Abstract
Introduction
Elastin fibers confer passive recoil to many tissues including the lung, skin, and arteries. In the penis, elastin is present in sinusoids, arterioles, and in the tunica albuginea. Although decreased penile elastin has been reported in men with erectile dysfunction, the exact role of elastin in physiologic processes integral to erection remains speculative.
Aim
The aim of this study was to characterize erectile function in elastin-deficient mice.
Methods
Elastin haploinsufficient mice (Eln+/−) and aged match Eln+/+ (Wt) mice were used. Cavernosum was removed from some mice for quantification of elastin, collagen, and smooth muscle actin. Ex vivo assessment of contractile force generation was performed by myography. In vivo assessment of intracorporal pressure normalized to mean arterial pressure in response to electrical stimulation of the cavernosal nerve was measured. Veno-occlusive function was determined by cavernosography.
Main Outcome Measures
The main outcome measures of this study were the in vitro and in vivo assessment of cavernosal vasoreactivity, veno-occlusive function and erection in mice deficient in elastin.
Results
Eln+/− mice exhibited ~33% less penile elastin than Wt mice, with no change in collagen. Cavernosal tissue from Eln+/− mice has a significantly heightened contractile response, explained in part by increased smooth muscle cell content. Veno-occlusive function was significantly altered in Eln+/− mice. Interestingly, erectile function was impaired only at submaximal voltage (1 V) stimulation (there was no impairment during the higher 2-V stimulus).
Conclusions
Eln+/− mice display a cavernosal phenotype consistent with developmental changes attributable to the loss of elastin. These alterations confer a degree of altered erectile function that is able to be overridden by maximal stimulatory input. Altogether, these data suggest that elastin is important for erectile function.
Keywords: Matrix, Animal Models, Sexual DysfunctionIntroduction
Introduction
The high degree of extensibility and recoil properties of corpora cavernosa are conferred by elastin fibers present in the sinusoids and the tunica albuginea. Elastin is an insoluble polymer found in the extracellular matrix of tissues subject to constant deformation. Encoded by a single copy gene on chromosome 7q11.2, elastin is secreted as a soluble precursor known as tropoelastin [1–3]. Mature elastic fibers are formed by deposition of tropoelastin on a scaffolding of fibrillin and fibrillin-rich microfibrils, resulting in extremely durable polymeric fibers [1–3]. Hydrophobic amino acids comprise 60% of elastin fibers and provide the quality of passive recoil.
Tissues rich in elastic fibers include lung, arterial vasculature, skin, certain ligaments, cartilages, and the penis [4,5]. The architectural orientation of elastin fibers is specific for different tissue types. In the corpora cavernosa, elastin is present in the cavernosal sinusoids, tunica albuginea, and veins. Histological sections demonstrate cavernosal sinusoids lined with endothelial cells and separated by a trabeculated network of collagen, elastin fibers and smooth muscle cells. Elastin fibers are also present in the tunica albuginea, where they are organized into an inner circular layer and an outer longitudinal layer [6].
Decreased elastin production can occur in heritable and acquired human diseases. Inherited conditions such as supravalvular aortic stenosis, cutis laxa, and William's syndrome all have defects in the elastin gene [7,8]. Degradation of elastin fibers occurs spontaneously as a consequence of aging and in chronic inflammatory conditions, particularly emphysema and aneurysms [9–11].
A significant loss of tunical elastin fibers in penile tissue from patients with venogenic erectile dysfunction (ED) has been reported [12]. Others studies similarly report a significant decrease in elastin and fibrillin in cavernosal biopsies from patients with ED [13,14]. Decreased elastin content in the cavernosum of type II diabetic mice that exhibit impaired erectile function has also been reported [15]. Although decreased elastin has been associated with ED in animal models and humans, the exact role of elastin in the physiologic processes integral to erection remains speculative. In this study, we utilized a transgenic mouse model of elastin deficiency to examine the role of elastin in penile erection.
Methods
Mice
Mice carrying a heterozygous deletion of exon 1 of the mouse elastin gene (Eln+/−) were used for the study [16]. Age-matched Eln+/+ mice (Wt) were used for controls. (Note: Eln−/− pups die shortly after birth because of apparent defects in their lung, and thus, cannot be used for these studies). Male mice were group housed in a temperature-controlled room with 12:12 hours light cycle and maintained with access to food and water ad libitum. All procedures were conducted with the approval of the Animal Care and Use Committee of the University of Washington and in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All mice were bred in-house and genotyped using the following primers: DL1: GGT TGT TCA GAC TAC AAT CTG ACC-3′, DL2: CAA CTT TGC CCA AAT GAC TCT CC-3′, DL5: GAG AGG TAT AGA GGG AAG ACT TGC-3′. Experiments were conducted in mice 12–16 weeks of age.
Histology
Cavernosum was excised and formalin fixed overnight with rehydration in 70% ethanol. Sections measuring 8 μm were stained with Gomeri's aldehyde fuchsin stain. Images were captured using a Leitz DMRB microscope at 4× and 10× magnification with an Image Pro system and Spot Insight digital camera (Leitz, Buffalo Grove, IL; Spot: Sterling Heights, MI, USA).
Desmosine and Hydroxyproline Quantification
Levels of cavernosal elastin and collagen were determined by desmosine and hydroxyproline content, respectively. Cavernosal tissue was isolated from each animal, homogenized in phosphate buffered saline with 6N HCl, and hydrolyzed at 110°C for 24 hours. Desmosine content was measured by competitive radioimmunoassay, and hydroxyproline was assessed in remaining hydrolyzed samples, as described [17,18] Results were expressed as picomoles desmosine or micrograms of hydroxyproline normalized per penis.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from mouse cavernosa by using Trizol (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized with RETROscript (Ambion, Austin, TX, USA) using random hexamers. Quantitative RT-PCR reactions employing SYBER green fluorescent reagent (Applied Biosystems, Carlsbad, CA, USA) were performed in a 7,500 Fast RT-PCR System (Applied Biosystems) with the following primers: tropoelastin (5′-Gcgtcttgctgatcctcttg-3′; 5′-GGGAACTCCACC AGGAAGTC-3′), smooth muscle actin (5′-CT GACAGAGGCACCACTGAA-3′; 5′-CATCTC CAGAGTCCAGCACA-3′), 18s rRNA (5′-CGG ACAGGATTGACAGATTG-3′; 5′-CAAATCG CTCCACCAACTAA-3′). The relative amounts of mRNAs were calculated from the values of comparative threshold cycle by using 18 seconds as a control.
Western Blot Analysis
Mouse cavernosal tissue was snap frozen, ground to powder, and lysed in sodium dodecyl sulfate (SDS) lysis buffer containing 1× Complete Protease Inhibitor Cocktail (Roche, Roche Applied Sciences: Indianapolis, IN, USA). The protein lysates were separated with SDS-polyacrylamide gel electrophoresis. The proteins were transferred onto Immobilon-P Transfer Membrane (Millipore, Billerica, MA, USA), and immunodetection carried out with either anti-smooth muscle actin (Sigma, St. Louis, MO, USA) or anti-vascular enothelial (VE) cadherin (Enzo Life Sciences, Farmingdale, NY, USA) antibodies and visualized with Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Developed films were scanned and band densitometry was performed with the Labworks program (UVP, Upland, CA, USA) to evaluate protein levels. To be certain that the difference in the band density is within linear range, overexpression of the film was avoided. The ratio of smooth muscle actin-to-VE cadherin was obtained. The mean value from Wt mice was set as 100, and the fold change of the ratio of smooth muscle actin-to-VE cadherin was calculated from each animal.
DNA Content
Mouse penile tissues were cut into small pieces with a fine scissor, and then put into 1 mL of 0.1N NaOH plus 0.025% saponin. The tissues were homogenized with a homogenizer, and were then incubated at 60°C for 1 hour. The samples were centrifuged and the supernatant was taken for DNA measurements. DNA was measured using the fluorescent compound H33258 (Sigma) using a 1,420 Multilabel Counter plate reader (Perkin-Elmer, Waltham, MA, USA) with calf thymus DNA (Sigma) as a standard.
Ex Vivo Vasoreactivity Assay
Ex vivo myography was performed similarly to as previously described [19]. Animals were anesthetized with 4% isoflurane and sacrificed by cervical dislocation. Cavernosa were obtained by removal of the corpus spongiosum and urethra then placed in a physiological salt solution (PSS [mM]: NaCl 130; KCl 4.7; MgSO4 1.17; KH2PO4 1.18; CaCl2 2.5; NaHCO3 14.9; ethylenediaminetetraacetic acid 0.03; dextrose 5.5). Using a dissecting microscope, each penile tissue was cut in half longitudinally with a razor blade and mounted on 0.2-mm mounting pins in the muscle bath (Multi Myograph model 610 M, Danish Myo Technologies). Penile tissues were incubated for 30 minutes at 37°C with 6 mL of PSS and gassed with 95% O2 and 5% CO2. At the end of the incubation period, the force transducer was zeroed and optimal passive tension was determined by exposing the sections to electrical field stimulation (EFS) (Grass S88X stimulator, Astro-Medical, West Warwick, RI, USA) at 20 Hz, 2 msec, and 10 V for 45 seconds. Penile contraction in response to phenylephrine (PE), at increasing one-half log doses, and EFS (for 45 seconds at 2 msec and 10 V every 5 minutes), at increasing frequencies of 0.62, 1.25, 2.5, 5, 10, 20, and 30 Hz was assessed. Force generation was monitored with an ADInstruments PowerLab 8/30 and interpreted by Chart 5.5.4 for Windows (ADInstruments, Colorado Springs, CO, USA). Contractile activity was normalized to tissue weight. In separate tissues, relaxation in response to acetylcholine (1 nM–10 μM) or EFS (0.015–20 Hz) stimulation were measured following plateau of PE-induced contraction. Dilatory responses are reported as a percent relaxation from the PE contraction. When applied on top of PE-induced contraction, we consistently observed that EFS stimulation results in a frequency-dependent relaxation. We verified that this relaxation is virtually abolished when nitric oxide synthase (NOS) is inhibited by L-nitro-arginine methyl ester (L-NAME) treatment (data not shown).
Murine Pharmaco-Cavernosography
Veno-occlusive function was assessed similar to a method previously described [20,21]. Following intracavernosal infusion of 40 μg (15 μL volume) papaverine HCl in H2O, the cavernosum was infused at a rate of 10 mL/minute until intracavernosal pressure (ICP) reached 200 mm Hg. For the next 20 minutes the rate of infusion required to maintain a minimum of 200 mm Hg ICP was assessed by systematically reducing the infusion rate until the ICP was maintained at a steady state (200–230 mm Hg). The initial maintenance rate was 3.0 μL/minute, followed by 2.5, 2.0, 1.75, 1.5, 1.25, 1.0, 0.9, and 0.8. The rate was changed as soon as it was apparent that ICP was not steady. The maintenance infusion rate was recorded as the final rate required to maintain steady pressure ≥200 mm Hg at the end of the 20 minutes.
In Vivo Erectile Function
Erectile function in vivo was studied for both Eln+/− and Wt mice. Mice were anesthetized with 4% isoflurane in O2 and intubated with a 22-gauge angiocatheter. Mice were placed on a thermoregulated surgical pad and ventilated using a small animal ventilator (IITC Inc., IITC Life Sciences: Woodland Hills, CA, USA) with tidal volume set at 0.22 mL/g at 120 breaths/minute and maintained on 1–2% isoflurane. For measurement of mean arterial pressure (MAP), the left carotid artery was cannulated with PE-10 tubing attached to a pressure transducer filled with heparinized saline (100 units/mL) (Kent Scientific, Torrington, CT, USA). To measure ICP, the penile shaft and right crus were then dissected from the surrounding skin and connective tissue. A 27-gauge needle filled with heparinized saline was connected to a pressure transducer using P-50 tubing and inserted into the right crus. The left cavernosal nerve and major pelvic ganglion were identified through a midline abdominal incision and stimulated with a bipolar platinum electrode (in-house design; Grass S48K nerve stimulator, and stimulus isolation unit SIU5; Grass Telefactor, Astro-Med, West Warwick, RI, USA) at increasing frequencies of 1, 2, 5, 10, and 20 Hz for 0.2 ms and 2 V for 1 minute at 2-minute intervals. MAP and ICP were transmitted to a data-acquisition program (Hem 3.2; Notocord, Newark, NJ, USA) and the erectile response was calculated from area under the curve (AUC, in mm Hg) for ICP divided by the AUC for the calculated MAP. The peak MAP value at the time of peak ICP was utilized to normalize the erectile response.
Statistics
Statistical significance between Wt and Eln+/− groups for desmosine and hydroxyproline levels, RT-PCR, Western blot, DNA content, and cavernosography were compared by Student's t-test. Ex vivo myography was analyzed by analysis of variance (anova) with Bonferroni post-hoc test and IC50 values calculated by nonlinear regression analysis followed by t-test. Statistical significance for in vivo studies was determined by anova with repeated measures followed by t-test for planned comparisons between groups for each dose. For all experiments, P < 0.05 denoted statistical significance. N = number of animals per group. Data expressed as mean ± standard error of the mean.
Results
Elastin Localization in Mouse Cavernosal tissue
Gomeri's aldehyde fusion staining of cavernosal cross-sections from Wt and Eln+/− mice, revealed the presence of elastin in the cavernosal sinusoidal matrix, cavernosal arterioles (both Figure 1A, B), as well as the surrounding tunica albuginea (Figure 1C, D).
Figure 1.
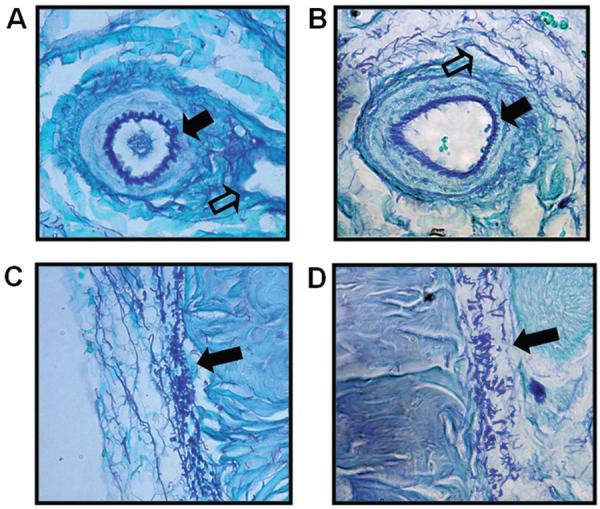
Localization of elastin in cavernosum from Eln+/− and Wt mice. Gomeri's aldehyde fuchsin stain shows elastic fibers (purple stain) A,B) dispersed throughout sinusoidal spaces (open arrow) and the elastin lamina in an arteriole (solid arrow) as well as C,D) elastin fibers in the outer tunica albuginea (solid arrow). (Wt = A and C; Eln+/− = B and D).
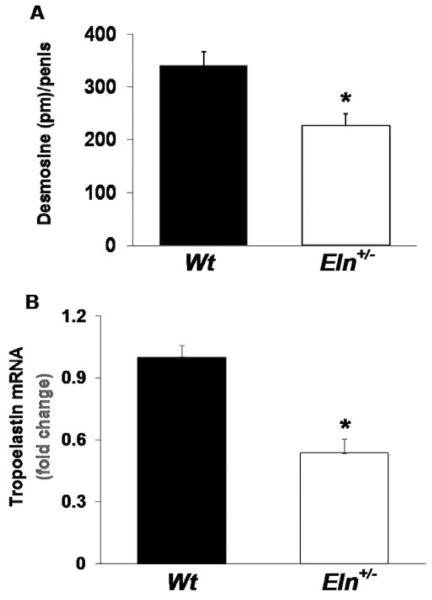
Penile Elastin Content
Desmosine/isodesmosine are covalently cross-linked lysine products found only in mature elastic fibers and are a reliable quantitative measure of the elastin content of a tissue [22]. Desmosine content was ~33% lower in penile tissue from Eln+/− mice (Figure 2A). Levels of tropoelastin mRNA, the elastin precursor, were also significantly decreased in penile tissue from Eln+/− vs. Wt mice (Figure 2B).
Figure 2.
Penile elastin content is decreased in Eln+/− vs. Wt mice. (A) Elastin content, as measured by desmosine quantification, was significantly lower in penile tissue from Eln+/− vs. Wt mice (N = 8–10/group, *P < 0.05, t-test). (B) Tropoelastin mRNA, as a fraction of total 18s rRNA, was significantly decreased in penile tissue from Eln+/− vs. Wt mice. The fold change of tropoelastin mRNA expression, referred to the Wt mice, is presented (N = 9/group, *P < 0.05, t-test).
Penile Collagen Content
To examine if changes in matrix were limited to elastin in Eln+/− mice, we measured penile collagen by hydroxyproline analysis. No significant differences were detected in penile collagen content between Eln+/− vs. Wt mice (hydroxyproline [μg]/penis: 216 ± 15 vs. 233 ± 7.7) (N = 9–11/group, P > 0.05, t-test).
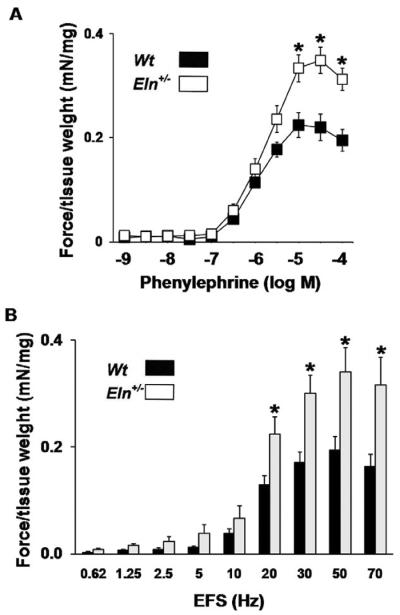
Contractile Force Generation of Isolated Cavernosum
Maximal contraction and sensitivity to PE was significantly heightened in penile tissue from Eln+/− vs. Wt (Figure 3B; -log EC50 for Wt and Eln+/− mice = 6.07 ± 0.08 vs. 5.82 ± 0.07, respectively, P < 0.05, t-test). EFS resulted in a frequency-dependent increase in force generation in Wt mice. The contractile response to EFS was significantly potentiated in cavernosal tissue from Eln+/− vs. Wt mice (Figure 3A). Altogether, these data are indicative of alterations at the smooth muscle, and not solely sympathetic nerve terminal, level.
Figure 3.
Heightened contractile force generation of isolated cavernosum from Eln+/− vs. Wt mice. (A) Contraction to phenylephrine was also significantly heightened in penile tissue from Eln+/− vs. Wt mice (N = 10/group, *P < 0.05, analysis of variance [ANOVA] with post-hoc test). (B) Ex vivo force generation in response to electrical field stimulation (EFS) was potentiated in cavernosum from Eln+/− vs. Wt mice. (N = 9–10/group, *P < 0.05, ANOVA with with post-hoc test).
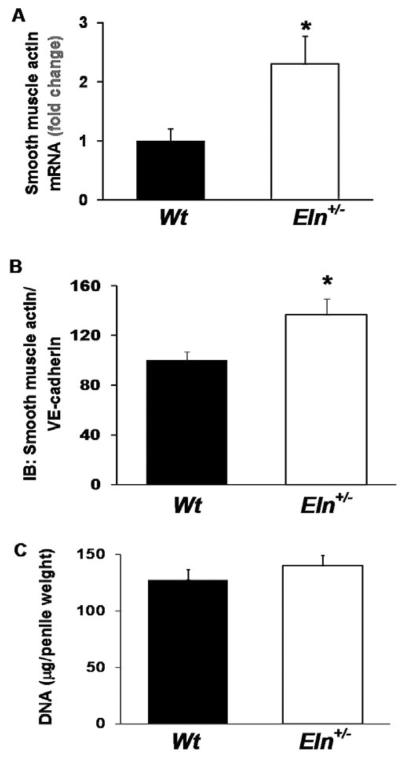
Penile Smooth Muscle Cell Content
To examine if the heightened force generation in Eln+/− mice reflected an increase in smooth muscle cell content, we measured smooth muscle actin mRNA and protein. Quantitative PCR revealed significantly elevated mRNA expression (>2-fold increase) of smooth muscle actin in penile tissue from Eln+/− vs. Wt mice (Figure 4A). Analysis of protein levels by Western blot indicated a significantly elevated ratio (~36% increase) of smooth muscle actin to the endothelial cell-specific protein, VE-Cadherin in penile tissue from Eln+/− mice (Figure 4B). To determine whether the increase in smooth muscle content in Eln+/− mice was due to cavernous smooth muscle hyperplasia or hypertrophy, we measured the total DNA content in the corpora cavernosa. No significant difference in total DNA content was detected between penile tissue from Eln+/− mice compared with that from Wt mice (Figure 4C), indicating that hypertrophy was the likely mechanism.
Figure 4.
Increased smooth muscle actin mRNA in cavernosum from Eln+/− vs. Wt mice. (A) Quantitative real-time polymerase chain reaction revealed significantly elevated mRNA expression of smooth muscle actin, as a ratio to 18s rRNA, in penile tissue from Eln+/− vs. Wt mice. The fold change of smooth muscle actin mRNA expression, referred to the Wt mice, is presented (N = 7/group, *P < 0.05, t-test). (B) Western blot analysis indicated an increased ratio of smooth muscle actin to VE-Cadherin in penile tissue from Eln+/− vs. Wt mice (N = 13–14/group, *P < 0.05, t-test). (C) Analysis of DNA content revealed no significant difference in total DNA in penile tissue from Eln+/− vs. Wt mice (N = 13–14/group, *P < 0.05, t-test).
Cavernosal Relaxation
The dilatory capacity of the tissue was determined by measurement of relaxation to activators of endothelial and neuronal NOS, acetylcholine and EFS, respectively. There was no significant change in dilatory capacity or sensitivity in response to ACh (Figure 5A; IC50 [-log] Wt = 7.26 ± 0.06 vs. Eln+/− = 7.42 ± 0.15; P > 0.05, t-test). Following pre-contraction with PE, EFS stimulation resulted in frequency-dependent relaxation of the cavernosal tissue. This relaxation response was virtually completely abolished in tissue treated with the NOS antagonist, L-NAME, supporting the relaxation response is due to activation of neuronal NOS (data not shown). There was no significant change in relaxation to EFS between Wt and Eln+/− groups (Figure 5B). Altogether, these data indicate that NOS-mediated relaxation, as relevant to erectile function, is unchanged in elastinhaploinsufficient mice.
Figure 5.
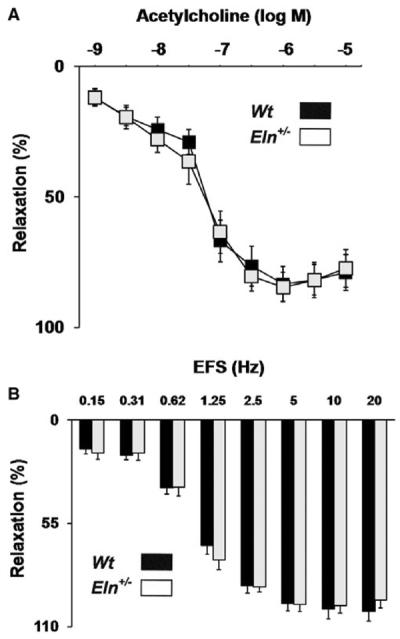
No change in relaxation to acetylcholine or electrical field stimulation in cavernosum from Eln+/− vs. Wt mice. (A) Relaxation to acetylcholine was not significantly changed in penile tissue from Eln+/− vs. Wt mice (N = 9–10/group, *P < 0.05, analysis of variance [ANOVA] with post-hoc test). (B) Similarly, ex vivo relaxation in response to electrical field stimulation (EFS) was unaltered in cavernosum from Eln+/− vs. Wt mice (N = 10/group, *P < 0.05, ANOVA with post-hoc test).
Veno-Occlusive Function
Pharmaco-cavernosography was used to assess veno-occlusive function. The flow rate required to maintain a set ICP (200 mm Hg) after the use of a smooth muscle relaxant, papaverine, was compared. Eln+/− mice required a higher flow rate to maintain 200 mm Hg ICP as compared with Wt mice (Figure 6).
Figure 6.
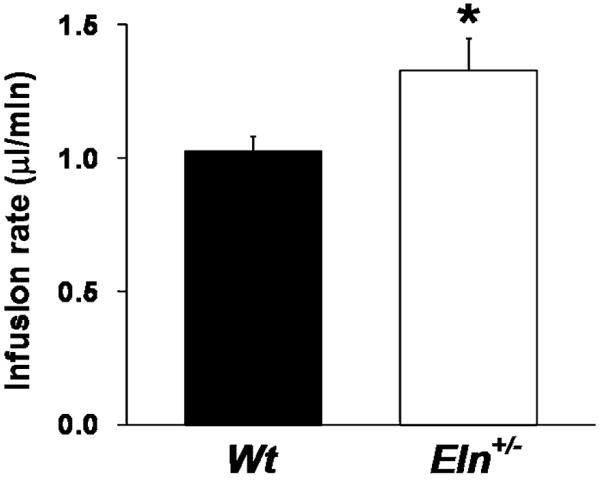
Impaired veno-occlusive function in Eln+/− mice. Pharmaco-cavernosography was used to assess venoocclusive function. The flow rate required to maintain a set intracavernosal pressure (200 mm Hg) after the use of a smooth muscle relaxant, papaverine, was compared. Eln+/− mice required a higher flow rate to maintain 200 mm Hg intracavernosal pressure as compared with Wt mice (N = 9–12/group, *P < 0.05, t-test).
In Vivo Erectile Function
At sub-maximal stimulus of the cavernosal nerve (1 V), Eln+/− mice demonstrated an attenuated increase in ICP/MAP as measured by AUC (Figure 7A). At a higher stimulation (2 V), the ICP/MAP response was intact in the Eln-deficient mice, suggesting that the overall ability to achieve elevated ICPs was intact (Figure 7B). Maximal ICP/MAP responses were not significantly impaired at sub-maximal or maximal stimulation, indicating that these mice are able to achieve high ICPs, but cannot maintain these pressures at lower stimulus (Figure 7B).
Figure 7.
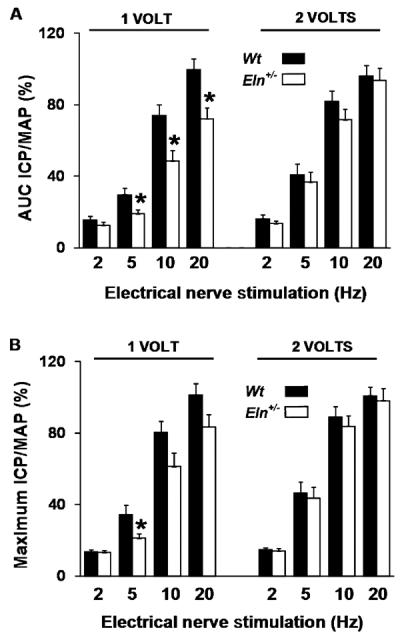
Intact intracavernosal pressure/mean arterial pressure (ICP/MAP) at maximal stimulus in vivo. (A) There was a significant decrease in the area under curve (AUC) ICP/MAP ratio in Eln+/− vs. Wt mice across a 1-V stimulus. Through a 2-V stimulus, erectile function was intact. (B) The maximum ICP/MAP response was not significantly different in Eln+/− vs. Wt mice at the 1 or 2-V stimulus (N = 19–25/group, *P < 0.05, ANOVA).
Discussion
Staining of cavernosal cross-sections revealed the presence of elastin throughout the sinusoids, arterioles, and surrounding tunica albuginea of both Wt and Eln+/− cavernosum (Figure 1). We were not able to detect quantifiable changes in the elastin content with this technique. Li et al. [8] similarly demonstrated that overall elastin deposition was reduced in aorta from tropoelastin haploinsufficient mice compared with Wt mice, but the reduction could not be appreciated by histology alone. Eln+/− mice typically express 30–40% less elastin in arterial vessels but have a normal lifespan [23–25]. In the present study, desmosine and RT-PCR analysis revealed a ~33% decrease in penile elastin content and significantly lower tropoelastin mRNA in Eln+/− vs. Wt mice (Figure 2), providing quantitative evidence for lowered elastin content in the Eln+/− mice. These data are consistent with findings in the aorta and pulmonary arteries [23,24]. Penile collagen, as measured by hydroxyproline, was unchanged in the Eln-haploinsufficient mice.
A striking phenotypic change in the Eln+/− mice was a significantly heightened contractile response of isolated cavernosum to both EFS and PE (Figure 3). These data suggest increased smooth muscle content and/or heightened contractile signaling in the Eln-deficient mice. A significant increase in smooth muscle actin mRNA was detected in cavernosal tissue from Eln+/− vs. Wt mice (Figure 4A). Further analysis indicated an increased ratio of smooth muscle to endothelial cells, as assessed by protein content of smooth muscle actin/VE-Cadherin (Figure 4B). This increase in penile smooth muscle content is consistent with studies indicating elastin is important for the regulation of smooth muscle cell phenotype. In vitro studies have suggested that elastin maintains smooth muscle cell quiescence, and degraded elastin (or non-polymerized elastin) promotes smooth muscle proliferation [26]. Changes observed in conduit vasculature from mice completely deficient in elastin (Eln−/−), have suggested that intact elastin is required to maintain smooth muscle cells in the differentiated state. Eln−/− mice die as newborns from obstructive arterial disease, which is characterized by an increase in arterial smooth muscle cell proliferation/content [16,27]. This compensatory increase likely occurs in an attempt to normalize wall pressures. It is tempting to speculate that this may be the case in the cavernosum as well. However, heightened smooth muscle presence and hypercontraction is often thought to be deleterious to the erectile response. With the added complications of veno-occlusive function, the unique functionality of sinusoids and the resistant barrier of the tunica, compensation in the cavernosum is likely more complex than in conduit arteries. The compensatory response in the aorta appears to occur only after a complete loss in elastin. Unlike in Eln−/− mice, arteries from Eln+/− mice did not exhibit increased smooth muscle cells. Although there were more lamellar units in the artery wall, no elevations in wall cell number were found [24]. There was similarly no change in total penile DNA content in our study, suggesting that the increase in penile smooth muscle content in Eln−/− mice is mainly a consequence of cell hypertrophy, and not hyperplasia. In a previous study, decreased cavernosal elastin content was seen alongside increased contractile force in type II diabetic mice [15,28]. Altogether, it is tempting to speculate the hyperplasia of the penile smooth muscle as a predominant contributor for the heightened force generation in this tissue, although we cannot rule out the possible contribution of potentiated contractile signaling. This could take place at the adrenergic receptor level or result from changes in intracellular signaling. Although out of the scope of this study, it is indeed plausible that increased activity of calcium-dependent contractile filament activation, or calcium sensitization through pathways such as RhoA/Rho-kinase and protein kinase C is present in the Eln+/− tissue. Signaling such as that mediated by the cytokine, tumor necrosis factor (TNFα) has also been shown to enhance contractile function [29]. However, TNFα infusion was reported to additionally impair dilatory signaling in mice, and TNF knockout mice in turn had enhanced dilatory function [29,30]. However, in our study, the vasoreactive changes were only seen in regards to contractile function. There was no change in relaxation to acetylcholine or EFS relaxation. This suggests no affect on eNOS or nNOS-mediated relaxation, respectively; however, we did not measure enzyme activity specifically (Figure 5). These data further support a specific linkage between elastin deficiency and heightened contraction.
Elastin is not just present in the cavernosal sinusoidal matrix and arterioles, but is also abundant in the tunica albuginea, and may play an important role in venous occlusion required for the maintenance of high ICPs. Pharmaco-cavernosography was used to assess veno-occlusive function in Eln+/− and Wt mice. The flow rate required to maintain a set ICP (200 mm Hg) after the use of a smooth muscle relaxant, papaverine, was compared. Eln+/− mice required a higher flow rate to maintain 200 mm Hg ICP as compared with Wt mice (Figure 6). However, it should be noted that the magnitude of difference in flow rate in our murine study (although statistically significant) was far less than the Δ flow rate used to define veno-occlusive order clinically [31]. It is unclear how these clinical measures translate to the mouse model. Nonetheless, the greater required flow rate in the Eln+/− mice is consistent with the idea that the elastic recoil property of elastin is essential to enable the opposing force of the tunica against the expanding cavernosum and thus venous occlusion. It is possible, however, that the heightened smooth muscle tone seen in cavernosum from Eln+/− mice can affect the cavernosography results. However, in our studies, at the time of infusion, the penis is fully erect (even visibly). Thus, we do not think smooth muscle tone is active at this point. That is, the ICP remains constant at the given infusion rates. Thus, although it is experimentally not feasible to dissect out the contribution of smooth muscle tone from veno-occlusive function completely, by the nature of our experimental design, it appears that venous-occlusion is being assessed during a point where smooth muscle tone is likely inactive.
The phenotypic changes of heightened cavernosal contractility and impaired veno-occlusive function, suggest that the Eln+/− mice may exhibit ED. Indeed, at sub-maximal stimulus of the cavernosal nerve, Eln+/− mice demonstrated an attenuated increase in ICP/MAP as measured by AUC (Figure 7A). However, at a higher stimulation threshold (2 V), the ICP/MAP response was not different in the Eln+/− vs. Wt mice, suggesting that the overall ability to achieve elevated ICPs is intact. Interestingly, the maximal ICP/MAP response was not significantly impaired at sub-maximal or maximal stimulation, indicating that these mice are able to achieve high ICPs, but not to maintain these pressures at lower stimulus parameters, further suggestive of venous leak (Figure 7B). Impaired erectile function at lower voltage stimulation may reflect both the heightened contractility and potentially increased “leakiness” of the cavernosum.
However, as erectile function is intact at higher voltage stimulation of the cavernosal nerves, it is apparent that the phenotypic impairments are able to be sufficiently overcome to achieve elevated ICP/MAP. These data are similar to findings of compensation seen in the arteries of Eln+/− mice where mice lead a normal lifespan despite having hypertension due to phenotypic changes in the cardiovascular system [23,24,32,33]. Interestingly, various studies point to an increased severity of disease phenotype when elastin levels are further decreased. One such study in the pulmonary circulation indicates increasing phenotypic abnormalities in mice with a graded decrease in elastin [23]. A set of transgenic mice engineered to express elastin at levels ranging from ~45 to 120% of the elastin content found in Wt mice, was examined. Elastin gene dosage was found to predispose the development of pulmonary arterial hypertension [23]. Similarly, the severity of cardiovascular defects in elastin-deficient mice was found to be inversely related to the elastin content of the various transgenic mice.
It is possible that a similar gradient effect of decreased elastin would affect erectile function. The mice used in this study lacked about 33% penile elastin content (Figure 2). The increased contractility and smooth muscle content (Figures 3 and 4) is likely reflective of the regulation of smooth muscle by elastin. Similarly, the impaired veno-occlusive function (Figure 6) could be speculated to reflect a decrease in tunical elastin content, although we are not able to quantify tunical elastin in the mouse. Future studies may examine whether the severity of these phenotypes, as well as overall erectile function, is worsened in engineered mice, which lack up to 60% of elastin content [27]. As elastin degradation occurs with aging, and decreased penile elastin content has been reported in men with ED, it is tempting to speculate that erectile function can be impaired beyond a particular threshold of elastin loss. It is also plausible that a decrease in elastin results in phenotypic changes consistent with ED, but that additional mechanistic alterations are required to worsen the severity of the erectile impairment.
A limitation of our study was the inability to differentiate the specific role of sinusoidal, arteriolar, and tunical elastin in erectile function. It is tempting to speculate that the localization of elastic fibers is important for selective mechanisms, such as regulation of arteriolar and sinusoidal blood flow and passive recoil for venous outflow, thus contributing to erectile function. Nonetheless, the present study does indicate an important role for elastin in the function of cavernosal tissue and suggests future studies to examine erectile function in mice with greater elastin deficiency.
Acknowledgments
This work was funded by the NIH (R01 DK082560). Histological analysis was performed by R. Browne, University of Washington. Desmosine/isodesmosine and hydroxyproline quantification was performed by B. Starcher, University of Texas Health Center.
Footnotes
-
(a)Conception and Design Kanchan Chitaley; Ian Luttrell; Xiaogang Jiang; Josephine Hidalgo-Tamola
-
(b)Acquisition of Data Ian Luttrell; Josephine Hidalgo-Tamola; Xiaogang Jiang
-
(c)Analysis and Interpretation of Data Ian Luttrell; Josephine Hidalgo-Tamola; Xiaogang Jiang; Kanchan Chitaley; Bob Mecham
-
(a)Drafting the Article Josephine Hidalgo-Tamola; Kanchan Chitaley
-
(b)Revising It for Intellectual Content Kanchan Chitaley; Ian Luttrell; Xiaogang Jiang; Josephine Hidalgo-Tamola
-
(a)Final Approval of the Completed Article Ian Luttrell; Kanchan Chitaley; Josephine Hidalgo-Tamola; Xiaogang Jiang; Dean Li
Conflict of Interest: None.
References
- 1.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–34. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson JM. Biochemistry and turnover of lung interstitium. Eur Respir J. 1990;3:1048–63. [PubMed] [Google Scholar]
- 3.Rucker RB, Dubick MA. Elastin metabolism and chemistry: Potential roles in lung development and structure. Environ Health Perspect. 1984;55:179–91. doi: 10.1289/ehp.8455179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parks WC, Pierce RA, Lee KA, Mecham RP. Elastin. In: Kleinman HK, editor. Advances in molecular and cell biology. Vol. 6. JAI Press, Inc.; Greenwich, CT: 1993. pp. 133–82. [Google Scholar]
- 5.Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41:494–7. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, Nunes L, Lue TF. Structural alterations in the tunica albuginea of the penis: Impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997;79:47–53. doi: 10.1046/j.1464-410x.1997.26511.x. [DOI] [PubMed] [Google Scholar]
- 7.Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–68. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 8.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–7. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce RA, Mariani TJ, Senior RM. Elastin in lung development and disease. Ciba Found Symp. 1995;192:199–212. doi: 10.1002/9780470514771.ch11. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–74. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso WV, Sekhon HS, Hyde DM, Thurlbeck WM. Collagen and elastin in human pulmonary emphysema. Am Rev Respir Dis. 1993;147:975–81. doi: 10.1164/ajrccm/147.4.975. [DOI] [PubMed] [Google Scholar]
- 12.Shafik A, Shafik I, El SO, Shafik AA. On the pathogenesis of penile venous leakage: Role of the tunica albuginea. BMC Urol. 2007;7:14. doi: 10.1186/1471-2490-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa WS, Carrerete FB, Horta WG, Sampaio FJ. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006;97:567–9. doi: 10.1111/j.1464-410X.2005.05917.x. [DOI] [PubMed] [Google Scholar]
- 14.Sattar AA, Wespes E, Schulman CC. Computerized measurement of penile elastic fibres in potent and impotent men. Eur Urol. 1994;25:142–4. doi: 10.1159/000475269. [DOI] [PubMed] [Google Scholar]
- 15.Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erec-tile dysfunction in the type II diabetic db/db mouse: Impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol. 2008;294:H2204–11. doi: 10.1152/ajpheart.00027.2008. [DOI] [PubMed] [Google Scholar]
- 16.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–80. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 17.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31:133–40. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- 18.Sims TJ, Bailey AJ. Quantitative analysis of collagen and elastin cross-links using a single-column system. J Chromatogr. 1992;582:49–55. doi: 10.1016/0378-4347(92)80301-6. [DOI] [PubMed] [Google Scholar]
- 19.Chitaley K, Luttrell I. Strain differences in susceptibility to in vivo erectile dysfunction following 6 weeks of induced hyperglycemia in the mouse. J Sex Med. 2008;5:1149–55. doi: 10.1111/j.1743-6109.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 20.Richenberg J, Rickards D. Radiological diagnosis of venous leak. In: Carson C, Kirby R, Goldstein I, editors. Textbook of Erectile Dysfunction. Isis Medical, Ltd.; Oxford, UK: 1999. pp. 225–37. [Google Scholar]
- 21.Motiwala HG. Dynamic pharmacocavernosometry: A search for an ideal approach. Urol Int. 1993;51:1–8. doi: 10.1159/000282501. [DOI] [PubMed] [Google Scholar]
- 22.Sato F, Wachi H, Ishida M, Nonaka R, Onoue S, Urban Z, Starcher BC, Seyama Y. Distinct steps of cross-linking, self-association, and maturation of tropoelastin are necessary for elastic fiber formation. J Mol Biol. 2007;369:841–51. doi: 10.1016/j.jmb.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Shifren A, Durmowicz AG, Knutsen RH, Faury G, Mecham RP. Elastin insufficiency predisposes to elevated pulmonary circulatory pressures through changes in elastic artery structure. J Appl Physiol. 2008;105:1610–9. doi: 10.1152/japplphysiol.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res. 2009;104:1217–24. doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP, Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 2008;11:97–112. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tukaj C. Enhanced proliferation of aortal smooth muscle cells treated by 1,25(OH)2D3 in vitro coincides with impaired formation of elastic fibres. Int J Exp Pathol. 2008;89:117–24. doi: 10.1111/j.1365-2613.2008.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad PJ, Osborne LR, Bendeck MP. Bouncing back from elastin deficiency. Circ Res. 2007;101:439–40. doi: 10.1161/CIRCRESAHA.107.160358. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo-Tamola J, Chitaley K. Review type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2009;6:916–26. doi: 10.1111/j.1743-6109.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- 29.Carneiro FS, Zemse S, Giachini FR, Carneiro ZN, Lima VV, Webb RC, Tostes RC. TNF-alpha infusion impairs corpora cavernosa reactivity. J Sex Med. 2009;6(suppl 3):311–9. doi: 10.1111/j.1743-6109.2008.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carneiro FS, Sturgis LC, Giachini FR, Carneiro ZN, Lima VV, Wynne BM, San MS, Brands MW, Tostes RC, Webb RC. TNF-alpha knockout mice have increased corpora cavernosa relaxation. J Sex Med. 2009;6:115–25. doi: 10.1111/j.1743-6109.2008.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Daum G, Chitaley K, Coats SA, Bowen-Pope DF, Eigenthaler M, Thumati NR, Walter U, Clowes AW. Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1403–8. doi: 10.1161/01.ATV.0000134705.39654.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1209–17. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wagenseil JE, Knutsen RH, Li DY, Mecham RP. Elastininsufficient mice show normal cardiovascular remodeling in 2K1C hypertension despite higher baseline pressure and unique cardiovascular architecture. Am J Physiol Heart Circ Physiol. 2007;293:H574–82. doi: 10.1152/ajpheart.00205.2007. [DOI] [PubMed] [Google Scholar]