Summary
Natural killer cells (NKs) are involved in every stage of hepatitis C viral (HCV) infection, from protection against HCV acquisition and resolution in the acute phase to treatment-induced clearance. In addition to their direct antiviral actions, NKs are involved in the induction and priming of appropriate downstream T-cell responses. In the setting of chronic HCV, overall NK cell levels are decreased, altered subset distribution is altered, and changes in NK receptor (NKR) expression have been demonstrated, although the contribution of individual NKRs to viral clearance or persistence remains to be clarified. Enhanced NK cell cytotoxicity accompanied by insufficient interferon-γ production may promote liver damage in the setting of chronic infection. Treatment-induced clearance is associated with activation of NK cells, and it will be of interest to monitor NK cell responses to triple therapy. Activated NK cells also have antifibrotic properties, and the same hepatic NK cell populations that are actively involved in control of HCV may also be involved in control of HCV-associated liver damage. We still have much to learn, in particular: how do liver-derived NKs influence the outcome of HCV infection? Do NK receptors recognize HCV-specific components? And, are HCV-specific memory NK populations generated?
Keywords: innate immunity, liver, human, interferon
Hepatitis C viral infection
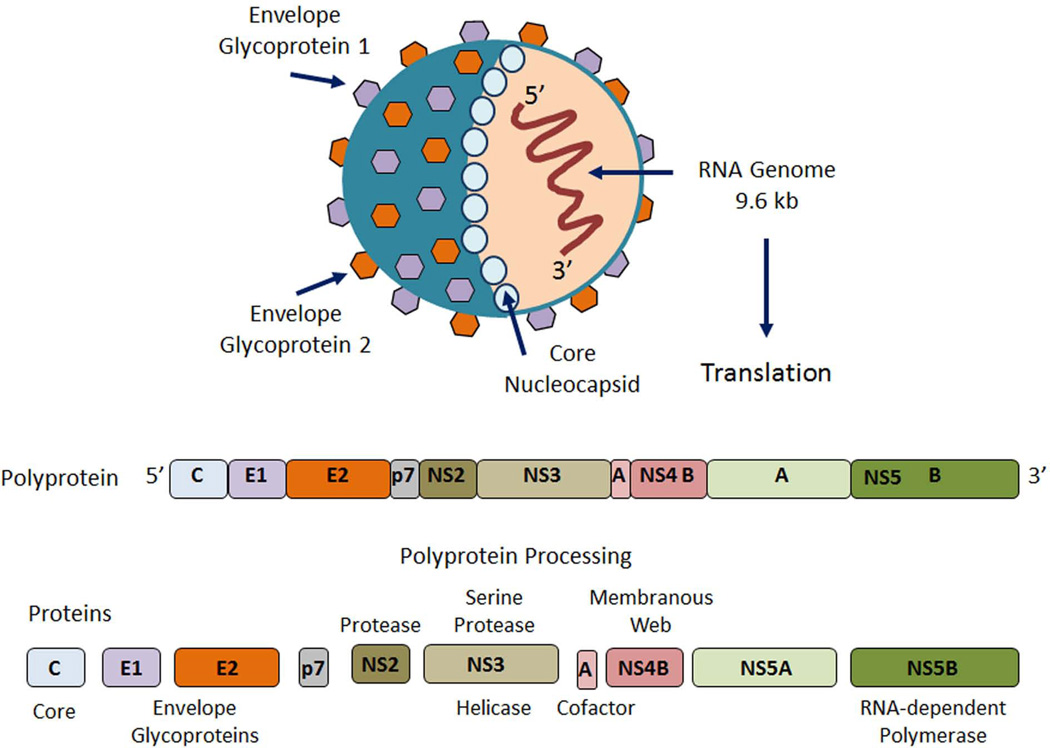
The hepatitis C virus (HCV) is a positive-stranded RNA enveloped virus, a member of the flavivirus family (1). The HCV genome is approximately 9600 nucleotides and encodes a single polyprotein precursor of approximately 3000 amino acids. The polyprotein is cleaved by both host and viral proteases into structural and nonstructural (NS) proteins (2) (Fig. 1). Replication is mediated by NS5B, the viral RNA-dependent RNA polymerase that is devoid of proof-reading capacity, resulting in a high mutation rate. The inherent sequence diversity of HCV represents one of the most substantial challenges to the development of an effective HCV vaccine. To date, seven major HCV genotypes demonstrating >30% nucleotide sequence divergence from each other and numerous subtypes have been identified.
Fig. 1. Hepatitis C virus (HCV).
HCV is an enveloped, positive-stranded RNA virus. The 9.6 kilo base (kb) genome is translated into a single polyprotein precursor of approximately 3000 amino acids. Cleavage of the polyprotein by viral and host-cell proteases yields structural viral proteins (core protein and envelope proteins E1 and E2) and nonstructural viral proteins (NS2 through NS5B), with a number of putative activities and functions.
Approximately 200 million people (an estimated 3% of the world’s population) throughout the world have chronic HCV infection (3); HCV persists in up to 80% of people infected, whereas only a minority (∼20%) of individuals exposed to HCV are able to spontaneously clear the infection. HCV, primarily transmitted via contaminated blood, is a leading cause of liver cancer and indication for liver transplantation (3). In the US alone, the burden over the next 10–20 years is expected to reach over $10 billion in direct medical costs and double this in overall societal costs (4).
Pegylated interferon-based regimens, with ribavirin, had been the standard of treatment over the past decade. When results are stratified according to genotype, sustained virologic response (SVR) was about 40% to 50% in patients with genotype 1 and 75% to 85% in patients with genotype 2/3 (5–7). Although recent advances in treatment, including the addition of NS3/4A protease inhibitors, have significantly enhanced SVR, drug toxicities and costs remain significant hurdles for many patients, and triple therapy may not be available for the majority of HCV-infected patients (8). The immune response to HCV is complex involving multi-cellular division of labor and includes components of innate and adaptive immunity (3). Enhanced understanding of HCV-host interactions and the mechanisms that regulate immunity within the liver is required to combat this virus and to develop improved therapies.
Natural killer cells
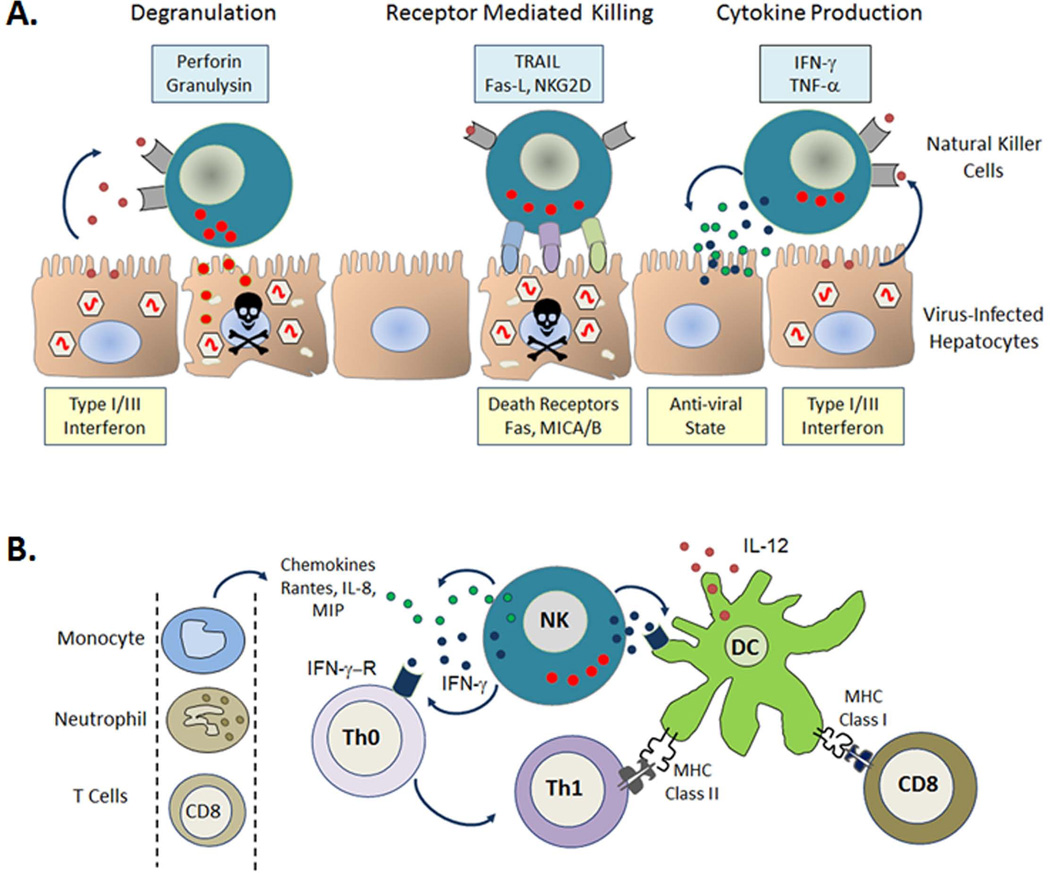
Natural killer (NK) cells are considered the principal innate effectors representing the first line of defense in the control of viral infections (9–11). They provide antiviral protection through surveillance of danger signals, downregulation of major histocompatibility complex (MHC) class I molecules ‘missing-self’ (12), and upregulation of MHC class I homologues ‘induced-self’ ligands (13), in addition to direct recognition of pathogen-associated molecules (14, 15). Their role may be direct, as NK cells can kill without prior sensitization via the release of granzyme and perforin containing cytotoxic granules (16). In addition, they produce cytokines such as interferon-γ (IFN-γ), which can limit viral replication (17). NKs also act indirectly by influencing the activation and/or trafficking of other key immune cell populations (18, 19) including dendritic cells (DCs) (20) and T cells (21, 22). An appreciation of this immune-regulatory function of NKs has provided insight into the critical role played by NKs in the crosstalk between innate and adaptive immunity and has highlighted their continuing role in chronic viral infections) (Fig. 2). The suggestion that NK memory responses develop and persist, at least in mice (23–25) and that human NK cells have functional memory-like properties after cytokine activation (26) adds a new level of complexity to this population opening up exciting possibilities for NK cell-based immunotherapy or vaccination in cases where more traditional approaches have been unsuccessful (27).
Fig. 2. Direct and indirect anti-viral effector mechanisms of natural killer (NK) cells.
In the setting of viral infection, infected cells produce Type I/III interferon (IFN). NK cells respond through direct mechanisms; degranulation and receptor mediated lysis of infected cells as well as production of anti-viral cytokines such as IFN-γ (A). NK cells also act indirectly to prime the adaptive immune response promoting dendritic cell (DC) maturation and the differentiation of immature helper T cells (Th0) towards an inflammatory phenotype (Th1). Production of chemokines by NK cells attracts other immune cells to sites of inflammation (B). TRAIL, TNF-related apoptosis-inducing ligand; Fas-L, Fas ligand; TNF-α, tumor necrosis factor α; IFN-γ-R, interferon-γ receptor; IL-8/12, interleukin-8/12; MIP, macrophage inflammatory protein; MHC, major histocompatibility complex.
Until recently, mainly because of the paucity of well described acute HCV cohorts and the perception that as part of the innate immune response NK cell function would impact only in the early stages of infection, the role of NK cells in HCV infection remained relatively unexplored. We hypothesized that NK cells were likely important at all stages of HCV infection, not just in the acute setting (28), and since then several studies have supported this premise. In this article, we review recent data from our own laboratory as well as others that have contributed significantly to our understanding of the role played by these complex and versatile immune effector cells in the setting of infection with HCV.
Natural killer cell activation
NK cell activity is stringently controlled by inhibitory NK receptors (NKRs), which in steady-state conditions override signals provided by engagement of activating receptors. Negative signaling induced by inhibitory receptors opposes NK cell activation and provides an important safeguard from NK cell reactivity toward normal, healthy cells (29). In the setting of viral infection, the balance favoring inhibition seen under normal conditions is shifted towards activation (30). The main classes of NKRs include the predominantly inhibitory killer immunoglobulin-like receptors (KIR), C-type lectin-like receptors of the CD94/ natural killer group 2 (NKG2) family comprising inhibitory (NKG2A) and activatory (NKG2C/D) isoforms, as well as the natural cytotoxicity receptors (NCRs) NKp30 (NCR3/CD337), NKp44 (NCR2/CD336), and NKp46 (NCR1/CD335) that deliver activation signals (15, 31–35). Additional surface receptors are involved in the activation of NK cells. Some of these are not exclusively expressed on NK cells and are mainly involved in NK cell adhesion to target cells. These include DNAX accessory molecule-1 (DNAM-1) and NKp80, also known as killer cell lectin-like receptor subfamily F, member 1 (KLRF1), involved in epithelial and myeloid cell interactions (36). Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL/Apo2L), responsible for extrinsic induction of cell death, can also be expressed on NK cells. TRAIL has been implicated in immunosuppressive, immunoregulatory, and immune-effector functions. With respect to pathological challenges, TRAIL and its receptors have been shown to play important roles in the immune response to viral infections (37). The majority of activating NKRs function as co-receptors requiring a second signal provided by loss of inhibition, cytokine stimulation, or a second activating receptor (38, 39).
As mentioned above, one of the main classes of NK inhibitory receptors is the KIR family. Diverse and polymorphic, they interact with highly polymorphic MHC class I ligands. Their main function lies in constitutive inhibition and in the generation of diversity in immune responses to pathogens and as such have received considerable attention as potential disease association markers (40). The other dominant NK inhibitory receptor is the evolutionary conserved NKG2A (38). This receptor forms dimers with CD94 and binds human leukocyte antigen-E (HLA-E), which presents leader peptides derived from classical MHC class I molecules (41). CD94/NGK2A serves to monitor appropriate expression of MHC class I and senses changes in overall MHC class I expression that may arise from viral infection (36). Another member of the NKG2 family, NKG2D, is a potent activating receptor on NK cells (42). Multiple inducible ligands, all of which are homologues of MHC class I molecules are recognized by NKG2D. Human ligands include MHC class I chain-related A and B (MICA, MICB) and UL16-binding proteins (ULBPs) (13, 43). Through recognition of ligands induced by stress or infection, NKG2D plays an important role in the control of viral infections. The importance of this receptor in host antiviral defense is emphasized by the multiple redundant mechanisms viruses, including human cytomegalovirus (HCMV) (44), human immunodeficiency virus (HIV) (45), and hepatitis B virus (HBV) (46), have evolved to counteract the NKG2D-dependent immune response. Another important group of activating receptors is the NCRs, which include NKp30, NKp44, and NKp46. All three NCRs are involved in the clearance of both tumor and virus-infected cells (35). Several tumor-specific and viral ligands of the NCRs have been described (35). B7-H6 expressed on transformed cells has been identified as a tumor-specific ligand for NKp30 (47, 48). BCL-2-associated athanogene 6 (BAG6), also known as HLA-B-associated transcript 3 (BAT3), released by tumor cells in exosomes binding to NKp30 can activate NK cells (49), and BAG6 expressed on the membrane of immature DCs (iDCs) is involved in the elimination of iDCs by NK cells (50). NKp30 also binds several viral ligands leading to inhibition including HCMV pp65 (51) and vaccinia virus haemagglutinin (HA) (52). Unlike NKp30 and P46 which are expressed on both resting and activated NK cells, NKp44 is detected only on activated NKs (35). Viral HA and HA-neuraminidase (HN) are activating ligands (53, 54), and tumor-derived recently proliferating cell nuclear antigen (PCNA) has been reported as an inhibitory ligand (55). Among the NCRs, NKp46 is the only receptor that has an orthologue in other species. This specific evolutionary conservation suggests that NKp46 is the primary NCR involved in tumor and pathogen recognition (35). NKp46 recognizes unknown ligands on pancreatic β-cells leading to the development of type I diabetes (56). Engagement of NKp46 by an as yet unidentified ligand on hepatic stellate cells protects from liver fibrosis (57). NKp46 is important for the recognition of HA from several viruses including influenza and sendai viruses (58).
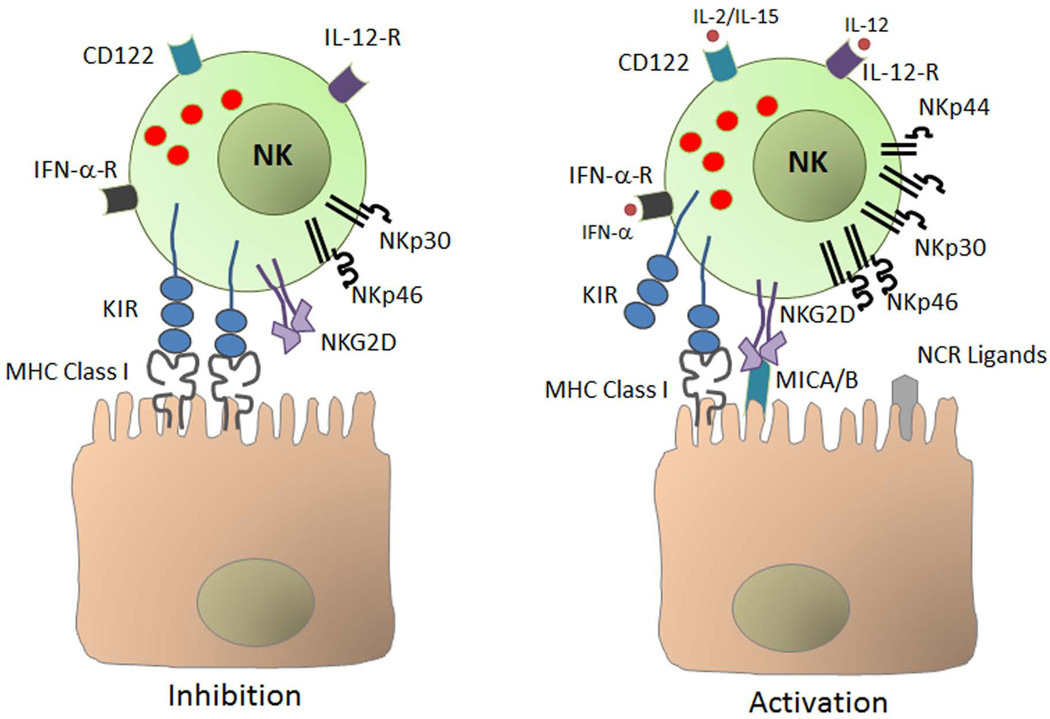
The current model of NK cell activation includes loss of constitutive inhibition through downregulation of MHC class I (59), upregulation of activating receptors and/or their ligands (30), cell adhesion and, response to inflammatory cytokines including IFNs induced by viral infection, interleukin-2 (IL-2), IL-15, and IL-12 (38, 60–64)(Fig. 3).
Fig. 3. Activation of natural killer (NK) cells.
Under normal conditions, NK cells are constitutively inhibited mainly through engagement of major histocompatibility complex (MHC) class I molecules on normal cells by NK cell-expressed killer immunoglobulin-like receptor (KIR). Under conditions of stress such as viral infection, loss of constitutive inhibition through downregulation of MHC class I, upregulation of activating receptors and/or their ligands, cell adhesion, and response to inflammatory cytokines including interferon-α (IFN-α) and interleukin-2 (IL-2), IL-12, and IL-15 results in activation of NK cells. MICA/B, MHC class I polypeptide-related sequence A/B; NCR, natural cytotoxicity receptor; TNF-α, tumor necrosis factor α; IFN-α-R, IFN-α receptor.
The role of natural killer cells early in HCV infection
Recent studies have implicated NK cells as important players in host defense in all stages of HCV infection and suggest that NKs may even protect from HCV acquisition. Genetic studies have linked KIR and MHC Class I polymorphisms to resistance to HCV (65, 66). These data suggest that heightened NK cell activity may prevent HCV infection in the setting of low-dose exposure. However, as KIR can also be expressed by T-cell subsets, the direct relevance of some of these data to NK cell biology remains to be fully established (67). We characterized NK cells in a unique cohort of prospectively collected peripheral blood samples from HCV-exposed injection drug users (IDUs). NK cell profiles in exposed individuals who remained uninfected despite being repeatedly exposed to HCV (n=11) were compared with pre-infection samples (median 90 days prior to HCV seroconversion) collected from 14 IDUs who were exposed and subsequently became infected. We demonstrated, in patients who remain protected from HCV infection, enrichment for CD56low effector NKs displaying enhanced IL-2 induced cytolytic activity against the NK-sensitive cell line K562 and higher levels of the NKp30 activating NCR (68). A role for NKs in preventing HCV infection is further supported by a recent study from Barbara Rehermann’s group at the National Institutes of Health (NIH) (69). Eleven healthcare workers with accidental percutaneous exposure to HCV-infected blood who remained negative for HCV RNA and HCV-antibodies were studied. All but one of these cases displayed increased multifunctional NK cell responses with enhanced NCR (NKp44, NKp46) and NKG2A expression, cytotoxicity (as determined by TRAIL and CD107a expression), and IFN-γ production (69). We have also demonstrated clear race- and gender-related differences in expression of NKp46, which correlates with differential HCV natural history, supporting the biological relevance of NKp46 in innate protection (70). NKp46 is considered the major human NCR involved in NK cell-mediated killing (58, 71). Taken together, these data support the hypothesis that NK cell activity contributes to anti-HCV defense in the earliest stages of infection, providing innate protection from HCV acquisition and point to a significant role for NCRs and enhanced cytotoxicity in this process.
Natural killers in the acute phase
Little is known of the role of NK cells in determining the outcome of acute HCV infection. It is thought that NK cells are activated early in acute HCV infection, although the precise role they play is unclear (72–74). Functionally, NK cell IFN-γ production (72) and cytotoxicity or degranulation were higher in individuals with acute HCV infection than in healthy controls (72, 73). In another study, the absolute percentage of circulating NK cells was significantly elevated in the acute phase of HCV infection compared to HCV-negative controls. In addition, NK cells from acutely infected patients showed increased degranulation in response K562 target cells (74). In the studies above, the activity of NK cells did not correlate with subsequent outcome. However, an indirect role for NKs through induction and priming of T-cell responses was suggested by the finding that peak NK cell activation and degranulation preceded peak T-cell responses and, of note, NK cell degranulation correlated with the magnitude of HCV-specific T-cell responses (73). Phenotypic alterations of NK cells in acute HCV infection have been reported but are difficult to interpret. Amadei et al. (72) observed an increased expression of NKG2D on NK cells, irrespective of the outcome, as compared with healthy controls which is consistent with activation. Alter et al. (74) showed that NK cells from acute infected patients demonstrated lower frequencies of NKp46- and NKp30-expressing NK cells, and these lower levels correlated with HCV clearance. This finding is somewhat counterintuitive, as high levels of NKp30 (68) and NKp46 (69) expression have recently been associated with protection against HCV infection in exposed uninfected individuals and as NKp46 expression correlates with anti-HCV activity in vitro (70, 75, 76). The authors suggest that activation-induced downregulation of NCRs may account for the diminished percentage of NK cells expressing NKp46 and NKp30 in patients who resolve acute infection and may reflect that early NK cell activation results in the onset of an effective innate immune response that participates in viral clearance (74). Further studies using well defined cohorts of patients with acute HCV infection are needed to define the contributions of individual NKRs to resolution.
Studies to date suggest direct involvement of NK cells in the acute phase of HCV infection; NK cell activation and phenotypic alterations have clearly been demonstrated. A direct role for NK cells in resolution of acute HCV infection has yet to be demonstrated. Activation of NK cells early in HCV infection likely favors induction and priming of downstream T-cell responses and HCV clearance (77).
Natural killer cell levels and phenotype in chronic HCV infection
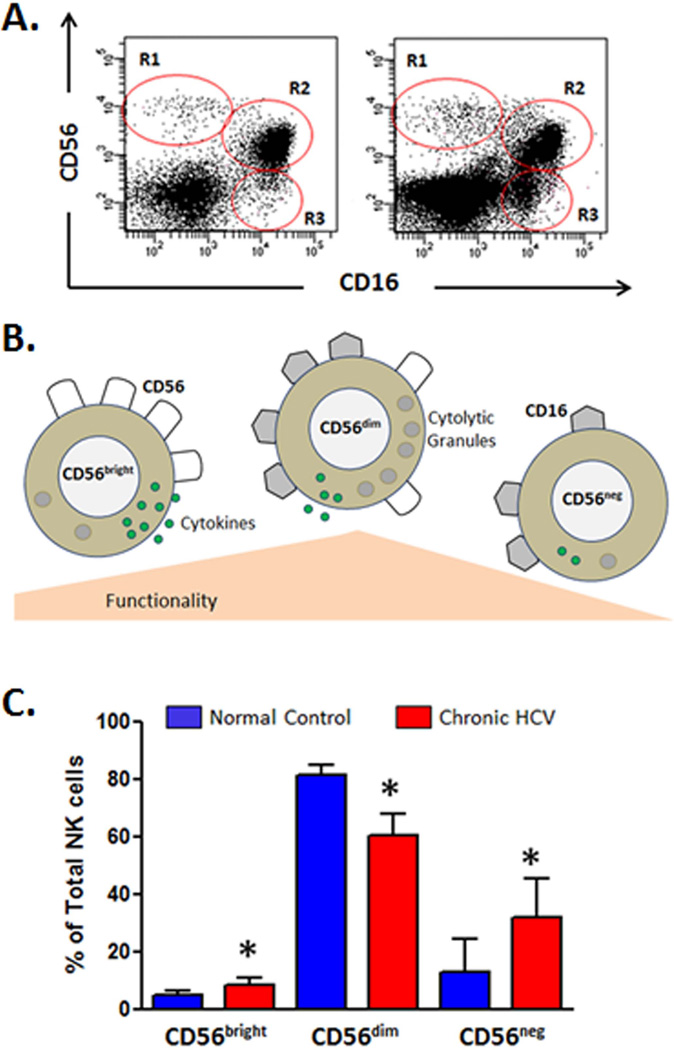
Significantly more is known of the role played by NK cells in the outcome of chronic HCV infection. NK cell frequency is reduced in chronic HCV compared to healthy controls (78–81). The reason for this decrease is currently unknown but is probably not due to NK cell recruitment to and compartmentalization in the liver as hepatic NK cell levels are also decreased (79, 82, 83). In humans, NKs can be identified by the expression of N-CAM (CD56) and relative expression of this antigen identifies functionally distinct immature/regulatory (CD56bright) and effector (CD56dim) NK subsets. The CD56dim subset, which are strongly cytolytic mature effector cells characterized by high perforin expression, account for the majority of circulating NK cells. In contrast, CD56bright NK cells are focused on production of cytokines such as IFN-γ (84). This subset is considered less mature and can give rise to the CD56dim NK cells (85). In addition to these conventional NK cell subsets, a highly dysfunctional subset of CD56negCD16pos NKs has been described that appears to be terminally differentiated, has impaired cytolytic function, and poor cytokine production (86). Altered subset distribution (decreased CD56dim and/or increased CD56bright) is a consistent finding in several chronic HCV cohorts (79, 87). Increased circulating levels of dysfunctional CD56negCD16pos have also been reported (88, 89) (Fig. 4). While changes in phenotype are clearly demonstrated in chronic HCV, conflicting data exist with respect to the expression of NKRs. These variances may arise from differences in methodologies, control groups used, the use of fresh or frozen blood samples, and small sample sizes (90). Increased NKG2A expression (79, 91–93) is a consistent findings in chronic HCV, which suggests inhibition of NK function, although this may simply reflect altered subset distribution as CD56bright NKs express high levels of this receptor. The evidence with respect to NCR expression in chronic HCV is conflicting as both decreased expression (94) and increased expression (91, 95, 96) have been reported. A significant role for the NKG2D pathway in the defense against HCV infection is suggested by several studies, although the overall contribution of the NKG2D pathway in the control of HCV infection is not fully elucidated (81, 91). The HCV-NS5A protein downregulates expression of NKG2D on NK cells via theTLR4 pathway, thus impairing their function. The suggested mechanism is that NS5A triggers IL-10 secretion from monocytes, which in turn promotes TGFβ production, which leads to downmodulation of NKG2D expression and impaired effector functions both IFN-γ and CD107a degranulation (97). In a direct infection system (HL7702 cells infected with HCV-positive serum), the HCV protease NS3/4A was shown to reduce the expression of NKG2D ligands MICA and MICB (98). Direct contact with HCV-infected cells impaired NK cell degranulation, lysis activity, and IFN-γ production, and this inhibition was associated with downregulation of NKG2D and NKp30 on NK cells. These observations suggest that direct cell-to-cell interaction between NK cells and HCV-infected hepatocytes may impair NK cell function in vivo and thereby contribute to the establishment of chronic infection (99). Augmentation of NKG2D activity may enhance immunity to some cancers or infections. For this to be possible, more research is needed to further understand mechanisms that regulate NKG2D function, expression, and signaling (43). NKG2D expression has been reported to be upregulated or downregulated or unchanged in HVC infection (91, 92, 95).
Fig. 4. Natural killer (NK) cell subset distribution is altered in HCV infection.
The expression patterns of CD56 and CD16 can identify three distinct NK cell subsets CD56brightCD16neg (R1), CD56dimCD16pos (R2), and CD56negCD16pos (R3). Representative flow cytometric dot plots of CD3neg lymphocytes (low forward and side scatter) from one normal control subject and one chronic HCV patient are shown (A). CD56bright NK cells do not display significant natural cytotoxicity and are focused on production of cytokines such as IFN-γ. This subset is considered less mature and can give rise to the CD56dim mature effector cells which are strongly cytolytic and characterized by high perforin-containing granule content. Although CD56dim NKs produce less cytokine than their CD56bright counterparts, because they represent the predominant NK subset in circulation, they are the main contributor to overall cytokine levels. The CD56negCD16pos NK subset is highly dysfunctional, has impaired cytotoxic function and poor cytokine production, and appears to be terminally differentiated (B). The bar chart shows the NK cell subset distribution which is altered in HCV infection. Median values and interquartile range are shown for normal control subjects (n = 5) compared to chronic HCV patients (n = 5). CD56dim mature effector NK cells account for the majority of circulating NK cells in both groups. Decreased CD56dim and increased CD56bright is a consistent finding in several chronic HCV cohorts (79, 87). Increased circulating levels of dysfunctional CD56negCD16pos are also evident. *p<0.05 calculated using a two sided Mann Whitney test.
The activity of natural killer cells in chronic HCV infection
In chronic HCV infection, overall levels of NK cells are decreased, the NK cell subset distribution is perturbed, and NKR expression is altered possibly reflecting activation in response to chronic virus-induced stimulation. The question remains, how do these changes impact on activity, and, does the functionality of NKs influence the outcome of infection. Several in vitro and ex vivo studies suggested that NK cell activity was inhibited in chronic HCV infection (100–104). However, the reported depressed activity of NK cells in chronic HCV may be a consequence of decreased levels of CD56dim effector cells, as more recent studies suggest that activity on a per cell basis is intact (78, 87). Several lines of evidence suggest that skewing or polarization of NK cell function away from IFN-γ production towards cytotoxicity may promote viral persistence and liver damage (81, 95, 105–106). Polarization of NK cell function towards cytokine production or cytotoxicity is clearly demonstrated in vitro. Cytokine stimulation (IL-12/IL-15) of isolated human NKs induces production of IFN-γ but not degranulation, whereas, phorbol myristate acetate (PMA) and calcium ionophore ionomycin stimulation induces degranulation (70). An NK cell polarization model as a mechanism of HCV evasion of effective NK cell responses and promotion of liver injury is supported by the available data. This phenomenon may be linked to the requirement for caspase activation for IFN-γ and TNF production, which is dispensable for cytotoxicity (107). Insufficient IFN-γ responses may result in increased viral replication, as IFN-γ has direct anti-viral properties and can control viral replication in vitro in a dose-dependent manner (108, 109). In addition to antiviral activity, IFN-γ is important for the differentiation and trafficking of appropriate helper T-cell responses (110, 111). Enhanced NK cell cytotoxicity accompanied by insufficient IFN-γ production may promote liver damage (95).
The above studies suggest that in addition to a decrease in overall levels of NK cells that NKR expression is altered reflecting activation in response to chronic virus-induced stimulation. The contribution of individual NKRs to viral clearance or persistence remains to be clarified. Data with respect to the functionality of NK cells in the setting of chronic HCV infection favors a polarization model. More research is needed to further understand mechanisms that regulate NK cells in the setting of HCV infection.
Natural killer cells in treatment
As antiviral therapy for chronic HCV infection continues to evolve, IFN-α remains as an integral component of current therapies. NK cells are one of the primary cell populations responding to IFN-α; therefore, it is logical to assume that NKs will be intimately involved in the response to antiviral therapy for chronic HCV. Human NK cells recognize HCV-infected hepatoma cells after IFN-α stimulation in a DNAM-1-dependent manner. Furthermore, interaction of IFN-α-stimulated NK cells with HCV-infected hepatoma cells efficiently reduces HCV replication (112). IFN-α also induces TRAIL expression on NK cells and increased expression of TRAIL on NK cells has been associated with control of HCV infection; these observations might account for the second-phase decline in HCV-RNA levels during pegylated-IFN-α therapy (113).
Different NK cell levels and phenotypic and functional features in patients with chronic hepatitis C treated with standard therapy (pegylated IFN-α) and ribavirin observed between non-responder versus SVR patients supports a role for NK cells in the response to treatment (114). Baseline frequencies of CD56dim NK cells and perforin content were significantly higher in SVR vs. non-responder subjects. NK cells were more activated, as evidenced by increased expression of CD69, in rapid virological responders (RVR). Moreover, higher natural and antibody-dependent NK cytolyticity were associated with SVR (114). We have demonstrated higher expression levels of inhibitory NKG2A in patients who failed to achieve SVR (89). Levels of NK cells and the IFN-γ expression upon stimulation with K562 were reversed after successful treatment with pegylated IFN-α and ribavirin; however, these skewed functions were not recovered in treatment-resistant patients (105). Circulating CD56neg functionally impaired NK cells are increased in chronic HCV-infected patients compared to uninfected controls with the highest levels seen in those who fail to respond to standard pegylated IFN-α and ribavirin therapy. Higher levels of these dysfunctional NK cells also correlate with poor early viral kinetics (viral decline < 1.4 log10 in the first 28 days of treatment) (89). Successful antiviral therapy restores NK cell levels in the liver. Analysis of paired liver biopsy samples has shown that SVR is associated with an increase in the total number of intrahepatic NK cells following treatment with IFN-α alone or combined with ribavirin (115, 116).
Taken together, the above studies suggest that activation of NK cells by IFN-α is important to achieve treatment-induced viral clearance. It will be of interest to monitor NK cell responses to triple therapy.
Hepatic NK cells and HCV
The human liver is relatively enriched in NK cells (117–119). The role of liver-derived NKs has been studied extensively in animal models, but their functions in human liver disease are largely unexplored (118, 119). Hepatic NKs comprise 30–50% of lymphocytes in the liver (117, 119). The relative enrichment and constitutive activation of NKs in normal liver reflects their role in immune surveillance and elimination of pathogens encountered in the liver (119). Phenotypic studies are limited but have shown that hepatic NK cells differ from peripheral NK cells in that the majority do not express CD16 (120). Increased CD94:NKG2A and decreased KIR expression is also characteristic of hepatic NK cells (121). Two recent comprehensive reviews highlight the paucity of data on human liver-derived NK cells (90, 122).
Intrahepatic NK cells may behave differently to NK cells in other areas due to the ‘tolerogenic’ environment in the liver (117). Murine intrahepatic NK cells express high levels of NKG2A and are hyporesponsive. They are less cytotoxic and have an altered cytokine profile producing lower levels of IFN-γ and greater levels of immunoregulatory cytokines, such as IL-10, compared to peripheral blood and splenic NK cells (123). This hyporesponsive state has been described in the early stages of hepatitis B virus infection and may contribute to the establishment of chronic viral infection (124). Similar studies on human liver-derived NK cells in chronic HCV infection have yet to be carried out. Much of what we know about hepatic NK cells in chronic HCV is inferred from our knowledge of the altered expression of important NK cell ligands in infected liver and cell culture systems. Direct contact with HCV-infected cells results in impaired NK cell degranulation and IFN-γ production. The observed inhibition was associated with a decrease in NK-activating receptor (NKG2D and NKp30) surface expression on NK cells. These observations suggest that direct interaction between NKs and HCV-infected hepatocytes may impair NK cell function in vivo contributing to the establishment of chronic infection (99). In HCV infection, there is an impairment of MIC-A/B expression which may result in lower levels of NK cell activation via the NKG2D ligand (97, 98, 125). The increased expression of NKG2A on hepatic NK cells (79) may be important, as HCV can upregulate HLA-E, the ligand for NKG2A, in vivo and in vitro thus representing a mechanism by which HCV may modulate the hepatic NK cell response (125, 126). HCV core protein can upregulate MHC class I expression on hepatocytes (127), which acts as a ligand for inhibitory KIR, another potential NK inhibitory strategy at play in the liver.
Hepatic NK cell numbers are decreased in chronic HCV and further decreased in cirrhosis (79, 82, 83). NK cells comprise 38% of lymphocytes in HCV-infected liver compared to 55% in non-HCV liver and have similar activation status, as evidenced by the expression of CD69. In chronic HCV infection, NK cell populations do not correlate with histological parameters (128) and decrease with histological progression (82, 129), suggesting they may not be directly involved in liver damage, making them an attractive population for immune intervention. NK cells are localized to necrotic areas in liver biopsy specimens in chronic HCV, but not chronic HBV (79). Increased proportions of CD56bright NK cells, increased expression of NKG2A in the liver of chronic HCV-infected patients (compared to chronic HBV infection) has been demonstrated which inversely correlated with viral load (79). Successful antiviral therapy restores the NK cell levels in the liver. Analysis of paired liver biopsy samples has shown that SVR is associated with an increase in the total number of intrahepatic NK cells following treatment with IFN-α alone or combined with ribavirin (115, 116).Activated NKs kill hepatocytes by releasing TRAIL in mice (130), and an HBV study suggests that this is also true for humans (131). IFN-α induces TRAIL expression on NK cells and increased expression of TRAIL on NK cells has been associated with control of HCV infection (113). Therefore TRAIL expression by hepatic NK cells may be important for HCV clearance (132). A recent study from Jacob Nattermann’s group (75) found an intrahepatic accumulation of highly cytolytic NK cells expressing high levels of NKp46. Of note, the frequency of intrahepatic NKp46High NK cells was inversely correlated with HCV-RNA levels. This observation suggests that hepatic NK cell populations are actively involved in control of HCV.
Natural killer cells and fibrosis
In the face of heightened immunity, control of immune responses to limit collateral damage is as important as sustaining anti-viral defense, and NK cells may be central to maintaining this balance (27, 133). Chronic liver injury leads to liver fibrosis that is associated with accumulation of collagen in the liver (134). Immune cells play important and opposing roles in the regulation liver fibrosis; CD8+ T cells promote (135) and NK cells inhibit fibrosis. In addition to their role in protection against pathogens and tumor transformation, intrahepatic NK cells have been demonstrated to have anti-fibrotic functions via inhibition of hepatic stellate cells (HSCs). They are capable of directly inducing HSC apoptosis in a TRAIL and NKG2D-dependent manner (136). Early activated stellate cells (HSCs) express increased levels of the NKG2D ligand MICA (136) (137) and upregulate TRAIL receptors upon activation (138). IFN-α treatment enhances while other factors (e.g. alcohol, TGF-β) attenuate the cytotoxicity of NK cells against HSCs, thereby differentially regulating liver fibrogenesis (118). Production of IFN-γ by NK cells directly inhibits HSC activation (139, 140). Inhibitory KIR knockdown stimulates NK cells and promotes their anti-fibrogenic activity in mice and in human cell co-cultures. These findings have implications for possible immune therapeutic strategies in patients with advanced liver disease (141). These studies have revealed that human NK cells can kill primary human HSCs. The ability of NK cells from HCV patients to kill HSCs is enhanced and correlates inversely with the stages of liver fibrosis (78). As mentioned above, an intrahepatic accumulation of NKp46high was demonstrated in chronic HCV. The frequency of this population not only inversely correlated with HCV-RNA levels but also with fibrosis stage (75). This finding suggests that the same hepatic NK cell populations that are actively involved in control of HCV may also be involved in control of HCV-associated liver damage.
Regulatory natural killer cells
The immune-regulatory role of NK cells appears to play a critical role in shaping subsequent T-cell responses. IFN-γ, produced by NK cells, can promote the development of appropriate inflammatory T-helper 1 (Th1) responses (142). NK cells through interaction with DCs may also be required for the initiation of T-cell responses (143). DCs can be broadly classified into two major subsets myeloid and plasmacytoid (mDC and pDCs, respectively), which play distinct roles in the immune system. As major antigen-presenting cells (APCs), mDCs are critical for the priming of virus-specific CD4+ and CD8+ T cells (144). Upon activation, they produce IL-12 and IL-15, which can activate NK cells (145, 146) in addition to promoting the differentiation of pathogen-specific CD4+ Th1 cells and cytotoxic CD8+ T cells (CTLS) (147). Upon pathogen sensing, pDCs produce type I and type III IFNs (148), which also play a role in the activation and expansion of NK cells (149). Crosstalk between NKs and DCs is bidirectional: DCs activate NK cells (150, 151), and NK cells induce the maturation of DCs (152). Maturation of immature DCs (iDCs) into efficient APCs capable of initiating effective T-cell responses is dependent on NK cells through cell surface contact and cytokine secretion (153, 154). Moreover, DCs might also activate NK cells indirectly by promoting the expansion of antigen-specific T cells, which secrete IL-2, which in turn activates NK cells. IL-2-activated NK cells eliminate iDCs and thus may also play a role in downregulation of immune responses, as IL-2 is primarily produced by activated inflammatory T cells (155, 156). Bidirectional crosstalk between DCs and NK cells is important for the priming, activation, and expansion of T-cell responses (157), and disruption of this pathway is emerging as an important immune evasion mechanism employed by several viruses and direct infection of DCs appears to be an important factor (158–161). It is unlikely that DCs support replication of HCV, although HCV RNA has been detected in both mDCs and pDCs (162, 163) while other studies have not been able to detect viral replication or protein synthesis in DCs after co-culture with infectious recombinant HCV (164). A recent study suggests that exosomal transfer of HCV RNA occurs between hepatocytes and DCs that would explain the presence of HCV RNA in cells that do not support replication (165). DCs have been shown to be defective in chronic HCV (166–170), although the mechanism has not been fully elucidated. Circumstantial evidence, such as downregulation of MICA/B and defective IL-15 production (125, 171) by DCs, suggests that dysregulation of NK:DC crosstalk may be involved further studies are required to provide direct evidence for dysregulation of this pathway in HCV infection. The numerous mechanisms evolved by viruses to inhibit NK cell activity may not be directed at the innate immune response but may represent a strategy to prevent effective induction of adaptive immune responses (172). Defective T-cell or DC activity observed in viral infection may represent a bystander effect of viral NK cell inhibition (28).
Memory NK cells
Evidence of NK cell memory populations involved in antiviral immunity is accumulating in murine studies (173–177). Adoptive transfer experiments demonstrate that hepatic NK cells are sufficient and required for anti-viral recall responses to vesicular stomatitis virus (VSV), influenza A, and HIV (174). NK cell memory of haptens and at least some viruses are dependent on CXCR6, a chemokine receptor on hepatic NK cells that was found to be required for the persistence of memory NK cells but not for antigen recognition. Thus, hepatic NK cells can develop adaptive immunity to structurally diverse antigens, an activity that may depend on NK cell-expressed CXCR6 (178). Murine cytomegalovirus (MCMV)-experienced NK cells confer 10-fold the level of protection from infection when transferred into newborn MCMV-susceptible mice. However, MCMV-specific NK cells are not confined to the liver (24). Identification of these long-lived memory NK cell populations in humans is more challenging and data is sparse (179). Human NK cells have functional memory-like properties after cytokine activation, which provides a novel rationale for integrating preactivation with combinations of cytokines into NK cell immunotherapy strategies (26). It has been suggested that in humans, that NKG2Cpos NK cells represent a CMV-specific memory NK cell population. NKG2Cpos NK cells transplanted from seropositive donors exhibit heightened function in response to a secondary CMV event compared with NKG2Cpos NK cells from seronegative donors (180).
NK cell vaccinations might provide new opportunities to immunize against pathogens that have proven difficult to control using conventional B- and T-cell vaccination strategies (181). Given the similarities with the NK cell response to virus in mice, it is not unreasonable to consider that antigen-specific stimulation of human NK cell receptors may lead to NK cell immunological memory. It has recently become appreciated that NK cells directly recognize pathogen-associated molecules (182–184). Direct pathogen recognition by NK cells adds a new dimension to a cell that is regulated not only by integration of a complex balance of inhibitory and activating receptor signals (15, 34, 185) but also by a wide array of cytokines (60–64). It is possible that NKRs may recognize HCV-specific components, as has been demonstrated for other flaviviruses, dengue virus (DV) and West Nile virus (WNV) (186). Human NK cell memory might pave the way for new vaccine approaches, not only against chronic virus infections but also cancer, through controlled exposure to NK cell-dependent antigens (27).
The observations that NK memory responses develop and persist at least in mice (23–25) and that inflammatory cytokines (25, 187) direct recognition of viral antigens and activating receptors are central to the generation and maintenance of NK memory suggests that NK cell vaccination may provide new opportunities to immunize against pathogens where conventional approaches have proved to be ineffective (181).
Concluding remarks
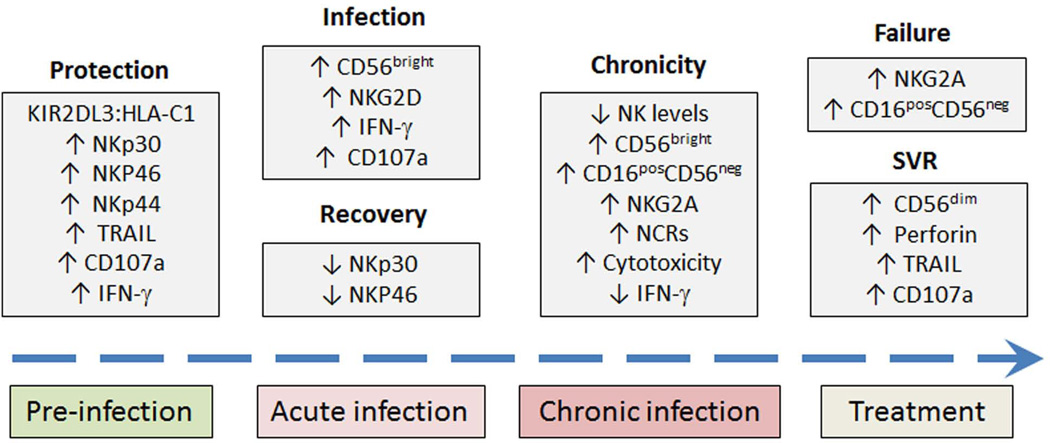
NK cells play important roles in every stage of HCV infection from protection against infection in IDUs to prediction of antiviral success or failure with IFN-based therapies (Fig. 5). They provide innate protection from HCV acquisition. Lack of constitutive inhibition and activation via NCRs are likely important in this process. NKs are activated early in HCV infection, and activation and phenotypic alterations have clearly been demonstrated. A direct role for NK cells in resolution of acute HCV infection has yet to be demonstrated. Activation of NK cells early in HCV infection likely favors induction and priming of downstream T-cell responses and HCV clearance. In the setting of chronic HCV, in addition to a decrease in overall NK cell levels and altered subset distribution, NKR expression is altered, reflecting activation in response to chronic virus-induced stimulation. The contribution of individual NKRs to viral clearance or persistence remains to be clarified. Data with respect to the functionality of NK cells in the setting of chronic HCV infection favor a polarization model with overactive cytotoxic and inadequate IFN-γ responses. Treatment-induced clearance is associated with activation of NK cells, and these activated NK cells may perform dual roles. On one hand, they are antiviral, but they may also be antifibrotic. We still have much to learn, in particular how liver-derived NK cells influence the outcome of HCV infection. The demonstration of NK cell antiviral memory and the possibility that NKRs may recognize HCV-specific components open up challenging but exciting avenues of investigation for the future.
Fig. 5. Natural killer (NK) cells play important roles in every stage of HCV infection.
NK cells play important roles in every stage of HCV infection from protection against infection in IDUs to prediction of antiviral success or failure with IFN-based therapies. Several NKRs and functional properties of NK cells have been implicated. Their association with natural history, stage of infection and treatment outcome are shown. KIR, killer immunoglobulin-like receptor; HLA, human leukocyte antigen; IFN-γ, interferon-γ; TRAIL, TNF-related apoptosis-inducing ligand; NCRs, natural cytotoxicity receptors; SVR, sustained virological response.
Acknowledgements
This work was supported by the following grants to HRR: U19 AI 1066328 (HCV Center Grant); VA Merit Review; RO1 DK60590 and RO1 DK071560.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra A, van der Laan LJ, Vanwolleghem T, Janssen HL. Experimental models for hepatitis C viral infection. Hepatology. 2009;50:1646–1655. doi: 10.1002/hep.23138. [DOI] [PubMed] [Google Scholar]
- 3.Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transplant. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of H. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10–12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. quiz 214–237. [DOI] [PubMed] [Google Scholar]
- 7.Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronsohn A, Jensen D. Distributive justice and the arrival of direct-acting antivirals: who should be first in line? Hepatology. 2011;53:1789–1791. doi: 10.1002/hep.24374. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. NNat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 11.Biron CA, Su HC, Orange JS. Function and Regulation of Natural Killer (NK) Cells during Viral Infections: Characterization of Responses in Vivo. Methods. 1996;9:379–393. doi: 10.1006/meth.1996.0043. [DOI] [PubMed] [Google Scholar]
- 12.Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunology today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature reviews Immunology. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 15.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 16.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–1211. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Eberlein J, Nguyen TT, Victorino F, Golden-Mason L, Rosen HR, Homann D. Comprehensive assessment of chemokine expression profiles by flow cytometry. J Clin Invest. 2010;120:907–923. doi: 10.1172/JCI40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83–88. doi: 10.1006/smim.1995.0012. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez NC, Flament C, Crepineau F, Angevin E, Vivier E, Zitvogel L. Dendritic cells (DC) promote natural killer (NK) cell functions: dynamics of the human DC/NK cell cross talk. Eur Cytokine Netw. 2002;13:17–27. [PubMed] [Google Scholar]
- 21.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol. 2005;42:451–454. doi: 10.1016/j.molimm.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: Rheostats for acute vs persistent infections. Virology. 2013;435:37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 24.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romee R, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biron CA. Expansion, maintenance, and memory in NK and T cells during viral infections: responding to pressures for defense and regulation. PLoS Pathog. 2010;6:e1000816. doi: 10.1371/journal.ppat.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transplan. 2006;12:363–372. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 29.Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protocol Immunol. 2010 doi: 10.1002/0471142735.im1109bs90. Chapter 11:pUnit 11 19B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol. 2011;1:497–512. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–234. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 32.Moretta A, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 33.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 35.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends iImmunol. 2013;34:182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Biassoni R. Human natural killer receptors, co-receptors, and their ligands. Curr Protocol Immunol. 2009 doi: 10.1002/0471142735.im1410s84. Chapter 14:pUnit 14 10. [DOI] [PubMed] [Google Scholar]
- 37.Falschlehner C, Schaefer U, Walczak H. Following TRAIL's path in the immune system. Immunology. 2009;127:145–154. doi: 10.1111/j.1365-2567.2009.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 40.Parham P. Influence of KIR diversity on human immunity. Adv Exp Med Biol. 2005;560:47–50. doi: 10.1007/0-387-24180-9_6. [DOI] [PubMed] [Google Scholar]
- 41.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 42.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 43.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 44.Jonjic S, Polic B, Krmpotic A. Viral inhibitors of NKG2D ligands: friends or foes of immune surveillance? Eur J Immunol. 2008;38:2952–2956. doi: 10.1002/eji.200838823. [DOI] [PubMed] [Google Scholar]
- 45.Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Cheng M, Tian Z. Hepatitis B virus down-regulates expressions of MHC class I molecules on hepatoplastoma cell line. Cell Mol Immunol. 2006;3:373–378. [PubMed] [Google Scholar]
- 47.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cell Mol Life Sci. 2011;68:3531–3539. doi: 10.1007/s00018-011-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Simhadri VR, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PloS One. 2008;3:e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnon TI, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 52.Jarahian M, et al. Modulation of NKp30- and NKp46-mediated natural killer cell responses by poxviral hemagglutinin. PLoS Pathog. 2011;7:e1002195. doi: 10.1371/journal.ppat.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Jarahian M, et al. Activation of natural killer cells by newcastle disease virus hemagglutinin-neuraminidase. 83. Journal of virology. 2009:8108–8121. doi: 10.1128/JVI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosental B, et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol. 2011;187:5693–5702. doi: 10.4049/jimmunol.1102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gur C, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol. 2010;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 57.Gur C, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012;61:885–893. doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- 58.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 59.Llano M, Guma M, Ortega M, Angulo A, Lopez-Botet M. Differential effects of US2, US6 and US11 human cytomegalovirus proteins on HLA class Ia and HLA-E expression: impact on target susceptibility to NK cell subsets. Eur J Immunol. 2003;33:2744–2754. doi: 10.1002/eji.200324182. [DOI] [PubMed] [Google Scholar]
- 60.Herberman RB. Augmentation of NK cell activity by IL-2. Progr Clin Biol Res. 1987;244:267–274. [PubMed] [Google Scholar]
- 61.Herberman RB, et al. Interferon and natural killer (NK) cells. Tes Rep Biol Med. 1981;41:590–595. [PubMed] [Google Scholar]
- 62.Carson WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. ldentification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 65.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 66.Romero V, et al. Interaction of NK inhibitory receptor genes with HLA-C and MHC class II alleles in Hepatitis C virus infection outcome. Mol Immunol. 2008;45:2429–2436. doi: 10.1016/j.molimm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werner JM, et al. Innate immune responses in hepatitis C virus exposed healthcare workers who do not develop acute infection. Hepatology. 2013 doi: 10.1002/hep.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golden-Mason L, Stone AE, Bambha KM, Cheng L, Rosen HR. Race- and gender-related variation in natural killer p46 expression associated with differential anti-hepatitis C virus immunity. Hepatology. 2012;56:1214–1222. doi: 10.1002/hep.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sivori S, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Amadei B, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pelletier S, Drouin C, Bedard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alter G, et al. Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J Hepatol. 2011;55:278–288. doi: 10.1016/j.jhep.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kramer B, et al. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology. 2012;56:1201–1213. doi: 10.1002/hep.25804. [DOI] [PubMed] [Google Scholar]
- 76.Heeg M, Thimme R. Natural killer cells and hepatitis C: natural killer p46 expression linked to antiviral and antifibrotic activity. Hepatology. 2012;56:1197–1200. doi: 10.1002/hep.25858. [DOI] [PubMed] [Google Scholar]
- 77.Marras F, Bozzano F, De Maria A. Involvement of activating NK cell receptors and their modulation in pathogen immunity. J Biomed Biotechnol. 2011;2011:152430. doi: 10.1155/2011/152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morishima C, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 79.Bonorino P, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51:458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 80.Meier UC, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliviero B, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. 1160, e1151–e1157. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 82.Kawarabayashi N, et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deignan T, et al. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101–108. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 84.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 85.Chan A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 86.Mavilio D, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golden-Mason L, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–1128. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez VD, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009;183:6612–6618. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 89.Golden-Mason L, et al. Natural killer inhibitory receptor expression associated with treatment failure and interleukin-28B genotype in patients with chronic hepatitis C. Hepatology. 2011;54:1559–1569. doi: 10.1002/hep.24556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut. 2011;60:268–278. doi: 10.1136/gut.2010.212555. [DOI] [PubMed] [Google Scholar]
- 91.De Maria A, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 92.Jinushi M, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 93.Takehara T, Hayashi N. Natural killer cells in hepatitis C virus infection: from innate immunity to adaptive immunity. Clin Gastroenterol Hepatol. 2005;3:S78–S81. doi: 10.1016/s1542-3565(05)00702-0. [DOI] [PubMed] [Google Scholar]
- 94.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahlenstiel G, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. e321–e322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harrison RJ, Ettorre A, Little AM, Khakoo SI. Association of NKG2A with treatment for chronic hepatitis C virus infection. Clin Exp Immunol. 2010;161:306–314. doi: 10.1111/j.1365-2249.2010.04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sene D, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. doi: 10.1371/journal.ppat.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wen C, et al. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell Mol Immunol. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon JC, Lim JB, Park JH, Lee JM. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85:12557–12569. doi: 10.1128/JVI.00838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crotta S, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonavita MS, et al. Normalization of depressed natural killer activity after interferon-alpha therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int J Tiss React. 1993;15:11–16. [PubMed] [Google Scholar]
- 103.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–457. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Par G, et al. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514–522. doi: 10.1016/s0168-8278(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 105.Dessouki O, et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: Reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331–337. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 106.Mondelli MU, Oliviero B, Mele D, Mantovani S, Gazzabin C, Varchetta S. Natural killer cell functional dichotomy: a feature of chronic viral hepatitis? Front Immunol. 2012;3:351. doi: 10.3389/fimmu.2012.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ussat S, Scherer G, Fazio J, Beetz S, Kabelitz D, Adam-Klages S. Human NK cells require caspases for activation-induced proliferation and cytokine release but not for cytotoxicity. Scan J Immunol. 2010;72:388–395. doi: 10.1111/j.1365-3083.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 108.Wang SH, et al. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepatitis. 2008;15:855–864. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183–190. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 110.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 111.Rotondi M, Chiovato L. The chemokine system as a therapeutic target in autoimmune thyroid diseases: a focus on the interferon-gamma inducible chemokines and their receptor. Curre Pharm Design. 2011;17:3202–3216. doi: 10.2174/138161211798157559. [DOI] [PubMed] [Google Scholar]
- 112.Stegmann KA, et al. Interferon alpha-stimulated natural killer cells from patients with acute hepatitis C virus (HCV) infection recognize HCV-infected and uninfected hepatoma cells via DNAX accessory molecule-1. J Infect Dis. 2012;205:1351–1362. doi: 10.1093/infdis/jis210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stegmann KA, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 114.Oliviero B, et al. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Van Thiel DH, Zhang X, Baddour N, Wright HI, Friedlander L, Gavaler JS. Intrahepatic mononuclear cell populations and MHC antigen expression in patients with chronic hepatitis C [correction of B]: effect of interferon-alpha. Digest Dis Sci. 1994;39:970–976. doi: 10.1007/BF02087546. [DOI] [PubMed] [Google Scholar]
- 116.Yamagiwa S, et al. Sustained response to interferon-alpha plus ribavirin therapy for chronic hepatitis C is closely associated with increased dynamism of intrahepatic natural killer and natural killer T cells. Hepatology Res. 2008;38:664–672. doi: 10.1111/j.1872-034X.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 117.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 118.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–1069. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leuk Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hata K, Van Thiel DH, Herberman RB, Whiteside TL. Natural killer activity of human liver-derived lymphocytes in various liver diseases. Hepatology. 1991;14:495–503. [PubMed] [Google Scholar]
- 121.Valiante NM, et al. Life, activation and death of intrahepatic lymphocytes in chronic hepatitis C. Immunol Rev. 2000;174:77–89. doi: 10.1034/j.1600-0528.2002.017417.x. [DOI] [PubMed] [Google Scholar]
- 122.Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851–863. doi: 10.1111/j.1365-2362.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 123.Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184:2693–2701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunn C, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 125.Jinushi M, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 126.Nattermann J, et al. The HLA-A2 restricted T cell epitope HCV core 35–44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herzer K, et al. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–8309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pernollet M, Jouvin-Marche E, Leroy V, Vigan I, Zarski JP, Marche PN. Simultaneous evaluation of lymphocyte subpopulations in the liver and in peripheral blood mononuclear cells of HCV-infected patients: relationship with histological lesions. Clin Exp Immunol. 2002;130:518–525. doi: 10.1046/j.1365-2249.2002.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tran A, et al. Phenotyping of intrahepatic and peripheral blood lymphocytes in patients with chronic hepatitis C. Digest Dis Sci. 1997;42:2495–2500. doi: 10.1023/a:1018856410794. [DOI] [PubMed] [Google Scholar]
- 130.Ochi M, et al. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–1331. doi: 10.1002/hep.20204. [DOI] [PubMed] [Google Scholar]
- 131.Dunn C, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Varchetta S, et al. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology. 2012;56:841–849. doi: 10.1002/hep.25723. [DOI] [PubMed] [Google Scholar]
- 133.Mengshol JA, Golden-Mason L, Rosen HR. Mechanisms of Disease: HCV-induced liver injury. Nat Clin Pract Gastroenterol Hepatol. 2007;4:622–634. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 134.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Safadi R, et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 136.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 137.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 139.Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. Involvement of natural killer cells in PolyI:C-induced liver injury. J Hepatol. 2004;41:966–973. doi: 10.1016/j.jhep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 140.Melhem A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 141.Muhanna N, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–98. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 142.Ge MQ, et al. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-gamma and perforin-dependent mechanisms. J Immunol. 2012;189:2099–2109. doi: 10.4049/jimmunol.1103474. [DOI] [PubMed] [Google Scholar]
- 143.Kos FJ, Engleman EG. Requirement for natural killer cells in the induction of cytotoxic T cells. J Immunol. 1995;155:578–584. [PubMed] [Google Scholar]
- 144.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 145.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 146.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 148.Stone AE, et al. Hepatitis C Virus Pathogen Associated Molecular Pattern (PAMP) Triggers Production of Lambda-Interferons by Human Plasmacytoid Dendritic Cells. PLoS Pathog. 2013;9:e1003316. doi: 10.1371/journal.ppat.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Romagnani C, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol. 2005;35:2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 150.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 152.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vitale M, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 155.Carbone E, et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 156.Terrazzano G, et al. Interaction between natural killer and dendritic cells: the role of CD40, CD80 and major histocompatibility complex class i molecules in cytotoxicity induction and interferon-gamma production. Scan J Immunol. 2004;59:356–362. doi: 10.1111/j.0300-9475.2003.01387.x. [DOI] [PubMed] [Google Scholar]
- 157.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 158.Sehgal M, Khan ZK, Talal AH, Jain P. Dendritic cells in HIV-1 and HCV infection: Can they help win the battle? Virology Res Treat. 2013;4:1–25. doi: 10.4137/VRT.S11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mandaric S, et al. IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathogens. 2012;8:e1002846. doi: 10.1371/journal.ppat.1002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marcenaro E, Carlomagno S, Pesce S, Moretta A, Sivori S. NK/DC crosstalk in anti-viral response. Adv Exp Med Biol. 2012;946:295–308. doi: 10.1007/978-1-4614-0106-3_17. [DOI] [PubMed] [Google Scholar]
- 161.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goutagny N, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951–1958. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 163.Rodrigue-Gervais IG, et al. Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients. J Virol. 2007;81:5537–5546. doi: 10.1128/JVI.01741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liang H, et al. Differential effects of hepatitis C virus JFH1 on human myeloid and plasmacytoid dendritic cells. J Virol. 2009;83:5693–5707. doi: 10.1128/JVI.02671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anthony DD, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. JImmunol. 2004;172:4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 167.Yonkers NL, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 168.Mengshol JA, et al. Impaired plasmacytoid dendritic cell maturation and differential chemotaxis in chronic hepatitis C virus: associations with antiviral treatment outcomes. Gut. 2009;58:964–973. doi: 10.1136/gut.2008.168948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ryan EJ, O'Farrelly C. The affect of chronic hepatitis C infection on dendritic cell function: a summary of the experimental evidence. J Viral Hepatitis. 2011;18:601–607. doi: 10.1111/j.1365-2893.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- 170.Dolganiuc A, Szabo G. Dendritic cells in hepatitis C infection: can they (help) win the battle? J Gastroenterol. 2011;46:432–447. doi: 10.1007/s00535-011-0377-y. [DOI] [PubMed] [Google Scholar]
- 171.Jinushi M, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 172.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nature reviews Microbiology. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 173.Pyzik M, Vidal SM. Natural killer cells: NK cells stroll down the memory lane. Immunol Cell Biol. 2009;87:261–263. doi: 10.1038/icb.2009.10. [DOI] [PubMed] [Google Scholar]
- 174.Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abdul-Careem MF, et al. Genital HSV-2 infection induces short-term NK cell memory. PloS One. 2012;7:e32821. doi: 10.1371/journal.pone.0032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. 2013;190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 180.Foley B, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun JC, Lanier LL. Versatility in NK cell memory. Immunol Cell Biol. 2011;89:327–329. doi: 10.1038/icb.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brown MG, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 183.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 184.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moretta L, et al. Human NK cells and their receptors. Microbes Infect. 2002;4:1539–1544. doi: 10.1016/s1286-4579(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 186.Hershkovitz O, et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183:2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]