Abstract
Purpose
To evaluate the efficacy of one intravitreal injection of dexamethasone (Ozurdex®; Allergan, Inc., Irvine, Calif., USA) in serous macular detachment (SMD) of one eye, associated with bilateral central retinal vein occlusion (CRVO) in a patient affected by Waldenström's macroglobulinemia (WM).
Patients and Methods
A female patient, affected by WM, complained of a progressive decrease in visual acuity, mainly in the left eye (LE). SMD in the LE associated with bilateral CRVO was diagnosed. One intravitreal injection of dexamethasone was administered in the LE and the patient was tested 1, 2, and 6 months after the injection.
Results
1, 2, and 6 months after the injection, the spectral domain optical coherence tomography (SD-OCT) showed a progressive slight reduction of foveal thickness that was not related to any improvement of visual function.
Conclusions
Treatment with dexamethasone (Ozurdex) induced a progressive slight reduction of SMD but no improvement of visual acuity, and it is possible that this is related to the condition of hematic hyperviscosity that is present in WM.
Key words: Waldenström's macroglobulinemia, Serous macular detachment, Intravitreal injection, Dexamethasone
Introduction
The World Health Organization defines Waldenström's macroglobulinemia (WM) as a lymphoplasmacytic lymphoma associated with a monoclonal immunoglobulin M (IgM) protein [1].
The ocular manifestations of hyperviscosity in WM can include central retinal vein occlusion (CRVO) that represents an uncommon complication of WM [2]. Irreversible optic nerve dysfunction and acute visual loss in CRVO, which is normally due to severe macular edema, are other uncommon complications [3]. Bilateral CRVO is an uncommon feature [4, 5].
Case Presentation
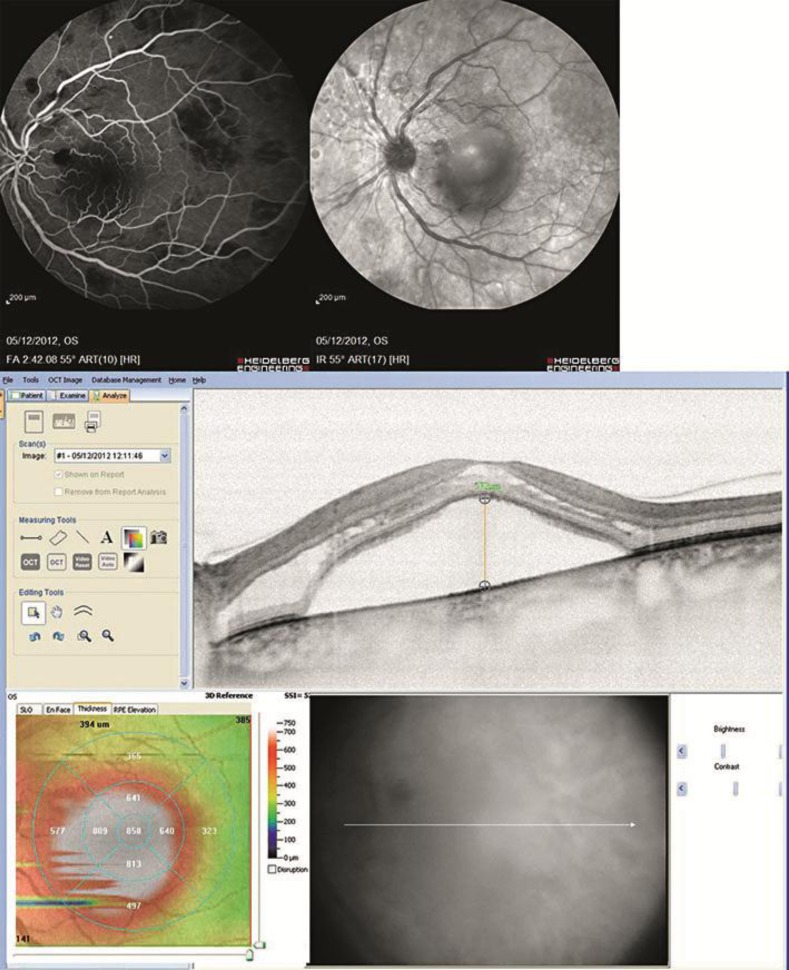
A female patient, aged 52 years, affected by WM since December 2010, came to our Department in November 2012 complaining of a decrease in visual acuity and floating vision in both eyes, which was more severe in the left eye, for the duration of about 1 month. The patient had been treated with systemic corticosteroid and was currently undergoing chemotherapy with Rituxan® (rituximab) and Velcade® (bortezomib). At the time of the visit, the best corrected visual acuity (BCVA) was 20/20 in the right eye and 20/30 in the left eye. At the slit lamp examination, the anterior segment was normal in both eyes, with no opacity of the lens and/or any signs of ocular inflammation. The intraocular pressure at Goldmann tonometry was 14 mm Hg in both eyes. A bilateral CRVO was diagnosed and confirmed by fluorescein angiography (FAG) and spectral domain optical coherence tomography (SD-OCT). FAG showed a bilateral CRVO with macular edema, complicated by serous macular detachment (SMD) in the left eye. SD-OCT (Cross Line, MM5, 3D Macular, RTVue® SD-OCT) showed a foveal thickness of 349 µm in the right eye and 858 µm in the left eye (fig. 1).
Fig. 1.
FAG, infrared reflectance, and SD-OCT of the left eye before treatment (foveal thickness 858 µm).
The left eye was treated with an intravitreal injection of dexamethasone (Ozurdex®; Allergan, Inc., Irvine, Calif., USA) 3 days after the diagnosis. Ozurdex is a biodegradable copolymer of lactic acid and glycolic acid containing micronized dexamethasone, which forms a matrix structure able to gradually release the total dose over time after the injection [6]. The most common local side effect can be an increase of intraocular pressure and cataract [7].
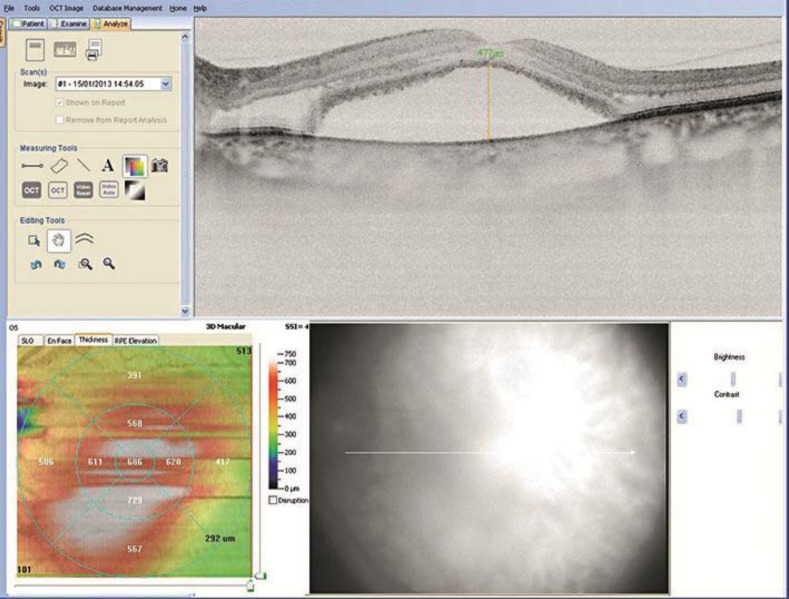
One month after the injection, the SD-OCT of the left eye showed a slight reduction of foveal thickness (686 µm) that was not related to any improvement of visual function (fig. 2).
Fig. 2.
SD-OCT of the left eye 1 month after Ozurdex (foveal thickness 686 µm).
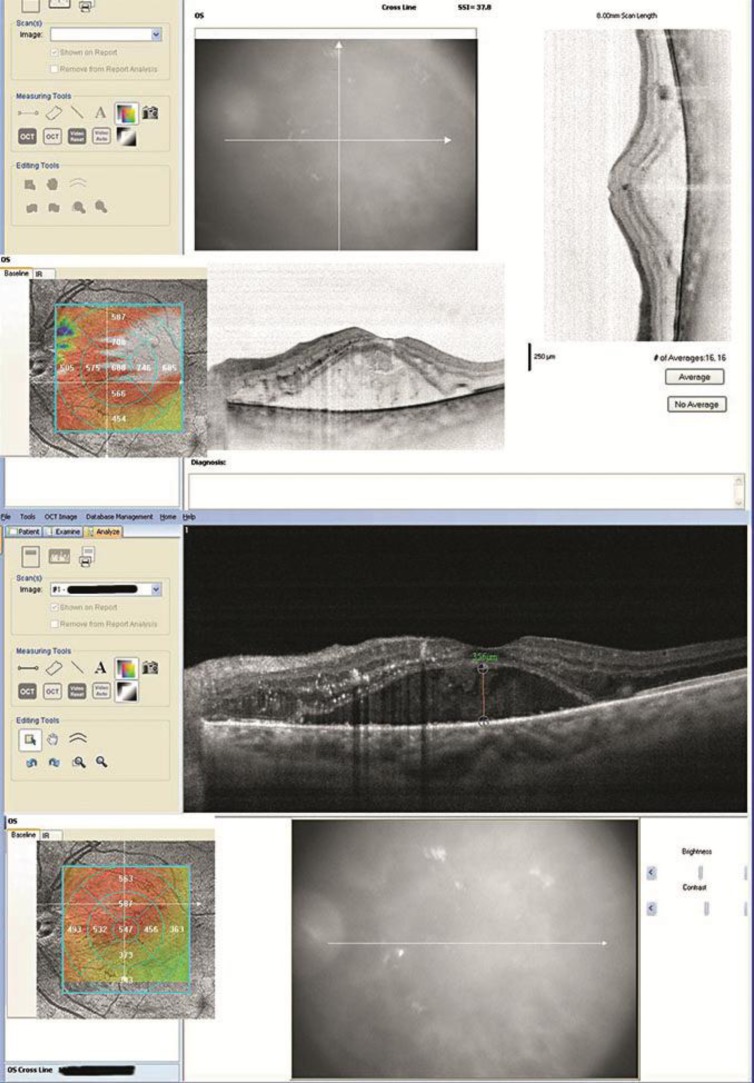
At 2 and 6 months after the injection, the SD-OCT showed a further mild reduction of foveal thickness that was 680 and 547 µm, respectively (fig. 3).
Fig. 3.
SD-OCT of the left eye 2 months (upper panel) and 6 months (lower panel) after Ozurdex (foveal thickness 680 µm and 547 µm, respectively).
This proves that there was a partial response to the injection of Ozurdex, although a complete reduction of foveal thickness is never achieved, unlike the usual response in non-WM-related CRVO [8].
At the time of the follow-ups, 1, 2, and 6 months after the injection, BCVA remained stable at 20/30, intraocular pressure was 14 mm Hg, and the patient did not show any sign of opacity of the lens or ocular inflammation.
It was not possible to repeat the FAG because the patient's general health condition deteriorated over time.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the consent is available for review.
Discussion
After one injection of Ozurdex, a progressive partial anatomical resolution of SMD associated with CRVO in WM is evident, although it is not related to any improvement of BCVA and is less pronounced than the resolution usually obtained in CRVO of treated patients that are not affected by WM [9]. This result is in accordance with a recent case report of a 65-year-old male patient affected by WM with a similar SMD that persisted despite repeated intravitreal injections of bevacizumab and corticosteroid [10].
In addition, 6 months after the injection of Ozurdex we did not notice any side effect such as an increase in intraocular pressure and cataract, other papers show instead cases of such side effect [11].
It is possible that the condition of hyperviscosity associated with WM, which causes retinopathy similar to retinal vein occlusion but with normal retinal blood flow [12], is an obstacle to any ocular intravitreal treatment of macular complications.
To improve the efficacy of treatment with dexamethasone implant in WM, a plasmapheresis could be considered. Recent articles showed that plasmapheresis in WM patients leads to a reduction of serum viscosity and IgM level, together with an improvement of retinopathy and normalization of retinal hemodynamics [13, 14] as evidenced by a reduction of the diameter of the retinal vein vessels [15].
Disclosure Statement
The authors have no commercial or other conflict of interest to declare.
References
- 1.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenström's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Avashia JH, Fath DF. Bilateral central retinal vein occlusion in Waldenström's macroglobulinemia. J Am Optom Assoc. 1989;60:657–658. [PubMed] [Google Scholar]
- 3.Fadilah SA, Muhaya M, Azlin I. Irreversible visual loss and optic nerve dysfunction associated with central retinal vein occlusion in Waldenström's macroglobulinemia. Med J Malaysia. 2007;62:349–351. [PubMed] [Google Scholar]
- 4.Casares PZ, Gillet DS, Verity DH, Rowson NR. Bilateral simultaneous central retinal vein occlusion (CRVO) caused by Waldenström's macroglobulinaemia with acquired Von Willebrand's disease. Br J Haematol. 2002;118:344–347. doi: 10.1046/j.1365-2141.2002.03576_1.x. [DOI] [PubMed] [Google Scholar]
- 5.Nabet L, Dufier JL, Cornu P, Junghers P, Chauvin JC, de Monteynard MS, et al. Bilateral occlusion of the central vein of the retina disclosing Waldenström's disease. Bull Soc Ophthalmol Fr. 1989;89:39–41. [PubMed] [Google Scholar]
- 6.Querques L, Querques G, Lattanzio R, Gigante SR, Del Turco C, Corradetti G, Cascavilla ML, Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica. 2013;229:21–25. doi: 10.1159/000342160. [DOI] [PubMed] [Google Scholar]
- 7.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28:351–366. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 8.Moisseiev E, Goldstein M, Waisbourd M, Barak A, Loewenstein A. Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond) 2013;27:65–71. doi: 10.1038/eye.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Besirli CG, Johnson MW: Immunogammopathy maculopathy associated with Waldenstrom macroglobulinemia is refractory to conventional interventions for macular edema. Retinal Cases and Brief Reports 2013, DOI: 10.1097/ICB.0b013e31828ef0dc. [DOI] [PubMed]
- 11.Meyer LM, Schönfeld CL: Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-up. J Ocul Pharmacol Ther 2013, E-pub ahead of print. [DOI] [PubMed]
- 12.Menke MN, Feke GT, McMeel JW, Treon SP. Effect of plasmapheresis on hyperviscosity-related retinopathy and retinal hemodynamics in patients with Waldenström's macroglobulinemia. Invest Ophthalmol Vis Sci. 2008;49:1157–1160. doi: 10.1167/iovs.07-1254. [DOI] [PubMed] [Google Scholar]
- 13.Alexander P, Flanagan D, Rege K, Foss A, Hingorani M. Bilateral simultaneous central retinal vein occlusion secondary to hyperviscosity in Waldenström's macroglobulinaemia. Eye (Lond) 2008;22:1089–1092. doi: 10.1038/eye.2008.193. [DOI] [PubMed] [Google Scholar]
- 14.Menke MN, Feke GT, McMeel JW, Branagan A, Hunter Z, Treon SP. Hyperviscosity-related retinopathy in Waldenström's macroglobulinemia. Arch Ophthalmol. 2006;124:1601–1606. doi: 10.1001/archopht.124.11.1601. [DOI] [PubMed] [Google Scholar]
- 15.Menke MN, Feke GT, McMeel JW, Treon SP. Ophthalmologic techniques to assess the severity of hyperviscosity syndrome and the effect of plasmapheresis in patients with Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9:100–103. doi: 10.3816/CLM.2009.n.027. [DOI] [PubMed] [Google Scholar]