. These experiments and others suggest a complex interplay between different tissues of the conceptus in the control and regulation of GSH status involving adequate supply of amino acid precursors and compartment-specific enzyme activities.
BSO selectively inhibits GCLc activity in the VYS, the most active region for GSH biosynthesis in the conceptus, in spatial and temporal patterns that appear to reflect relative rates of GSH turnover. These interactions are of significant interest from a purely embryo-protective standpoint, due to the importance of GSH and Cys in maintaining adequate antioxidant defenses, but also show that BSO is capable of producing significant GSH depletion in tissues over a short duration, making it a good candidate for disruption of developmental signaling. Large net increases of total glutathione and total cysteine (Table 1) seen in control conceptuses after 6 hr are preceded by a slight decrease in concentration at 1 hr and are believed to be a consequence of the saturation of WEC culture medium with 95%O2/5%CO2 (per normal WEC protocols) just prior to initiation of the BSO exposure time course (0 hr).
Cysteine/cystine, as the most abundant soluble thiol couple in the extracellular milieu, plays an important role in defining the extracellular signaling environment. In control VYS and EMB, total intracellular cysteine follows the same general pattern of increase as seen for total glutathione, although the relative increase in the EMB is much greater compared to VYS. This suggests that the lower rates of GSH synthesis in the EMB are not due to lack of its rate-limiting precursor (Cys) but may be influenced by other regulatory factors. Responses to BSO treatment are relatively small for both tissues across the 6 hr time course. In fluid compartments such as the YSF, control total cysteine concentrations exceed total glutathione concentrations by 23-fold. Treatment with BSO produces a trend of increased total cysteine in both YSF and AF fluid compartments at 6 hr because the processes of protein uptake and proteolytic digestion that supply the Cys continue unabated, during a time when the amino acid is not being incorporated into new GSH. Previous reports have suggested that the YSF serves as an important storage depot for excess amino acids in the conceptus [35] and GSH itself is an important physiological storage sink for Cys [48].
As total GSH and Cys concentrations change spatially and temporally within cells and in extracellular fluid compartments, so do their respective Eh values, which are able to regulate the respective redox nodes in the signaling pathways that control key developmental events. In control conceptuses Eh values for the GSSG/GSH redox couple follow an order from negative to positive (reducing to oxidizing) of VYS>EMB>YSF>AF. Over the 6 hr time course evaluated, Eh conditions become progressively reduced due to the robust adaptive response in GSH biosynthesis initiated by the saturation of culture medium by 95% O2. Control of Eh in individual compartments of the conceptus appear to be tightly regulated under steady state conditions because Eh for VYS, EMB, and YSF remain relatively unchanged over the 6 hr period in spite of the fact that total glutathione and total cysteine concentrations nearly doubled. Inhibition of GSH synthesis with BSO results in a significant change of VYS and EMB Eh towards the positive, indicating increased intracellular oxidation. A 55% decrease in GSH concentrations in the VYS (3 hr) and a 71% decrease in the EMB (6 hr) were required, however, before significant increases (oxidation) of Eh were observed. Studies in cell cultures have shown that relatively small >18 mV shifts in Eh to the positive are of sufficient magnitude to oxidize key protein thiols within the EMB [49–51]. Of the many redox-sensitive signaling nodes that may be critical for developmental programming and pattern formation, only a few have been identified and shown to be impacted by chemical and environmental cues. These include transcription factors such as, NFkB, AP-1, and Nrf2 [6, 52, 53]. Compared to VYS and EMB, the fluid compartments tend to have greater variability of Eh, both in the normal steady state and following exposure to chemical and environmental insults. These results collectively suggest that redox conditions within the conceptus are independently regulated and change dynamically in response to external stimuli.
Evidence for the direct involvement of redox signaling in the regulation of developmental growth and differentiation implicate both ROS as signaling molecules and Eh for GSH and Cys redox couples as significant factors in interpreting environmental cues that are necessary for regulation of normal development. Growth factor receptors such as EGFR are known to actively induce ROS production to prolong and enhance their signaling capacity [54] by redox inhibition of the phosphatases needed to reverse kinase-activated signals. Redox signaling mechanisms involve the participation of several different redox couples, many of which are kinetically independent. Because the receptors and sensors responsible for the redox regulation of these processes are found both intracellularly and extraxcellularly, a characterization of tissues and the fluids that bathe them is essential.
The nature and extent of post-translational protein modifications that occur in response to conditions of oxidative stress are often underestimated because of the use of selective “oxidative stress” expression arrays that focus on a subset of target genes known to be involved in control of redox regulation in cells. Lost, are the myriad of other redox-sensitive proteins that are found in virtually every other critical developmental pathway. Only a relatively small number of redox-sensitive regulatory proteins involved in developmental signaling and control have been identified, although an ever-increasing number of growth factors, transcription factors, and other regulatory elements have been well characterized in mechanistically relevant cancer studies [55, 56]. The BIAM analysis has allowed us to assess the potential general impact of altered Eh in terms of post-translational oxidative modification of protein thiols in the EMB following BSO treatment. A comparison of BIAM binding to reduced protein–SH groups in the EMB between 2D gel blots from control and BSO treated conceptuses show a clear reduction in overall label intensity in the treatment group, including the near disappearance of some protein spots. This analysis indicates the presence of numerous EMB proteins that are redox sensitive and that may have a role in developmental signaling and functional regulation. Work is now underway to identify and characterize the specific proteins involved.
Proteomic ICAT procedures provide the means to directly compare changes in relative abundance between large numbers of proteins from treated and control samples. Considering only the 100–300 most abundant EMB proteins labeled with the ICAT reagent, individual proteins map to four pathways that were found to be significantly enriched following BSO exposure (6 hr) based on the KEGG library of functional genes (Figure 2). Proteomic analysis of EMB response to conditions of oxidative stress provides a snapshot of real changes in the concentrations of specific proteins but does not make any predictions regarding their possible regulation at the transcriptional level. Significantly enriched pathways are listed in descending order (Table 2), beginning with those having the greatest number of enriched proteins found in the Splicesome, Glycolysis/Gluconeogenesis, Antigen Processing and Presentation, and Aminoacyl-tRNA Biosynthesis pathways. While these pathways may seem superficially unrelated, examination of the specific proteins identified provides instruction regarding the adaptive and protective strategies being employed by the EMB in response to the effects of BSO exposure. The adaptive processes of RNA processing, stabilization, translation, protein synthesis, protein folding and stabilization are all represented in the activities of proteins mapped to these four pathways.
The proteins and enzymes of the Spliceosome are responsible for creating much of the functional complexity of the genome by cutting different splice variants from common mRNAs which code for functionally diverse protein isoforms [57]. Alternative splicing is known to occur frequently during early embryonic development and involves the modification of a number of growth factors, transcription factors, and other regulatory elements [58]. The fibroblast growth factors (FGF), for example, are a highly conserved class of essential mammalian developmental growth regulators that show a remarkable diversity of function. In the mouse, Fgf8 has been shown to be responsible for proper gastrulation, establishment of the mesoderm, brain and spinal cord development and patterning, and limb development [59–61]. In mammals Fgf8s are known to exist as several different splice variants that also possess very different functions. Fgf8b is the splice form required for posterior mesoderm development [61] and the functional loss of Fgf8a or Fgf8a/Fgf8b decreases growth and alters patterning in the hindbrain and spinal cord. In cultured NIH3T3 cells, human and mouse FGF8B/Fgf8b will induce robust transformation, while FGF8A/Fgf8a will not [62, 63]. The implications of the present work are that disruptions of conceptal redox environments may affect critical developmental regulators. By changing the relative composition and function of the Spliceosome, for example, the quantity and selection of essential splice variants that are known to be critical for establishing specificity in developmental signaling and response will also change.
From the Glycolysis/Gluconeogenesis pathway, the protein glyceraldehyde-3 phosphate dehydrogenase (GAPDH) is known to be induced through a variety of redox signaling molecules. Highly responsive to cellular conditions, including hypoxia, the mouse 5′ GAPDH flaking region contains several important response elements that are relevant to developmental signaling and are redox regulated. These include the AP1, SP1, HIF-1, XRE, MARE, GRE and RARE elements that also regulate the expression of other developmentally relevant genes such as VEGF [64, 65]. GAPDH has also recently been shown to have a direct role in cellular function as a mediator of RNA stability. S-thiolation of GAPDH on its active site, Cys 152, prevents mRNA unwinding and, thus, enhances mRNA stability under oxidative conditions [66]. The heat shock proteins and protein disulfide isomerases from the Antigen Processing and Presentation pathways have well defined roles in stabilizing and refolding proteins necessary for the maintenance of cellular homeostasis in oxidizing environments[67]. Proteins of the Aminoacyl-tRNA Biosynthesis pathway support de novo protein biosynthesis as an important adaptive and protective function during the recovery from oxidative conditions [68]. Post-translational protein modifications of the aminoacyl-tRNA synthetases at critical Cys sites reduce fidelity and can result in mistranslated and misfolded proteins. Thus, the specific proteins found to be elevated in all four of the significantly enriched pathways identified in the ICAT proteomic analysis can be shown to have related functions in the processing and stabilization of mRNA and proteins. Other proteins showing significant increases due to BSO treatment, but not mapping to enriched pathways, provide additional insights into the extended EMB responses to changes in Eh and the expected effects on redox regulation. Considerable additional work will be required to verify the mechanistic associations between these observed protein changes and their redox-related post-translational modifications and control of developmental growth and differentiation.
CONCLUSIONS
In conclusion, GSH and Cys concentrations change in tissue and fluid compartments of the developing conceptus in differential spatial and temporal patterns. Redox potentials are maintained within a relatively narrow range in control VYS and EMB even under adaptive conditions where total glutathione and total cysteine concentrations are increased by nearly two-fold. Relatively large decreases in GSH and Cys concentrations, as produced by BSO, are required before the corresponding Eh is shifted significantly toward the positive and greater oxidation. When GSH synthesis is inhibited by BSO, the Cys normally incorporated into new GSH is shuttled into the YSF fluid compartment and is increased intracellularly in the tissues of the EMB, changing the Eh of fluid and tissue compartments toward greater oxidation. The BSO-induced positive Eh of the EMB leads to the oxidation of a large number of protein thiols, indicating a high potential for altered redox signaling. Proteomic analysis using ICAT also shows that BSO treatment increases the concentrations of a number of EMB proteins belonging to regulatory pathways such as the Spliceosome that are critical for normal signaling and growth regulation during development as well as a number proteins that essentially act as ‘kinetic traps’ to help stabilize the cellular environment during recovery. An understanding of the regulation and control of dynamic redox environments within compartments of the viable, intact developing conceptus is critical for our ability to unravel the complexities of developmental signaling and pattern formation.
Figure 1.

Bubble diagram and light micrograph showing the location and spatial relationships of tissue and fluid compartments sampled for GSH, GSSG, cys, and cySS in the GD 11 rat conceptus. The visceral yolk sac (VYS) encloses the entire conceptus and must be first traversed by any nutrient, chemical, or environmental factor before reaching the embryo (EMB) proper. The underlying fluid compartment, bounded by the VYS and amnion (dashed lines), is designated yolk sac fluid (YSF). The amniotic fluid (AF) bathes the embryo proper and is contained within the amnion. The tissue and fluid sampling procedure was described in detail in the Methods section.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Gu YZ. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. Journal of Internal Medicine. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burhans WC, Heintz NH. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radical Biology and Medicine. 2009;47:1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JM, Harris C. REDOX CONTROL OF TERATOGENESIS. Reproductive Toxicology. doi: 10.1016/j.reprotox.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hansen JM. Thalidomide modulates nuclear redox status and preferentially depletes glutathione in rabbit limb versus rat limb. The Journal of pharmacology and experimental therapeutics. 2002;300:768–776. doi: 10.1124/jpet.300.3.768. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JM. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxidants & redox signaling. 2004;6:1–14. doi: 10.1089/152308604771978291. [DOI] [PubMed] [Google Scholar]
- 7.Gius D. Redox signaling in cancer biology. Antioxidants & redox signaling. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants & redox signaling. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterfield DA. Redox Proteomics in Selected Neurodegenerative Disorders: From Its Infancy to Future Applications. Antioxidants & redox signaling. 2012;120118110231009 doi: 10.1089/ars.2011.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabens Liedhegner EA. Mechanisms of altered redox regulation in neurodegenerative diseases-focus on S-glutathionylation. Antioxidants & redox signaling. 2012;16:543–566. doi: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmastro MM, Piganelli JD. Oxidative Stress and Redox Modulation Potential in Type 1 Diabetes. Clinical and Developmental Immunology. 2011;2011 doi: 10.1155/2011/593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakoli S. Reactive Oxygen Species and Thiol Redox Signaling in the Macrophage Biology of Atherosclerosis. Antioxidants & redox signaling. 2012;120611124142004 doi: 10.1089/ars.2012.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turan B. Role of antioxidants in redox regulation of diabetic cardiovascular complications. Current pharmaceutical biotechnology. 2010;11:819–836. doi: 10.2174/138920110793262123. [DOI] [PubMed] [Google Scholar]
- 14.Go Y-M. Cysteine/cystine redox signaling in cardiovascular disease. Free radical biology & medicine. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles RL. Redox signalling in cardiovascular disease. Proteomics Clinical applications. 2008;2:823–836. doi: 10.1002/prca.200780104. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JM. Oxidative stress as a mechanism of teratogenesis. Birth defects research Part C Embryo today. 2006;78:293–307. doi: 10.1002/bdrc.20085. [DOI] [PubMed] [Google Scholar]
- 17.Finkel T. Signal transduction by reactive oxygen species. The Journal of Cell Biology. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray PD. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y. Redox control of the survival of healthy and diseased cells. Antioxidants & redox signaling. 2011;15:2867–2908. doi: 10.1089/ars.2010.3685. [DOI] [PubMed] [Google Scholar]
- 20.Valko M. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Dennery PA. Effects of oxidative stress on embryonic development. Birth defects research Part C Embryo today. 2007;81:155–162. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 Redox Control: Regulation of Cytoplasmic Activation by Glutathione and DNA Binding by Thioredoxin-1. Toxicological Sciences. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 23.Kamata T, Ambo H, Puzon-McLaughlin W, Tieu KK, Handa M, Ikeda Y, Takada Y. Critical cysteine residues for regulation of integrin alpha IIb beta 3 are clustered in the epidermal growth factor domains of the beta 3 subunit. PORTLAND PRESS; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanic B, Katsuyama M, Miller FJ. An Oxidized Extracellular Oxidation-Reduction State Increases Nox1 Expression and Proliferation in Vascular Smooth Muscle Cells Via Epidermal Growth Factor Receptor Activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2234–2241. doi: 10.1161/ATVBAHA.110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-κB and AP-1. Toxicology Letters. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 26.Harris C, Fantel AG, Juchau MR. Differential glutathione depletion by l-buthionine-S, R-sulfoximine in rat embryo versus visceral yolk sac in vivo and in vitro. Biochemical Pharmacology. 1986;35:4437–4441. doi: 10.1016/0006-2952(86)90760-4. [DOI] [PubMed] [Google Scholar]
- 27.Harris C, Dixon M, Hansen JM. Glutathione depletion modulates methanol, formaldehyde and formate toxicity in cultured rat conceptuses. Cell Biology and Toxicology. 2004;20:133–145. doi: 10.1023/b:cbto.0000029466.08607.86. [DOI] [PubMed] [Google Scholar]
- 28.Harris C. Rodent whole embryo culture. Methods in molecular biology (Clifton, NJ) 2012;889:215–237. doi: 10.1007/978-1-61779-867-2_13. [DOI] [PubMed] [Google Scholar]
- 29.Jones DP. [11] Redox potential of GSH/GSSG couple: Assay and biological significance. In: Helmut S, Lester P, editors. Methods in Enzymology. Academic Press; 2002. pp. 93–112. [DOI] [PubMed] [Google Scholar]
- 30.Harris C. Oxidative stress, thiols, and redox profiles. Methods in molecular biology (Clifton, NJ) 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- 31.Kirlin WG. Glutathione redox potential in response to differentiation and enzyme inducers. Free radical biology & medicine. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 32.Hansen JM, Moriarty-Craige S, Jones DP. Nuclear and cytoplasmic peroxiredoxin-1 differentially regulate NF-κB activities. Free Radical Biology and Medicine. 2007;43:282–288. doi: 10.1016/j.freeradbiomed.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu C, Hu J, Liu T, Ago T, Sadoshima J, Li H. Quantitative Analysis of Redox-Sensitive Proteome with DIGE and ICAT. Journal of Proteome Research. 2008;7:3789–3802. doi: 10.1021/pr800233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go Y-M. Quantification of redox conditions in the nucleus. Methods in molecular biology (Clifton, NJ) 2009;464:303–317. doi: 10.1007/978-1-60327-461-6_17. [DOI] [PubMed] [Google Scholar]
- 35.Rowe PB. Serine metabolism in rat embryos undergoing organogenesis. Journal of embryology and experimental morphology. 1985;87:137–144. [PubMed] [Google Scholar]
- 36.Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Nrf2 Represses FGF21 During Long-Term High-Fat Diet–Induced Obesity in Mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris C. Hypoxia and Altered Redox Status in Embryotoxicity. In: Kavlock R, Daston G, editors. Drug Toxicity in Embryonic Development I. Springer; Berlin Heidelberg: 1997. pp. 519–548. [Google Scholar]
- 38.Nkabyo YS. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. The Journal of nutrition. 2006;136:1242–1248. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 39.Iyer SS, Accardi CJ, Ziegler TR, Blanco RA, Ritzenthaler JD, Rojas M, Roman J, Jones DP. Cysteine Redox Potential Determines Pro-Inflammatory IL-1β Levels. PloS one. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriarty-Craige SE. Extracellular thiols and thiol/disulfide redox in metabolism. Annual review of nutrition. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 41.Harris C, Hiranruengchok R, Lee E, Berberian RM, Eurich GE. Glutathione status in chemical embryotoxicity: Synthesis, turnover and adduct formation. Toxicology in Vitro. 1995;9:623–631. doi: 10.1016/0887-2333(95)00072-g. [DOI] [PubMed] [Google Scholar]
- 42.Hansen JM, Lee E, Harris C. Spatial Activities and Induction of Glutamate-Cysteine Ligase (GCL) in the Postimplantation Rat Embryo and Visceral Yolk Sac. Toxicological Sciences. 2004;81:371–378. doi: 10.1093/toxsci/kfh154. [DOI] [PubMed] [Google Scholar]
- 43.Harris C. Glutathione Biosynthesis in the Postimplantation Rat Conceptus in Vitro. Toxicology and Applied Pharmacology. 1993;120:247–256. doi: 10.1006/taap.1993.1109. [DOI] [PubMed] [Google Scholar]
- 44.Ottaviano FG. Redox regulation in the extracellular environment. Circulation journal: official journal of the Japanese Circulation Society. 2008;72:1–16. doi: 10.1253/circj.72.1. [DOI] [PubMed] [Google Scholar]
- 45.Brent RL, Beckman DA, Jensen M, Koszalka TR. Experimental yolk sac dysfunction as a model for studying nutritional disturbances in the embryo during early organogenesis. Teratology. 1990;41:405–413. doi: 10.1002/tera.1420410406. [DOI] [PubMed] [Google Scholar]
- 46.Brent RL. Experimental yolk sac dysfunction as a model for studying nutritional disturbances in the embryo during early organogenesis. Teratology (Philadelphia) 1990;41:405–413. doi: 10.1002/tera.1420410406. [DOI] [PubMed] [Google Scholar]
- 47.Burton GJ. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. The journal of clinical endocrinology and metabolism. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 48.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express. gamma.-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- 49.Hutter DE, Till BG, Greene JJ. Redox State Changes in Density-Dependent Regulation of Proliferation. Experimental Cell Research. 1997;232:435–438. doi: 10.1006/excr.1997.3527. [DOI] [PubMed] [Google Scholar]
- 50.Sen CK. Antioxidant and redox regulation of gene transcription. The FASEB journal. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radical Biology and Medicine. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 52.Harris C, Hansen JM. Nrf2-Mediated Resistance to Oxidant-Induced Redox Disruption in Embryos. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2012;95:213–218. doi: 10.1002/bdrb.21005. [DOI] [PubMed] [Google Scholar]
- 53.Ozolinš TRS. Regulation and control of AP-1 binding activity in embryotoxicity. Methods in molecular biology (Clifton, NJ) 2012;889:291–303. doi: 10.1007/978-1-61779-867-2_18. [DOI] [PubMed] [Google Scholar]
- 54.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal Growth Factor (EGF)-induced Generation of Hydrogen Peroxide. Journal of Biological Chemistry. 1997;272:217–221. [PubMed] [Google Scholar]
- 55.Acharya A. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxidative medicine and cellular longevity. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Current pharmaceutical design. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 57.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revil T, Gaffney D, Dias C, Majewski J, Jerome-Majewska L. Alternative splicing is frequent during early embryonic development in mouse. BMC Genomics. 2010;11:399. doi: 10.1186/1471-2164-11-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Q, Li JYH. Distinct functions of the major Fgf8 spliceform, Fgf8b, before and during mouse gastrulation. Development. 2007;134:2251–2260. doi: 10.1242/dev.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Q, Li K, Sunmonu NA, Li JYH. Fgf8b-containing spliceforms, but not Fgf8a, are essential for Fgf8 function during development of the midbrain and cerebellum. Developmental Biology. 2010;338:183–192. doi: 10.1016/j.ydbio.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh AK. Molecular cloning and characterization of human FGF8 alternative messenger RNA forms. Cell growth & differentiation. 1996;7:1425–1434. [PubMed] [Google Scholar]
- 63.MacArthur CA. FGF-8 isoforms differ in NIH3T3 cell transforming potential. Cell growth & differentiation. 1995;6:817–825. [PubMed] [Google Scholar]
- 64.Reyes-Hernández OD, Mejía-García A, Sánchez-Ocampo EM, Castro-Muñozledo F, Hernández-Muñoz R, Elizondo G. Aromatic hydrocarbons upregulate glyceraldehyde-3-phosphate dehydrogenase and induce changes in actin cytoskeleton. Role of the aryl hydrocarbon receptor (AhR) Toxicology. 2009;266:30–37. doi: 10.1016/j.tox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Higashimura Y, Nakajima Y, Yamaji R, Harada N, Shibasaki F, Nakano Y, Inui H. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Archives of Biochemistry and Biophysics. 2011;509:1–8. doi: 10.1016/j.abb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez-Pascual F. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Molecular and Cellular Biology. 2008;28:7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Advanced Drug Delivery Reviews. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Ling J. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proceedings of the National Academy of Sciences - PNAS. 2010;107:4028–4033. doi: 10.1073/pnas.1000315107. [DOI] [PMC free article] [PubMed] [Google Scholar]