Abstract
Hand, foot and mouth disease (HFMD) is a highly contagious viral infection characterized by typical maculopapular or vesicular eruptions on the hands and feet and in the oral cavity. It affects predominantly children and/or immunocompromised adults. It usually follows a benign and self-limiting course. However, HFMD cases with severe or lethal complications such as encephalitis, meningitis, pulmonary edema and myocarditis have also been reported, mostly in children, but also in adults. High infectivity of HFMD has contributed to several large outbreaks of this disease in recent decades in East and Southeast Asia, the United States and Finland. The most common pathogens were Coxsackievirus A16, Enterovirus 71 and, recently, also Coxsackieviruses A6 and A10. Differences in the course of HFMD have been observed, depending on the virus type. Recently, many cases of atypical HFMD have been described in the literature with unusual morphology and/or localization of skin lesions. Atypical HFMD manifestations including vesiculobullous exanthema, often on the trunk or extremities, and perioral zone involvement were often caused by Coxsackievirus A6 infections. We present 3 cases of familial transmission of HFMD caused by Coxsackievirus A6 with some atypical features, benign course and complete recovery among immunocompetent adults.
Key Words: Hand, foot and mouth disease; Coxsackievirus A6; Enterovirus; Immunocompetent adult; Onychomadesis
Introduction
Hand, foot and mouth disease (HFMD) is a highly contagious viral infection that affects predominantly children. HFMD typically occurs in summer and early autumn. The disease has an incubation period of 3–6 days. The prodromal symptoms include low-grade fever, malaise and sore throat. This initial phase is usually followed by enanthem and erythematous, papular or vesicular skin lesions, localized predominantly on palms and soles. Less commonly the lateral and dorsal surface of hands and feet, and perioral skin can be affected [1].
Since HFMD is predominantly a childhood or immunodeficiency-associated disease, few cases have been reported so far in immunocompetent adults [2]. In the present paper, we describe 3 cases of HFMD associated with Coxsackievirus A6, with a familial route of transmission, benign course and complete recovery among immunocompetent adults.
Case Reports
Family 1
Case 1: A 35-year-old man, computer technician, with irrelevant past medical history, was admitted to our outpatient clinic at the beginning of October 2012 with a 2-day history of papular and vesicular skin rash. He complained of fever with chills, malaise and sore throat 1 day before the onset of cutaneous symptoms. Moreover, the patient reported close contact with a child suffering from HFMD, during a family party, 1 week before symptoms developed.
Physical examination revealed erythematous papulovesicular lesions on the posterior oropharyngeal structures and multiple erythematous papules followed by vesicular lesions on his palms, soles and perioral zone (fig. 1a, b, fig. 2a). A complete blood count revealed no abnormalities. The erythrocyte sedimentation rate was increased to 20 mm/h. Blood chemistry tests were normal, except for the level of C-reactive protein (CRP), which was elevated to 12 mg/l. Syphilis, HIV and hepatitis B and C serologies were negative. The pattern of serum protein electrophoresis was normal, and the initial chest X-ray and ECG demonstrated no pathologic findings.
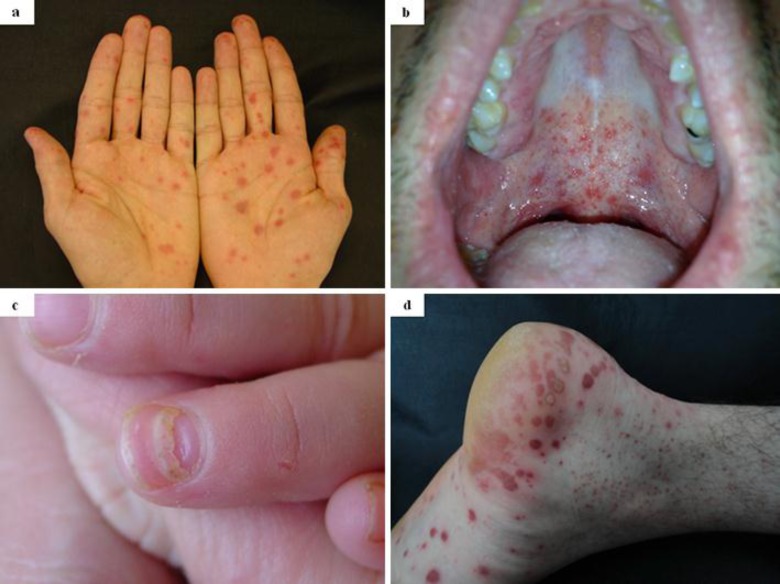
Fig. 1.
a Family 1, case 1: erythematous, papular lesions of the palms mimicking erythema multiforme or secondary syphilis. b Family 1, case 1: papular enanthem of the oral cavity. c Family 1, the child: onychomadesis of a fingernail 2 months after Coxsackievirus A6 infection. d Family 2, case 3: erythematous, papulovesicular lesions of the right foot.
Fig. 2.
a Family 1, case 1: impetigo-like clinical picture with perioral, erythematous, papulovesicular and scaly skin lesions. b Family 2, case 3: erythema multiforme-like clinical picture with perioral and perinasal, erythematous papular and vesiculobullous lesions.
Viral ribonucleic acid was extracted from vesicular fluid samples (both from the throat and skin) and from plasma. Afterwards, it was amplified with reverse transcription-seminested polymerase chain reaction (PCR) assay as described elsewhere [3], using primers that allow amplification of all known human enteroviruses and identification of the serotypes by amplicon sequencing. The primers used for the seminested step amplified a 376-bp portion of the capsid viral protein 1 (VP1) gene. The amplicon sequencing and comparison to gene database identified Coxsackievirus A6, both from vesicles and plasma.
Histological examination of the skin biopsy from a palmar papule showed orthokeratotic hyperkeratosis with areas of confluent parakeratosis and presence of singular basal and suprabasal keratinocyte necrosis. Moreover, a lymphocytic inflammatory infiltrate was present in the superficial dermis up to dermo-epidermal junction. The histological features were consistent with a viral infection.
The patient was diagnosed with HFMD based on characteristic clinical manifestations, positive results of enterovirus PCR analysis, molecular typing for Coxsackievirus A6 and histological findings. Symptomatic treatment with oral paracetamol and lidocaine oral spray was introduced. During a 7-month follow-up, no complications were observed. Skin and mucosal lesions resolved completely. No nail changes have been observed so far, but the child who infected the patient with Coxsackievirus A6 developed onychomadesis 2 months later (fig. 1c).
Case 2: A 37-year-old woman, sister of the patient described above, clothing store manager, with an unremarkable past medical history, was admitted to our outpatient clinic in the middle of October 2012, with a 1-day history of characteristic palm and sole rash. The symptoms were preceded by a sore throat and low-grade fever. Ten days before the onset of the first symptoms, she had contact with her brother, who at the time of the meeting was asymptomatic. The day after, the male patient developed symptoms of HFMD. He was infected by his niece suffering from HFMD 1 week before the siblings’ meeting. The female patient was absent from the family party, where the child suffering from HFMD was present. Physical examination revealed singular erythematous macular and papular lesions on the patient's soles and palms. The oral cavity was spared. No other diagnostic exam was executed due to the patient's refusal. Considering characteristic clinical manifestations and recent contact with a person suffering from HFMD, the diagnosis of HFMD was made. No treatment was prescribed as the patient did not complain of any disturbances. Complete resolution of the disease was observed within 2 weeks.
Family 2
Case 3: A 21-year-old man, student, with irrelevant medical history, was admitted to our Dermatology Department in April 2013 in compromised physical condition, with a 2-day deteriorating papular and vesicular skin rash accompanied by a 3-day high fever, chills and malaise. Two weeks before developing the symptoms, during a family meeting, the patient had contact with a niece suffering from HFMD. Physical examination showed numerous erythematous papular and vesiculobullous lesions spread throughout the perioral and perinasal zones, oral cavity, on his palms and soles, as well as on the distal lower extremities, up to the knees (fig. 1d, fig. 2b). A complete blood count revealed lymphocytopenia (1.29 g/l), and the erythrocyte sedimentation rate was increased to 26 mm/h. Blood chemistry tests showed an elevated CRP level (56 mg/l). Syphilis, HIV, Epstein-Barr virus, cytomegalovirus, measles and rubella serologies were negative. The PCR assay confirmed Coxsackievirus A6 both in plasma and in plantar vesicle fluid. Only symptomatic treatment was introduced. After a 3-day hospitalization, the patient's conditions as well as inflammatory parameters improved and he was discharged. After 2 weeks, all the lesions resolved completely. During a 2-month follow-up, no complications were observed.
Discussion
The term HFMD derives from typical maculopapular or vesicular lesions involving the skin of the hands, feet and oral mucosa. The prodromal phase including low-grade fever, malaise and sore throat is commonly observed. In adults, the clinical picture of the mucocutaneous lesions can mimic erythema multiforme or other infectious diseases, such as syphilis. In the majority of cases, HFMD presents a benign and self-limiting course. The skin lesions usually resolve within 1–2 weeks with no residual scars. Enanthem usually precedes the onset of the skin rash. Additionally, oral lesions may appear in the absence of cutaneous symptoms. Here, we describe a case of HFMD without apparent mucosal lesions at the time of the physical examination (case 2). Similar cases of HFMD without oral involvement have rarely been reported in the literature [2]. During recent outbreaks of HFMD in Spain and Taiwan, caused by Coxsackievirus A6, the disease had a broader spectrum of manifestations [4]. In addition to the typical papulovesicular rash on the palms and soles and in the oral cavity, skin lesions were also present on the buttocks, trunk and perioral zone. Some lesions were vesiculobullous, bigger and present on different sites of the body. Both our male patients infected with Coxsackievirus A6 presented numerous papulovesicular lesions in the perioral zone. Moreover, the case 3 patient had similar but bigger lesions also on the lower extremities, up to his knees. To our knowledge, this is the first published report of atypical HFMD in adults caused by Coxsackievirus A6. The exact pathogenesis of this atypical clinical manifestation has not been revealed so far. It is not known whether Coxsackievirus A6 might have transformed into a more virulent strain. However, these uncommon manifestations may be helpful for early diagnosis of Coxsackievirus A6 infection and can sometimes spare unnecessary and expensive molecular analysis.
The association between onychomadesis and HFMD was proposed for the first time by Clementz and Mancini [5] in 2000 and Bernier et al. [6] in 2001. The exact pathomechanism of nail shedding has not been elucidated so far. This spontaneous separation of the nail plate from the matrix, starting at the proximal edge has been observed in some cases of HFMD, 1–2 months after the onset of the disease. The nail changes were temporary, with subsequent nail regrowth within 1–4 months. Although there are some publications concerning onychomadesis related to Coxsackievirus A6 infection, no clear correlation between virus type and HFMD-related nail changes has been demonstrated [7]. So far, none of our patients has presented the above nail changes, but the child who infected the case 1 patient with Coxsackievirus A6 presented onychomadesis 2 months after the onset of HFMD.
High infectivity of HFMD has contributed to several large outbreaks of this disease which occurred in the last 2 decades in East and Southeast Asia, the United States and Finland [8, 9, 10]. During these outbreaks, most of the cases of HFMD followed a benign and self-limiting course. However, HFMD cases with severe or lethal complications such as encephalitis, meningitis, pulmonary edema and myocarditis, requiring hospitalization and in some cases extracorporeal life support techniques, have been reported, mostly in children, but also in adults [7, 11].
The causative agents of HFMD include a non-polio enterovirus genus (family Picornaviridae). Within this genus, Coxsackievirus A16, Enterovirus 71 and, recently, also Coxsackieviruses A6 and A10 are the most common pathogens of HFMD [8, 12]. Genetic typing, in order to distinguish the exact virus strain, is usually not necessary to confirm the HFMD diagnosis. Nonetheless, in some cases of HFMD information on the exact type of the virus is crucial for appropriate disease management and for reliable assessment of the risk of potential complications. In the literature, significant differences are reported in the course of HFMD depending on the pathogen; a significantly greater frequency of fatalities and serious complications of HFMD (especially pulmonary and neurological) was observed in association with Enterovirus 71 when compared to Coxsackievirus A16 [13]. Hence, it is of great importance to develop rapid and reliable diagnostic methods in order to differentiate the exact type of HFMD virus.
Treatment of HFMD is usually symptomatic and does not influence the course of the infection. Children and immunocompromised adults are the most susceptible to HFMD. Other at-risk categories include elderly people and pregnant women. To our knowledge, only a few HFMD cases have been described in the literature in immunocompetent adults [2, 8, 14]. Disease transmission among family members occurs commonly and involves fecal-oral and/or respiratory routes [2]. Children are usually infected by asymptomatic or mildly symptomatic adults. However, in our case, close contact with mildly symptomatic children suffering from HFMD initiated a strong, symptomatic infection in the immunocompetent adults (cases 1 and 3). One of the adults transmitted the disease to another immunocompetent adult (case 2). The literature shows many cases of child-to-child or/and adult-to-child HFMD household transmission [15]. To our knowledge, this is the first report of symptomatic HFMD transmission among immunocompetent adults.
Conclusion
Being a highly contagious disease with rare but severe complications, especially in small children, the elderly or immunocompromised people, early and accurate diagnosis of HFMD is crucial. HFMD associated with Coxsackievirus A6 may have an atypical skin presentation with facial involvement and vesiculobullous lesions on the body. Hence, in order to prevent HFMD from spreading and to recognize in time the possible complications, all dermatologists should have a profound knowledge of this disease and should be aware that it can also occur in immunocompetent adults.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Miller GD, Tindall JP. Hand-foot-and-mouth disease. JAMA. 1968;203:827–830. [PubMed] [Google Scholar]
- 2.Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol. 2010;22:216–218. doi: 10.5021/ad.2010.22.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang WC, Huang LM, Lu CY, Cheng AL, Chang LY. Atypical hand-foot-mouth disease in children: a hospital-based prospective cohort study. Virol J. 2013;10:209. doi: 10.1186/1743-422X-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7–11. doi: 10.1046/j.1525-1470.2000.01702.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernier V, Labrèze C, Bury F, Taïeb A. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649–651. doi: 10.1007/s004310100815. [DOI] [PubMed] [Google Scholar]
- 7.Jiang M, Wei D, Ou WL, Li KX, Luo DZ, Li YQ, Chen E, Nong GM. Autopsy findings in children with hand, foot, and mouth disease. N Engl J Med. 2012;367:91–92. doi: 10.1056/NEJMc1110981. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6 – Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213–214. [PubMed] [Google Scholar]
- 9.Lin TY, Twu SJ, Ho MS, Chang LY, Lee CY. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg Infect Dis. 2003;9:291–293. doi: 10.3201/eid0903.020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485–1488. doi: 10.3201/eid1509.090438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 12.Blomqvist S, Klemola P, Kaijalainen S, Paananen A, Simonen ML, Vuorinen T, Roivainen M. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49–54. doi: 10.1016/j.jcv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JP, Jr, Baden L, Pallansch MA, Anderson LJ. Enterovirus 71 infections and neurologic disease – United States, 1977–1991. J Infect Dis. 1994;169:905–908. doi: 10.1093/infdis/169.4.905. [DOI] [PubMed] [Google Scholar]
- 14.Shea YF, Chan CY, Hung IF, Chan KH. Hand, foot and mouth disease in an immunocompetent adult due to Coxsackievirus A6. Hong Kong Med J. 2013;19:262–264. doi: 10.12809/hkmj133692. [DOI] [PubMed] [Google Scholar]
- 15.Chang LY, Tsao KC, Hsia SH, Shih SR, Huang CG, Chan WK, Hsu KH, Fang TY, Huang YC, Lin TY. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA. 2004;291:222–227. doi: 10.1001/jama.291.2.222. [DOI] [PubMed] [Google Scholar]