Abstract
Transcriptional regulation of expression of the human mitochondrial thiamine pyrophosphate transporter (the product of the SLC25A19 gene) is unknown. To understand this regulation, we cloned and characterized the 5′-regulatory region of the SLC25A19 gene (1,080 bp). The cloned fragment was found to possess promoter activity in transiently transfected human-derived liver HepG2 cells. 5′- and 3′-deletion analysis has identified the minimal region required for basal SLC25A19 promoter activity to be between −131 and + 20 (using the distal transcriptional start site as +1). The minimal promoter lacks typical TATA motif and contains two inverted CCAAT boxes (binding sites for NF-Y transcriptional factor). By means of mutational analysis, the critical role of both the upstream and downstream CCAAT boxes in basal SLC25A19 promoter activity was established; however, each of these boxes alone was found to be unable to support promoter activity. EMSA and supershift EMSA (with the use of specific antibodies against NF-Y subunits) studies, as well as chromatin immunoprecipitation assay, demonstrated the binding of NF-Y to both CCAAT boxes in vitro and in vivo, respectively. The requirement for NF-Y in SLC25A19 promoter activity in vivo was directly confirmed by the use of a dominant negative NF-YA mutant in transiently transfected HepG2 cells. These studies report for the first time the characterization of the SLC25A19 promoter and demonstrate an essential role for NF-Y in its basal activity.
Keywords: SLC25A19, promoter, transcription regulation, thiamine pyrophosphate, mitochondria
1. Introduction
Thiamine (vitamin Bl) is an important micronutrient required for life. In its biologically active forms (mainly thiamine pyrophosphate, TPP, a derivative that accounts for ~ 85–90% of the total forms of thiamine in cells) the vitamin acts as a cofactor for critical cytoplasmic (transketolase) and mitochondrial (pyruvate dehydrogenase, α-ketoglutarate dehydrogenase and branched-chain α-ketoacid dehydrogenase) enzymes that catalyse reactions related to carbohydrate and energy metabolism (Berdanier, 1994; Bettendorff et al., 2009; Singleton and Martin, 2001). Thiamine also plays an important role in reducing cellular oxidative stress via maintaining normal cellular redox state (Calingasan et al., 1999; Portari et al., 2008; Singleton and Martin, 2001; Todd and Butterworth, 1999). Thus, low intracellular levels of thiamine leads to impairment in oxidative energy metabolism (acute energy failure) and to a propensity for oxidative stress (Calingasan et al., 1999; Portari et al., 2008; Singleton and Martin, 2001; Todd and Butterworth, 1999; Gangolf et al., 2010); it also leads to impairment in the structure and function of mitochondria (Bettendorff et al., 1995), an organelle that maintains close to 90% of cellular TPP. At the clinical level, thiamine deficiency in humans leads to serious consequences that include cardiovascular and neurological abnormalities (Said, 2011; Tanphaichirt, 1994; Victor et al., 1989).
All mammalian cells cannot synthetize thiamine de novo, and thus, they capture it from their surrounding environment via transport across cell membrane. It is well established now that cellular uptake of free thiamine occurs via a specialized carrier-mediated mechanism that involves the plasma membrane high-affinity thiamine transporter-1 and -2 (THTR-1 and THTR-2; for humans these systems are referred to as hTHTR-1 and hTHTR-2) (Diaz et al., 1999; Eudy et al., 2000). In the cell, free thiamine is converted to TPP via an enzymatic process that takes place exclusively in the cytoplasm (Gangolf et al., 2010; Dues and Blum, 1970; Cusaro et al., 1977); there is no synthesis of TPP in the mitochondria (Barile et al., 1990). Most of the cytoplasmic TPP (~ 90%) is then transported into the mitochondria (Bettendorff, 1994; Bettendorff, 1995) for utilization in the metabolic reactions mentioned earlier. Uptake of TPP by mitochondria occurs by a specific, carrier-mediated process that involves the mitochondrial TPP transporter (MTPPT; product of the SLC25A19 gene) (Iacobazzi et al., 2001; Dolce et al., 2001; Kang et al., 2008; Lindhurst et al., 2006). This carrier protein (320 amino acids) is like all other members of the mitochondrial carrier family (MCF) of proteins consists of three tandem repeats (three modules) of approximately 100 amino acids each, and is predicted to have six transmembrane domains. The SLC25A19 gene is conserved among species from bacteria to mouse and humans, and mutations in this gene lead to drastic depletion in mitochondrial TPP level and to the development of Amish congenital lethal microcephaly (an autosomal recessive disorder) (Lindhurst et al., 2006; Siu et al., 2010), and of neuropathy and bilateral striatal necrosis (Spiegel et al., 2009). There is little currently known about how the MTPPT system is regulated at the transcriptional and post-transcriptional levels. Addressing these issues is physiologically important because MTPPT is responsible for supplying the mitochondria with an important cofactor needed for activity of a number of enzymes in the energy metabolism/production pathways. As a first step to this knowledge, we aimed in this study at examining the basic transcriptional activity of the SLC25A19 gene. Thus, we cloned and characterized the 5′-regulatory region (promoter) of this gene and showed that this promoter is TATA-less and contains two inverted CCAAT boxes in close proximity to the transcriptional start site(s). Our results also show a critical role for the NF-Y transcriptional factor in regulating the basal activity of the SLC25A19 promoter.
2. Materials and Methods
2.1. Chemicals and reagents
The primers and synthetic oligonucleotides for EMSA were purchased from Sigma Genosys (Woodlands, TX). The NF-YA dominant-negative expression vector (Δ4YAm29dn) was kindly provided by Dr. Roberto Mantovani (University of Milan, Milan, Italy). Anti-NF-YA, anti-NF-YB, anti-NF-YC antibody, and normal rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Routine biochemicals, cell culture reagents, transfection reagents were all of molecular biology quality and were purchased from Fisher Scientific (Tustin, CA), Sigma (St. Louis, MO), and Life Technologies (Rockville, MD).
2.2. 5′-Rapid amplification of the cDNA ends
Transcriptional start site (TSS) for SLC25A19 in HepG2 cells were identified with the rapid amplification of the cDNA ends (RACE) technique using 5′-RACE kit (Life Technologies). The sequence information for the transcript variants 1, 2 and 3 of the human SLC25A19 deposited in GenBank (accession no. NM_001126121, NM_021734, and NM_001126122) was used as a guide for primer design. 3.5 micrograms of total RNA isolated from HepG2 cells was used with gene-specific reverse primer 5′-GCGAGACAGGCGCTCATGCT-3′ (from exon 5) in the initial RT-PCR. The first-strand cDNAs were isolated and tailed. The PCR of tailed cDNAs was then performed by using the gene-specific reverse primers 5′-CTGGAAACGGATCTTGATGA-3′ and 5′-GATGACGTCGAAGGGACTGAT-3′ (from exon 4, both) and the manufacturer’s Abridged Anchor forward primer. A subsequent nested amplification was performed by using the gene-specific reverse primer 5′-GGCCCACACAATGTCCATCAGT-3′ (from exon 4) and the manufacturer’s Abridged Universal Amplification forward primer. PCR products were analyzed on a 2% agarose gel and subcloned into the pGEM-T Easy vector (Promega, Madison, WI). The DNA sequence was verified by the Laragen Sequencing Facility (Los Angeles, CA).
2.3. Cloning of the 5′-regulatory region for the SLC25A19 gene
The sequence information for the SLC25A19 gene and flanking sequence deposited in GenBank (accession no. NG_008274) was used to design PCR cloning primers. A PCR was performed by using two gene-specific primers (forward: 5′-CGCTGGGCAGCGCGTGCGAGGTAGGT-3′ and reverse: 5′-GAGATGCTTCTCTGGTCTGGTGTGACA -3′) and 100 ng of human genomic DNA (Clontech, Mountain View, CA). PCR conditions were denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 3 min, and then a final extension at 72°C for 10 min. The 1,080-bp product was isolated on a 0.7% agarose gel, cloned into the pGEM-T Easy vector, digested with XhoI and HindIII (these sites were designed into the primers), and subcloned into the XhoI/HindIII cut pGL3-Basic luciferase reporter vector (Promega) upstream of the luciferase gene. The DNA sequence was verified by the Laragen Sequencing Facility.
2.4. Construction of luciferase reporter plasmids
5′- and 3′-deletion clones were obtained by PCR using gene-specific primers with XhoI (forward primer) and HindIII (reverse primer) sites (Table 1), followed by subcloning of the PCR fragments into polycloning site of pGL3-basic vector. Mutations of cis-elements were obtained by site-specific mutagenesis with QuickChangeII XL site-directed mutagenesis kit, using wild-type minimal promoter (−131nt to + 20 nt region) as a template and primers containing mutated nucleotides (Table 2). The nucleotide sequence of the DNA fragments inserted into the pGL3-basic vector was verified by sequencing (Laragen Sequencing Facility) before use.
Table 1.
Sequence of primers used in PCR for generation of 5′- and 3′-deletions of SLC25A19 promoter
| Sequence (5′–3′) | Position |
|---|---|
| CCCTCGAGAACCGTGACGCTAGTTGTGTGCG | −631 to −609 |
| CCCTCGAGAGGTCGCAGCCCGGCTTCGCAAG | −271 to −249 |
| CCCTCGAGGTCCAGCTGTCCTGCCCTTGGTC | −171 to −149 |
| CCCTCGAGCCGGTGATATCTTGCGGGAGG | −131 to −111 |
| CCCTCGAGTCTGGCGGGGAGCTGCACGGTG | −71 to −50 |
| AAAAGCTTGAGATGCTTCTCTGGTCTGGTGTGACA | +194 to +220 |
| AAAAGCTTGGTAGAGGTTACTCACACGGCTCGGCGGGT | +53 to +82 |
| AAAAGCTTAGCGCTCTCCGCTCTCTCTAGCTC | +17 to +40 |
| AAAAGCTTGCTCCTCGCGCAGTCTTAACAG | −2 to +20 |
XhoI and HindIII restriction sites are shown in bold and underlined. The relative position of the primer is based on numbering the distal start of transcription initiation of SLC25A19 as position +1.
Table 2.
Sequence of primers used for site-directed mutagenesis of the minimal SLC25A19 promoter
| Upstream CCAAT box (−83 to −79) | |
| Mut 1 Forward | 5′-CCTCAAGCGCACAACCATTAATCCAGCGTCTGGCGGGGAGC-3′ |
| Mut 1 Reverse | 5′-GCTCCCCGCCAGACGCTGGATTAATGGTTGTGCGCTTGAGG-3′ |
| Mut 2 Forward | 5′-CCTCAAGCGCACAACCATCGATCCAGCGTCTGGCGGGGAGC-3′ |
| Mut 2 Reverse | 5′-GCTCCCCGCCAGACGCTGGATCGATGGTTGTGCGCTTGAGG-3′ |
| Mut 3 Forward | 5′-CCTCAAGCGCACAACCATTGATCCAGCGTCTGGCGGGGAGC-3′ |
| Mut 3 Reverse | 5′-GCTCCCCGCCAGACGCTGGATCGATGGTTGTGCGCTTGAGG-3′ |
| Downstream CCAAT box (−49 to −45) | |
| Mut 1 Forward | 5′-GGAGCTGCACGGTGATTAACCCAGGAGCCCGCCAGTTTCG-3′ |
| Mut 1 Reverse | 5′-CGAAACTGGCGGGCTCCTGGGTTAATCACCGTGCAGCTCC-3′ |
| Mut 2 Forward | 5′-GGAGCTGCACGGTGATCAGCCCAGGAGCCCGCCAGTTTCG-3′ |
| Mut 2 Reverse | 5′-CGAAACTGGCGGGCTCCTGGGCTGATCACCGTGCAGCTCC-3′ |
| Mut 3 Forward | 5′-GGAGCTGCACGGTGAGTTGCCCAGGAGCCCGCCAGTTTCG-3′ |
| Mut 3 Reverse | 5′-CGAAACTGGCGGGCTCCTGGGCAACTCACCGTGCAGCTCC-3′ |
| Outside CCAAT boxes (−64 to −63) | |
| Forward | 5′-CATTGGTCCAGCGTCTGGCGAAGAGCTGCACGGTGATTGGC-3′ |
| Reverse | 5′-GCCAATCACCGTGCAGCTCTTCGCCAGACGCTGGACCAATG-3′ |
Location of mutation is shown in bold and underlined.
2.5. Cell growth, transfection, and reporter gene assay
The human-derived liver HepG2 cells (passages 10–25, American Type Culture Collection, Manassas, VA) were grown in DMEM growth medium containing 10% fetal bovine serum, glutamine (0.29 g/l), sodium bicarbonate (3.6 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l). HepG2 cells (70–80% confluency in each well of 12-well plate) were cotransfected with 2 μg of test promoter construct and 100 ng of the Renilla transfection control plasmid Renilla luciferase-thymidine kinase (pRL-TK) (Promega) with Lipofectamine reagent (Life Technologies) according to the manufacturer’s instructions. In experiments involving NF-YA dominant negative mutant, HepG2 cells were cotransfected with 2 μg of SLC25A19 minimal promoter construct or pGL3-Control as described above along with varying amounts of eukaryotic expression vector for NF-YA dominant negative mutant protein. Renilla-normalized firefly luciferase activity was measured forty eight hours after transfection by using the Dual Luciferase Assay system (Promega). Data are presented as means ± SE of at least three independent experiments and given as fold over pGL3-Basic expression set arbitrarily at one.
2.6. EMSA
Nuclear extract from HepG2 cells was obtained with the commercially optimized NE-PER Nuclear and Cytoplasmic extraction reagents (Thermo Scientific Pierce, Rockford, Il) according to the manufacturer’s instructions. A 64-bp region of the minimal promoter spanning a sequence between −98 and −35 (wild-type or mutated in CCAAT sites) was used for EMSA. Wild-type promoter region had a sequence: 5′-CTCAAGCGCACAACCATTGGTCCAGCGTCTGGCGGGGAGCTGCACGGTGATTGGCC CAGGAGCC-3′. Mutated promoter fragment contained mutations either in the upstream/downstream CCAAT box or in the both boxes had a sequence: 5′-CTCAAGCGCACAACCATCGATCCAGCGTCTGGCGGGGAGCTGCACGGTGATCAGCC CAGGAGCC-3′ (mutations are underlined and shown in bold). Promoter fragment was biotin-labeled using Biotin 3′ end DNA labeling kit (Thermo Scientific Pierce). EMSA was performed using LightShift Chemiluminescent EMSA kit (Thermo Scientific Pierce) according to the manufacturer’s instructions. Binding reactions were carried out in 20 μl which contained 5 μg of nuclear extract, 20 fmol of biotin end-labeled DNA fragment, and 50 ng/μl poly (dI · dC), for 20–30 min at room temperature. Oligonucleotide competition analysis was conducted with a 100-fold molar excess of commercial CBF/NF-Y competitor oligonucleotides, consensus or mutant, (Santa Cruz Biotechnologies) or a 200-fold molar excess of unlabeled SLC25A19 DNA fragment. For the supershift assays, the nuclear extract was pretreated with 1–2μg anti-NF-YA, anti-NF-YB, anti-NF-YC antibody (or combination of the antibodies against all three subunits, 0.5 μg each) or 1μg normal rabbit IgG. DNA-protein complexes were separated on 6% DNA retardation gel(Invitrogen Life Technologies) in 0.5× Tris borate-EDTA at 100V, followed by electrophoretic transfer of binding reactions to nylon membrane and crosslink of transferred DNA to membrane. Biotin-labeled DNA was detected by chemiluminescence using Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific Pierce).
2.7. Chromatin immunoprecipitation (ChIP)
ChIP was performed with the ChIP assay kit (Millipore, Temecula, CA) according to manufacturer’s protocol. Briefly, HepG2 cells (1×106 cells) were treated with 1% formaldehyde (final concentration) for 10 min at 37°C (to crosslink proteins to DNA), lysed in SDS lysis buffer, followed by sonication of cell lysates to shear DNA to fragments of 200–1000 bp in length. Sonicated cell supernatant was incubated with 10 μg of NF-YA, NF-YB or normal rabbit IgG overnight at 4°C, followed by precipitation of immunocomplex on protein A agarose/salmon sperm DNA for 1 hr at 4°C. Immunoprecipitates were eluted and incubated for 4 hrs at 65°C (to reverse protein/DNA crosslinks). Samples were treated with proteinase K; DNA was purified by phenol/chloroform extraction and ethanol precipitation and then used for PCR. Primers used for amplification of the SLC25A19 promoter region from −271 to + 82 were 5′-AGGTCGCAGCCCGGCTTCGCAAG-3′(forward) and 5′ GGTAGAGGTTACTCACACGGCTCGGCGGGT - 3′(reverse). Primers used for amplification of the promoter region from −822 to −376 were 5′-GAAACAAGATTGCCCCATGAGTCG -3′ (forward) and 5′-GCCAGAACCCGGATAATCTCAAC -3′ (reverse). PCR conditions were denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 30 sec.
3. Results
3.1. Mapping of the transcriptional start site(s) of the SLC25A19 gene in HepG2 cells
As a first step in identifying the promoter region of SLC25A19, we determined the TSS of SLC25A19 in HepG2 cells using the rapid amplification of the cDNA ends (RACE) technique. Several clones for two prominent PCR products were obtained from 5′-RACE and were sequenced. The results showed the potential existence of two distinct TSSs (both represented by a guanosine) for SLC25A19 in HepG2 cells. Sites were identified as G at 5031and G at 5055 position using RefSeqGene information for the SLC25A19 gene deposited in GenBank (accession no. NG_008274). For convenience of our further data presentation the G of distal TSS will be mapped as +1 and the G from proximal TSS as + 25.
3.2. Cloning and identification of the SLC25A19 promoter
The originally reported sequence information for the SLC25A19 gene and its flanking sequence was used as a guide for the primer design, and a 1,080-bp genomic fragment was cloned from human genomic DNA. The identity of the cloned genomic DNA fragment was determined by sequencing. The fragment contains the full exon 1 of the SLC25A19 gene including two identified TSSs, part of the intron 1 (154 bp downstream from the exon 1) and extends to −860 bp upstream of the identified distal transcription initiation site (using this distal TSS as +1). Activity of the cloned putative promoter (mapped as −860/+220) was examined in a transient transfection experiment, using the Firefly luciferase reporter construct and the HepG2 cells. As shown in Fig. 1A, the cloned 5′-flanking region of the SLC25A19 gene was found to have significant promoter activity in transiently transfected HepG2 cells. Indeed, when compared to pGL3-basic vector, the −860/+220 reporter construct generated over 60-fold increase in luciferase activity.
Figure 1. Activity of the SLC25A19 promoter in HepG2 cells and deletion analysis.
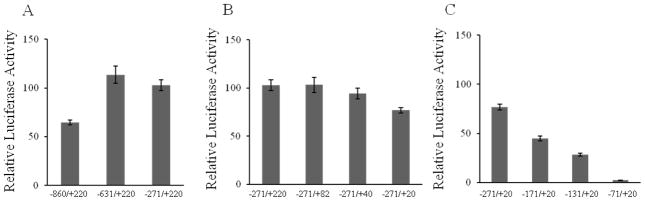
The cloned 1,080-bp 5′-regulatory region of the SLC25A19 (−860/+220) was used for 5′-deletion (A and C) and 3′-deletion (B) analysis as described in Materials and Methods. Promoter constructs in pGL3-Basic were transiently expressed in HepG2 cells for luciferase assays. Data are reported as relative firefly luciferase activity normalized to Renilla luciferase activity and represent means ± SE of at least 3 independent experiments, each performed in triplicate.
3.3. Determination of the minimal SLC25A19 promoter
We used a 5′- and a 3′-deletion analysis to determine the minimal genomic region required for basal activity of the SLC25A19 promoter. A series of 5′ and 3′ deletion reporter constructs were generated by PCR amplification and used for transient transfection of HepG2 cells. Initial 5′-deletion analysis using constructs with a variable 5′end, but a common 3′end at nucleotide +220 showed that deletion of nucleotides up to −271 does not reduce a promoter activity (Fig. 1A). Furthermore, we detected that genomic region −271/+220 has a promoter activity significantly higher (~1.6-fold) than the cloned −860/+220 region but comparable to the −631/+220 region. We concluded the presence of possible repressing elements between nucleotides −860 and −631. We then used region −271 to + 220 for further identification of the minimal promoter region. 3′-deletion analysis using constructs with a variable 3′ end, but a common 5′ end at nucleotide −271 showed that deletion of nucleotides from + 220 to + 20 slightly reduces luciferase activity (to ~ 75% compared to −271/+220 region) (Fig.1B). Construct −271/+20 retained the distal TSS and conferred ~120% of the activity of the cloned 1,080-bp promoter fragment. The further characterization of SLC25A19 promoter region from nucleotide −271 to + 20 was performed by 5′-deletion analysis (Fig. 1C). Deletion of nucleotides −271 to −131 significantly reduced luciferase activity (to ~37% compared to −271 to +20 region). Ablation of additional 60 nucleotides virtually abolished the reporter activity. We concluded that the minimal 5′-flanking region of SLC25A19 gene required for basal promoter activity is localized in a sequence between −131 and +20. The identified 151-bp minimal promoter contains distal TSS and represents ~ 44% of the activity of the cloned 1,080-bp promoter fragment.
3.4. Identification of potential cis-elements (transcription factors binding sites) and establishment of a key role of CCAAT boxes in promoter activity
The minimal SLC25A19 promoter sequence from nucleotides −131 to + 20 was analyzed for potential transcription factor(s) binding sites using MatInspector computational software (www.genomatix.de). The analysis revealed lack of the typical TATA motif and presence of two putative inverted CCAAT boxes (binding sites for NF-Y) as well as a number of putative cis-regulatory elements for several transcriptional factors including GATA-binding factor 2, transcriptional repressor CDP, Smad3 transcriptional factor, kidney-enriched kruppel-like factor, KLF15.
Since the CCAAT box is a prototypical promoter element required for transcriptional activation of many eukaryotic genes (Dorn et al., 1987; Dolfini et al., 2012), in our further characterization of the minimal SLC25A19 promoter we focused on two putative CCAAT boxes identified in its sequence. Figure 2 represents a genomic sequence of the minimal SLC25A19 promoter region and location of CCAAT boxes. As can be seen from the Figure 2, both CCAAT promoter elements are located in close proximity to TSS at about −80 and −50 from TSS with a distance between them at about 34 nucleotides. The distance and orientation of CCAAT elements in SLC25A19 promoter with respect to TSS as well as the distance between these CCAAT boxes are in perfect agreement with the Position Specific Frequency Matrix–PSFM, described for NF-Y (Dolfini et al., 2009).
Figure 2. Genomic sequence of the SLC25A19 minimal promoter region.

Schematic presentation depicting the minimal promoter sequence (−131 to +20). +1 refers the distal transcription start site mapped on human MTPPT mRNA. Two inverted CCAAT boxes are shown in bold text and underlined.
To study the functional contribution of the putative CCAAT boxes in governing the basal activity of the SLC25A19 promoter, we introduced point mutations into the core pentanucleotide sequence of the wild-type promoter (by means of site-directed mutagenesis) and examined the effect of these mutations on promoter activity in transiently transfected HepG2 cells. We generated a series of mutant reporter plasmids carrying specific mutations in the upstream, downstream, or in both CCAAT boxes (Fig. 3A). The results showed that mutating either CCAAT box individually or together leads to a significant (P < 0.01) decrease in activity of the minimal SLC25A19 promoter (Fig. 3B). In the case of mutating the CCAAT sites together the reduction in promoter activity was close to 90 % (compared to activity of the wild-type minimal promoter). In contrast to above, mutating two nucleotides (the two Gs at positions −64 and −63 to As) outside the core of CCAAT boxes (negative control) showed no significant effect on promoter activity. These findings suggest a key role of the two identified CCAAT boxes in basal activity of the SLC25A19 promoter. The data also suggests that both CCAAT boxes contribute significantly to promoter activity but each box on its own is unable to support basal promoter activity.
Figure 3. Mutational analysis of the CCAAT cis-regulatory elements in the SLC25A19 minimal promoter region.

(A) By means of site-directed mutagenesis mutations were introduced into the core pentanucleotide sequence of the upstream or downstream CCAAT box, or into the core sequence of both CCAAT boxes. Altered nucleotides are shown in bold and underlined. (B) The mutated minimal promoter constructs in pGL3-Basic were transiently expressed in HepG2 cells for luciferase assays. Data are reported as relative firefly luciferase activity normalized to Renilla luciferase activity and represent means ± SE of at least 3 independent experiments, each performed in triplicate. Construct entitled as Unrelated mutations represents mutating of two nucleotides (G at position −64 and G −63 to A at −64 and −63) outside the core of CCAAT boxes (negative control).
3.5. NF-Y binding in the SLC25A19 minimal promoter
CCAAT boxes are widely distributed cis-elements that known to be bound by the transcription factor NF-Y (Dolfini et al., 2012). NF-Y is a trimeric protein complex composed of three subunits, NF-YA, NF-YB, and NF-YC, which recognizes the CCAAT core and flanking nucleotides and binds to this CCAAT cis-element with high specificity and affinity (Bi et al., 1997; Romier at al., 2003).
In order to identify the transcription factors that bind to the SLC25A19 minimal promoter, the electromobility shift assay was performed using a 64-bp region of the minimal promoter spanning a sequence between −98 and −35 and nuclear extract from HepG2 cells. The EMSA revealed the formation of two major DNA/protein complexes resulting in two closely located major bands with the decreased gel mobility (bands 1 and 2) (Fig. 4; lane 2). Both complexes were specifically inhibited by unlabeled probe (200-fold molar excess; lane 3) and unlabeled consensus CBF/NF-Y oligonucleotides (100–fold molar excess; lane 5) resulting in disappearance of both bands on a gel. In contrast, the mutated CBF/NF-Y oligonucleotides (a 100–fold molar excess) was found to be insufficient in competing with a 64-bp minimal promoter region for formation of both complexes (Fig. 4; lane 6).
Figure 4. EMSA with oligonucleotide competition.
Gel shift assay was performed with the biotin-labeled a 64-bp region of the SLC25A19 minimal promoter spanning a sequence between −98 and −35 and nuclear extract from HepG2 cells as described in Materials and Methods. A 200-fold molar excess of unlabeled fragment (self), 100-fold molar excess of NF-Y consensus oligonucleotide (NF-Y), or 100-fold molar excess of NF-Y mutated oligonucleotide (NF-Y mut) was used for oligonucleotide competition. Two major DNA/protein complexes are numbered and indicated with arrows.
Direct evidence of NF-Y binding to the SLC25A19 minimal promoter was obtained from super-shift analysis with the use of specific antibodies against NF-Y subunits. HepG2 nuclear extracts were pre-incubated with the individual antibodies against A, B, or C subunit of NF-Y trimer and with the mixture of antibodies against all three subunits. As shown in Fig. 5, NF-YA and NF-YB antibodies (but not NF-YC for reason that could be due to inability of this particular antibody to work in our experimental conditions) as well as a mixture of all three subunit antibodies caused a supershift (i. e., a dramatic decrease in mobility of both DNA/protein complexes with simultaneous complete disappearance of band 1 and 2). A nonspecific IgG antibody that served as a negative control in our studies had no effect on mobility of band 1 and 2. These results suggest that NF-Y can bind to the −98 and−35 region of the SLC25A19 minimal promoter using its inverted CCAAT boxes as targets for binding.
Figure 5. Binding of NF-Y transcription factor to theSLC25A19 minimal promoter.
Gel shift assay was performed with the biotin-labeled a 64-bp region of the SLC25A19minimal promoter spanning a sequence between −98 and −35 and the nuclear extract from HepG2 cells as described in Materials and Methods. Binding reaction was performed in the absence of antibody (no antibody), in the presence of nonspecific IgG (IgG), or in the presence of antibody against subunit A (NF-YA), subunit B (NF-YB), subunit C (NF-YC), or combination of antibodies against all three subunits of NF-Y (NF-YA, B and C). Arrows and numbers indicate two major DNA/protein complexes with arrows at top showing the supershifted bands.
In another study, we introduced point mutations into CCAAT core sequences of a 64-bp promoter fragment and used it in EMSA. Our aim here is to gain more information about the relative contribution of CCAAT boxes to basal promoter activity. Promoter fragment that contained mutations either in the upstream or downstream CCAAT box revealed the same mobility shift pattern as wild-type fragment (with formation of DNA/protein complex 1 and 2) (Fig. 6) indicating that both CCAAT are functional in respect of NF-Y binding. When both CCAAT boxes inside the promoter fragment were mutated, no complexes were detected by EMSA.
Figure 6. DNA/protein profile from the EMSA using the mutated SLC25A19 minimal promoter.
Gel shift assay was performed with the biotin-labeled a 64-bp region of the SLC25A19 minimal promoter (−98 to −35) and the nuclear extract from HepG2 cells. Binding reaction was performed with the wild type promoter fragment (WT) or with the promoter fragment harboring mutations in the distinct CCAAT box (upstream or downstream) and in both CCAAT boxes (both). Two major DNA/protein complexes are numbered and indicated with arrows.
3.6. In vivo binding of NF-Y to the SLC25A19 promoter through the CCAAT boxes
In order to examine the binding of NF-Y to the SLC25A19 promoter in vivo, chromatin immunoprecipitation (ChIP) analysis was performed using the cross-linked chromatin prepared from HepG2 cells. Chromatin was immunoprecipitated with the antibody against NF-YA or NF-YB, and after cross-link reversal, the DNA was used for PCR amplification of promoter region harboring both the upstream and downstream CCAAT boxes (region −271 to + 82). In parallel, as a negative control, the same DNA was used for PCR amplification of promoter region that does not contain any CCAAT cis-elements (region −822 to −376). The amplification of the region −271 to + 82 was detected in immunoprecipitates obtained with antibodies against NF-YA or NF-YB, but not with the rabbit normal IgG (Fig. 7; PCR product of 352 bp). In contrast, in case of the region −822 to −376 amplification was detected only in the input sample, but not in the any immunoprecipitate (Fig. 7; PCR product of 446 bp), indicating the specificity of the ChIP analysis. These data demonstrate that anti-NF-Y antibodies are able to specifically precipitate the minimal SLC25A19 promoter fragment, which, as established in our in vitro studies, is a target for NF-Y, and provide a direct confirmation of NF-Y association with the minimal SLC25A19 promoter in vivo.
Figure 7. Binding of NF-Y transcription factor to the SLC25A19 promoter in vivo.
ChIP assay was performed using the HepG2 cells and the antibody against subunit A (NF-YA) or subunit B (NF-YB) of NF-Y. The same immunoprecipitated DNA was PCR amplified using primers specific for the region containing CCAAT boxes (−271 to + 82) or unrelated part of the promoter region (−822 to +376). A control reaction with normal IgG (IgG) served as a negative control.
3.7. Direct evidence for the requirement of NF-Y for basal SLC25A19 promoter activity in vivo
The role of NF-Y binding toward basal activity of the SLC25A19 promoter in vivo was tested with the use of a dominant negative NF-YA mutant. Overexpression of mutant form of NF-YA subunit is known to cause the inhibition of NF-Y dependent transcription due to formation of defective NF-Y trimer complexes which are unable to bind the DNA (Mantovani et al., 1994). In these studies, we transiently transfected HepG2 cells with the promoter-luciferase construct containing the minimal SLC25A19 promoter along with the varying amounts of NF-YA mutant expression plasmid. The results of luciferase assay showed that addition of the NF-YA mutant expression vector led to a significant (P < 0.01) decrease in SLC25A19 promoter activity and this decrease was found to be dose-dependent (Fig. 8A). Indeed, 2.5 μg and 5.0 μg of a dominant negative NF-YA mutant caused decrease in promoter activity by 47% and 69%, respectively, compared to control (i.e., minimal SLC25A19 promoter-luciferase construct). To verify the specificity of the observed effect, we performed co-transfection of HepG2 cells with the NF-YA mutant expression plasmid together with the pGL3-Control plasmid, where the luciferase reporter gene expression is driven by the SV40 promoter. As can be seen from Fig. 8B, high amount of NF-YA mutant plasmid (5.0 μg) did not cause the reduction in the SV40 promoter activity, suggesting that the observed NF-YA mutant-mediated inhibition of SLC25A19 promoter is specific. These data provide the direct evidence of the important role of a functional NF-Y trimer complex in maintaining of basal SLC25A19 promoter activity in vivo.
Figure 8. Essential role of NF-Y in the SLC25A19 promoter functionality in vivo.

HepG2 cells were cotransfected with 2 μg of SLC25A19 minimal promoter construct (A) or pGL3-Control (B) along with varying amounts of eukaryotic expression vector for NF-YA dominant negative mutant protein (NF-YA dn), followed by luciferase assay. Data are reported as relative firefly luciferase activity normalized to Renilla luciferase activity and represent means ± SE of 3 independent experiments, each performed in triplicate.
4. Discussion
There is nothing currently known about how MTPPT is regulated. Meantime, knowledge of how MTPPT is transcriptionally/post-transcriptionally regulated is of great importance since this carrier mediates transport of an important co-factor from the cytoplasm into mitochondria, and thus, plays a critical role in functionality of mitochondrial TPP-requiring enzymes and overall mitochondria/cell functions and health. As a first step to understanding how the MTPPT system is transcriptionally regulated, we cloned the 5′-regulatory region of the SLC25A19 gene, identified the minimal promoter region needed for basal activity, and characterized important transcriptional factors that are required for promoter activity.
To locate the putative SLC25A19 promoter, we mapped the TSS(s) in the region upstream of the translational start codon in human liver HepG2 cells and identified two TSSs (both guanosine) in close proximity to each other (with a distance in between them of 25 nucleotides). Both sites were located inside exon 1 of SLC25A19 (exon 1 is similar for all three reported transcript variants (Iacobazzi et al., 2001)). However, we were unable to detect TSS matching the first nucleotide of the exon 1 that could be due to the low abundance of the longer transcript in HepG2 cells. Guided by location of TSSs, we then amplified and cloned a genomic region of 1,080 bp from human chromosome 17 upstream of the translation start site for SLC25A19. Because the translation initiator ATG sequence is located inside exon 4 of the SLC25A19 gene, the cloned putative promoter region did not contain the translation initiation site. Partial sequence from intron 1 was included in the cloned fragment based on the information obtained from the promoter prediction program (http://www-bimas.cit.nih.gov/cgi-bin/molbio/proscan) showing the existence of this intron-containing sequence inside the predicted promoter region. The cloned genomic region that extended to −860 bp upstream of the distal transcription initiation site (using the G of distal TSS as +1) demonstrated significant promoter activity in transiently transfected HepG2 cells. Using both 5′-deletion and 3′-deletion analysis we further characterized the cloned promoter and identified the minimal region required for basal SLC25A19 promoter activity, which retained ~44% of the activity of the cloned 1,080-bp promoter fragment. This region (between −131 and +20) was found to be TATA-less, GC-rich, and contained a number of putative cis-regulatory elements, including two CCAAT boxes.
CCAAT box represents one of the most ubiquitous promoter elements found in promoters of many eukaryotic genes, including housekeeping, inducible, cell-cycle-regulated, developmentally controlled, and tissue-specific genes (Berry et al., 1992; Kabe et al., 2005; Manni et al., 2001; Ronchi et al., 1996; Roy and Lee, 1995). It is recognized by the NF-Y transcriptional factor that binds to CCAAT box with high specificity and affinity, and, through synergistic interactions with the nearby transcriptional factors, plays a crucial role in transcription initiation (Dolfini et al., 2012). The NF-Y is heterotrimeric protein complex with histone-like features, composed of three subunits, NF-YA, NF-YB, and NF-YC (all three subunits are required for DNA binding). Numerous CCAAT harboring promoters have been reported to contain two or more CCAAT boxes, particularly for cell-cycle-regulated genes (Farina et al., 1999; Manni et al., 2001). The two CCAAT boxes that we identified in the minimal SLC25A19 promoter are in inverted orientation and located in the region between −83 and −79 nt (upstream box) and in between −49 and −45 nt (downstream box) from the distal TSS. These our findings are in a good agreement with the bioinformatics analysis performed on a great number of eukaryotic promoters that showed either orientation of CCAAT boxes inside the promoter and location of CCAAT boxes within 600 bp from a TSS with the preferable location in the −80 region (Dolfini et al., 2009). A distance between two CCAAT boxes inside the SLC25A19 promoter was found to be about 34 nt. This our finding also fits with the statistical analysis (Dolfini et al., 2009) that reported the respective distance to be about 32 nt, which corresponds to three turns of the double DNA helix. NF-Y is known to have an absolute requirement for each of the CCAAT pentanucleotides from the core sequence as well as a strong preference for the specific flanking nucleotides (10 nucleotides upstream and 15 nucleotides downstream from the CCAAT core). In agreement with this, the consensus sequences of both CCAAT boxes inside SLC25A19 promoter indeed demonstrate a conservation of almost all nucleotides (from pentanucleotide core as well as from flanks) required for NF-Y binding.
We examined the role of CCAAT boxes in regulating the activity of the SLC25A19 promoter by mutating the CCAAT pentamer and observed that the mutations of either CCAAT box individually as well as the mutations of both boxes almost abolished the activity of the minimal SLC25A19 promoter. These findings led us to a conclusion that both upstream and downstream CCAAT boxes are the key cis-elements important for the SLC25A19 promoter activity.
Binding of NF-Y to the SLC25A19 promoter through its CCAAT boxes was demonstrated in our in vitro EMSA and supershift EMSA studies and then confirmed in vivo by ChIP analysis. The promoter region used in our EMSA studies was designed to contain two inverted CCAAT boxes including their core pentanucleotides and their flanking nucleotides (10 nucleotides upstream and 15 nucleotides downstream from the CCAAT core). Major DNA/protein complexes were competed away with unlabeled consensus NF-Y oligonucleotides, but not with the mutated oligonucleotides. In supershift experiments performed with the use of specific antibodies against NF-Y subunits we observed the clear supershift of the identified DNA/protein complexes that allowed us to conclude that NF-Y indeed binds to the CCAAT boxes of SLC25A19 minimal promoter. The direct functional evidence for NF-Y requirement in SLC25A19 promoter activity in vivo was obtained by the use of a dominant negative NF-YA mutant. Overexpression of mutant form of NF-YA subunit is known to cause the inhibition of NF-Y dependent transcription due to formation of defective NF-Y trimer complexes which are unable to bind the DNA (Mantovani et al., 1994). Using cells transiently transfected with dominant negative NF-YA mutant, we demonstrated dramatic reduction of SLC25A19 promoter activity.
We extended our knowledge of the SLC25A19 promoter functioning with respect to relative contribution of CCAAT boxes by using CCAAT-mutated promoter fragment in EMSA. Mutations in either the upstream or downstream CCAAT box did not change the mobility shift pattern indicating that each box on its own can bind the NF-Y although with differences in binding affinity; indeed, we observed a better binding of NF-Y to the downstream CCAAT box. Mutations in downstream box led to a marked decrease in detection of both bands (longer exposure was required to detect complexes), suggesting that the upstream box behaves as a weak cis-element and preferable cis-element for NF-Y binding is the downstream CCAAT box. In contrast, mutations of both CCAAT boxes led to complete abolishment of DNA/protein formation in EMSA studies. Given that each CCAAT box on its own can bind the NF-Y but is unable to maintain the basal promoter activity (as we observed in our luciferase assay studies), we hypothesize that the SLC25A19 promoter activity requires the cooperative interaction between NF-Y molecules bound to separate CCAAT sites. Distance in about 34 nucleotides between two CCAAT boxes found in the structure of SLC25A19 promoter supports possibility of cooperative interaction for NF-Y molecules by providing precise stereospecific alignment in a way it was discussed in the literature previously (Dolfini et al., 2009).
In conclusion, this study represents the first characterization of the SLC25A19 promoter and reports the identification of NF-Y as a key transcriptional factor involved in regulation of basal promoter activity. These findings should serve as base for future studies of factors that regulate the expression and functionality of the MTPPT with respect to maintenance of mitochondrial and overall cellular thiamine/TPP homeostasis.
HIGHLIGHTS.
Study provides identification of SLC25A19 minimal promoter.
NF-Y transcription factor plays a key role in SLC25A19 basal promoter activity.
Study is important for understanding of transcriptional regulation of SLC25A19.
Acknowledgments
This work was supported by grants from the Dept. of Veterans Affairs and the NIH (DK-56061 and AA-18071).
List of abbreviations
- A
Adenosine
- bp
Base pair(s)
- C
Cytidine
- cDNA
DNA complementary to RNA
- Chip
Chromatin immunoprecipitation
- DMEM
Dulbecco’s Modified Eagle Medium
- EDTA
Ethylenediaminetetraacetic acid
- EMSA
Electromobility shift assay
- G
Guanosine
- IgG
Immunoglobulin G
- kb
Kilobase
- MTPPT
Mitochondrial thiamine pyrophosphate transporter
- MCF
Mitochondrial carrier family
- nt
Nucleotide(s)
- NF-Y
Nuclear factor Y
- PCR
Polymerase chain reaction
- RACE
Rapid amplification of the cDNA ends
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SDS
Sodium dodecyl sulphate
- SE
Standard error of mean
- SLC25A19
Solute carrier family 25 (mitochondrial thiamine pyrophosphate carrier), member 19
- T
Thymidine
- THTR-1
Thiamine transporter-1
- THTR-2
Thiamine transporter-2
- TPP
Thiamine pyrophosphate
- Tris
2-Amino-2-hydroxymethyl-propane-1,3-diol
Footnotes
Author Contributions
S.M.N. and H.M.S. designed research, analyzed data and wrote the paper; S.M.N. and J.E.V. performed the experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barile M, Passarella S, Quangliariello E. Thiamin pyrophosphate uptake into isolated rat liver mitochondria. Arch Biochem Biophys. 1990;280:352–357. doi: 10.1016/0003-9861(90)90341-u. [DOI] [PubMed] [Google Scholar]
- Berdanier CD. Advanced Nutrition Micronutrients. CRC Press; Boca Raton, FL: 1998. pp. 80–88. [Google Scholar]
- Berry M, Grosveld F, Dillon N. A single point mutation is the cause of the Greek form of hereditary persistence of fetal haemoglobin. Nature. 1992;358:499–502. doi: 10.1038/358499a0. [DOI] [PubMed] [Google Scholar]
- Bettendorff L. The compartmentation of phosphorylated thiamin derivatives in cultured neuroblastoma cells. Biochim Biophys Acta. 1994;1222:7–14. doi: 10.1016/0167-4889(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Bettendorff L. Thiamin homeostasis in neuroblastoma cells. Neurochem Int. 1995;26:295–302. doi: 10.1016/0197-0186(94)00123-c. [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamin deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem. 1995;64:2013–2021. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Wins P. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009;76:2917–2925. doi: 10.1111/j.1742-4658.2009.07019.x. [DOI] [PubMed] [Google Scholar]
- Bi W, Wu L, Coustry F, de Crombrugghe B, Maity SN. DNA binding specificity of the CCAAT-binding factor CBF/NF-Y. J Biol Chem. 1997;272:26562–26572. doi: 10.1074/jbc.272.42.26562. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamin deficiency. J Neuropath Exp Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Cusaro G, Rindi G, Sciorelli G. Subcellular distribution on thiamin-pyrophosphokinase and thiamin-pyrophosphatase activities in rat isolated enterocytes. Int J Vit Nutr Res. 1977;47:99–106. [PubMed] [Google Scholar]
- Deus B, Blum H. Subcellular distribution of thiamin pyrophosphokinase activity in rat liver and erythrocytes. Biochim Biophys Acta. 1970;219:489–492. doi: 10.1016/0005-2736(70)90229-4. [DOI] [PubMed] [Google Scholar]
- Diaz GA, Banikazemi M, Oishi K, Desnick RJ, Gelb BD. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat Genet. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- Dolce V, Fiermonte G, Runswick MJ, Palmieri F, Walker JE. The human mitochondrial deoxynucleotide carrier and its role in the toxicity of nucleoside antivirals. Proc Natl Acad Sci U S A. 2001;98:2284–2288. doi: 10.1073/pnas.031430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfini D, Zambelli F, Pavesi G, Mantovani R. A perspective of promoter architecture from the CCAAT box. Cell Cycle. 2009;8:4127–4137. doi: 10.4161/cc.8.24.10240. [DOI] [PubMed] [Google Scholar]
- Dolfini D, Gatta R, Mantovani R. NF-Y and the transcriptional activation of CCAAT promoters. Crit Rev Biochem Mol Biol. 2012;47:29–49. doi: 10.3109/10409238.2011.628970. [DOI] [PubMed] [Google Scholar]
- Dorn A, Bollekens J, Staub A, Benoist C, Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987;50:863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Eudy JD, Spiegelstein O, Barber RC, Wlodarczyk BJ, Talbot J, Finnell RH. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol Genet Metab. 2000;71:581–590. doi: 10.1006/mgme.2000.3112. [DOI] [PubMed] [Google Scholar]
- Farina A, Manni I, Fontemaggi G, Tiainen M, Cenciarelli C, Bellorini M, Mantovani R, Sacchi A, Piaggio G. Down-regulation of cyclin B1 gene transcription in terminally differentiated skeletal muscle cells is associated with loss of functional CCAAT-binding NF-Y complex. Oncogene. 1999;18:2818–2827. doi: 10.1038/sj.onc.1202472. [DOI] [PubMed] [Google Scholar]
- Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L. Thiamin status in humans and content of phosphorylated thiamin derivatives in biopsies and cultured cells. Plos One. 2010;5:e13616. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobazzi V, Ventura M, Fiermonte G, Prezioso G, Rocchi M, Palmieri F. Genomic organization and mapping of the gene (SLC25A19) encoding the human mitochondrial deoxynucleotide carrier (DNC) Cytogenet Cell Genet. 2001;93:40–42. doi: 10.1159/000056945. [DOI] [PubMed] [Google Scholar]
- Kabe Y, Yamada J, Uga H, Yamaguchi Y, Wada T, Handa H. NF-Y is essential for the recruitment of RNA polymerase II and inducible transcription of several CCAAT box-containing genes. Mol Cell Biol. 2005;25:512–522. doi: 10.1128/MCB.25.1.512-522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Samuels DC. The evidence that the DNC (SLC25A19) is not the mitochondrial deoxyribonucleotide carrier. Mitochondrion. 2008;8:103–108. doi: 10.1016/j.mito.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, Chen A, Castegna A, Verhoeven N, Mathews CK, Palmieri F, Biesecker LG. Knockout of Slc25a19 causes mitochondrial thiamin pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci U S A. 2006;103:15927–15932. doi: 10.1073/pnas.0607661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni I, Mazzaro G, Gurtner A, Mantovani R, Haugwitz U, Krause K, Engeland K, Sacchi A, Soddu S, Piaggio G. NF-Y mediates the transcriptional inhibition of the cyclin B1, cyclin B2, and cdc25C promoters upon induced G2 arrest. J Biol Chem. 2001;276:5570–5576. doi: 10.1074/jbc.M006052200. [DOI] [PubMed] [Google Scholar]
- Mantovani R, Li XY, Pessara U, Hooft van Huisjduijnen R, Benoist C, Mathis D. Dominant negative analogs of NF-YA. J Biol Chem. 1994;269:20340–20346. [PubMed] [Google Scholar]
- Portari GV, Marchini JS, Vannucchi H, Jordao AA. Antioxidant effect of thiamin on acutely alcoholized rats and lack of efficacy using thiamin or glucose to reduce blood alcohol content. Basic Clin Pharmacol Toxicol. 2008;103:482–486. doi: 10.1111/j.1742-7843.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- Romier C, Cocchiarella F, Mantovani R, Moras D. The NF-YB/NF-YC structure gives insight into DNA binding and transcription regulation by CCAAT factor NF-Y. J. Biol. Chem. 2003;278:1336–1345. doi: 10.1074/jbc.M209635200. [DOI] [PubMed] [Google Scholar]
- Ronchi A, Berry M, Raguz S, Imam A, Yannoutsos N, Ottolenghi S, Grosveld F, Dillon N. Role of the duplicated CCAAT box region in gamma-globin gene regulation and hereditary persistence of fetal haemoglobin. EMBO J. 1996;15:143–149. [PMC free article] [PubMed] [Google Scholar]
- Roy B, Lee AS. Transduction of calcium stress through interaction of the human transcription factor CBF with the proximal CCAAT regulatory element of the grp78/BiP promoter. Mol Cell Biol. 1995;15:2263–2274. doi: 10.1128/mcb.15.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton CK, Martin PR. Molecular mechanisms of thiamin utilization. Curr Mol Med. 2001;1:197–207. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- Spiegel R, Shaag A, Edvardson S, Mandel H, Stepensky P, Shalev SA, Horovitz Y, Pines O, Elpeleg O. SLC25A19 Mutation as a cause of Neuropathy and Bilateral Striatal Necrosis. Ann Neurol. 2009;66:419–424. doi: 10.1002/ana.21752. [DOI] [PubMed] [Google Scholar]
- Siu VM, Ratko S, Prasad AN, Prasad C, Rupar CA. Amish microcephaly: long-term survival and biochemical characterization. Am J Med Genet A. 2010;152A:1747–1751. doi: 10.1002/ajmg.a.33373. [DOI] [PubMed] [Google Scholar]
- Tanphaichirt V. Modern Nutrition in Health and Disease. Lea and Febiger; New York: 1994. pp. 359–375. [Google Scholar]
- Todd KG, Butterworth RF. Early microglial response in experimental thiamin deficiency: an immunohistochemical analysis. Glia. 1999;25:190–198. doi: 10.1002/(sici)1098-1136(19990115)25:2<190::aid-glia9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: Davis; 1989. [Google Scholar]






