Abstract
This study examined discharge rate modulation at respiratory (0–0.5 Hz) and beta (16–32 Hz) frequencies in trapezius motor units active during voluntary contractions and during periods of instructed rest under conditions of low and high psychosocial stress. In separate sessions, single motor unit activity was recorded from the trapezius muscle of healthy women during low-intensity voluntary contractions and during periods of instructed muscle rest that followed voluntary contractions. The level of psychosocial stress during periods of instructed muscle rest was manipulated using a verbal math task combined with social evaluative threat which increased perceived anxiety, heart rate, and blood pressure (P ≤ 0.002). Discharge rate modulation was quantified by the mean power of motor unit discharge rate profiles within frequency bands of interest. Under low stress conditions, motor units active during instructed rest had greater power at 0–0.5 Hz (P = 0.002) and less power at 16–32 Hz (P = 0.009) compared to those active during voluntary contraction. Exposure to the stressor increased the amount of motor unit activity during instructed rest (P = 0.021) but did not alter the power of discharge rate modulation at 0–0.5 Hz (P = 0.391) or 16–32 Hz (P = 0.089). These results indicate that sustained motor unit activity during periods of instructed muscle rest has a lesser contribution from inputs at beta frequencies and a greater contribution from inputs at respiratory frequencies than present during low-intensity voluntary contractions. Furthermore, increases in motor unit activity when exposed to stressors during periods of instructed rest are not caused by changes in inputs at respiratory or beta frequencies.
Keywords: Motor unit, Trapezius, Beta oscillation, Respiration, Stress, Electromyography
Introduction
Occupational studies suggest that rest periods in trapezius muscle activity throughout the workday may be important to avoid the development of neck pain (Veiersted et al. 1993; Hagg and Astrom 1997; Sandsjo et al. 2000). It has even been suggested that an inability of the muscle to relax following active contractions may be a better predictor of injury than the absolute magnitude or frequency of muscle activation (Lundberg 1999). However, activation of trapezius motor units has been observed experimentally even when no explicit motor task is being performed (Kitahara et al. 2000; Lundberg et al. 2002). Furthermore, acute exposure to psychosocial stressors has been shown to reduce the frequency of rest periods in the trapezius muscle (McLean and Urquhart 2002; Schleifer et al. 2008). The mechanisms underlying sustained activation of trapezius motor units in the absence of physical task demands and during exposure to psychosocial stressors are not known.
One potential explanation for sustained muscle activity in the absence of physical task demands may involve respiratory inputs to the trapezius motor neuron pool. Phase relations between trapezius muscle activity and chest wall movement during singing (Pettersen et al. 2005; Pettersen and Westgaard 2005) and modulation of motor unit discharge rates at respiratory frequencies during tasks performed with the upper extremities (Westgaard et al. 2006) suggest that the trapezius muscle receives respiratory inputs during voluntary motor tasks. However, it is not known whether respiratory inputs contribute to motor unit activity during periods of intended rest, or whether these inputs differ from those received during voluntary contractions. A second potential explanation for sustained trapezius activity may involve the postural role of this muscle. The trapezius is an axioscapular muscle, which acts bilaterally to help stabilize the neck and shoulder girdle (Culham and Peat 1993), and may therefore remain active in a postural role even in the absence of other physical task demands. It has been proposed that the maintenance of a stable postural set is facilitated by tonic descending oscillations from pyramidal tract neurons in the beta frequency range (Gilbertson et al. 2005; Androulidakis et al. 2006, 2007; Kristeva et al. 2007). Beta oscillations from the motor cortex can be detected as modulation of motor unit discharge rates at beta (16–32 Hz) frequencies (Conway et al. 1995; Baker et al. 1997) and are most pronounced while holding an isometric contraction or maintaining a steady limb position (Sanes and Donoghue 1993; Conway et al. 1995; Baker et al. 1997; Baker 2007). Although these observations suggest a functional role for beta oscillations in maintaining posture, modulation of trapezius motor units at beta frequencies has not previously been examined.
Acute exposure to psychosocial stressors has been shown to reduce the frequency of rest periods in trapezius muscle activity (McLean and Urquhart 2002; Schleifer et al. 2008). Although respiration is under the control of the sympathetic nervous system and often increases in response to stress, the one study that previously examined changes in respiratory modulation of motor unit activity during exposure to a stressor reported decreased modulation at these frequencies (Westgaard et al. 2006). However, the decrease in respiratory modulation observed in this study may have been confounded by changes in the motor task across stress conditions.
Postural inputs to the motor neuron pool during exposure to a stressor have received even less attention. Beta oscillations in the activity of pyramidal tract neurons are inversely related to the excitability of the motor cortex (Chen et al. 1999; Baker and Baker 2003; Maki and Ilmoniemi 2010), such that increases in cortical excitability are accompanied by decreases in cortical oscillations at beta frequencies. There is evidence that stress can influence the excitability of the motor cortex (Wassermann et al. 2001; Centonze et al. 2005), suggesting that stress might also affect cortical oscillations at beta frequencies. This hypothesis is supported by evidence that corticomuscular coherence at beta frequencies is reduced in the presence of a cognitive load (Kristeva-Feige et al. 2002); however, these results were obtained during voluntary contraction of a forearm muscle and may not explain stress-evoked increases in trapezius muscle activity during periods of intended muscle rest.
The first aim of this study was to compare the modulation of trapezius motor unit discharge rates at respiratory (0–0.5 Hz) and beta (16–32 Hz) frequencies between motor units active in the absence of physical task demands and motor units active during steady volitional contractions. The second aim of this study was to determine whether acute exposure to psychosocial stressors in the absence of physical task demands alters the modulation of trapezius motor unit discharge rates at these frequencies. We hypothesized that the discharge of trapezius motor units would be modulated at 0–0.5 and 16–32 Hz, consistent with respiratory and beta contributions to tonic and voluntary activation of the trapezius muscle. We further hypothesized that discharge rate modulation at these frequencies would be altered both by voluntary contraction and by acute exposure to psychosocial stressors, reflecting a state-dependent shift in the distribution of synaptic inputs to the motor neuron pool. Specifically, we expected to observe (1) greater modulation at 16–32 Hz and reduced modulation at 0–0.5 Hz for motor units active during voluntary contractions compared to motor units active in the absence of physical task demands due to a greater requirement for postural control during voluntary force generation; (2) greater modulation at 0–0.5 Hz during acute exposure to a stressor due to enhanced sympathetic activation of respiration; and (3) reduced modulation at 16–32 Hz during acute exposure to a stressor reflecting an increase in cortical excitability.
Methods
Subjects
Motor units active in the absence of physical task demands were recorded from 15 healthy women ranging from 23 to 55 years of age (mean (SD) 35.5 (10.8) years). Motor units active during low-intensity voluntary contractions were recorded from nine healthy women ranging from 23 to 50 years of age (mean (SD) 31.4 (9.3) years). Six women participated in both experiments. All subjects were free of neck pain at the time of the study and reported no history of neck pain or other neurological or orthopedic impairments in the upper limbs. All participants provided informed consent in accordance with procedures approved by of the Colorado Multiple Institutional Review Board. These procedures included additional protections for a partial waiver of consent required for the stress manipulation as described below.
Experimental protocol
For all experiments, subjects were seated upright with their hip and knee joints flexed approximately 90° and their feet resting comfortably on a foot rest. The arms were abducted approximately 45° and positioned in line with the trunk, with the elbows flexed and the forearms supported on arm rests parallel to the floor. Custom restraints were placed over each acromion preventing movement of the shoulders. Each restraint consisted of a padded force transducer (1112 N range; 7.6 mV/N; P310, Cooper Instruments, Warrenton, VA, USA) mounted on a vertical metal restraint that was adjusted to fit securely over the acromion with the shoulders in a relaxed position. Elevation of the shoulders exerted a compressive force on the restraints. The force exerted by the left shoulder was displayed in real time on a feedback screen positioned at eye level approximately one meter in front of the subject. The position of the screen and the gain of the visual feedback were kept constant across experimental conditions.
Data from motor units active in the absence of physical task demands were obtained from women who participated in a previous study regarding the effects of acute exposure to psychosocial stressors on motor unit discharge behavior during voluntary ramp contractions (Stephenson and Maluf 2010). In this study, subjects performed five brief voluntary ramp contractions of the trapezius muscle during exposure to low and high stress conditions. Each contraction was followed by a 60-s rest period in which visual feedback of shoulder elevation force was removed, and subjects were instructed to relax their shoulders. These rest periods are subsequently referred to as periods of instructed muscle rest. Subjects began counting at the start of each voluntary contraction and continued counting throughout the 60-s rest period such that the stress manipulation (see below) was maintained during periods of instructed muscle rest between successive voluntary contractions. Despite specific instructions to relax the muscle, motor unit activity was observed during periods of instructed muscle rest in 15 of the 21 subjects. These data comprise the present study sample.
The voluntary ramp contractions reported in Stephenson and Maluf (2010) were performed while matching a triangular force template, such that the voluntary command to the motor neuron pool varied with time. Furthermore, the voluntary contractions reached intensities of up to 30% of maximum force, and the mean discharge rate was much higher than that observed during periods of instructed muscle rest. Therefore, these voluntary contractions were not suitable for comparison with the tonic motor unit discharge observed during instructed rest, and an additional experiment was conducted to examine motor unit discharge behavior during steady-state voluntary contractions. For these experiments, subjects performed a low-intensity isometric contraction of the trapezius muscle by matching shoulder elevation force to a steady target displayed on the feedback screen. The target force was initially set to a level that resulted in regular discharge of an isolated motor unit, and was then reduced every 30 s until motor unit discharge was no longer observed (see Fig. 1). The lowest contraction level in which regular motor unit discharge was observed was selected for analysis. To enable comparison with the motor unit activity recorded during periods of instructed muscle rest, these experiments were performed during exposure to the low stress condition as described below.
Fig. 1.
Representative example of the procedure used to identify minimum motor unit discharge rates during voluntary contractions. The target force was initially set to a level that resulted in regular discharge of an isolated motor unit and was then reduced every 30 s until motor unit discharge was no longer observed. The lowest contraction level at which regular motor unit discharge was observed (indicated by vertical dashedlines) was selected for analysis. In this example, the contraction level corresponding to the minimum discharge rate was 1.9% maximum, and the subject could not reduce the contraction intensity further without fully relaxing, whereby the motor unit ceased to discharge
Psychosocial stress manipulation
Levels of arousal were manipulated using a psychosocial stressor comprising a verbal math task (Noteboom et al. 2001; Lundberg et al. 2002) combined with social evaluative threat (Dickerson and Kemeny 2004). Subjects were informed that the purpose of the study was to examine the effects of mental concentration and therefore remained naïve to the stress manipulation until the debriefing that followed the high stress condition. To control for attention effects, a low stress condition was performed in which subjects were asked to verbally count backwards by five from a four-digit number that was evenly divisible by five. Subjects were provided with a new number prior to each contraction and were required to continue counting throughout the 60-s rest period. Subjects were told that these were practice trials in which their performance would not be monitored. Encouragement and positive feedback were provided during the low stress condition regardless of actual performance.
The low stress condition was followed by a high stress condition in which the diYculty of the math task was increased by asking the subject to verbally count backwards by a randomly selected one- or two-digit number from a randomly selected four-digit number. Prior to this condition, subjects were informed that it was extremely important to perform the test as fast and accurately as possible. Subjects were instructed that their performance would be videotaped and graded against other participants and were offered a monetary incentive for good performance. The high stress condition was administered by a different examiner with whom the subject was not familiar, and no positive feedback was provided regardless of actual performance. Immediately after completing the high stress condition, all subjects were fully debriefed regarding the stress manipulation. Subjects were assured that that their performance had not been permanently recorded on videotape and that they would receive the full monetary compensation regardless of performance.
Data acquisition
The interference EMG signal from the left upper trapezius muscle was recorded using biploar surface electrodes (silver-silver chloride; 8 mm electrode diameter; In Vivo Metric, Healdsburg, CA, USA). Electrodes were placed with 15 mm interelectrode distance, centered 20 mm lateral to the midpoint between C7 and the posterior lateral border of the acromion, and the reference electrode was placed over a bony portion of the clavicle. Bioamplifiers (Coulbourn Instruments, Allentown, PA, USA) were used to amplify (1,000×) and band-pass filter (13–1,000 Hz) the signal prior to sampling and storage at 2,000 samples/s (Power 1401, 16-bit resolution, Cambridge Electronic Design, Cambridge, UK). Single motor unit activity was recorded from the left upper trapezius muscle using a bipolar intramuscular electrode. Intramuscular electrodes were custom made and consisted of two Formvar-insulated stainless steel wires (50 μm diameter; California Fine Wire Co., Grover Beach, CA, USA) with the cross-sectional area exposed for recording. A 30-gauge needle was used to insert the wires between the two surface electrodes. The needle was removed after placement of the intramuscular wires, and the signals were examined online to verify the detection of motor unit action potentials during low-intensity voluntary contractions. The reference electrode for the intramuscular recordings was a surface electrode placed over the clavicle. Intramuscular EMG signals were amplified (1,000×) and band-pass filtered (20–8,000 Hz) prior to sampling and storage at 20,000 samples/s.
Physiologic arousal was assessed at the beginning of the experimental session (baseline) and at the end of each block of test contractions using an automated oscillometric cuff (Coulbourn V series module) placed around the right arm to measure the heart rate and mean arterial pressure. Perceived anxiety was assessed at baseline and at the end of each block of test contractions using the state anxiety portion of the Spielberger State-Trait Anxiety Index (Spielberger et al. 1970).
To confirm the frequency content of respiration, chest wall movement was measured using an aneroid pressure transducer (Coulbourn Instruments, Allentown, PA, USA) during experiments that examined steady-state voluntary contractions of the trapezius muscle (N = 9). No instructions with regard to breathing were provided, and respiratory data were sampled and stored at 100 samples/s as subjects breathed naturally throughout the experiment.
Quantification of motor unit behavior
Single motor unit action potentials were discriminated from the intramuscular EMG recording using Spike2 software (v5.14; Cambridge Electronic Design, Cambridge, UK). Due to small amplitude or similar shapes of motor unit action potentials, it was not possible to reliably discriminate the discharge profile of every active motor unit. Therefore, the amount of motor unit activity present during periods of instructed rest was quantified by counting the number of times the intramuscular EMG recording crossed a threshold that was manually selected to exceed baseline noise for each subject (Fig. 2). Each time a MUAP was discharged, the threshold was crossed, and the intramuscular EMG signal was not assessed for the following 5 ms to avoid multiple phases of the same MUAP being counted more than once. This analysis was performed for the 50 s following the end of each voluntary contraction. The final 10 s of the rest period was excluded to avoid anticipatory or preparatory activity associated with the subsequent trial. Any activity associated with obvious voluntary movement (e.g. postural adjustments) was also excluded. The amount of motor unit activity present during instructed rest was expressed as MUAP/s and was averaged across the five rest periods in each stress condition for each subject. Root mean square (RMS) amplitude of the interference EMG signal was quantified over the same periods.
Fig. 2.
Representative example of the procedure used to quantify the amount of motor unit activity present during instructed muscle rest. The top two panels show the intramuscular EMG recorded in the 50 s following a voluntary contraction under low stress (left) and high stress (right) conditions in the same subject. The bottom panel shows an enlarged view with greater time resolution (1 s duration) for the same signal, enabling individual motor unit action potentials (MUAP) to be identified. Each time the EMG signal crossed a threshold that was manually positioned to exceed baseline noise (horizontal dashed lines) a MUAP was identified, as indicated by the event raster above each plot. The amount of motor unit activity was quantified as the total number of MUAP events identified in the rest period, divided by the duration of the rest period (expressed as MUAP/s)
Discriminated motor unit action potentials were analyzed using custom software written in Matlab (v7.5.0.342, MathWorks Inc., Natick, MA, USA). The instantaneous discharge rate of each motor unit was determined as the reciprocal of the time interval between each MUAP and the MUAP preceding it, expressed in pulses per second (pps). Regular discharge was defined as interspike intervals <600 ms, and the mean and coefficient of variation (CV) of instantaneous discharge rates were calculated for periods of regular firing. The modulation of motor unit discharge rate was quantified by calculating the frequency content of the motor unit discharge rate profiles as described by Rosenberg et al. (1989). Briefly, the discharge times were partitioned into contiguous, non-overlapping epochs of 1.28 s that each comprised 256 bins. Each 5-ms bin was then given a value of 1 when it contained a discharge time, and a value of 0 when it did not. These time-series data were converted into the frequency domain using a fast Fourier transform with a resolution of 0.125 Hz (sampling frequency = 256 Hz and window size = 2,048). The resulting power spectra were quantified by calculating the mean power across 0–0.5 and 16–32 Hz (see Fig. 4a). These bands correspond with previous reports indicating modulation of motor unit discharge rate at 0–0.5 Hz associated with respiration (Westgaard et al. 2006), and at 16–32 Hz associated with oscillations in cortical activity (Farmer et al. 1993a; Farmer et al. 1993b; Conway et al. 1995; Gross et al. 2000). The frequency content of chest wall movement confirmed that the 0–0.5 Hz modulation of motor unit discharge was within the same frequency range as respiration (see Fig. 4). Mean values were analyzed to reflect the overall effect of each condition within the frequency bands of interest, rather than detailed variations of peaks within these bands.
Fig. 4.
a Representative examples of power spectra derived from the instantaneous discharge rate profiles of motor units active during instructed muscle rest (light gray line) and voluntary contraction (dark gray line) in the same subject. Dashed vertical lines indicate the frequency bands over which the mean power was quantified. Note that peaks in the power spectra at 8–12 Hz which correspond to mean dis charge rates are shifted toward higher values for the voluntary contraction. b, c Group averages for the mean power at respiratory (0–0.5 Hz) and beta (16–32 Hz) frequencies. Error bars represent 95% confidence intervals. d A representative example of the power spectra derived from respiratory chest wall movement for the same subject as in panel A
Statistical analysis
It was not possible to discriminate the same motor units across low and high stress conditions and variables based on motor unit discriminations were therefore analyzed as independent samples. Normality of data was assessed using the Kolmogorov–Smirnov test. No variables were significantly different from the normal distribution. All variables are reported in the text as mean (SD) and in figures as mean ± 95% CI. The level of significance for all comparisons was set at P ≤ 0.05. All statistical analyses were performed with SPSS software (v16.0.1, Chicago, IL, USA).
Contraction intensity was quantified by RMS amplitude of the interference EMG signal and compared across periods of instructed rest and voluntary contractions using an independent t-test. The mean and CV of instantaneous discharge rate profiles, and the power in motor unit discharge rate profiles at 0–0.5 Hz and 16–32 Hz were compared (1) across motor units active during periods of instructed rest and voluntary contractions, and (2) across low and high stress conditions for motor units active during periods of instructed rest using independent t-tests. Heart rate, blood pressure, and perceived anxiety were compared across time (baseline; low stress; high stress) using a repeated measures one-way analysis of variance. The amount of motor unit activity and RMS EMG during periods of instructed muscle rest were compared across stress conditions (low stress; high stress) using a paired t-test. The association between the amount of motor unit activity present during instructed rest and each measure of arousal was assessed using Pearson's correlation.
Results
Comparison of motor unit activity between instructed rest and volitional contractions
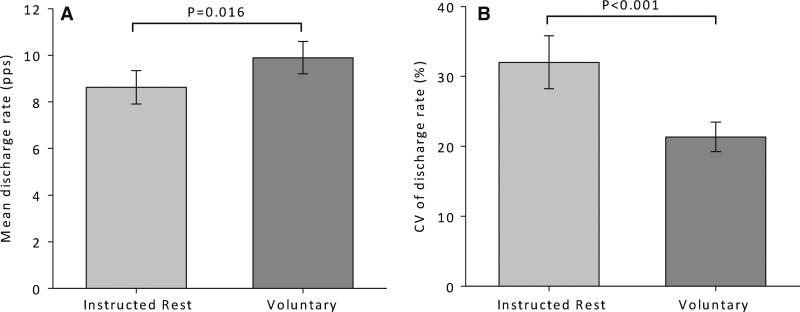
Twenty-seven motor units (from 12 subjects) were discriminated during periods of instructed muscle rest in the low stress condition. Eighteen motor units (from 9 subjects) were discriminated during the voluntary contractions performed under low stress conditions. RMS EMG during the voluntary contractions (13.7 (12.5) % maximum) was similar to that observed during instructed rest (11.5 (7.8) % maximum; P = 0.591). However, the discharge rate of motor units active during periods of instructed muscle rest was lower (P = 0.016) and more variable (P < 0.001) than that of motor units active during voluntary contractions performed at the lowest contraction intensity at which motor unit activity could be observed (Fig. 3). The mean power of discharge rate modulation at 0–0.5 Hz was greater for motor units active during periods of instructed muscle rest compared to those active during voluntary contractions (Fig. 4b, P = 0.002). Conversely, the mean power of discharge rate modulation at 16–32 Hz was less for motor units active during periods of instructed rest compared to those active during voluntary contractions (Fig. 4c, P = 0.009). There was a positive association between mean discharge rates and power at 0–0.5 Hz (r2 = 0.18; P < 0.001), power at 16–32 Hz (r2 = 0.59; P < 0.001), and total power from 0 to 32 Hz (r2 = 0.60; P < 0.001). Therefore, we expressed the power at each frequency as a percentage of the total power to account for differences in the influence of mean discharge rates on the power spectra across experimental conditions. Despite a significantly lower mean discharge rate during instructed rest, the mean relative power of discharge rate modulation remained greater at 0–0.5 Hz (0.17 (0.16) % vs. 0.06 (0.04) %; P = 0.002) and less at 16–32 Hz (0.44 (0.01) % vs. 0.46 (0.01) %; P = 0.001) during instructed rest compared to voluntary contractions.
Fig. 3.
The mean (a) and coefficient of variation (b) of discharge rate for motor units active during periods of instructed muscle rest and during low-amplitude voluntary contractions performed under low stress conditions. Error bars represent 95% confidence intervals
Comparison of motor unit activity during instructed rest under low and high stress conditions
The experimental stressor successfully increased heart rate, mean arterial blood pressure, and perceived anxiety during the high stress condition compared to the low stress condition (Heart rate = 81.5 (18.0) vs. 69.0 (8.7) bpm; blood pressure = 102.2 (12.4) vs. 92.5 (11.0) mmHg; perceived anxiety = 46.3 (11.6) vs. 32.0 (10.2); P ≤ 0.002). Values during the low stress condition were not significantly different from baseline values (Heart rate = 69.0 (8.7) vs. 69.1 (8.1) bpm; blood pressure = 92.5 (11.0) vs. 87.1 (6.8) mmHg; perceived anxiety = 32.0 (10.2) vs. 26.4 (6.1); P > 0.05). The amount of motor unit activity present during periods of instructed rest was greater in the high stress condition than in the low stress condition (P = 0.021; Fig. 5a). This was paralleled by an increase in RMS EMG during the high stress condition (13.3 (9.6) vs. 11.5 (7.8) % maximum in the low stress condition; P = 0.043). Changes in motor unit activity across stress conditions were correlated with changes in mean arterial blood pressure (r2 = 0.45; P = 0.006; Fig. 5b) and heart rate (r2 = 0.28; P = 0.044; Fig. 5c), but not with changes in perceived anxiety (r2 = 0.00; P = 0.850; Fig. 5d).
Fig. 5.
a Group average for the amount of motor unit activity (MUAP/s) present during instructed muscle rest under low and high stress conditions. Error bars represent 95% confidence intervals. b–d Relations between the change in the amount of motor unit activity and the change in blood pressure (b), heart rate (c) and state anxiety (d) from low to high stress conditions
Twenty-seven motor units (from 12 subjects) were discriminated during periods of instructed muscle rest in the low stress condition, and 30 motor units (from 14 subjects) were discriminated during periods of instructed rest in the high stress condition. The duration of sustained activity for these motor units was similar across low and high stress conditions (41.6 (11.0) s and 36.7 (15.0) s, respectively; P = 0.205). Motor unit discharge rate was similar across low and high stress conditions (P = 0.701; Fig. 6a); however, the variability of this discharge was higher during the low stress condition (P = 0.015; Fig. 6b). The mean power of discharge rate modulation at 0–0.5 Hz (P = 0.391) and 16–32 Hz (P = 0.089; Fig. 6c, d) was also similar across low and high stress conditions.
Fig. 6.
The mean (a) and coefficient of variation (b) of discharge rate, and the mean power at respiratory (0–0.5 Hz) (c) and beta (16–32 Hz) (d) frequencies for motor units active during periods of instructed muscle rest during low and high stress conditions. Error bars represent 95% confidence intervals
Discussion
This study examined the frequency modulation of motor unit discharge rates in the trapezius muscle of healthy women and is the first study to demonstrate modulation at 0–0.05 and 16–32 Hz for motor units with sustained activity during periods of instructed muscle rest that followed voluntary contractions. Furthermore, the results showed that discharge rate modulation at 16–32 Hz was reduced whereas modulation at 0–0.5 Hz was increased in motor units active during instructed muscle rest compared to voluntary contraction. These differences were accompanied by a slower and more variable pattern of motor unit discharge during instructed muscle rest. Acute exposure to psychosocial stressors increased the amount of trapezius motor unit activity during periods of instructed muscle rest; however, this activity was not associated with changes in discharge rate modulation at either 0–0.5 or 16–32 Hz.
Source of the observed discharge rate modulation
Modulation of trapezius motor unit discharge rates at 0–0.5 Hz has been observed previously during active motor tasks and has been attributed to respiratory inputs to the motor neuron pool (Westgaard et al. 2006). Although a respiratory origin for this low-frequency modulation has not been directly demonstrated, the power spectrum is remarkably similar to that of chest wall movement (see Fig. 4d) and discharge rate profiles have been shown to be phased locked to the respiration signal with peak discharge rates most often occurring at the transition from inspiration to expiration (Westgaard et al. 2006). Baweja et al. (2009) recently reported that visual force feedback during low-intensity force-matching tasks increases fluctuations in the force trace at 0–1 Hz, suggesting that visuomotor corrections may contribute to oscillations at these frequencies. However, if visuomotor corrections contributed to oscillations in trapezius motor unit discharge at 0–0.5 Hz, it would be expected that the magnitude of these oscillations would be greater during voluntary contractions performed with visual force feedback than during periods of instructed muscle rest in which no force feedback was provided. We observed the opposite result, suggesting that oscillations due to visuomotor corrections did not obscure respiratory inputs at low frequencies.
Modulation of discharge rates at 16–32 Hz has not previously been reported for trapezius motor units, although is well studied in the motor units of more distal muscles where it is known to arise from beta frequency oscillations in the activity of pyramidal tract neurons within the motor cortex (Conway et al. 1995; Baker et al. 1997, 2003; Jackson et al. 2002). Although differences in the strength of direct cortical projections to distal and proximal muscles have been reported (Clough et al. 1968; Porter and Hore 1969; Jankowska et al. 1975), evoked responses to transcranial electrical and magnetic stimulation occur at a latency that is consistent with direct projections from pyramidal tract neurons to the trapezius motor neuron pool (Gandevia and Applegate 1988; Berardelli et al. 1991; Strenge and Jahns 1998; Truffert et al. 2000; Bawa et al. 2004; Alexander et al. 2007). Additional studies using electro- or magnetoencephalography to determine the coherence of cortical and muscle activity at beta frequencies are needed to confirm a cortical origin of the 16–32 Hz oscillations in motor unit discharge rates observed in the present study and to compare the strength of this input between muscles with different functional roles. However, a cortical origin of the observed beta modulation is supported by the finding that this modulation was greatest when subjects were holding a voluntary isometric contraction, similar to what has been reported for the more distal muscles (Sanes and Donoghue 1993; Conway et al. 1995; Baker et al. 1997; Baker 2007).
There is some evidence from distal muscles suggesting that corticomuscular coherence at beta frequencies may not arise solely from cortical output pathways (Halliday et al. 1998; Baker and Baker 2003; Riddle and Baker 2005), and it has been postulated that feedback from the periphery may also contribute to beta oscillations in motor unit discharge (Baker 2007). The proposed functional role of this peripheral contribution is to assist with proprioceptive processing. This view is compatible with evidence that beta oscillations represent a cortical state that promotes the maintenance of a steady motor output (Gilbertson et al. 2005; Androulidakis et al. 2006, 2007; Kristeva et al. 2007). Our interpretation of results from the present study assumes that beta oscillations from the motor cortex facilitate tonic motor output, which assists with maintaining a steady level of force during the voluntary force-matching task, as well as maintaining a stable upright posture during periods of instructed muscle rest. However, our results are also consistent with the proposal that beta oscillations play a role in proprioceptive processing, as voluntary contractions that involve a steady level of force production presumably require greater knowledge of the peripheral state than do periods of quiet rest. Whatever the functional role of beta oscillations in motor unit discharge, our results provide the first evidence that these oscillations may be present in axial muscles and are not constrained to the more commonly studied limb muscles.
Motor unit activity during instructed muscle rest
The results of this study support previous reports of trapezius motor unit activity during periods of intended muscle rest in low stress conditions (Kitahara et al. 2000) and suggest that both respiratory and beta inputs may contribute to this activity. Compared to voluntary contractions maintained at a constant target force, the modulation of motor unit discharge rates at beta frequencies was reduced. It has been proposed that beta inputs to the motor neuron pool facilitate the maintenance of a stable postural set (Gilbertson et al. 2005; Androulidakis et al. 2006, 2007; Kristeva et al. 2007), and the higher level of beta inputs to the trapezius motor neuron pool when subjects were instructed to maintain a stable voluntary contraction is consistent with this functional role. Although of lesser magnitude, discharge rate modulation at beta frequencies was still present during instructed muscle rest, consistent with the hypothesis that maintaining a stable upright posture may contribute to activation of the trapezius muscle during periods of instructed rest. In contrast, the modulation of motor unit discharge rates at respiratory frequencies was greater during periods of instructed muscle rest than during voluntary contractions, suggesting that respiratory inputs to trapezius motor neurons may be suppressed during the performance of an explicit motor task with high accuracy demands.
Motor units active during periods of instructed muscle rest that followed voluntary contractions had a lower mean discharge rate and higher discharge variability than motor units active during a steady-state voluntary contraction performed with similar cognitive demands. This occurred despite the fact that motor units from the voluntary contraction were analyzed at the lowest discharge rate that was possible under voluntary control as tested in these experimental conditions (see Fig. 1). The observation that motor neuron discharge could not be voluntarily maintained at the low rates observed during instructed rest suggests that different mechanisms may contribute to trapezius muscle activation during voluntary and involuntary contractions.
The decrease in discharge rate modulation at 16–32 Hz during instructed rest could be explained by a reduction in the absolute amplitude of the power spectra at the lower discharge rates observed in this condition. However, the lower discharge rates cannot explain concurrent increases in discharge modulation at 0–0.5 Hz, and differences in discharge rate modulation persisted for both frequency bands even after accounting for differences in the total power of discharge rates across the two experimental conditions. Thus, it appears unlikely that the observed differences in discharge rate modulation at respiratory and beta frequencies were confounded by differences in the mean discharge rate.
Effects of acute exposure to a psychosocial stressor
An increase in the amount of motor unit activity when exposed to a stressor during periods of instructed muscle rest that followed voluntary contractions supports previous reports of increased trapezius muscle activation in response to stress (Lundberg et al. 1994, 2002; Bansevicius et al. 1997; McLean and Urquhart 2002; Bloemsaat et al. 2005; Nilsen et al. 2007; Schleifer et al. 2008). The order of the low and high stress conditions was not counter-balanced across subjects, with the low stress condition always being performed first. It is therefore possible that differences in motor unit activity between the low and high stress condition may have been influenced by testing order. However, the relation between the amount of motor unit activity and stress-induced changes in heart rate and blood pressure suggests a physiologic mechanism for the observed difference. Despite an increase in the amount of motor unit activity during the high stress condition, modulation of the motor unit discharge was similar to that observed during the low stress condition. This indicates that inputs to the motor neuron pool at respiratory and beta frequencies are similar across stress conditions, suggesting that these inputs do not underlie the observed increases in motor unit activity.
Contrary to our findings, Westgaard et al. (2006) reported reduced respiratory modulation of discharge rates with stress. However, the Westgaard study compared motor unit activity during a low-amplitude voluntary contraction performed in the absence of stress to motor unit activity during a stress-inducing keyboard task that required subjects to press buttons with their fingers. Although the physical demands imposed on the trapezius muscle by the keyboard task were considered to be minimal, it is not clear whether the reduced proportion of motor units exhibiting respiratory modulation was caused by exposure to the stressor, or a change in the motor task. The comparison of low and high stress conditions with no physical task demands in the present study indicates that respiratory inputs to the trapezius motor neuron pool are not altered by exposure to a psychosocial stressor that causes a moderate cardiovascular response typical of that encountered in the workplace.
Although Kristeva-Feige et al. (2002) previously reported reduced corticomusclar coherence at beta frequencies during performance of a mental arithmetic task, this study examined the flexor digitorum superficialis muscle during voluntary contractions with visual force feedback. Therefore, the observed changes in coherence may have been specific to the muscle examined or the type of contraction performed. Alternatively, any effects of the mental task may be confined to corticomuscular coherence and not extend to the power of beta oscillations in the muscle. Discrepancies between these measures have been reported previously (e.g. Baker and Baker 2003; Witte et al. 2007; Chakarov et al. 2009) and may reflect peripheral influences on the coherence measure.
It is possible that the frequency content of motor unit discharge rate profiles is not sensitive enough to detect changes in inputs to the motor neuron pool across conditions; however, the comparison of motor unit activity during instructed muscle rest and voluntary contraction suggests that this is not the case. Trapezius motor neurons also receive input from other sources, for example from spinal reflex pathways (Alexander and Harrison 2002, 2003; Alexander et al. 2007). Although there is no evidence supporting an a priori hypothesis that spinal inputs to the trapezius muscle are altered by psychosocial stress, this possibility remains to be examined. Alternatively, it is possible that inputs to the motor neuron pool are not altered by acute exposure to stressors, and it is the processing properties of the motor neuron itself that change. One candidate mechanism for altered processing of synaptic inputs is the dendritic persistent inward current (Heckman et al. 2008b), the magnitude of which is influenced by norepinephrine (Conway et al. 1988; Lee and Heckman 1999). Noradrenergic input to the spinal cord is modulated with stress (Aston-Jones et al. 2001), and it remains possible that the magnitude of persistent inward currents and, consequently, the input–output gain of the motor neuron pool increase during exposure to psychosocial stressors. We previously showed evidence for the presence of persistent inward currents in trapezius motor neurons (Stephenson and Maluf 2010). Although indirect estimates of the magnitude of these currents were similar in low and high stress conditions, it is likely that they were tightly controlled by inhibitory inputs (Heckman et al. 2008a) to meet the requirements of accurate force generation during the target-matching task. Thus, the results cannot be generalized to conditions in which force feedback is removed, and the relative role of altered synaptic inputs to spinal motor neurons versus altered processing of these inputs in response to acute exposure to a stressor is yet to be determined.
Although the mean discharge rate of active motor units was similar between low and high stress conditions, the variability of discharge rate was significantly lower during exposure to the stressor. The magnitude of this difference was small and may reflect slightly greater net excitation of the motor neuron pool in the high stress condition resulting in a more regular discharge of action potentials. Although greater net excitation would also be expected to increase motor unit discharge rate, trapezius motor units are known to be unresponsive to small changes in excitation (Westgaard and De Luca 2001), and discharge variability may be more sensitive than mean discharge rate to small changes in depolarization of the motor neuron membrane.
Conclusion
Sustained trapezius motor unit activity during periods of instructed muscle rest that follow voluntary contractions has a lesser contribution from inputs at beta frequencies and a greater contribution from inputs at respiratory frequencies than present during low-intensity voluntary contractions. Acute exposure to a psychosocial stressor increases the amount of trapezius motor unit activity present during these periods of instructed muscle rest, but does not alter the magnitude of inputs to the motor neuron pool at respiratory or beta frequencies. Further study is therefore required to determine the contribution of other sources of synaptic input and altered processing of these inputs to the increase in net excitation of the trapezius motor neuron pool during acute exposure to psychosocial stressors. Such studies may suggest new therapeutic strategies to target sources of tonic muscle activity that may contribute to or exacerbate symptoms of chronic neck pain.
Acknowledgments
This research was supported by NIH award R21-AR054181 to KSM.
Contributor Information
Jennifer L. Stephenson, Department of Physical Medicine & Rehabilitation, Applied Neuromuscular Physiology Lab, Physical Therapy Program, University of Colorado Denver School of Medicine, MS C244, Education 2 South, Bldg #L28, 13121 E. 17th Ave, Room 3106, Aurora, CO 80045, USA
Evangelos A. Christou, Department of Applied Physiology and Kinesiology, Neuromuscular Physiology Lab, University of Florida, Gainesville, FL 32511, USA
Katrina S. Maluf, Department of Physical Medicine & Rehabilitation, Applied Neuromuscular Physiology Lab, Physical Therapy Program, University of Colorado Denver School of Medicine, MS C244, Education 2 South, Bldg #L28, 13121 E. 17th Ave, Room 3106, Aurora, CO 80045, USA
References
- Alexander CM, Harrison PJ. The bilateral reflex control of the trapezius muscle in humans. Exp Brain Res. 2002;142:418–424. doi: 10.1007/s00221-001-0951-2. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Harrison PJ. Reflex connections from forearm and hand afferents to shoulder girdle muscles in humans. Exp Brain Res. 2003;148:277–282. doi: 10.1007/s00221-002-1256-9. [DOI] [PubMed] [Google Scholar]
- Alexander C, Miley R, Stynes S, Harrison PJ. Differential control of the scapulothoracic muscles in humans. J Physiol. 2007;580:777–786. doi: 10.1111/j.1469-7793.2000.t01-1-02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Gilbertson TP, Brown P. Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. Eur J Neurosci. 2006;24:3299–3304. doi: 10.1111/j.1460-9568.2006.05201.x. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Yarrow K, Litvak V, Gilbertson TP, Brown P. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur J Neurosci. 2007;25:3758–3765. doi: 10.1111/j.1460-9568.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol. 2007;17:649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501(Pt 1):225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. effect of oscillatory activity on corticospinal output. J Neurophysiol. 2003;89:1941–1953. doi: 10.1152/jn.00832.2002. [DOI] [PubMed] [Google Scholar]
- Bansevicius D, Westgaard RH, Jensen C. Mental stress of long duration: EMG activity, perceived tension, fatigue, and pain development in pain-free subjects. Headache. 1997;37:499–510. doi: 10.1046/j.1526-4610.1997.3708499.x. [DOI] [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res. 2004;158:385–390. doi: 10.1007/s00221-004-2031-x. [DOI] [PubMed] [Google Scholar]
- Baweja HS, Kennedy DM, Vu J, Vaillancourt DE, Christou EA. Greater amount of visual feedback decreases force variability by reducing force oscillations from 0–1 and 3–7 Hz. Eur J Appl Physiol. 2009;108:935–943. doi: 10.1007/s00421-009-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Priori A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Corticobulbar and corticospinal projections to neck muscle motoneurons in man. A functional study with magnetic and electric transcranial brain stimulation. Exp Brain Res. 1991;87:402–406. doi: 10.1007/BF00231857. [DOI] [PubMed] [Google Scholar]
- Bloemsaat JG, Meulenbroek RG, Van Galen GP. Differential effects of mental load on proximal and distal arm muscle activity. Exp Brain Res. 2005;167:622–634. doi: 10.1007/s00221-005-0066-2. [DOI] [PubMed] [Google Scholar]
- Centonze D, Palmieri MG, Boffa L, Pierantozzi M, Stanzione P, Brusa L, Marciani M, Siracusano A, Bernardi G, Caramia M. Cortical hyperexcitability in post-traumatic stress disorder secondary to minor accidental head trauma: a neurophysiologic study. J Psychiatry Neurosci. 2005;30:127–132. [PMC free article] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol. 2009;102:1115–1120. doi: 10.1152/jn.91095.2008. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Clough JF, Kernell D, Phillips CG. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968;198:145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489(Pt 3):917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham E, Peat M. Functional anatomy of the shoulder complex. J Orthop Sports Phys Ther. 1993;18:342–350. doi: 10.2519/jospt.1993.18.1.342. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol. 1993a;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Applegate C. Activation of neck muscles from the human motor cortex. Brain. 1988;111(Pt 4):801–813. doi: 10.1093/brain/111.4.801. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Tass PA, Salenius S, Hari R, Freund HJ, Schnitzler A. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol. 2000;527(Pt 3):623–631. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg GM, Astrom A. Load pattern and pressure pain threshold in the upper trapezius muscle and psychosocial factors in medical secretaries with and without shoulder/neck disorders. Int Arch Occup Environ Health. 1997;69:423–432. doi: 10.1007/s004200050170. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol. 2008a;586:1225–1231. doi: 10.1113/jphysiol.2007.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron Wring patterns. Neuroscientist. 2008b;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Spinks RL, Freeman TC, Wolpert DM, Lemon RN. Rhythm generation in monkey motor cortex explored using pyramidal tract stimulation. J Physiol. 2002;541:685–699. doi: 10.1113/jphysiol.2001.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Projections of pyramidal tract cells to alpha-motoneurones innervating hind-limb muscles in the monkey. J Physiol. 1975;249:637–667. doi: 10.1113/jphysiol.1975.sp011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T, Schnoz M, Laubli T, Wellig P, Krueger H. Motor-unit activity in the trapezius muscle during rest, while inputting data, and during fast finger tapping. Eur J Appl Physiol. 2000;83:181–189. doi: 10.1007/s004210000277. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage. 2007;36:785–792. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113:124–131. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Stress responses in low-status jobs and their relationship to health risks: musculoskeletal disorders. Ann N Y Acad Sci. 1999;896:162–172. doi: 10.1111/j.1749-6632.1999.tb08113.x. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Kadefors R, Melin B, Palmerud G, Hassmen P, Engstrom M, Dohns IE. Psychophysiological stress and EMG activity of the trapezius muscle. Int J Behav Med. 1994;1:354–370. doi: 10.1207/s15327558ijbm0104_5. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Forsman M, Zachau G, Eklof M, Palmerud G, Melin B, Kadefors R. Effects of experimentally induced mental and physical stress on motor unit recruitment in the trapezius muscle. Work & Stress. 2002;16:166–178. [Google Scholar]
- Maki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2009.11.078. [DOI] [PubMed] [Google Scholar]
- McLean L, Urquhart N. The influence of psychological stressors on myoelectrical signal activity in the shoulder region during a data entry task. Work & Stress. 2002;16:138–153. [Google Scholar]
- Nilsen K, Sand T, Stovner L, Leistad R, Westgaard R. Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculoskeletal Disorders. 2007;8:81. doi: 10.1186/1471-2474-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol. 2001;91:821–831. doi: 10.1152/jappl.2001.91.2.821. [DOI] [PubMed] [Google Scholar]
- Pettersen V, Westgaard RH. The activity patterns of neck muscles in professional classical singing. J Voice. 2005;19:238–251. doi: 10.1016/j.jvoice.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Pettersen V, Bjorkoy K, Torp H, Westgaard RH. Neck and shoulder muscle activity and thorax movement in singing and speaking tasks with variation in vocal loudness and pitch. J Voice. 2005;19:623–634. doi: 10.1016/j.jvoice.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Porter R, Hore J. Time course of minimal corticomotoneuronal excitatory postsynaptic potentials in lumbar motoneurons of the monkey. J Neurophysiol. 1969;32:443–451. doi: 10.1152/jn.1969.32.3.443. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol. 2005;566:625–639. doi: 10.1113/jphysiol.2005.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Sandsjo L, Melin B, Rissen D, Dohns I, Lundberg U. Trapezius muscle activity, neck and shoulder pain, and subjective experiences during monotonous work in women. Eur J Appl Physiol. 2000;83:235–238. doi: 10.1007/s004210000284. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local Weld potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer LM, Spalding TW, Kerick SE, Cram JR, Ley R, Hatfield BD. Mental stress and trapezius muscle activation under psychomotor challenge: a focus on EMG gaps during computer work. Psychophysiology. 2008;45:356–365. doi: 10.1111/j.1469-8986.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Stephenson JL, Maluf KS. Discharge behaviors of trapezius motor units during exposure to low and high levels of acute psychosocial stress. J Clin Neurophysiol. 2010;27:52–61. doi: 10.1097/WNP.0b013e3181cb81d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenge H, Jahns R. Activation and suppression of the trapezius muscle induced by transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1998;38:141–145. [PubMed] [Google Scholar]
- Truffert A, Rosler KM, Magistris MR. Amyotrophic lateral sclerosis versus cervical spondylotic myelopathy: a study using transcranial magnetic stimulation with recordings from the trapezius and limb muscles. Clin Neurophysiol. 2000;111:1031–1038. doi: 10.1016/s1388-2457(00)00292-3. [DOI] [PubMed] [Google Scholar]
- Veiersted KB, Westgaard RH, Andersen P. Electromyographic evaluation of muscular work pattern as a predictor of trapezius myalgia. Scand J Work Environ Health. 1993;19:284–290. doi: 10.5271/sjweh.1472. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiatry. 2001;50:377–382. doi: 10.1016/s0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, De Luca CJ. Motor control of low-threshold motor units in the human trapezius muscle. J Neurophysiol. 2001;85:1777–1781. doi: 10.1152/jn.2001.85.4.1777. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Bonato P, Westad C. Respiratory and stress-induced activation of low-threshold motor units in the human trapezius muscle. Exp Brain Res. 2006;175:689–701. doi: 10.1007/s00221-006-0587-3. [DOI] [PubMed] [Google Scholar]
- Witte M, Patino L, Andrykiewicz A, Hepp-Reymond MC, Kristeva R. Modulation of human corticomuscular beta-range coherence with low-level static forces. Eur J Neurosci. 2007;26:3564–3570. doi: 10.1111/j.1460-9568.2007.05942.x. [DOI] [PubMed] [Google Scholar]