Abstract
Oxidation of polyunsaturated fatty acids (PUFAs) releases α, β-unsaturated aldehydes that modify deoxyguanosine (dG) to form cyclic 1,N2-propanodeoxyguanosine adducts. One of the major adducts detected in vivo is acrolein-derived 1,N2-propanodeoxyguanosine (Acr-dG). We used a chemical model system to examine the effects of four antioxidants known to inhibit fatty acid oxidation on the formation of Acr-dG and 8-oxodeoxyguaonsine (8-oxodG) from the PUFA docosahexaenoic acid (DHA) under oxidative conditions. We found that epigallocatechin gallate (EGCG) and dihydrolipoic acid (DHLA) inhibit both Acr-dG and 8-oxodG formation. In contrast, ascorbic acid and α-tocopherol actually increase Acr-dG at high concentrations and do not show a concentration-dependant inhibition of 8-oxodG. We also studied their effects on blocking Acr-dG formation directly from Acr. EGCG and DHLA can both effectively block Acr-dG formation, but ascorbic acid and α-tocopherol show weak or little effect. These results highlight the complexity of antioxidant mechanisms and also reveal that EGCG and DHLA are effective at suppressing lipid-peroxidation induced Acr-dG and 8-oxodG formation as well as blocking the reaction of dG with Acr.
Introduction
The oxidation of cellular membrane polyunsaturated fatty acids (PUFAs) produces a variety of reactive α, β-unsaturated aldehydes (enals) that can cause DNA and protein damage (1, 2). For example, enals can react directly with DNA or proteins to form pro-mutagenic cyclic adducts or protein carbonyls, both of which have been detected in tissues of rodents and humans. The damage to DNA and protein caused by enals is believed to be etiologically involved in degenerative diseases like atherosclerosis, cancer and aging (3-5).
Acrolein (Acr) is one of the most reactive aldehyde products of lipid peroxidation (6). The chemical reaction of Acr and deoxyguanosine (dG) has been well studied (7). This reaction yields three isomers of cyclic 1,N2-propanodeoxyguanosine (Acr-dG): Acr-dG 1 and 2, later designated as α-hydroxy Acr-dG (α-OH-Acr-dG), and Acr-dG 3, later designated as γ-hydroxy Acr-dG (γ-OH-Acr-dG) (7, Fig. 1). γ-OH-Acr-dG was the predominant adduct detected in rodent and human tissues by an HPLC-based 32P-postlabeling method (8). Under oxidative conditions, reactions of docosahexaenoic acid (DHA) and arachidonic acid (AA) with dG in Tris buffer yielded Acr-dG as a major product, although DHA produced more Acr than AA (9). Consistent with in vivo results, γ-OH-Acr-dG was the predominant isomer formed in the reactions (9). The reason for the preferential formation of one isomer over the other is presently unclear. However, it should be noted that a recent study using an HPLC/tandem mass spectrometry method detected more α-OH-Acr-dG than γ-OH-Acr-dG in lung DNA of smokers and ex-smokers (10).
Fig. 1.
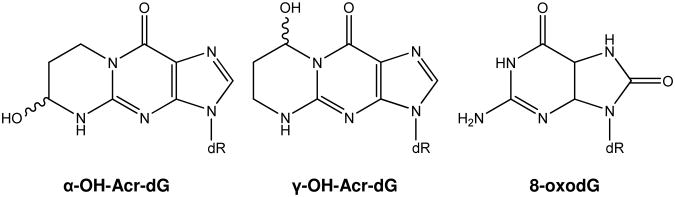
Structures of α-OH-Acr-dG, γ-OH-Acr-dG and 8-oxodG.
Acr was mutagenic in Salmonella tester strains TA 100 and 104, and Acr-dG adducts were detected in the DNA isolated from these strains under the conditions in which mutation occurred (11, 12). Acr adducts were also detected by immunoassay in the DNA of Chinese hamster ovary cells incubated with Acr (13). Site-specific mutagenesis studies have shown that 1,N2-propanodG adducts are pro-mutagenic, leading to base substitution and frame-shift mutations (14). However, evidence also shows that γ-OH-Acr-dG incorporated into E. coli DNA does not miscode due to opening of the propano ring and subsequent excision by nucleotide excision repair (15, 16). While γ-isomer was not mutagenic in E. coli, it was in mammalian cells in single-, but not double-stranded DNA (14-16). Results from the in vitro studies are likely to depend on many factors, such as structures of adducts, sequence context, single- vs. double-stranded DNA and the host system used. Acr has been shown to induce urinary bladder cancer in rats (17). A metabolite of cyclophosphamide, a widely used cancer therapeutic agent, Acr is thought to be the causative agent for the secondary bladder cancer in humans (18). In addition, Acr is a major component of tobacco smoke (19) and the levels of Acr-dG are significantly higher in the oral tissues of smokers than non-smokers (20). More recently, it has been shown that Acr preferentially binds to human p53 gene at the mutational hotspots detected in human lung cancer (21). Together, these results suggest that Acr and its cyclic DNA adducts may play an etiological role in certain human cancers.
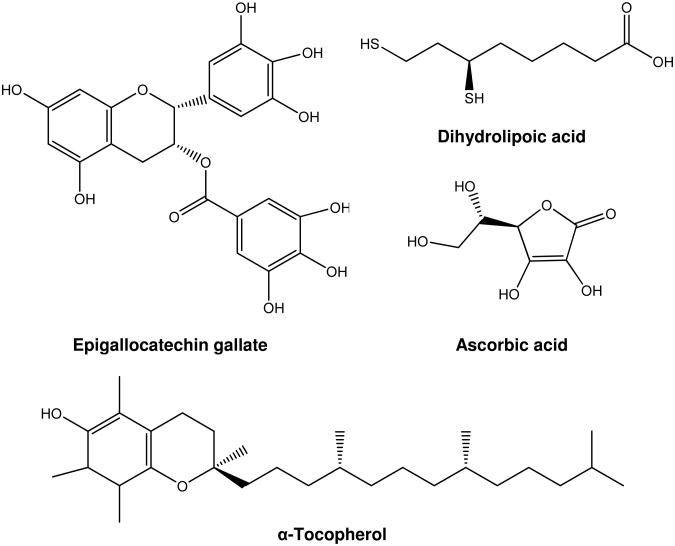
Limited information is available regarding the effect of antioxidants on the formation of cyclic adducts from lipid peroxidation. In this study, we investigated the effects of four different classes of antioxidants on the formation of Acr-dG from DHA as a source of Acr under oxidative conditions. These compounds are shown in Fig. 2. Epigallocatechin gallate (EGCG), the most abundant green tea catechin, is believed to be beneficial to human health due to its strong antioxidant activities (22). Ascorbic acid (water soluble) and α-tocopherol (fat soluble) are two of the most studied antioxidant vitamins. Dihydrolipoic acid (DHLA), the reduced form of lipoic acid, has been shown to exhibit chelating activity, inhibit lipid peroxidation and increase intracellular glutathione (GSH) (23). We examined their effects on the formation of Acr-dG and 8-oxodeoxyguanosine (8-oxodG), a commonly used marker for oxidative DNA damage from DHA. In addition to their antioxidant activities, the ability of these compounds to directly block the reaction of dG with Acr was also investigated.
Fig. 2.
Structures of the antioxidant compounds studied.
Materials and Methods
Materials
Acr was purchased from Alpha Aesar (Ward Hill, MA), Chelex 100 resin was purchased from Bio-Rad Laboratories (Hercules, CA), DHA was purchased from Cayman Chemical Company (Ann Arbor, MI), and 8-oxodG was purchased from Sigma-Aldrich Co. (St. Louis, MO). EGCG was a generous gift from Dr. C. T. Ho (Rutgers University, Piscataway, New Jersey). All other chemicals and reagents were from Sigma-Aldrich Co. (St. Louis, MO) and Fisher Chemical (Fair Lawn, NJ). Acr-dG standard was synthesized and its identity confirmed using previously published procedures (7).
HPLC systems
The HPLC system used in adduct analysis consisted of two LC-10 AD VP pumps, an SCL-10A VP controller, a SPD-M10A VP photodiode array detector with the detection wavelength at 254 nm (Shimadzu, Kyoto, Japan), and a Prodigy ODS 3 C18 reversed-phase column (5 μm, 250 mm × 4.6 mm; Phenomenex Torrance, CA) eluted with a linear gradient program.
The solvent systems used were as follows: System 1: (A) 50 mM NaH2PO4, pH 5.8, (B) MeOH-H2O (3:1); the flow rate was 1 mL/min; the solvent gradient was 30% B in 40 min, System 2: (A) 5.0 mM sodium citrate, pH 6.0, (B) MeOH-H2O (1:1); the flow rate was 0.7 mL/min; the solvent gradient was 30% B in 75 min, System 3: (A) 5.0 mM sodium citrate, pH 6.0, (B) MeOH-H2O (1:1); the flow rate was 0.7 mL/min; the solvent gradient was 45% B in 60 min. In between analyses, the HPLC system was washed in 100% solvent B for 10 min.
Incubation of DHA and FeSO4 with dG in the presence of antioxidants
Various amounts of EGCG (in water), ascorbic acid (in water), α-tocopherol (in DMSO), or DHLA (in DMSO) were added to a reaction mixture containing 3 mM DHA, 15 mM dG, 25 μM FeSO4 and 50 μL DMSO. The total reaction volume was made up to one mL by adding Chelex-treated tris-HCl (100 mM, pH 7.4). The mixture was incubated in a 37°C shaking water bath for 18 h. After removal from the water bath, the reaction mixtures were extracted with 2 mL chloroform for 5 min, centrifuged in a microcentrifuge at 12,000 rpm for 10 min, and the aqueous phase separated and stored at -20°C until analysis.
Reaction of Acr with dG in the presence of antioxidants
Various amounts of EGCG, ascorbic acid, α-tocopherol or DHLA were added to 0.5 mM Acr and 0.5 mM dG. Total reaction volume was made up to one mL with Chelex-treated phosphate buffer (100 mM, pH 7.4). The mixture was incubated in a 37°C shaking water bath for 18 h and stored at -20°C until analysis.
HPLC analysis of Acr-dG and 8-oxodG
For quantification of dG adducts, 100 μL of the extract from the reaction mixture were analyzed using HPLC System 1, with the exception of reaction with DHLA. Analysis of the extract from the reaction with DHLA using HPLC System 1 showed an interference peak near Acr-dG which hindered its accurate quantification. Therefore, the DHLA samples were analyzed by HPLC System 2. For the analysis of the reaction mixtures of Acr and dG, an aliquot of 250 μL was analyzed using HPLC System 3. This aliquot was not extracted because only a small amount of Acr was used in the reaction. In all analyses, the Acr-dG and 8-oxodG adduct peaks were detected by UV at 254 nm and identified by comparing their retention times and characteristic UV spectra with those of the standards. The adduct levels were quantified using the peak areas and compared to those from known amounts of standards.
Statistical analysis
Adduct levels are expressed as mean ± standard deviation. Statistical analysis was carried out using Student's t-test.
Results
Formation of Acr-dG and 8-oxodG in reactions with DHA in the presence of FeSO4
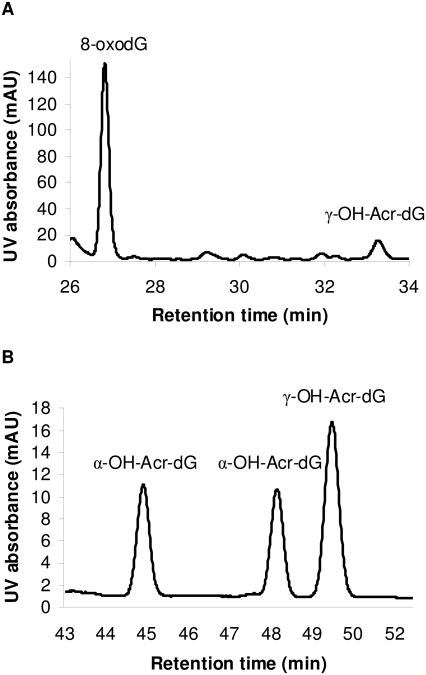
We detected Acr-dG and 8-oxodG formation only when both DHA and FeSO4 are present in the reaction, suggesting that these adducts are originated from lipid peroxidation. Both were well-separated using HPLC System 1 with retention times at 33.3 and 26.7 min, respectively (Fig. 3A). The identities of these products were confirmed by comparing their retention times and characteristic UV spectra with those of the standards. Typical yields under the reaction conditions ranged from 6 to 8 nmol for Acr-dG and 22 to 38 nmol for 8-oxodG. The predominant Acr-dG isomer detected in these reactions was γ-OH-Acr-dG with little or no α-OH-Acr-dG, as previously reported (9).
Fig. 3.
Typical HPLC chromatograms showing the analysis of the reaction mixture of DHA (3 mM) and dG (15 mM) in the presence of FeSO4 (Panel A) using System 1. The peaks at 26.7 and 33.3 min represent 8-oxodG and γ-OH-Acr-dG, respectively. α-OH-Acr-dG was not detected in this reaction. Panel B shows the analysis of the reaction mixture of Acr (0.5 mM) and dG (0.5 mM) using HPLC System 3. The peaks at 45.0 and 48.2 and 49.5 min represent α-OH-Acr-dG and γ-OH-Acr-dG, respectively.
Effects of antioxidants on the formation of Acr-dG and 8-oxodG from DHA
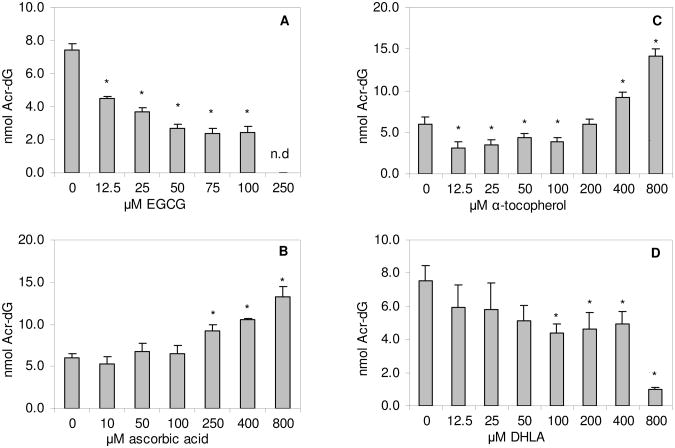
Addition of EGCG to the reaction mixture in a concentration range from 0 to 250 μM caused a concentration-dependent decrease in Acr-dG formation (Fig. 4A). At 250 μM, EGCG completely inhibited the formation of Acr-dG from DHA. However, ascorbic acid up to 100 μM did not affect Acr-dG formation and it actually significantly enhanced Acr-dG formation at higher concentrations (250 to 800 μM, Fig. 4B). Similarly, higher concentrations of α-tocopherol (≥400μM) also increased Acr-dG formation. However, from 12.5 to 100 μM, α-tocopherol significantly reduced Acr-dG formation by 28.2 to 48.7% in a concentration-independent manner (Fig. 4C). Like EGCG, DHLA exerted a concentration-related antioxidant effect on Acr-dG formation, but was considerably weaker than EGCG. Only at concentrations greater than 100 μM did DHLA cause a significant decrease in the formation of Acr-dG (Fig. 4D). Interestingly, the formation of Acr-dG was sharply decreased by 87% with DHLA at 800 μM.
Fig. 4.
Effects of various concentrations of EGCG (A), ascorbic acid (B), α-tocopherol (C) and DHLA (D) on the formation of γ-OH-Acr-dG in the reaction of DHA with dG in the presence of FeSO4. The values are mean ± standard deviation from 3-4 experiments. *significantly different from the control; n.d., not detectable
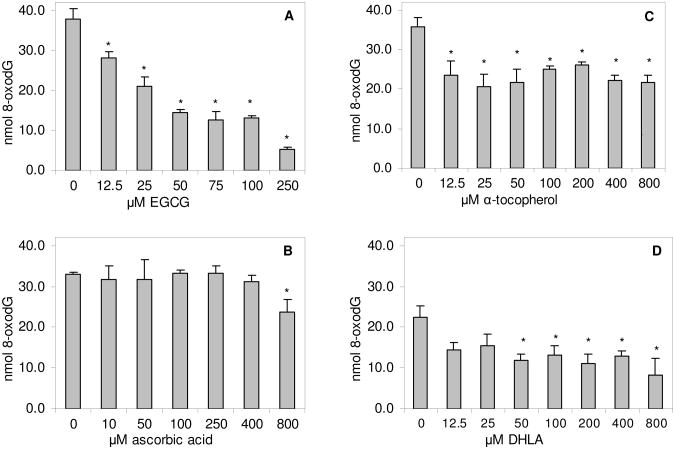
The effects of antioxidants on 8-oxodG formation from DHA were examined in the same reaction mixtures. Again, EGCG caused a concentration-dependent inhibition of 8-oxodG. The yields of 8-oxodG were reduced from 38 nmol without EGCG to 6 nmol with 250 μM EGCG (Fig. 5A). While ascorbic acid had no effect on 8-oxodG levels with concentrations up to 400 μmol, it caused a small, yet significant, inhibition at 800 μM (Fig. 5B). α-Tocopherol in the same concentration range significantly decreased 8-oxodG formation by 27.3 to 42.3% (Fig. 5C), but the inhibition was not concentration-dependent. Similarly, DHLA inhibited 8-oxodG formation (Fig. 5D) without a clear concentration effect. The results showed that among the antioxidants studied, EGCG was the most potent inhibitor against the formation of both dG adducts derived from DHA.
Fig. 5.
Effects of various concentrations of EGCG (A), ascorbic acid (B), α-tocopherol (C) and DHLA (D) on the formation of 8-oxodG in the reaction of DHA with dG in the presence of FeSO4. The values are mean ± standard deviation from 3-4 experiments. *significantly different from the control
The blocking effects of antioxidants on the formation of Acr-dG from Acr
As nucleophiles, the antioxidants could conjugate directly with Acr via Michael addition reaction. Therefore, an important question is whether the antioxidants can block Acr-dG formation directly from Acr. Antioxidant was added to the reaction mixture containing Acr and dG in pH 7.4 phosphate buffer. Under these conditions, dG reacts with Acr yielding both α-OH-Acr-dG and γ-OH-Acr-dG in almost equal amounts, shown in Fig. 3B, as previously reported (7). The first two peaks at 45.0 and 48.2 min were identified as α-OH-Acr-dG and the third peak at 49.5 min as γ-OH-Acr-dG by comparing their retention times and UV spectra with those of the standards.
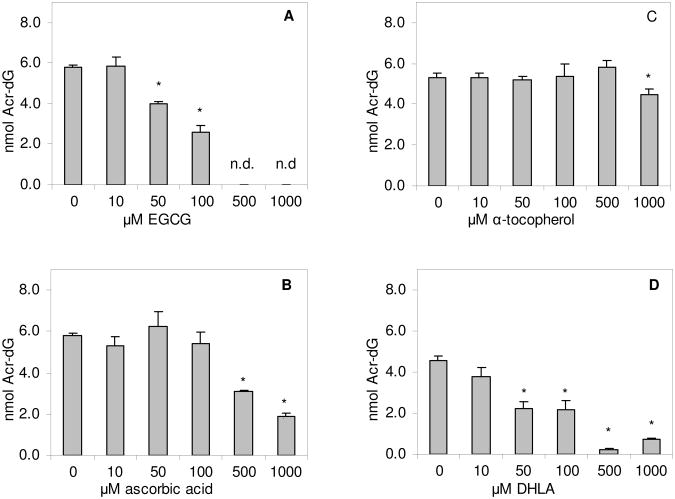
Because we found that the direct blocking effects of antioxidants toward Acr-dG formation were not stereo-specific, the yields of α-OH Acr-dG and γ-OH Acr-dG in the reactions were combined. EGCG showed the most effective blockage with a clear concentration-dependent inhibition showing complete inhibition at 500 μM (Fig. 6A). Interestingly, ascorbic acid, a known scavenger of Acr (24), did not show any significant decrease until its concentration reached 500 μM (Fig. 6B). In contrast, α-tocopherol had little or no blocking effect on Acr-dG formation (Fig. 6C). As expected, DHLA, a strong nucleophile containing sulfhydryls, effectively reduced the formation of Acr-dG with a nearly complete inhibition at the high concentrations (Fig. 6D).
Fig. 6.
Effects of various concentrations of EGCG (A), ascorbic acid (B), α-tocopherol (C) and DHLA (D) on the formation of Acr-dG adducts (total of α-OH-Acr-dG and γ-OH-Acr-dG) in the reaction of Acr with dG. The values are mean ± standard deviation from 3-4 experiments. *significantly different from the control; n.d., not detected
Discussion
Both Acr-dG and 8-oxodG are potential pro-mutagenic DNA lesions that can be formed from lipid peroxidation (14-16, 25). The discovery of antioxidants that protect against their formation provides useful tools for studying the roles of these DNA adducts in cancer. It is hoped that the promising compounds may eventually apply to cancer prevention. The purpose of this study is to better understand the effects of antioxidants on these endogenous DNA damages in a well-defined chemical system using DHA as a source of Acr. The results show that not all antioxidants behave in the same manner toward the formation of Acr-dG and 8-oxodG by lipid peroxidation. While EGCG and DHLA effectively inhibited the formation of both adducts over a wide concentration range, ascorbic acid had little inhibitory effect. On the contrary, ascorbic acid induced Acr-dG formation at higher concentrations. This observation is not entirely unexpected, as it has been reported that ascorbic acid can act as a prooxidant under certain reaction conditions (26). It is possible that at higher concentrations, ascorbic acid actually stimulates the oxidation of DHA. An intriguing observation is the biphasic effect of α-tocopherol on Acr-dG; while the Acr-dG formation was inhibited by α-tocopherol at lower concentrations, its formation was enhanced at higher concentrations. The dual role of α-tocopherol as antioxidant vs. prooxidant toward lipid peroxidation has been reported previously (27). In fact, EGCG has also been shown to have prooxidant effects (28). However, under the conditions we used, such prooxidant effects were not observed. Another important finding is that EGCG and DHLA can effectively block the formation of Acr-dG from Acr, presumably by its conjugation via Michael addition reaction. Our studies show that EGCG and DHLA are versatile compounds; they can not only inhibit oxidative DNA damages as antioxidants, but also block DNA adduction directly from Acr. These results suggest the potential of both compounds to inhibit DNA damage caused by Acr from endogenous lipid peroxidation as well as environmental sources, such as cigarette smoke.
It seems paradoxical that ascorbic acid increased DHA-derived Acr-dG formation at concentrations where it blocked Acr-dG formation directly from Acr. However, the results may be explained by the fact that ascorbic acid can also act as a prooxidant in the presence of ferrous ion and once reduced, ascorbic acid is no longer able to conjugate with Acr. It is possible that only when the concentration of ascorbic acid reached 500 μM is there excess unreduced form to block Acr-dG by Acr. Furthermore, the lack of or weak inhibitory effect of ascorbic acid on 8-oxodG formation suggests that two distinct pathways may be involved in the formation of 8-oxodG and Acr-dG from lipid peroxidation. This notion is also supported by the finding that α-tocopherol was a relatively weak inhibitor of 8-oxodG formation while it displayed a clear biphasic effect on Acr-dG formation from DHA. A similar pattern of increase in Acr-dG formation from DHA caused by ascorbic acid and α-tocopherol at concentration range of 200 to 800 μM (Fig. 4B and Fig. 4C) suggests a comparable prooxidant potency of these two compounds under these conditions. Like ascorbic acid, α-tocopherol inhibited Acr-dG formation directly from Acr only when its concentration reached 1000 μM. However, its blocking effect was much weaker compared with that of ascorbic acid. This observation again suggests that while α-tocopherol can block the reaction of Acr with dG, at lower concentrations it primarily acts as an antioxidant. Although DHLA and EGCG have also been reported to possess prooxidant activity under certain conditions (23, 28, 29), they did not show a biphasic effect in the concentration range we studied. Taken together, our results underscore the complexity in the mechanisms of these agents as antioxidants to suppress lipid-peroxidation induced DNA damage.
Although the exact mechanism by which DHA generates Acr under oxidative conditions is not fully understood, one of the first steps appears to involve the formation of several mono and polyhydroperoxides of DHA (30). In the presence of iron, fatty acid hydroperoxides are capable of initiating the formation of enals and other reactive species such as epoxides (31). It is conceivable that antioxidants could decrease DNA damage by several mechanisms, including blocking one or more steps during fatty acid oxidation, scavenging reactive oxygen species and aldehydes and chelating iron. EGCG and DHLA are known to chelate metal ions and ascorbic acid has been shown to bind to Acr (22, 23, 24). It was also reported that EGCG effectively binds to and removes α, β-unsaturated aldehydes like Acr and 4-hydroxynonenal as conjugates (32). Furthermore, a notable difference in our reactions with DHA vs. Acr was the formation of stereoisomers of Acr-dG; the former yielded predominantly γ-OH-Acr-dG, while the latter produced an almost equal amount of both α and γ isomers. The reason for the preferential formation of γ stereoisomer with DHA is presently unclear. It is plausible that this stereoselectivity is due to the different buffers used in these reactions because it has been shown that Tris buffer has a profound effect on dG adducts formed by malondialdehyde (33).
We used 25 μM FeSO4 in our reactions to mimic the concentrations in human plasma (34,35). We did not use H2O2 because studies in cells enriched with DHA showed that iron-oxygen complexes are more potent than H2O2 in initiating free radical-mediated oxidation (36). Although a chemical model system was used in this study, it may be of interest to compare the concentrations of antioxidants to those found in human plasma. It should be noted that the EGCG concentrations used in our study are considerably higher than those found in human plasma after drinking green tea (37). However, ascorbic acid, α-tocopherol and lipoic acid levels in plasma after supplementation were comparable to the lower concentrations that we used (38-40). Studies have shown that concentrations of ascorbic acid and α-tocopherol could be higher in tissues than in plasma (41, 42). Therefore, the antioxidant concentrations used in the present study may be considered relevant to those found in cells or tissues. Cells are adapted to protect against reactive oxygen species through endogenous antioxidants like GSH, glutathione peroxidase, superoxide dismutase and catalase. Evidence shows that these endogenous antioxidants act in concert with dietary antioxidants to protect DNA and other macromolecules from damage (43). Although our model studies do not provide information on the intricate interplay of exogenous and endogenous antioxidants and other dietary substances, they do generate useful and potentially important data regarding the effects of antioxidants on two major types of endogenous DNA damages induced by lipid peroxidation. Like other in vitro studies, caution needs to be taken in extrapolating the results to cells or tissues. Nevertheless, we have shown for the first time how these widely used antioxidants in their distinct manners affect the cyclic Acr-dG adduct formation from DHA or Acr, a ubiquitous environmental pollutant. Because EGCG and DHLA are effective inhibitors against both Acr-dG and 8-oxodG formation over a wide range of concentration, studies in vivo using these two compounds seem warranted.
Acknowledgments
This study was supported by National Cancer Institute (NCI) grant CA043159. We thank Dr. Chi-Tang Ho of Rutgers University for providing EGCG.
References
- 1.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Refsgaard HHF, Tsai L, Stadtman ER. Modifications of proteins by polyunsaturated fatty acid peroxidation products. Proc Natl Acad Sci U S A. 2000;97:611–616. doi: 10.1073/pnas.97.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair J, De Flora S, Izzotti A, Bartsch H. Lipid peroxidation-derived etheno-DNA adducts in human atherosclerotic lesions. Mutat Res. 2007;621:95–105. doi: 10.1016/j.mrfmmm.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181-182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 6.Esterbauer J, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 8.Nath RG, Chung FL. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc Natl Acad Sci U S A. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem Res Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, et al. Naturally occurring carbonyl compounds are mutagens in salmonella tester strain TA104. Mutat Res. 1985;148:25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 12.Foiles PG, Akerkar SA, Chung FL. Application of an immunoassay for cyclic acrolein deoxyguanosine adducts to assess their formation in DNA of Salmonella typhimurium under conditions of mutation induction by acrolein. Carcinogenesis. 1989;10:87–90. doi: 10.1093/carcin/10.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Foiles PG, Akerkar SA, Miglietta LM, Chung FL. Formation of cyclic deoxyguanosine adducts in Chinese hamster ovary cells by acrolein and crotonaldehyde. Carcinogenesis. 1990;11:2059–2061. doi: 10.1093/carcin/11.11.2059. [DOI] [PubMed] [Google Scholar]
- 14.Yang IY, Chan G, Miller H, Huang Y, Torres MC, et al. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 15.Yang IY, Hossain M, Miller H, Khullar S, Johnson F, et al. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J Biol Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 16.VanderVeen LA, Hashim MF, Nechev LV, Harris TM, Harris CM, et al. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J Biol Chem. 2001;12:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- 18.Khan MA, Travis LB, Lynch CF, Soini Y, Hruszkewycz AM, et al. p53 mutations in cyclophosphamide-associated bladder cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:397–403. [PubMed] [Google Scholar]
- 19.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, et al. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Nath RG, Ocando JE, Guttenplan JB, Chung FL. 1,N2-Propanodeoxyguanosine adducts: potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998;58:581–584. [PubMed] [Google Scholar]
- 21.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 23.Moini H, Packer L, Saris NEL. Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 24.Fodor G, Arnold R, Mohacsi T, Karle I, Flippen-Anderson J. A new role for L-ascorbic acid: Michael donor to α,β-unsaturated carbonyl compounds. Tetrahedron. 1983;39:2137–2145. [Google Scholar]
- 25.Park JW, Floyd RA. Lipid peroxidation products mediate the formation of 8-hydroxydeoxyguanosine in DNA. Free Radic Biol Med. 1992;12:245–250. doi: 10.1016/0891-5849(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 26.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Niki E. Interaction of α-tocopherol with iron: antioxidant and prooxidant effects of α-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim Biophys Acta. 1988;958:19–23. doi: 10.1016/0005-2760(88)90241-x. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (-)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem Pharmacol. 2003;66:1769–78. doi: 10.1016/s0006-2952(03)00541-0. [DOI] [PubMed] [Google Scholar]
- 29.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Lyberg AM, Adlercreutz P. Monitoring monohydroperoxides in docosahexaenoic acid using high-performance liquid chromatography. Lipids. 2006;41:67–76. doi: 10.1007/s11745-006-5072-z. [DOI] [PubMed] [Google Scholar]
- 31.Chen HJC, Chung FL. Epoxidation of trans-4-hydroxy-2-nonenal by fatty acid hydroperoxides and hydrogen peroxide. Chem Res Toxicol. 1996;9:306–312. doi: 10.1021/tx9501389. [DOI] [PubMed] [Google Scholar]
- 32.Beretta G, Furlanetto S, Regazzoni L, Zarrella M, Facino RM. Quenching of alpha,beta-unsaturated aldehydes by green tea polyphenols: HPLC-ESI-MS/MS studies. Pharm Biomed Anal. 2008;48:606–11. doi: 10.1016/j.jpba.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Niedernhofer LJ, Riley M, Schnetz-Boutaud N, Sanduwaran G, Chaudhary AK, et al. Temperature-dependent formation of a conjugate between tris(hydroxymethyl)aminomethane buffer and the malondialdehyde-DNA adduct pyrimidopurinone. Chem Res Toxicol. 1997;10:556–561. doi: 10.1021/tx960191c. [DOI] [PubMed] [Google Scholar]
- 34.Pruchnicki MC, Coyle JD, Hoshaw-Woodard S, Bay WH. Effect of phosphate binders on supplemental iron absorption in healthy subjects. J Clin Pharmacol. 2002;42:1171–1176. doi: 10.1177/009127002401382669. [DOI] [PubMed] [Google Scholar]
- 35.Najean Y, Castro-Malaspina H, Colonna P, Dresch C. The influence of circadian variations in plasma iron on the measure of plasma iron turnover. Eur J Nucl Med. 1977;2:189–191. doi: 10.1007/BF00257279. [DOI] [PubMed] [Google Scholar]
- 36.Schafer FQ, Qian SY, Buettner GR. Iron and free radical oxidations in cell membranes. Cell Mol Biol. 2000;46:657–662. [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 38.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatam LJ, Kayden HJ. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res. 1979;20:639–645. [PubMed] [Google Scholar]
- 40.Teichert J, Tuemmers T, Achenbach H, Preiss C, Hermann R, et al. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J Clin Pharmacol. 2005;45:313–328. doi: 10.1177/0091270004270792. [DOI] [PubMed] [Google Scholar]
- 41.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr, Wang Y, et al. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14:1133–1139. doi: 10.1023/a:1012186203165. [DOI] [PubMed] [Google Scholar]
- 42.Blatt DH, Leonard SW, Traber MG. Vitamin E kinetics and the function of tocopherol regulatory proteins. Nutrition. 2001;17:799–805. doi: 10.1016/s0899-9007(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 43.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]