Abstract
Drug molecules with lack of specificity and solubility lead patients to take high doses of the drug to achieve sufficient therapeutic effects. This is a leading cause of adverse drug reactions, particularly for drugs with narrow therapeutic window or cytotoxic chemotherapeutics. To address these problems, there are various functional biocompatible drug carriers available in the market, which can deliver therapeutic agents to the target site in a controlled manner. Among the carriers developed thus far, mesoporous materials emerged as a promising candidate that can deliver a variety of drug molecules in a controllable and sustainable manner. In particular, mesoporous silica nanoparticles are widely used as a delivery reagent because silica possesses favourable chemical properties, thermal stability and biocompatibility. Currently, sol-gel-derived mesoporous silica nanoparticles in soft conditions are of main interest due to simplicity in production and modification and the capacity to maintain function of bioactive agents. The unique mesoporous structure of silica facilitates effective loading of drugs and their subsequent controlled release. The properties of mesopores, including pore size and porosity as well as the surface properties, can be altered depending on additives used to fabricate mesoporous silica nanoparticles. Active surface enables functionalisation to modify surface properties and link therapeutic molecules. The tuneable mesopore structure and modifiable surface of mesoporous silica nanoparticle allow incorporation of various classes of drug molecules and controlled delivery to the target sites. This review aims to present the state of knowledge of currently available drug delivery system and identify properties of an ideal drug carrier for specific application, focusing on mesoporous silica nanoparticles.
Keywords: Mesoporous silica nanoparticle, targeted drug delivery, controlled release, sol-gel process, chemotherapy
Introduction
Drugs are essential components in our lives in terms of eliciting a therapeutic outcome in various disease states. Due to ageing population, the consumption of drugs as well as the amount of reported side effects has consistently increased from 1980.1,2 There are various reasons for the occurrence of side effects such as practice error,3 impaired homeostasis in elderly4 or lack of compliance due to polypharmacy.1 Aside from these, the fundamental cause of adverse drug reactions can be due to the properties of the drug molecule itself. Lack of specificity of a drug molecule will lead to high-dosage regimen and ultimately cause undesired interactions of a drug with healthy tissues or cells. To attenuate this, dose reduction is required, but sub-concentration and lack of therapeutic efficacy can be another issue. This issue is often confronted when using a drug with a narrow therapeutic window, and chemotherapeutics.5–7 For example, vancomycin requires a high-loading dose to have an acquired effectiveness, but it was observed that more than 4 g of it can cause severe nephrotoxicity.6 In addition, there are few drugs which cannot be administered through its ideal route due to its hydrophobicity, such as camptothecin7 and paclitaxel.8 Thus, achieving effective concentration of therapeutic agents at its target site is not always feasible.
To address these problems, it is important to develop functional biocompatible drug carriers. During the last three decades, various polymeric devices have been fabricated for the delivery of a variety of drugs or bioactive agents.9 These devices include, for example, parenteral depot systems,10 microspheres,11,12 nanoparticles13 and implants.14,15 For instance, antibiotics,16 osteoconductive proteins17 and peptides10 have been incorporated into polymeric devices to treat the infection and speed up the recovery of injured bone. The release of the drug or bioactive agent incorporated into these devices can be tailored to allow both targeted (e.g. for the treatment of tumour) as well as sustained (varies from days up to years) release.18,19 In both cases, controlled therapeutic concentrations of the drug would be released in the local vicinity of the delivery device, thereby reducing possible systemic side effects or undesired interactions of the drug with healthy tissues and cells as well as avoiding unnecessary drug metabolism or inactivation that would reduce its effectiveness. The controlled release of the drug has been mainly controlled by controlling the molecular weight of the polymeric device20 and/or the drug-loading content.21 When the device is used purely as a drug delivery carrier, the polymer must not interact with the drug, and the completeness of device degradation must coincide with the end of the drug release.18
Altering drug chemical constituents or developing pro-drug is another way to reduce side effects, but altering physicochemical properties of drug molecule can distort its effectiveness, and its complex preparation, high cost and less stable formulation restrict its appliance.8,22 Thus, delivering biomolecules to the specific site by a controlled drug delivery system is considered as an ideal way to improve quality use of medicine by reducing dose and frequency of drug intake,23–25 taking into account that effective drug release rates and durations require careful assessment of target site pharmacokinetics, drug delivery vehicle design, the selection of clinically effective drug according to the clinical context, effective dosage and drug release kinetics requirements.26
As one of the most promising nanocarriers, mesoporous silica nanoparticles (MSNs) are reviewed here focusing on their physicochemical properties and targeting drug delivery applications.
Currently available drug delivery systems
The high or frequent dosing, systemic absorption in unrelated sites and suboptimal concentration of bioactive agents in target site contribute to the restriction in accessibility of therapeutic agents. By developing drug delivery systems, the function of drugs can be significantly improved which could also render huge economical benefits. For example, Wong et al.22 estimated that US$8 billion could be saved by only developing more effective drug delivery systems for hydrophobic drugs. Thus, many studies investigated different forms of drug delivery vehicles, and the most popular systems are listed in Table 1.
Table 1.
Different types of drug delivery system.
| Drug delivery system | Structure | Chemical properties | References |
|---|---|---|---|
| Liposomes |  |
Consists of hydrophobic tail and hydrophilic head group | 27–29 |
| Forms closed vesicles with an aqueous core | |||
| Internal aqueous domain between the lipid bilayers | |||
| Encapsulation of drugs occurs either in the aqueous space or intercalated into the bilayer | |||
| Dendrimers |  |
Hyper branched and globular macromolecules | 30–32 |
| Well defined core, backbone and multivalent periphery | |||
| By hydrophobic and electrostatic interactions, incorporate biomolecules | |||
| Convergent – endo-receptor | |||
| Divergent – exo-receptor | |||
| Carbon nanotubes |  |
Rolling up a single layer of grapheme sheet – single walled | 33–37 |
| Rolling up many layers to form concentric cylinders – multi-walled | |||
| Gold nanoparticles |  |
Gold nanoparticle serves as core | 8,38 |
| Photosensitive | |||
| Iron oxide nanoparticles |  |
Superparamagnetic particles | 8,39 |
| Need trigger to release biomolecules, for example, laser irradiation | |||
| Titanium dioxide nanoparticles |  |
Self-ordered | 40–42 |
| Nano-tubular structure | |||
| Photodynamic therapy | |||
| Silica nanoparticles |  |
Mesoporous structure | 7, 23, 25 |
| Honeycomb-like structure | |||
| Active surface |
The listed drug carriers have different physicochemical properties which make them suitable for different drugs. The common goal of the carrier is to transport drug molecules to the target site in a controlled manner. Ideally, they should be biocompatible, not cause any immunogenic or cellular reactions and release drug molecule controllably at the target sites without altering its therapeutic effects.25
Liposomes
Liposome is a spherical self-closed structure made up of one or several lipid bilayer(s), that is, similar to cell membrane, surrounding an inner hollow aqueous core. The drug molecules can be loaded within the lipid bilayer or in the aqueous core or at the interface between them. Since the lipid is an essential biomolecule for most living tissues and has an amphiphilic nature, that is, ability to spontaneously self-assemble into a variety of microstructures, liposome is widely used as a temperature or pH-sensitive drug delivery vehicle particularly for cytotoxic anti-cancer drugs. Being amphiphilic, liposomes have hydrophilic head and hydrophobic tail, and in the presence of either polar or non-polar liquid, lipids can be self-assembled into different microstructures.43 Hydrophobic drugs, for example, amphotericin B, taxol or annamycin, can be passively incorporated into liposomes with 100% trapping efficiencies. For hydrophilic drugs, however, active loading is required to get this level of entrapment.44 Furthermore, the drug encapsulated in liposomes can be transported to the target site without rapid degradation and minimum side effect. Liposomes also have a unique ability to deliver the entrapped drug into cells by fusion or endocytosis, and therefore, any drug can be loaded into the liposome regardless of its solubility.11,45 As shown in Table 2, liposomes have comparably low-drug-loading and sustained release capacity.46 Thus, liposomes generally require additional functionalisation such as polyethylene glycol (PEG) to provide long-term stability and to address problems related to short shelf-life, poor solubility and rapid clearance.28 PEG is hydrophilic, biocompatible and non-toxic, which can delay hydrolysis or enzymolysis.29 However, PEG can hinder the binding of the liposome to the delivery site.27
Table 2.
Comparison between liposome and nanoparticle.46
| Nanosystem | Smallest size | Drug loading | Sustained release | Targeting | In vivo stability | Biocompatibility | Low cost/complexity |
|---|---|---|---|---|---|---|---|
| Liposome | + | + | + | ++ | + | +++ | ++ |
| Nanoparticle | ++ | ++ | +++ | ++ | ++ | ++ | +++ |
: low; ++: moderate; high: +++.
Dendrimers
Dendrimers can be utilised in various areas such as material science, catalysis and drug delivery.30 This versatility is related to its branched structure with multiple targeting.32 Also, due to its distinctive structure, it can selectively host biomolecules and deliver them to the target sites.31 For example, the most common type of dendrimer is polyamidoamine dendrimers which can selectively host methotrexate.47 However, the toxicity of dendrimers has been of concern.32 The non-degradable dendrimers produced side effects with repeated administration.30,47 Thus, the modification of cationic dendrimers is essential to prevent its accumulation in the liver and to inhibit nonspecific toxicity.32 Due to their low biocompatibility,46 change in chemical compositions is required, such as polyester-based dendrimer.31,47
Carbon nanotubes
Carbon nanotubes (CNTs) are low dimensional sp2 carbon nanomaterials, and their flexibility is produced by their various physicochemical properties35 that can be used in the transportation of various therapeutic agents such as vaccine, protein, antibiotics37 and anti-cancer and anti-inflammatory agents.34 However, the insolubility of CNTs can pose health complications.33–36 For example, CNTs without functionalisation can accumulate in the lungs, which leads to pulmonary toxicity and inflammation.35 This perniciousness is highly dependent on material preparation and administration route of CNTs. As with liposomes and dendrimers, a biocompatible coating such as PEGlyation can remarkably reduce in vivo toxicity of CNTs.36
Gold and iron oxide nanoparticles
Gold and iron oxide are widely used in controlled drug release, especially in anti-cancer therapy. They are mostly used in combination with other biomolecules. For example, magnetic iron oxide provides the core of the particle, while the shell is composed of silica, dextran or gold attached via cross-linkers.48 The advantage of using gold nanoparticle is that it can release drug molecule in a controlled manner by absorbing heat and increasing kinetic energy to release drug molecules. Similarly, controlled release of drug molecule is possible with iron oxide under the influence of an external magnetic field. This can ultimately reduce dose and systemic absorption of cytotoxic drugs by guiding them to the target tumour cells.39 However, in real practice, there are many parameters to be considered such as magnetic properties, field strength and field geometry, depth of target, blood flow, body weight and vascular supply.49 For gold nanoparticles, the accumulation and excretion profiles are not well understood,8 and the accumulation within bloodstream can block blood flow. Also, the cost of gold nanomaterials needs to be considered. Iron oxide needs surface functionalisation due to poor solubility.8
Titanium dioxide
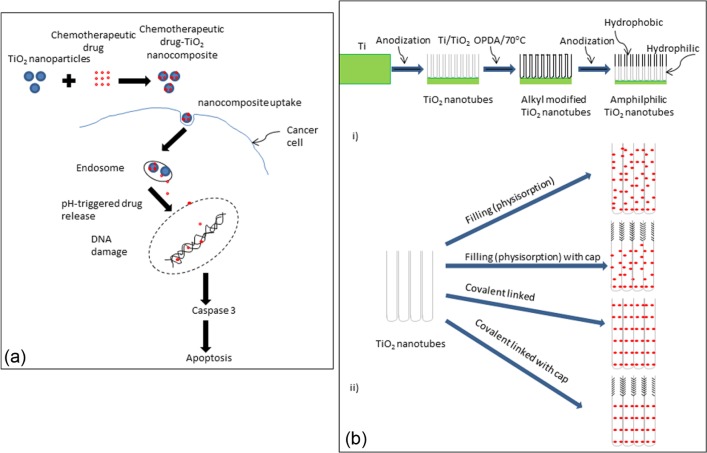
Micro- and nanoporous titanium dioxide (TiO2) film, applied on the surface of titanium implant using micro-arc oxidation and anodic titanium oxide treatments, respectively, has been employed as a container for antibiotic-loaded sol-gel-derived silica xerogel. The presence of micro- and nanoporous TiO2 film enhanced the drug-loading efficiency of sol-gel-derived silica xerogel and provided controlled release of antibiotic.50 TiO2 is also a potential photosensitiser, which can catalyse DNA damage; the release of drugs or active molecules can be triggered by ultraviolet light or X-ray radiation.51 TiO2 is chemically inert and is ideal for use in chemotherapy as it can inhibit tumour growth.42 Recently, the development of ‘smart’ pH-responsive drug delivery vehicle based on TiO2 nanoparticles for intelligent and enhanced delivery of chemotherapeutic drug has been attempted. The ‘smart’ TiO2 nanoparticles only release the anti-cancer drug under acidic pH, that is, in the vicinity of the tumour tissue, and this is a desirable characteristic for tumour-targeted drug delivery22,52 (see Figure 1(a)).
Figure 1.
Diagrammatic presentation showing (a) the chemotherapeutic agent release from ‘smart’ pH-responsive TiO2 nanoparticles (adopted from Zhang et al.2) and (b) (i) the fabrication process of TiO2 nanotube and (ii) different methods of drug loading into TiO2 nanotubes using HRP, involving immersion without surface modification of nanotubes (physisorption), immersion after OPDA modification of the upper nanotube layer (physisorption with hydrophobic cap), covalently linked HRP over the entire tubes (covalently linked nanotubes) and OPDA cap modified upper nanotube layer and HRP covalently linked lower nanotube layer (covalently linked with cap) (adopted from Song et al.5).
HRP: horseradish peroxidise; OPDA: octadecylphosphonic acid.
Self-organised, highly ordered TiO2 nanotubes with regular hollow structure and large surface area provided both initial burst and sustained release of drug.53 Amphiphilic TiO2 nanotubes have also been fabricated by a double anodisation method combined with organic monolayer grafting (see Figure 1(b)). These amphiphilic nanotubes could be used as ‘capped’ biomolecule vehicles with loading efficiency of ~4.4 × 10−11 nmol/tube; the presence of amphiphilic characteristics with the hydrophobic site outside counteracts nonspecific protein adsorption.33,54
Due to its photocatalytic activity, TiO2 nanoparticles in combination with photodynamic therapy (PDT) could be employed as a novel therapeutic strategy for the treatment of tumours, for example, glioma.51 Photoexcited TiO2 nanoparticles have strong oxidation and reduction activity, and then, they could drive various chemical changes.55 Photoexcited TiO2 nanoparticles damage DNA of the tumour cells through inflammation and generation of reactive oxygen species such as hydrogen peroxide and free hydroxyl radicals.41 It has been also observed that ultrasound irradiation (i.e. sonodynamic therapy (SDT)) of TiO2 nanoparticles was effective in producing OH radicals that induce apoptosis of cancer cells.56
TiO2, however, has potential to pose health problems such as respiratory tract cancer,57,58 as long-term exposure to TiO2 nanoparticles can induce pulmonary inflammation and cause lung tumour.
MSNs
Encouraged by the exciting discovery of the new family of molecular sieves generally called M41S in the early 1990s,59 MSNs have emerged as a promising drug vehicle, primarily due to their unique mesopore structure that while preserving a level of chemical stability, surface functionality and biocompatibility ensures the controlled release of a variety of drug molecules.
In fact, silica is widespread in living nature, from single-celled organisms to higher plants, which can be used for various purposes.12 Compared to other metal oxides such as titania and iron oxide, silica is considered to have better biocompatibility60 and can be safely taken up by living cells through endocytosis.61,62 The abundant presence of silanol groups in silica can have an affinity to phospholipids, which can be actively taken up by the cells.63 Additionally, its active surface property allows developing MSN with various surface properties through surface functionalisation with different molecule, which consequently allows targeted delivery of different types of therapeutic agents. This will be further explained later in this review. Due to its strong Si–O bond,63 silica nanoparticles are more stable to external stimuli such as mechanical stress and degradation compared to liposomes and dendrimers, eliminating the need for any additional stabilisation such as covalent linkers used in other delivery systems.25,64
The mesoporous form of silica has unique properties, particularly in loading of therapeutic agents at high quantities and in the subsequent releases.64,65 The mesopore structure such as pore size and porosity can be tuned to the size and type of drugs.64 Another distinctive advantage of MSNs is that they have well-defined surface properties that allow easy functionalisation of the silanol-containing surface to control drug loading and release.23,61–63,66–70 The surface functionalisation is generally needed to load proper type of drug molecules (hydrophobic/hydrophilic or positive/negative charged), specific actions can also be endowed by the functionalisation through chemical links with other materials such as stimuli-responsive, luminescent or capping materials, leading to smart and multifunctional properties.64,66,71,72
Sol-gel processing of MSNs
Generally, a simple process called ‘sol-gel process’ is used to produce MSNs with controlled mesopore structure and surface properties (Figure 2). This procedure is not multi-step and does not require many excipients. Due to its simplicity, a low-cost synthesis procedure is achievable.70,73–75 There are two main stages in the sol-gel process: hydrolysis and condensation reactions. In aqueous solution, colloidal particles are produced through hydrolysis, which can be stimulated at acidic or alkaline pH. In contrast, neutral pH accelerates the condensation reaction, which creates gel-like three-dimensional (3D) network by cross-linking sol particles through siloxane bonds. The condensation reaction is reversible, so the silica can be restructured easily. After drying at ambient temperature, the different biomolecules can be embedded in the matrix of silica gel and controllably released, depending on structure and porosity of MSN. The first reported material, mobile crystalline material-41 (MCM-41), is micrometre-sized without a well-defined shape containing hexagonally ordered channels. Recently, several sol-gel methods have been developed to control the morphology (spheres, rods, twisted columns and kidney bean-shaped) and size (60–1000 nm).76,77 Different pore structures and porosity of MSN are possible by modulating parameters such as pH, temperature, raw materials, solvents, catalysts, precursor and additives in different concentrations.23,61
Figure 2.
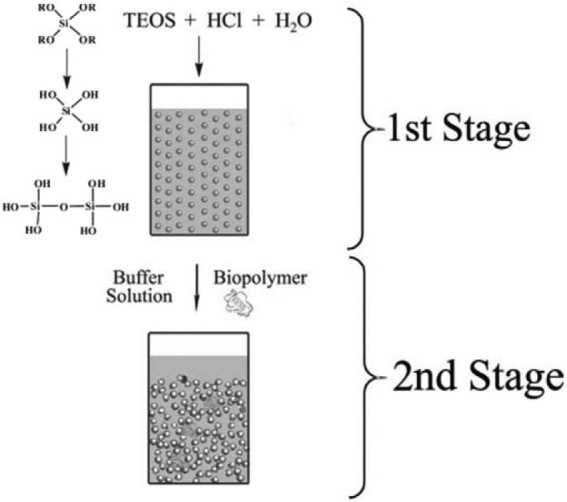
Illustration of the sol-gel process in the synthesis of MSN.73
MSN: mesoporous silica nanoparticle; TEOS: tetraethyl orthosilicate.
The MSNs with different particle sizes and pore structures exploited thus far are given in Table 3. The following section will cover silica precursors and additives as well as the effect of temperature as the main parameters required during MSN production.
Table 3.
Overview of MSNs exploited to have different particle size and pore structure.
| Silica source | Surfactant | Particle size (TEM, nm) | Pore structure | Synthesis pH condition | Size control strategy | Surfactant removal method | Reference |
|---|---|---|---|---|---|---|---|
| TEOS | C16TAB | 60–100 | Ordered 2D hexagonal | Basic (NaOH) | Quench by water dilution and neutralisation | Calcination | 78 |
| TEOS | C16TAB | 100–2500 | Ordered 2D hexagonal | Basic (NaOH) | Dilute conditions | Calcination | 79 |
| TEOS | C16TAB | 200–250 | Ordered 2D hexagonal | Basic (NaOH) | Dilute conditions | Ethanolic acid extraction | 80 |
| TEOS | C16TAB | 30–280 | Ordered 2D hexagonal | Basic (NH4OH) | Dilute conditions and NH4OH concentration adjustment | Ethanolic acid extraction | 81 |
| TEOS | C16TAB | 100–300 | Disordered | Neutral | Propanetriol as co-surfactant and co-solvent | Ethanolic acid extraction | 82 |
| TMOS | C16TAB | <20 | Disordered | In basic (TEA) | Ratio of C16TAB/Si | Dialysis in ethanol/acetic acid solution | 83 |
| TEOS and APTES | C16TAB | 100–220 | Ordered 3D cubic | Basic (NH4OH) | Dye incorporation and pore expander addition | Ethanolic acid extraction | 84 |
| TEOS | C16TAB | 42 | Ordered 2D hexagonal | Basic (NH4OH) | Dilute condition and PEG modification with hydrothermal treatment | Ethanolic acid/salt extraction | 85 |
| TEOS | C16TAB, n-dodecylamin | 60–740 | Ordered 2D hexagonal | Basic (NH4OH) | Dilute and co-solvent conditions | Calcination | 86 |
| Sodium silicate | CnTAX (n = 14, 16, 18; X = Br, Cl) | 30–70 | Disordered | Basic (NaOH) | low concentration of CnTMAX-sodium silicate | Calcination | 87 |
| TEOS | C16TAC | 20–500 | Ordered 2D hexagonal | Basic (NH4OH) | Pluronic F127 controls particle growth | Calcination | 88 |
| TMOS 18) | CnTAC (n = 14, 16,18) | 150–860 | Ordered 2D hexagonal | Basic (NaOH) | Co-solvent/water ratio | Calcination | 89 |
| TEOS | Pluronic F127, P65, P123 and F108 | 100–300 | Ordered 3D cubic | Acidic (HCl) | Fluorocarbon surfactant suppresses particle growth | Calcination | 90 |
| TEOS | C16TAC | 45–150 | Worm like | Basic (TEA) | TEOS/TEA ratio | Ethanolic extraction | 91 |
| TEOS P123 | Pluronic | 50–300 | Ordered 2D hexagonal | Acidic (HCl) | H2O amount and salt addition | Calcination | 92 |
MSN: mesoporous silica nanoparticle; TEOS: tetraethyl orthosilicate; TEM: transmission electron microscopy; TMOS: tetramethoxysilane; APTES: (3-aminopropyl)triethoxysilane; TEA: tetraethylammonium; PEG: polyethylene glycol; 2D: two-dimensional; 3D: three-dimensional.
Silica precursor
Organically modified precursors are not susceptible to hydrolysis because an organic group is linked directly to a silicon atom, which does not need oxygen bridge.73 It is conceded that organo-silica nanoparticles consist of better properties, including large surface area, less condensed siloxane structure and low density.93,94 However, they are only appropriate for particular cases.73 The limited accessibility and high cost of organic template lead to its restricted use in practical applications.75 Examples of silica precursors which are commonly used and accordingly will be discussed in this review include glycerol-derived polyol-based silanes, orthosilicic acid, sodium metasilicate, tetraethyl orthosilicate (TEOS) or tetramethoxysilane (TMOS) and tetrakis (2-hydroxyethyl) orthosilicate (THEOS).
Glycerol-derived polyol-based silane precursors are not pH dependent but very sensitive to the ionic strength of the sol. This can accordingly form optically clear, monolithic mesoporous silica. The residuals can be either removed or retained; therefore, the shrinkage during long-term storage can be minimised.95 However, Shchipunov’s73 study stated that it has high viscous solution, complicated procedure and insufficient porosity. This was confirmed by Brook et al.’s95 study, and these factors limit its usage in real practice. Orthosilicic acid was used as a silica precursor in the past, but due to the extensive time consumption and requirement of freshly prepared acid, it is not widely used anymore.73
Sodium metasilicate is another precursor to sol-gel-derived silica. Formation of sodium chloride was investigated, which can pose a problem if significant amount is generated. Latter researches suggested removing this salt formulation by dialysis, but this is a time- and cost-consuming procedure. Due to this reason, alkoxides, pure alkoxysilanes, are currently used broadly.73,93
TEOS or TMOS was commonly used in MSN synthesis. However, their poor water solubility requires additional organic solvent and alcohol and needs extreme conditions of pH and high temperature, which restricts their use.73,96,97
THEOS had been investigated to address the problems associated with TEOS and TMOS. It is now used in many studies as MSN precursor because it is more biocompatible with biopolymers and more water soluble than TEOS and TMOS, and can process jellification at ambient temperature with a catalyst.51–56,59–74,97,98
Additives
The careful selection of additives is paramount to produce MSNs with desired characteristics for drug delivery. The commonly used additives are listed in Table 4.
Table 4.
Common chemical constituents used in the synthesis of MSN.
| Substrate | Function | References |
|---|---|---|
| N-dodecanoyl-β-alanine | Surfactant with an amino acid residue | 73 |
| Self-assemble into fibrils in aqueous solution | ||
| Tween-80 | Surfactant | 62 |
| CTAB | Surfactant | 63,98 |
| Increase water solubility of hydrophobic ligand | ||
| Structure-directing agent | ||
| PEO | Detergent and phase separation | 62,95 |
| Induce hydration | ||
| PEO/sol ratio regulates pore size | ||
| Poly ethylene glycol | Improve biocompatibility | 73 |
| Improve functional characteristics of silica matrix | ||
| Poly vinyl alcohol | Settle gel down in THEOS-containing solution | 73 |
| Hydrogen fluoride | Catalyst | 96,97 |
| Sodium hydroxide | Catalyst | 67 |
| Hydrogen chloride | Catalyst | 67, 70,96 |
| Ammonium nitrate | Surfactant removal | 63 |
| Trihydroxysilylpropyl methylphosphate | Surface agents | 63 |
| Prevent inter-place aggregation | ||
| Methanol | Solvent in TMOS | 67,97 |
| Remove surfactant | ||
| Ethanol | Solvent in TEOS | 97 |
| Hexane | Solvent | 66 |
| Water | Solvent | 66 |
| Non-ionic triblock copolymer | Structure-directing agent | 66 |
CTAB: N-cetyltrimethylammonium bromide; PEO: polyethylene oxide; MSN: mesoporous silica nanoparticle; TEOS: tetraethyl orthosilicate; TMOS: tetramethoxysilane; THEOS: tetrakis (2-hydroxyethyl) orthosilicate.
The two main chemical components used in MSN production are a surfactant and a catalyst. Surfactant is usually required when the synthesis takes place at an ambient temperature. For example, if temperature is under 25°C and pH is around 6, fibrous aggregates can be formed, which requires surfactants. Increasing temperature or shifting pH can disintegrate these aggregates but may also damage the incorporated therapeutic agents.97 Additionally, using an appropriate surfactant is crucial as it may optimise the function of drug loading and release by a complex interaction between drug molecules and matrix.62,73 Depending on the chain length of the surfactant, different pore sizes can be obtained.66 For example, surfactants such as hexadecyltrimethylammonium bromide (C16TAB) and dodecyltrimethylammonium bromide (C12TAB) are important in controlling pore size, particle morphology and thus loading dose.61,64,99 The longer chain length of C16TAB used in MCM-41 increased pore size and released high dose of therapeutic agent, ibuprofen (68%), than shorter chain length of C12TAB, which only released 55% of ibuprofen.66 Similarly, the addition of surfactant, N-cetyltrimethylammonium bromide (CTAB), prolonged the release of cancer agents, which enhanced the effects of cytotoxicity.100 Also, structure-directing ability of CTAB can speed up the process of synthesis.69 In order to incorporate drug molecules into the pores, this surfactant needs to be removed from mesopores by mixing with appropriate solvent under high vacuum.63,67
Catalyst is another fundamental variable responsible for mesopores channel formation. As hydrolysis and condensation are dependent on pH, addition of hydrochloride or sodium dioxide can catalyse or inhibit different reactions.70 For example, by adding hydrogen chloride, the pH will decline, and degree of particle repulsion will also decline accordingly. This leads to siloxane cross-linkage formation between the nanoparticles.24,70
Specific additives used in different biomolecules entrapments are significant because the porosity can be tuned selectively by adding different additives and manipulating synthesis conditions like pH. This produces unique ordered mesopore channel structure, and physical characteristics of MSNs.23,25,61,62,64,73
Temperature
Traditionally, MSNs are produced at high temperature, and there are some problems associated with it. High temperature (≥100°C) in calcination or spray drying can alter the textural properties of MSN and damage biomolecules.60,62 Spray drying after sol-gel process can drastically change the properties of sol-gels, which can even result in non-porous structure.24 At high temperatures, the shrinkage of mesopores is significant, which results in inability to control morphology of MSN,25,75 and the template cannot be recovered or re-used, which results in economic disadvantages. It can even secrete noxious gases, which can cause environmental problems.25,75
Thus, recent MSN synthesis procedures were carried out at mild and ambient conditions in aqueous media. At ambient conditions, sol-gel-derived MSN has better composition range and bioactivity than melt-derived MSN. For example, high-loading dose and greater surface area, porosity and functionality in bone bonding rate are achievable by sol-gel process at ambient condition.62 Liong et al.’s.63 study conceded that optimal temperature range is 65°C–80°C, which will produce spherical particles of diameter in the range 100–200 nm with minimal loss of biopolymers from vaporisation.101 Even at a low temperature, chemical reactions can take place easily by using appropriate chemical components and that homogenous suspension can be achieved in a short time at a molecular level.101
Properties of MSNs
Different parameters of MSN fabrication contribute to different delivering mechanisms of active agents. The parameters that control the kinetics of drug release from MSN are outlined in Table 5.
Table 5.
Summary of different factors that regulate controlled release of MSN.99
| Adsorption | Release | |||
|---|---|---|---|---|
| Host–guest interactions and controlled adsorption and release kinetics | Textural properties | Mesopore diameter | Size selectivity | Rate modulator |
| Surface area | Enhanced adsorption | |||
| Mesopores volume | Higher drug loading | |||
| Chemical properties | Surface functionalisation | Allow loading | Slow down | |
| Increase loading |
MSNs: mesoporous silica nanoparticles.
Textual properties
The size of the drug delivery carrier is an important determinant which can be divided into three scales: macro, micro and nano. ‘Macro’-sized delivering agents are used to transport biomolecules to organs, whereas ‘micro’-scaled carriers target tissue delivery. With respect to intracellular drug delivery, the large-sized carrier is limited, as it cannot be engulfed by mammalian cells via endocytosis, which may ultimately cause accumulation of drug vehicle. Also, it was shown that larger sized materials are more likely to trigger an acute immune response in vivo, as it is within the size window of bacteria.46 Therefore, particle size in micrometer range is unfavourable in drug delivery.94 In biomedical applications, ‘nano’-scaled delivery carriers should be employed in order to deliver therapeutic agents at a cellular level such as in the cell membrane, cytoplasm or nucleus by facile endocytosis.23,25,46,66
As particle size increases, the efficiency of uptake by the cell decreases.65 From Table 6, it can be concluded that the diameter of MSN can be tuned controllably in the range 20–500 nm. It was stated that particle size between 50 and 300 nm can be engulfed by living animal cells without causing any cytotoxicity, while MSN of diameter <300 nm is desirable from a biomedical point of view.25,60 Slowing et al.’s65 study conceded that particle size around 200 nm or smaller will have highest efficiency and particle size larger than 1000 nm will cause little uptake. Similarly, another study confirmed that nanoparticles below 200 nm will induce endocytosis,102 whereas nanoparticles with larger size may be internalised by phagocytosis or not internalised at all.105
Table 6.
Textural properties of MSNs.
| Diameter | Surface area | Pore volume | Pore size | Reference |
|---|---|---|---|---|
| 50–300 nm | >900 m2/g | >0.9 cm3/g | 2–6 nm | 25 |
| 20–500 nm | 2–6 nm | 60 | ||
| 180 nm | 61 | |||
| 50–100 nm | > 1000 m2/g | ~1 cm3/g | 91 | |
| >700 m2/g | >1 cm3/g | 2–10 nm | 102 | |
| 55–440 nm | > 800 m2/g | 2–10 nm | 103 | |
| > 1000 m2/g | ~1 cm3/g | 104 |
MSNs: mesoporous silica nanoparticles.
The term ‘mesoporous’ refers to the sizes between 2 and 50 nm.106 The pore size can be tuned selectively with a narrow distribution65 between 2 and 6 nm in diameter with pore volume of around 1 cm3/g, depending on the type of drug molecules that will be incorporated.25,61 Larger pore size is suitable to load a high dose of drug molecules.65 In addition, large surface area allows the high adsorption of therapeutic agents on the surface of MSN, which is related to high-loading dose of therapeutic agents.25,91,104
Internal structure
The internal mesopore structure of MSNs is the most relevant and fascinating property. Mesopores are not randomly distributed, but rather specifically aligned and structured presenting honeycomb-like structures with hundreds of empty channels. The channels are considered individual reservoir of drugs without interconnections between channels.25 The internal structure of the MSNs, including size, volume and aligned structure of mesopores, can be controlled by the initial reagents or the surfactant. Several reviews have discussed this aspect of MSNs in detail.76,107,108 Apart from this, the most commonly used MSNs (MCM-41, MCM-48, Santa Barbara–type mesoporous particle-15 (SBA-15), SBA-16) are summarised in Table 7.
Table 7.
Different types of MSNs with different pore diameter and internal structure.
| Type | Pore diameter | Internal structure | Reference |
|---|---|---|---|
| MCM-41 | 1.5–3.5 nm | 2D hexagonal | 105 |
| MCM-41 | 3.70 nm | Hexagonal structure with unidimensional pore structure | 106 |
| MCM-41 | 2–5 nm | 2D hexagonal | 109 |
| SBA-15 | 6.0–10.0 nm | 2D hexagonal | 105 |
| SBA-15 | 7.80 nm | 2D hexagonal | 106 |
| SBA-15 | 5–10 nm | 2D hexagonal | 110 |
| SBA-16 | 4–9 nm | 3D-cubic cage like | 105 |
| MCM-48 | 2.5–3.0 nm | 3D cubic | 105 |
| MCM-48 | 3.49 nm | A cubic structure with a 3D pore system | 106 |
MSNs: mesoporous silica nanoparticles; MCM: mobile crystalline material; SBA: Santa Barbara–type mesoporous particle; 2D: two-dimensional; 3D: three-dimensional.
Surface functionalisation
MSNs possess well-defined structure and high density of surface silanol groups, which can be modified with a wide range of organic functional group.111 The surface functional groups can play several roles in biomedical applications of MSNs: (a) to control the surface charge of MSNs, (b) to chemically link with functional molecules inside or outside the pores and (c) to control the size of pore entrance for entrapping molecules in the nanopores. There are three methods of surface functionalisation for MSNs: co-condensation, post-synthesis grafting and surfactant displacement methods. In the one-pot co-condensation process, organosilanes are added directly in the synthesising gel solution together with a silica source.112 Then, the surfactant molecules can be removed by ion exchange with an ethanolic solution of ammonium nitrate.113,114 The advantages of co-condensation include simple operation, uniformity in distribution of functionalisation and achievable high loading. Controlling surface charge allows the effective and selective loading of drugs at high quantity. Along with charge property, hydrophobic surface treatment using chemical groups such as phenyl reduces wettability of MSNs so that aqueous medium will not easily penetrate into the mesopores, delaying the drug release from the matrix.66 Length of molecules to be linked to the mesopore surface can determine the pore entrance size, leading to selective-sized drug loading. More promising aspect of the surface functionalisation is to link specific molecules that have special functionality, such as stimuli-responsiveness, fluorescence imaging and capping/blocking the pore entrance to allow on-demand smart actions of drug delivery, diagnosis-assisted therapeutics and sustainable release of loaded drugs. These unique properties of MSNs can facilitate hosting of various drug molecules and delivering them to the target site in a controllable and sustainable manner.64
Drug delivery applications of MSNs
Controlled release of therapeutics to the specific site over an extended period of time is an important determinant in delivery of biologically active agents. This local delivery will reduce potential side effects as well as maximise therapeutic effects by obtaining the optimal concentration of biologically active agents in local sites.23,24,64 The release of bioactive molecules from MSN is dependent on the size of pores, which can be controlled by the processing parameters as we have previously described. The controlled release of biomolecules is available through narrow mesoporous channels and nanoporous structure, which allows kinetics of drug release to be carried out with high precision.24,61 For example, in Radin et al.’s24 study, controlled release of bioactive agents such as antibiotics, proteins and growth factor was observed through MSN channels. In addition, in order to prevent premature release, capping systems have been developed such as cadmium sulphide (CdS) cap (Figure 3), which will be detailed later.
Figure 3.
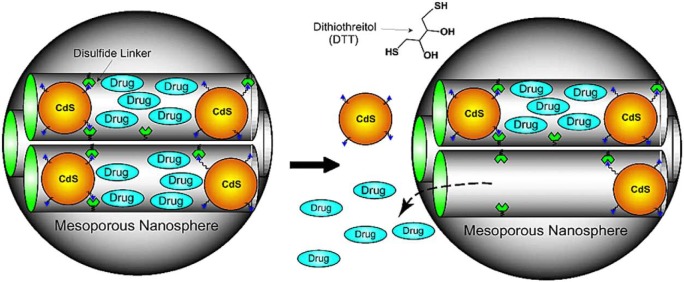
MSNs showing capping structure useful for controlled release.80
CdS: cadmium sulphide.
Because of the tuneable intrinsic properties as well as the flexibility in functionality, MSNs have been applied in delivering various types of therapeutic molecules, and the examples studied with most representative forms of MSNs are summarised in Table 8.
Table 8.
Different classes of drugs delivered by MSNs.
| Classes | Drug | Delivery vehicles | Ref |
|---|---|---|---|
| Anti-inflammatory | Ibuprofen | MCM-41 | 23,99 |
| Ibuprofen | SBA-15 | 23,99 | |
| Naproxen | MCM-41 | 99 | |
| Naproxen | Amine-modified MCM-41 | 99 | |
| Naproxen | SBA-15 | 99 | |
| Antibiotics | Amoxicillin | Si-SBA-15 | 23 |
| Erythromycin | SBA-15 | 23 | |
| Erythromycin | Octadecyl-functionalised SBA-15 | 23 | |
| Vancomycin | CdS-capped MCM-41 | 80 | |
| Osteogenic | Alendronate | MCM-41 | 69,115 |
| Alendronate | Amine-modified MCM-41 | 69,115 | |
| Alendronate | SBA-15 with phosphorus | 93,116 | |
| Chemotherapy | Camptothecin | Galactose-functionalised MSN | 109 |
| Doxorubicin | Folic-acid conjugated MSN | 67 | |
| Doxorubicin | DOX-hydrazone-MSN-FA | 67 |
MSNs: mesoporous silica nanoparticles; MCM: mobile crystalline material; SBA: Santa Barbara–type mesoporous; CdS: cadmium sulphide; FA: folate.
Anti-inflammatory
There are two main conventional mesoporous silica materials: MCM-41 and SBA-15. MCM-41 and SBA-15 have hexagonal one-dimensional (1D) channel internal structure but have different pore sizes, 2–5 nm and 5–10 nm, respectively (Table 7). Normally, as the pore size decreases, the delivery rate decreases accordingly, and smaller pore size stabilises drug molecules from hydrolysis.23,99 However, due to other factors, faster ibuprofen release rate was observed in MCM-41 compared to SBA-15. There is high potential for fluids to penetrate inside the mesochannels in MCM-41 compared to SBA-15 due to its lower pore wall thickness with larger external surfaces and lower intensity of drug assembly.23,99 Consequently, due to enhanced wettability of MCM-41 and higher number of pores on the surface, larger and faster release rate of ibuprofen was investigated in MCM-41 than SBA-15.23
Further functional development in these conventional structures has been achieved by the addition of functional groups on MSNs’ active surface. In MCM-41 or SBA-15, mesoporous channel walls only consist of silanol groups, which can form weak intermolecular hydrogen bonds with drug molecules, and this is not sufficient for controlled and sustained release of drug molecules.23 The substitution of amino-group will increase the interaction between amine groups and acidic groups of ibuprofen, facilitating the slower release of ibuprofen.99 Thus, controlled and prolonged ibuprofen release over a period of time was observed with amine-modified MCM-41.102
Similarly, higher amount of naproxen release was observed in the amine-modified sample than unmodified MCM-41 due to its immobilisation of drug on the external surface. In addition, due to the additional interaction between amine group and naproxen, sustained release of naproxen was observed in amine-modified MCM-41.99
Osteogenesis
Alendronate belongs to the biphosphate family, which is used for bone repair and regeneration. An increase in adsorption of alendronate was observed from 1% to 40% by local delivery. Similar to anti-inflammatory agents, amino-modified MCM-41 illustrated three times higher loading capacity due to chemical interactions between phosphate groups in alendronate with the silanol and amino groups of MSN.69,115
SBA-15 with phosphorus also illustrated enhanced alendronate loading capability and had better sustained release kinetics compared to conventional SBA-15 due to increased interaction between PO4 units and host molecule. MSN is not only delivering bioactive molecules, but it also can enhance osteoconductivity by slight minimal changes in composition.117 This is often referred as ‘bioglass’ and is composed of CaO-SiO2P2O5 with mesopore channels and used in bioactive bone grafts application.69,118 Both CaO and P2O5 can have an effect on mesopore morphology, and this enables it to achieve higher surface area and pore volume.69 They are bioactive as they develop an apatite-like layer on their surfaces. Thus, chemically modified SBA-15 can exert dual effects as they deliver therapeutic agents as a local treatment of bone disease and simultaneously enhance bone resorption, and this is ideal in tissue engineering biotechnology.116
Antibiotics
In order to prevent resistance and achieve sufficient therapeutic effects, controlled and targeted release is important in antibiotics usage. Different morphologies of MSN have different release kinetics. It was observed that hexagonal internal structure releases amoxicillin slower than disk form.75,95 Also faster release rate of amoxicillin was observed in powder compared to disk formulation.119
Figure 3 illustrates CdS cap, which had been applied in vancomycin. Due to this covalently capped CdS, premature release of vancomycin was so negligible that less than 1.0% of premature release was investigated. This cap can be removed and drug molecules can be released through cleavage of the disulphide linker from chemical stimulation of dithiothreitol (DTT) and mercaptoehtanol (ME).80
Change in release kinetics by surface functionalisation was observed in erythromycin incorporated in SBA-15. For example, by functionalising the surface of SBA-15 with hydrophobic long-chain hydrocarbon moieties, such as octadecyl-functionalised SBA-15, the release rate of erythromycin was impeded.8 This is another evidence of how functionalisation controls the release kinetics.103
Chemotherapeutics
Among many different therapeutic agents, selective intracellular delivery of cancer therapy agents seems particularly important because they are extremely cytotoxic to normal cells, making ‘zero premature release’ significant.25 Also, many anti-cancer agents are hydrophobic, such as camptothecin, so the drug delivery system is essential. The specific drug delivery to the target cells will increase therapeutic efficacy as well as minimise the systemic absorption, which will greatly reduce side effects such as nausea.104 So far, there were many studies done in relation to incorporate chemotherapeutic agents within sol-gel matrix of MSN and deliver it to the cancerous cells to stimulate apoptosis. In order to target cancer cells specifically, the difference between cancer and normal cells should be acknowledged. There are various differences between cancer and normal cells such as receptor expression and pH.
Many cancer cells over-express folic acid and galactose receptors. Thus, the addition of folic acid or galactose on the surface of the MSN was investigated. Gary-Bobo et al.’s109 study observed effective delivery to the colorectal cancer cells by galactose-functionalised MSN, and in Klichko et al.,61 Fan et al.67 and Kratz et al.’s120 studies, effective delivery of anti-cancer agents to the cancer cells with over-expressed alpha-folate receptor63 was investigated by covalently conjugating folic acid on MSN’s surface.
In terms of pH, cancerous cells usually have acidic pH around 5.3. The normal blood pH is around 7. Thus, functionalisation with acid-cleavable compound will enhance uptake of MSN by cancer cells. For example, MSN functionalised with acid-sensitive carboxylic hydrazone linker produced higher doxorubicin uptake by cancer cells.67
Surface functionalisation MSNx
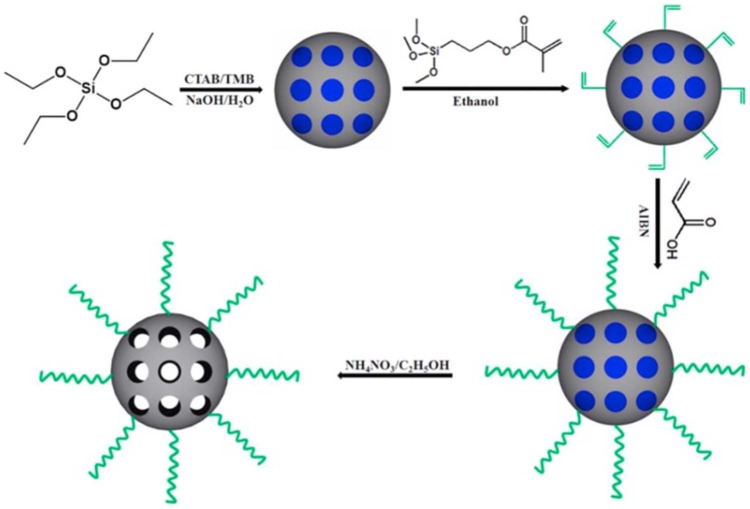
Recently, Li et al.’s study established a core MSN cross-linked with a poly(acrylic acid) (PAA) through disulphide linkage, which acted as a drug release switcher (Figure 4). The anti-cancer agent, DOX, was loaded into this particle. DOX was released to the medium by the dissociation of disulphide linkage achieved by a reduction-responsive reaction. Additionally, controlled release was achieved by varying the concentration of the reductant. This study observed DOX release in two different mediums (phosphate-buffered saline (PBS) of pH 7.4), one with 2 mM of glutathione, simulating environment of cancer cells, and one without glutathione, corresponding to normal human cells. The significantly higher release rate was observed with glutathione medium; 49.4% and 16.9% release rates were observed with glutathione and without glutathione, respectively (Figure 5). Through in vitro assays, this study confirmed that DOX-loaded MSN-PAA cross-linking only produced remarkable cytotoxic effects on human cancer cells (HeLa cells), and relatively lower cytotoxicity was observed with normal human cells (293 cells). This recent research proves that controlled and targeted release of cytotoxic agents can be achieved by surface functionalisation of MSNs.
Figure 4.
Schematic presentation of PAA-MSN preparation process achieved from Li et al.121
PAA: poly(acrylic acid); MSN: mesoporous silica nanoparticle; CTAB: N-cetyltrimethylammonium bromide; TMB: trimethylbenzene.
Figure 5.
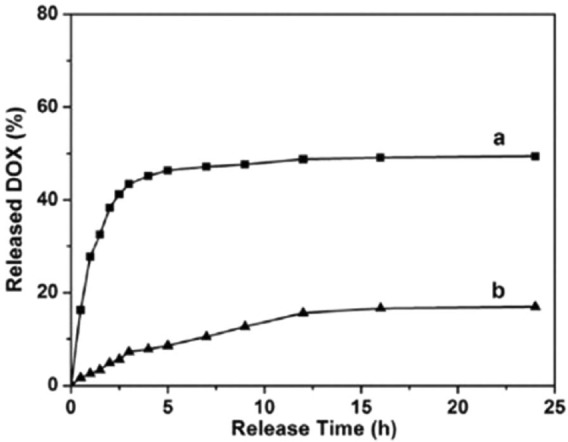
The graph showing greater DOX release from DOX in MSN-PAA in (a) 2 mM of glutathione medium compared to (b) without glutathione medium.121
PAA: poly(acrylic acid); MSN: mesoporous silica nanoparticle.
Stimuli-responsive mesoporous silica drug carriers
MSNs have been widely used due to their unique structure of parallel pores that enable achieving zero-order release kinetics for compounds incorporated into their structure. Often, it is critical to be able to release the drug only in specific time and location. Such a feature is particularly important for the delivery of highly potent drugs, such as chemotherapeutics. Several mechanisms to induce the release have been established, including systems that respond to light, pH, electric current and mechanical stimuli. The key targets in the design of effective systems are to release the drug substantially, decrease the nonspecific release from enzymatic hydrolysis, increase the cell uptake by modifying the surface charge and increase the loading and release ability.71
pH
An interesting approach is the use of nanovalves in the surface of the MSN, which are able to open according to the surrounding pH. Huang et al.122 demonstrated that by fabricating smart bi-functionalising MSNs with two pH-dependent polymer types (poly(2-diethylaminoethyl methacrylate) (PDEAEMA)), controlled release of doxorubicin was possible.
Nanovalves can contain alpha cyclodextrin (CD) ring on a stalk that is tethered to the pore opening of the MSN, which relies on the hydrogen bonding interaction between the CD and the stalk. At neutral pH, the CD is complexed with the stalk and the cyclic component is located near the pore opening, not allowing for the release of the cargo. When protonation takes place at lower pH, the affinity binding decreased and the CD is released allowing for the cargo to be released as well.123
Temperature
Currently, temperature-sensitive polymer poly(N-isopropylacrylamide) (pNIPAM) has become a material of choice when temperature-controlled stimulation is considered. Critical solution temperature for pNIPAM is 31°C; therefore, at higher temperatures it swells, which in turn triggers the drug cargo release, typically at higher temperatures, for example, at 37°C. It was demonstrated that pNIPAM-coated MSNs have a temperature-dependent release profile,124,125 and it was shown that DOX release at 37°C was significantly greater than at room temperature.126
Similarly, paraffin was shown to enable the temperature-dependent drug releases, which were directly related to its transition temperature. The benefit of using paraffin is that it creates a hydrophobic layer covering the silica nanoparticles that inhibits the release of cargo from the inside. Nevertheless, when the temperature is increased and the paraffin melts, the cargo can easily be released.126
Further approaches include the use of DNA as a coating on the mesoporous particles. Denaturation of DNA at higher temperature enabled the release of the drug cargo.127
Magnetic
A combination of MSNs with magnetic nanoparticles, in order to control drug release in the presence of an external trigger (magnetic and electromagnetic fields) has also been demonstrated. To attach magnetic nanoparticles to MSN, a single-stranded DNA was immobilised first on the MSN surface, and then magnetic beads were tethered to the DNA via a complementary sequence. Upon hybridisation of DNA strands, the pores were capped with the magnetic nanoparticles. Increase in the local temperature, obtained using alternating magnetic field, melts the double-stranded DNA, uncapping the MSN pores and releasing the model drug, for example, fluorescein. Another bonus of this technology is that it combines the release of chemotherapeutics and increases the temperature of the surrounding tissue (hyperthermia). These two effects can simultaneously enhance effects of anti-cancer therapy and lead to lower doses of drugs required.128
Light
Irradiation of the surface of functionalised MSN with a light of characteristic wavelength is another possible strategy for controlling the release of the cargo from the particles. For example, when MSNs were functionalised with mercaptopropyl, and the pores are loaded with sulforhodamine 101, exposure to visible light triggered the release of the entrapped molecules due to photodegradation of the chemical groups of mercaptopropyl. It was suggested that this approach can be utilised to deliver anti-cancer drugs.129
Enzyme
The use of enzyme-responsive materials has been explored to obtain controlled drug release. MSNs have been coated with polymers that degrade in the presence of specific enzymes. A protease-sensitive polymer, such as polyethylene glycol diacrylate (PEGDA), a peptide macromer possessing matrix metalloproteinase (MMP) substrate polypeptides, was coated on MSNs. The system was shown to release the drug only in vitro (fibroblast cells) and in vivo when enzymes were present.126 MCM-41 was functionalised with starch derivatives and loaded with a dye; the release of the dye was demonstrated only in the presence of the enzyme β-d-galactosidase. Efficacy of the system was tested in vitro using HeLa and LLC-PK1; tests demonstrated that the nanoparticles were devoid of unspecific toxicity, while for DOX-loaded particles, cell viability significantly decreased due to the release of the drug.130
Chemical reactions
Another type of stimuli-responsive release is based on the reaction of bond-reducing molecules that cleave certain bonds and therefore allows the release of specific molecules. This approach was used for MSNs, which were capped with CdS nanocrystals. Vancomycin and adenosine triphosphate (ATP) were loaded in MSNs, and the release was controlled by bond-reducing molecules, such as DTT and ME. These MSNs were shown to be biocompatible with neuroglial cells in vitro.131
In different studies, Luo131 used collagen which was attached to the pores of the MSNs by the use of molecules such as dl-dithiothreitol, which is a reducing agent, through the disulphide bonds. Lactobionic acid was used as a cell-targeting molecule and the release of the model drug, fluorescein isothiocyanate, was shown to be controlled by cleavage of the disulphide bonds.
The bio-safety study on the silica-based mesoporous nanoparticles
The justification of bio-safety of MSNs is extremely complicated because it can be varied depending on different factors such as different routes of administration, weight and size of particles and different formulations. As a result, the recent studies on bio-safety of MSN are controversial. One of recent studies observed bio-safety of silica material through nuclear magnetic resonance (NMR)-based metabolomic analysis. This study observed that silica nanoparticles induced increase in lipids, which can cause membrane modification. Its result shows that the toxicological effects due to high dose of silica nanoparticles can be associated with elevated levels of ATP and adenosine diphosphate (ADP), or due to utilisation of glucose and amino acids. Also, this study points out that the production of metabolic end products through silica nanoparticles can cause toxicity.132
Additionally, another study observed significant increases in liver and spleen weight and splenocyte proliferation after MSNs were intraperitoneally administrated in female BALB/c mice for 4 weeks. The study result indicates MSNs can affect immune systems through the dysregulation of the spleen. However, in this study, inconsistent results were observed between in vivo and in vitro data, and in vitro data showed low cytotoxicity of MSN, which indicates that further investigations are required.
In addition, bio-safety of MSN can be associated with different routes of administration. In Fu et al.’s133 study, despite the fact that silica nanoparticles could cross different biological barriers into the liver, low absorption rate of silica nanoparticles were observed when they were administrated by the intramuscular injection. In contrast, silica nanoparticles were well absorbed into the intestinal tract and persisted in the liver when it administered through oral route. Additionally, silica nanoparticles were mainly present in the liver and spleen when they were injected intravenously. In contrast to other studies, this study found that most of silica nanoparticles were excreted through urine and faeces after different routes of administration, which indicates that silica nanoparticle is reasonably biocompatible and can be used for different biomedical applications.134 Further studies need to be conducted to justify bio-safety of silica nanoparticles and to discover MSNs with maximal bio-safety.
Summary and perspectives
In conclusion, drug delivery system is significant to maximise therapeutic efficacy and minimise side effects of many bioactive molecules. It can address the problems with poor solubility, stability and lack of specificity of drug molecules. This review presented a great potential of MSN in drug delivery. Compared to the other drug delivery systems, MSN offers some advantages such as biocompatibility, ease in modifying structure through active silanol group surface, controlled release and simple synthesis procedure called sol-gel process, which can bring economical benefits as well. Depending on what chemical substituents are used in sol-gel procedure, different morphology of MSN can be produced. Distinctive mesoporous structure and active surface of MSN can incorporate various therapeutic agents and deliver them without altering their therapeutic effectiveness. As MSN is in its developmental stage, there are high expectations on its usage and function in drug delivery.
Despite many studies illustrating the effectiveness of sol-gel-processed MSN, there are not many studies done in vivo, and this can be challenging for MSN in clinical applications. For example, intervariability such as different blood volume and circulation and different immunogenicity may affect the effectiveness of MSN drug delivery. Also, more studies need to be conducted with regard to MSN precursor because the slight shrinkage of MSN during the drying process needs to be addressed. Finally, there are insufficient studies done for the biodegradability and long-term biocompatibility of MSN. Biodegradability is essential to minimise accumulation of MSN. Thus, further research is required to address the limitations and develop more effective drug delivery MSN system.
MSN has been found to be an attractive candidate as a gene (plasmid DNA) delivery carrier to human cells, that is, as an important therapeutic option for the treatment of genetically caused diseases, for example, cancers. The MSN with plasmid DNA readily entered into human cells without supplementary polymers, for example, cationic dendrimers. MSN with large pore size (>15 nm) could efficiently protect plasmids from nuclease-mediated degradation, leading to high transfection efficiency of the plasmids encoding luciferase (pLuc) and green fluorescent protein (pGFP). Furthermore, production of non-spherical MSN ellipsoids with tuneable aspect ratios for magnetic field–assisted assembly into ordered arrays/structures found a potential for drug/gene delivery applications. The use of MSN as gene delivery carrier is another avenue which requires further research.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was supported by a grant, Priority Research Centers Program (grant#: 2009-0093829), through the National Research Foundation (NRF), Republic of Korea.
References
- 1. Jyrkka J, Mursu J, Enlund H, et al. Polypharmacy and nutritional status in elderly people. Curr Opin Clin Nutr 2012; 15(1): 1–6 [DOI] [PubMed] [Google Scholar]
- 2. Zhang M, Holman CDJ, Preen DB, et al. Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980–2003. Brit J Clin Pharmaco 2007; 63(2): 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferner RE. Medication errors. Brit J Clin Pharmaco 2012; 73(6): 912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004; 56(2): 163–184 [DOI] [PubMed] [Google Scholar]
- 5. Plank C, Anton M, Rudolph C, et al. Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opin Biol Th 2003; 3(5): 745–758 [DOI] [PubMed] [Google Scholar]
- 6. Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Ch 2008; 52(4): 1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu J, Liong M, Zink JI, et al. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007; 3(8): 1341–1346 [DOI] [PubMed] [Google Scholar]
- 8. Liu YJ, Zhang B, Yan B. Enabling anticancer therapeutics by nanoparticle carriers: the delivery of paclitaxel. Int J Mol Sci 2011; 12(7): 4395–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000; 21(23): 2475–2490 [DOI] [PubMed] [Google Scholar]
- 10. Kim YJ, Choi S, Koh JJ, et al. Controlled release of insulin from injectable biodegradable triblock copolymer. Pharmaceut Res 2001; 18(4): 548–550 [DOI] [PubMed] [Google Scholar]
- 11. Arshady R. Preparation of biodegradable microspheres and microcapsules: 2. Polyactides and related polyesters. J Control Release 1991; 17(1): 1–21 [Google Scholar]
- 12. Brannon-Peppas L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm 1995; 116(1): 1–9 [Google Scholar]
- 13. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliver Rev 2003; 55(3): 329–347 [DOI] [PubMed] [Google Scholar]
- 14. Sanders LM, Kell BA, McRae GI, et al. Prolonged controlled-release of nafarelin, a luteinizing hormone-releasing hormone analogue, from biodegradable polymeric implants: influence of composition and molecular weight of polymer. J Pharm Sci 1986; 75(4): 356–360 [DOI] [PubMed] [Google Scholar]
- 15. Kailasam S, Wise DL, Gangadharam PRJ. Bioavailability and chemotherapeutic activity of clofazimine against Mycobacterium avium complex infections in beige mice following a single implant of a biodegradable polymer. J Antimicrob Chemoth 1994; 33(2): 273–279 [DOI] [PubMed] [Google Scholar]
- 16. Li LC, Deng J, Stephens D. Polyanhydride implant for antibiotic delivery – from the bench to the clinic. Adv Drug Deliver Rev 2002; 54(7): 963–986 [DOI] [PubMed] [Google Scholar]
- 17. Saito N, Okada T, Toba S, et al. New synthetic absorbable polymers as BMP carriers: plastic properties of poly-d,l-lactic acid-polyethylene glycol block copolymers. J Biomed Mater Res 1999; 47(1): 104–110 [DOI] [PubMed] [Google Scholar]
- 18. Göpferich A, Tessmar J. Polyanhydride degradation and erosion. Adv Drug Deliver Rev 2002; 54(7): 911–931 [DOI] [PubMed] [Google Scholar]
- 19. Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release 2001; 73(2–3): 121–136 [DOI] [PubMed] [Google Scholar]
- 20. Tojo K, Aoyagi H, Kurita T. Surface dissolution-bulk erosion model of drug release from biodegradable polymer rods. J Chem Eng Jpn 1998; 31(4): 648–651 [Google Scholar]
- 21. Hedberg EL, Tang A, Crowther RS, et al. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release 2002; 84(3): 137–150 [DOI] [PubMed] [Google Scholar]
- 22. Wong J, Brugger A, Khare A, et al. Suspensions for intravenous (IV) injection: a review of development, preclinical and clinical aspects. Adv Drug Deliver Rev 2008; 60(8): 939–954 [DOI] [PubMed] [Google Scholar]
- 23. Wang S. Ordered mesoporous materials for drug delivery. Micropor Mesopor Mat 2009; 117(1–2): 1–9 [Google Scholar]
- 24. Radin S, Chen T, Ducheyne P. The controlled release of drugs from emulsified, sol gel processed silica microspheres. Biomaterials 2009; 30(5): 850–858 [DOI] [PubMed] [Google Scholar]
- 25. Slowing II, Vivero-Escoto JL, Wu CW, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliver Rev 2008; 60(11): 1278–1288 [DOI] [PubMed] [Google Scholar]
- 26. Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006; 27(11): 2450–2467 [DOI] [PubMed] [Google Scholar]
- 27. Barani H, Montazer M. A review on applications of liposomes in textile processing. J Liposome Res 2008; 18(3): 249–262 [DOI] [PubMed] [Google Scholar]
- 28. Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. P Natl Acad Sci USA 1988; 85(18): 6949–6953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Qi XR, Maitani Y, et al. PEG-PLA diblock copolymer micelle-like nanoparticles as all-trans-retinoic acid carrier: in vitro and in vivo characterizations. Nanotechnology 2009; 20(5): 055106. [DOI] [PubMed] [Google Scholar]
- 30. Matthews OA, Shipway AN, Stoddart JF. Dendrimers – branching out from curiosities into new technologies. Prog Polym Sci 1998; 23(1): 1–56 [Google Scholar]
- 31. Soliman GM, Sharma A, Maysinger D, et al. Dendrimers and miktoarm polymers based multivalent nanocarriers for efficient and targeted drug delivery. Chem Commun 2011; 47(34): 9572–9587 [DOI] [PubMed] [Google Scholar]
- 32. McNerny DQ, Leroueil PR, Baker JR. Understanding specific and nonspecific toxicities: a requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010; 2(3): 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamashita T, Yamashita K, Nabeshi H, et al. Carbon nanomaterials: efficacy and safety for nanomedicine. Materials 2012; 5(2): 350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vashist SK, Zheng D, Pastorin G, et al. Delivery of drugs and biomolecules using carbon nanotubes. Carbon 2011; 49(13): 4077–4097 [Google Scholar]
- 35. Liu Z, Robinson JT, Tabakman SM, et al. Carbon materials for drug delivery & cancer therapy. Mater Today 2011; 14(7–8): 316–323 [Google Scholar]
- 36. Zhang SA, Yang K, Liu ZA. Carbon nanotubes for in vivo cancer nanotechnology. Sci China Chem 2010; 53(11): 2217–2225 [Google Scholar]
- 37. Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 2005; 9(6): 674–679 [DOI] [PubMed] [Google Scholar]
- 38. Le Guevel X, Daum N, Schneider M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 2011; 22(27): 275103. [DOI] [PubMed] [Google Scholar]
- 39. Dobson J. Magnetic nanoparticles for drug delivery. Drug Develop Res 2006; 67(1): 55–60 [Google Scholar]
- 40. Losic D, Simovic S. Self-ordered nanopore and nanotube platforms for drug delivery applications. Expert Opin Drug Del 2009; 6(12): 1363–1381 [DOI] [PubMed] [Google Scholar]
- 41. Trouiller B, Reliene R, Westbrook A, et al. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 2009; 69(22): 8784–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirakawa K, Mori M, Yoshida M, et al. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radical Res 2004; 38(5): 439–447 [DOI] [PubMed] [Google Scholar]
- 43. Bergstrand N. Liposomes for drug delivery [Elektronisk resurs] from physico-chemical studies to applications. PhD Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2003 [Google Scholar]
- 44. Chonn A, Cullis PR. Recent advances in liposomal drug-delivery systems. Curr Opin Biotech 1995; 6(6): 698–708 [DOI] [PubMed] [Google Scholar]
- 45. Goyal P, Goyal K, Vijaya Kumar SG, et al. Liposomal drug delivery systems – clinical applications. Acta Pharmaceut 2005; 55(1): 1–25 [PubMed] [Google Scholar]
- 46. Rangaramanujam MK, Omathanu PP, Sujatha K. Biomedical applications of nanotechnology: dendrimers and hyperbranched polymers for drug delivery. In: V Labhasetwar V, Leslie-Pelecky DL. (eds) Biomedical applications of nanotechnology. NJ: John Wiley & Sons, 2007, pp. 107–129 [Google Scholar]
- 47. Majoros IJ, Williams CR, Becker A, et al. Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2009; 1(5): 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lubbe AS, Bergemann C, Brock J, et al. Physiological aspects in magnetic drug-targeting. J Magn Magn Mater 1999; 194(1–3): 149–155 [Google Scholar]
- 49. Neuberger T, Schopf B, Hofmann H, et al. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater 2005; 293(1): 483–496 [Google Scholar]
- 50. Han C-M, Lee E-J, Kim H-E, et al. Porous TiO2 films on Ti implants for controlled release of tetracycline-hydrochloride (TCH). Thin Solid Films 2011; 519(22): 8074–8076 [Google Scholar]
- 51. Yamaguchi S, Kobayashi H, Narita T, et al. Novel photodynamic therapy using water-dispersed TiO2-polyethylene glycol compound: evaluation of antitumor effect on glioma cells and spheroids in vitro. Photochem Photobiol 2010; 86(4): 964–971 [DOI] [PubMed] [Google Scholar]
- 52. Zhang H, Wang C, Chen B, et al. Daunorubicin-TiO2 nanocomposites as a ‘smart’ pH-responsive drug delivery system. Int J Nanomed 2012; 7: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang CY, Xiao XF, Mao D, et al. The study of using TiO2 nanotube arrays as a drug delivery for alendronate. In: Kim YH, Yarlagadda P, Zhang X, et al. (eds) Advanced materials research, vols 335–336 Trans Tech Publications, 2011, pp. 1469–1472 [Google Scholar]
- 54. Song YY, Schmidt-Stein F, Bauer S, et al. Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. J Am Chem Soc 2009; 131(12): 4230–4232 [DOI] [PubMed] [Google Scholar]
- 55. Cai R, Kubota Y, Shuin T, et al. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res 1992; 52(8): 2346–2348 [PubMed] [Google Scholar]
- 56. Miyoshi N, Kyo K, Kotaro T, et al. Application of titanium dioxide (TiO2) nanoparticles in photodynamic therapy (PDT) of an experimental tumor. In: 4th nanoscience and nanotechnology symposium, vol. 1415 (eds Iskandar F, Khairurrijal, Abdullah M.), Bali, Indonesia, 23–25 September 2011, pp. 21–23 Melville, NY: American Institute of Physics [Google Scholar]
- 57. Boffetta P, Soutar A, Cherrie JW, et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Cause Control 2004; 15(7): 697–706 [DOI] [PubMed] [Google Scholar]
- 58. Fryzek JP, Chadda B, Marano D, et al. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J Occup Environ Med 2003; 45(4): 400–409 [DOI] [PubMed] [Google Scholar]
- 59. Kresge CT, Leonowicz ME, Roth WJ, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992; 359: 710–712 [Google Scholar]
- 60. Shchipunov YA, Burtseva YV, Karpenko TY, et al. Highly efficient immobilization of endo-1,3-beta-d-glucanases (laminarinases) from marine mollusks in novel hybrid polysaccharide-silica nanocomposites with regulated composition. J Mol Catal B: Enzym 2006; 40(1–2): 16–23 [Google Scholar]
- 61. Klichko Y, Liong M, Choi E, et al. Mesostructured silica for optical functionality, nanomachines, and drug delivery. J Am Ceram Soc 2009; 92(1): S2–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tourne-Peteilh C, Begu S, Lerner DA, et al. Sol-gel one-pot synthesis in soft conditions of mesoporous silica materials ready for drug delivery system. J Sol-Gel Sci Techn 2012; 61(3): 455–462 [Google Scholar]
- 63. Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008; 2(5): 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Trewyn BG, Slowing II, Giri S, et al. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Accounts Chem Res 2007; 40(9): 846–853 [DOI] [PubMed] [Google Scholar]
- 65. Slowing II, Trewyn BG, Giri S, et al. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater 2007; 17(8): 1225–1236 [Google Scholar]
- 66. Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Edit 2007; 46(40): 7548–7558 [DOI] [PubMed] [Google Scholar]
- 67. Fan JQ, Fang G, Wang XD, et al. Targeted anticancer prodrug with mesoporous silica nanoparticles as vehicles. Nanotechnology 2011; 22(45): 455102. [DOI] [PubMed] [Google Scholar]
- 68. Vivero-Escoto JL, Slowing II, Trewyn BG, et al. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010; 6(18): 1952–1967 [DOI] [PubMed] [Google Scholar]
- 69. Vallet-Regi M, Izquierdo-Barba I, Colilla M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos T Roy Soc A 2012; 370(1963): 1400–1421 [DOI] [PubMed] [Google Scholar]
- 70. Sakai-Kato K, Hasegawa T, Takaoka A, et al. Controlled structure and properties of silicate nanoparticle networks for incorporation of biosystem components. Nanotechnology 2011; 22(20): 205702. [DOI] [PubMed] [Google Scholar]
- 71. Lee C-H, Cheng S-H, Huang I-P, et al. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew Chem Int Ed 2010; 49: 8214–8219 [DOI] [PubMed] [Google Scholar]
- 72. Meng HA, Xue M, Xia TA, et al. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc 2010; 132: 12690–12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shchipunov YA. Entrapment of biopolymers into sol-gel derived silica nanocomposites. In: Ruiz-Hitzky E, Ariga K, Lvov YM. (eds) Bio-inorganic Hybrid Nanomaterials: strategies, syntheses, characterization and applications. Weinheim: Wiley-VCH Verlag GmbH & Co, KGaA, 2008, pp. 75–111 [Google Scholar]
- 74. Shchipunov YA, Kojima A, Imae T. Polysaccharides as a template for silicate generated by sol-gel processes. J Colloid Interf Sci 2005; 285(2): 574–580 [DOI] [PubMed] [Google Scholar]
- 75. Wang YG, Huang SJ, Kang SF, et al. Low-cost route for synthesis of mesoporous silica materials with high silanol groups and their application for Cu(II) removal. Mater Chem Phys 2012; 132(2–3): 1053–1059 [Google Scholar]
- 76. Slowing II, Vivero-Escoto JL, Trewyn BG, et al. Mesoporous silica nanoparticles: structural design and applications. J Mater Chem 2010; 20: 7924–7937 [Google Scholar]
- 77. Wu S-H, Hung Y, Mou C-Y. Mesoporous silica nanoparticles as nanocarriers. Chem Commun 2011; 47: 9972–9985 [DOI] [PubMed] [Google Scholar]
- 78. Fowler CE, Khushalani D, Lebeau B, et al. Nanoscale materials with mesostructured interiors. Adv Mater 2001; 13: 649–652 [Google Scholar]
- 79. Cai Q, Luo Z-S, Pang W-Q, et al. Dilute solution routes to various controllable morphologies of MCM-41 silica with a basic medium. Chem Mater 2001; 13: 258–263 [Google Scholar]
- 80. Lai C-Y, Trewyn BG, Jeftinija DM, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc 2003; 125: 4451–4459 [DOI] [PubMed] [Google Scholar]
- 81. Lu F, Wu S-H, Hung Y, et al. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009; 5: 1408–1413 [DOI] [PubMed] [Google Scholar]
- 82. He Q, Cui X, Cui F, et al. Size-controlled synthesis of monodispersed mesoporous silica nano-spheres under a neutral condition. Micropor Mesopor Mat 2009; 117: 609–616 [Google Scholar]
- 83. Urata C, Aoyama Y, Tongawa A, et al. Dialysis process for the removal of surfactants to form colloidal mesoporous silica nanoparticles. Chem Commun 2009; 34: 5094–5096 [DOI] [PubMed] [Google Scholar]
- 84. Suteewong T, Sai H, Cohen R, et al. Highly aminated mesoporous silica nanoparticles with cubic pore structure. J Am Chem Soc 2011; 133: 172–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lin Y-S, Abadeer N, Haynes CL. Stability of small mesoporous silica nanoparticles in biological media. Chem Commun 2011; 47: 532–534 [DOI] [PubMed] [Google Scholar]
- 86. Nooney RI, Thirunavukkarasu D, Chen Y, et al. Synthesis of nanoscale mesoporous silica spheres with controlled particle size. Chem Mater 2002; 14: 4721–4728 [Google Scholar]
- 87. Lin H-P, Tsai C-P. Synthesis of mesoporous silica nanoparticles from a low-concentration CnTMAX-sodium silicate components. Chem Lett 2003; 32: 1092–1093 [Google Scholar]
- 88. Suzuki K, Ikari K, Imai H. Synthesis of silica nanoparticles having a well-ordered mesostructure using a double surfactant system. J Am Chem Soc 2004; 126: 462–463 [DOI] [PubMed] [Google Scholar]
- 89. Yano K, Fukushima Y. Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J Mater Chem 2004; 14: 1579–1584 [Google Scholar]
- 90. Han Y, Ying JY. Generalized fluorocarbon-surfactant-mediated synthesis of nanoparticles with various mesoporous structure. Angew Chem Int Edit 2005; 44: 288–292 [DOI] [PubMed] [Google Scholar]
- 91. Möller K, Kobler J, Bein T. Colloidal suspensions of nanometer-sized mesoporous silica. Adv Funct Mater 2007; 17: 605–612 [Google Scholar]
- 92. Berggren A, Palmqvist AEC. Particle control of colloidal suspensions of mesostructured silica. J Phys Chem C 2008; 112: 732–737 [Google Scholar]
- 93. Du HW, Hamilton PD, Reilly MA, et al. A facile synthesis of highly water-soluble, core-shell organo-silica nanoparticles with controllable size via sol-gel process. J Colloid Interf Sci 2009; 340(2): 202–208 [DOI] [PubMed] [Google Scholar]
- 94. Chen Y, Chen HR, Ma M, et al. Double mesoporous silica shelled spherical/ellipsoidal nanostructures: synthesis and hydrophilic/hydrophobic anticancer drug delivery. J Mater Chem 2011; 21(14): 5290–5298 [Google Scholar]
- 95. Brook MA, Chen Y, Guo K, et al. Proteins entrapped in silica monoliths prepared from glyceroxysilanes. J Sol-Gel Sci Techn 2004; 31(1–3): 343–348 [Google Scholar]
- 96. Shchipunov YA, Karpenko TY, Krekoten AV. Hybrid organic-inorganic nanocomposites fabricated with a novel biocompatible precursor using sol-gel processing. Compos Interface 2005; 11(8–9): 587–607 [Google Scholar]
- 97. Colby MW, Osaka A, Mackenzie JD. Effects of temperature on formation of silica-gel. J Non-Cryst Solids 1986; 82(1–3): 37–41 [Google Scholar]
- 98. Shchipunov YA, Karpenko TY, Krekoten AV, et al. Gelling of otherwise nongelable polysaccharides. J Colloid Interf Sci 2005; 287(2): 373–378 [DOI] [PubMed] [Google Scholar]
- 99. Halamova D, Zelenak V. NSAID naproxen in mesoporous matrix MCM-41: drug uptake and release properties. J Incl Phenom Macro 2012; 72(1–2): 15–23 [Google Scholar]
- 100. He QJ, Shi JL, Chen F, et al. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010; 31(12): 3335–3346 [DOI] [PubMed] [Google Scholar]
- 101. Mackenzie JD. Applications of the sol-gel process. J Non-Cryst Solids 1988; 100(1–3): 162–168 [Google Scholar]
- 102. Manzano M, Aina V, Arean CO, et al. Studies on MCM-41 mesoporous silica for drug delivery: effect of particle morphology and amine functionalization. Chem Eng J 2008; 137(1): 30–37 [Google Scholar]
- 103. Doadrio JC, Sousa EMB, Izquierdo-Barba I, et al. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J Mater Chem 2006; 16(5): 462–466 [Google Scholar]
- 104. Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003; 2(5): 347–360 [DOI] [PubMed] [Google Scholar]
- 105. Vivero-Escoto JL, Luis J. Surface functionalized mesoporous silica nanoparticles for intracellular drug delivery. Diss Abstr Int 2009; 71: 0638 [Google Scholar]
- 106. Selvam P, Bhatia SK, Sonwane CG. Recent advances in processing and characterization of periodic mesoporous MCM-41 silicate molecular sieves. Ind Eng Chem Res 2001; 40(15): 3237–3261 [Google Scholar]
- 107. Wan Y, Zhao DY. On the controllable soft-templating approach to mesoporous silicates. Chem Rev 2007; 107: 2821–2860 [DOI] [PubMed] [Google Scholar]
- 108. Hoffmann F, Cornelius M, Morell J, et al. Silica-based mesoporous organic – inorganic hybrid. Angew Chew Int Edit 2006; 45: 3216–3251 [DOI] [PubMed] [Google Scholar]
- 109. Gary-Bobo M, Hocine O, Brevet D, et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int J Pharm 2012; 423(2): 509–515 [DOI] [PubMed] [Google Scholar]
- 110. Yiu HHP, Wright PA. Enzymes supported on ordered mesoporous solids: a special case of an inorganic-organic hybrid. J Mater Chem 2005; 15(35–36): 3690–3700 [Google Scholar]
- 111. Huh S, Wiench JW, Trewyn BG, et al. Tuning of particle morphology and pore properties in mesoporous silicas with multiple organic functional groups. Chem Commun 2003; 18: 2364–2365 [DOI] [PubMed] [Google Scholar]
- 112. Huh S, Wiench JW, Yoo JC, et al. Organic functionalization and morphology control of mesoporous silicas via a co-condensation synthesis method. Chem Mater 2003; 15: 4247–4256 [Google Scholar]
- 113. Lang N, Tuel A. A fast and efficient ion-exchange procedure to remove surfactant molecules from MCM-41 materials. Chem Mater 2004; 16: 1961–1966 [Google Scholar]
- 114. Burleigh MC, Markowitz MA, Spector MS, et al. Direct synthesis of periodic mesoporous organosilicas: functional incorporation by co-condensation with organosilanes. J Phys Chem B 2001; 105: 9935–9942 [Google Scholar]
- 115. Balas F, Manzano M, Horcajada P, et al. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J Am Chem Soc 2006; 128(25): 8116–8117 [DOI] [PubMed] [Google Scholar]
- 116. Colilla M, Izquierdo-Barba I, Vallet-Regi M. Phosphorus-containing SBA-15 materials as bisphosphonate carriers for osteoporosis treatment. Micropor Mesopor Mat 2010; 135(1–3): 51–59 [Google Scholar]
- 117. Vallet-Regi MA, Ruiz-Gonzalez L, Izquierdo-Barba I, et al. Revisiting silica based ordered mesoporous materials: medical applications. J Mater Chem 2006; 16(1): 26–31 [Google Scholar]
- 118. Wu CT, Chang J. Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012; 2(3): 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vallet-Regi M, Doadrio JC, Doadrio AL, et al. Hexagonal ordered mesoporous material as a matrix for the controlled release of amoxicillin. Solid State Ionics 2004; 172(1–4): 435–439 [Google Scholar]
- 120. Kratz F, Muller IA, Ryppa C, et al. Prodrug strategies in anticancer chemotherapy. Chemmedchem 2008; 3(1): 20–53 [DOI] [PubMed] [Google Scholar]
- 121. Li H, Zhang JZ, Tang Q, et al. Reduction-responsive drug delivery based on mesoporous silica nanoparticle core with crosslinked poly(acrylic acid) shell. Mater Sci Eng C Mater Biol Appl 2013; 33(6): 3426–3431 [DOI] [PubMed] [Google Scholar]
- 122. Huang X, Hauptmann N, Appelhans D, et al. Source: synthesis of hetero-polymer functionalized nanocarriers by combining surface-initiated ATRP and RAFT polymerization. Small 2012; 8(23): 3579–3583 [DOI] [PubMed] [Google Scholar]
- 123. Du L, Liao S, Khatib HA, et al. Controlled-access hollow mechanized silica nanocontainers. J Am Chem Soc 2009; 131: 15136–15142 [DOI] [PubMed] [Google Scholar]
- 124. Ruhland TM, Reichstein PM, Majewski AP, et al. Superparamagnetic and fluorescent thermo-responsive core-shell-corona hybrid nanogels with a protective silica shell. J Colloid Interf Sci 2012; 374: 45–53 [DOI] [PubMed] [Google Scholar]
- 125. Singh N, Karambelkar A, Gu L, et al. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J Am Chem Soc 2011; 133: 19582–19585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Aznar E, Mondragón L, Ros-Lis JV, et al. Finely tuned temperature-controlled cargo release using paraffin-capped mesoporous silica nanoparticles. Angew Chem Int Edit 2011; 50: 11172–11175 [DOI] [PubMed] [Google Scholar]
- 127. Chen C, Geng J, Pu F, et al. Polyvalent nucleic acid/mesoporous silica nanoparticle conjugates: dual stimuli-responsive vehicles for intracellular drug delivery. Angew Chem Int Edit 2011; 50: 882–886 [DOI] [PubMed] [Google Scholar]
- 128. Ruiz-Hernández E, Baeza A, Vallet-Regi M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011; 5: 1259–1266 [DOI] [PubMed] [Google Scholar]
- 129. Wu C, Chen C, Lai J, et al. Molecule-scale controlled-release system based on light-responsive silica nanoparticles. Chem Commun 2008; 23: 2662–2664 [DOI] [PubMed] [Google Scholar]
- 130. Bernardos A, Mondragon L, Aznar E, et al. Enzyme-responsive intracellular controlled release using nanometric silica mesoporous supports capped with ‘Saccharides’. ACS Nano 2010; 4: 6353–6368 [DOI] [PubMed] [Google Scholar]
- 131. Luo Z, Cai K, Hu Y, et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Edit 2011; 50: 640–643 [DOI] [PubMed] [Google Scholar]
- 132. Feng J, Li J, Wu H, et al. Metabolic responses of HeLa cells to silica nanoparticles by NMR-based metabolomic analyses. Metabolomics 2013; 9: 874–886 [Google Scholar]
- 133. Fu C, Liu T, Li L, et al. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013; 34(10): 2565–2575 [DOI] [PubMed] [Google Scholar]
- 134. Lee S, Kim M, Lee D, et al. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomed 2013; 8: 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]