Viable cysticercosis lesions can be missed by computed tomography. Antigen enzyme-linked immunosorbent assay (Ag-ELISA) detected 7 patients with viable cysts among 39 patients with apparently calcified cysticercosis only. Patients with a positive Ag-ELISA were 27 times more likely to have viable cysts.
Keywords: neurocysticercosis, antigen detection, calcifications, epilepsy, Peru
Abstract
Background. Computed tomography (CT) remains the standard neuroimaging screening exam for neurocysticercosis, and residual brain calcifications are the commonest finding. Magnetic resonance imaging (MRI) is more sensitive than CT but is rarely available in endemic regions. Enzyme-linked immunoelectrotransfer blot (EITB) assay uses antibody detection for diagnosis confirmation; by contrast, enzyme-linked immunosorbent assay (ELISA) antigen detection (Ag-ELISA) detects circulating parasite antigen. This study evaluated whether these assays predict undetected viable cysts in patients with only calcified lesions on brain CT.
Methods. Serum samples from 39 patients with calcified neurocysticercosis and no viable parasites on CT were processed by Ag-ELISA and EITB. MRI was performed for each patient within 2 months of serologic testing. Conservatively high ELISA and EITB cutoffs were used to predict the finding of viable brain cysts on MRI.
Results. Using receiver operating characteristic–optimized cutoffs, 7 patients were Ag-ELISA positive, and 8 had strong antibody reactions on EITB. MRI showed viable brain cysts in 7 (18.0%) patients. Patients with positive Ag-ELISA were more likely to have viable cysts than Ag-ELISA negatives (6/7 vs 1/32; odds ratio, 186 [95% confidence interval, 1–34 470.0], P < .001; sensitivity 85.7%, specificity 96.9%, positive likelihood ratio of 27 to detect viable cysts). Similar but weaker associations were also found between a strong antibody reaction on EITB and undetected viable brain cysts.
Conclusions. Antigen detection, and in a lesser degree strong antibody reactions, can predict viable neurocysticercosis. Serological diagnostic methods could identify viable lesions missed by CT in patients with apparently only calcified cysticercosis and could be considered for diagnosis workup and further therapy.
Computed tomography (CT) or magnetic resonance imaging (MRI) constitute the main pillar of neurocysticercosis (NCC) diagnosis [1, 2]. Both neuroimaging techniques are highly informative regarding the number and topography of lesions, their stage of involution, and the degree of inflammatory reaction of the host against the parasites. In general, MRI provides better image detection and definition. The availability of several image protocols, multiplanar reconstruction of images, its capability to visualize the posterior fossa without bone artefacts, and its high-contrast resolution allow MRI to recognize many forms of cysticercosis not visualized on CT, without using ionizing radiation [3]. However, MRI has a low sensitivity for detecting calcified lesions, which are by far the most common neuroimaging finding in endemic populations; it is also expensive and rarely available in many endemic regions [4–6]. Thus, CT remains the screening neuroimaging procedure of choice for patients with suspected neurocysticercosis whereas MRI is the best option for the evaluation of patients with intraventricular cysticercosis, brainstem cysts, and small cysts located over the convexity of cerebral hemispheres [3, 7].
Serological assays complement imaging procedures by detecting circulating antibodies against Taenia solium or by detecting T. solium antigens [8–10]. These immunodiagnostic tests have been used with variable results [11–13]. Antibody detection by enzyme-linked immunoelectrotransfer blot (EITB) assay is the assay of choice for serodiagnosis and is highly sensitive and specific in comparison to previous assays [14]. However, antibody detection indicates exposure to the parasite but not necessarily the presence of established, viable infection. Moreover, antibodies may persist long after the parasite has been eliminated through immune mechanisms and/or drug therapy [12, 15].
Antigen detection by enzyme-linked immunosorbent assay (Ag-ELISA) is associated with the presence of at least 1 viable parasite [8, 9, 12, 16, 17]. Our group reported the presence of parasitic antigen in half the patients with hydrocephalus (without evidence of viable lesions in CT) and postulated that this could be due to the presence of viable parasites not detected by CT. However, MRI was not done in this study and the hypothesis could not be proven. A consistently negative Ag-ELISA test has been associated to CT scans showing calcifications only [18].
We conducted this study to address whether Ag-ELISA or EITB could predict the presence of viable brain cysts in patients whose CT scans show only calcified lesions. This could help identify patients who would benefit from more advanced imaging and eventually require a different clinical management, as in contrast to patients with only calcifications, patients with viable parasites may benefit from antiparasitic treatment or require surgical procedures [19]. In the particular case of extraparenchymal NCC, early detection should avoid disease progression, saving serious morbidity and reducing mortality risks.
MATERIALS AND METHODS
Samples
Consecutive symptomatic patients with NCC attending the Cysticercosis Unit in the Instituto Nacional de Ciencias Neurologicas in Lima, Peru, were invited to participate if they had intraparenchymal brain calcifications (1 or more) but no viable parasites on brain CT scan. Exclusion criteria included the presence of hydrocephalus, images suspected to correspond to viable intra- or extraparenchymal cysticerci, or patients who had received antiparasitic treatment following CT.
A thorough physical and funduscopic examination was conducted on each participant to rule out subcutaneous or ocular cysticercosis. Variables such as time of disease, previous antiparasitic treatment, and time since last seizure were recorded as well. Appropriate informed consent procedures were followed including signature of a written consent form. The study protocol and consent forms were reviewed and approved by the institutional review boards of the Universidad Peruana Cayetano Heredia and the Instituto Nacional de Ciencias Neurologicas in Lima, Peru.
Study Procedures
A 5-cc blood sample was collected from each patient by venipuncture. Samples were processed by the B158/B60 monoclonal antibody (mAb)–based ELISA for the detection of circulating antigens and an EITB assay. Patients had a brain MRI done within 2 months of serologic testing, and the proportion of cases with viable brain cysticercosis lesions were compared between positive and negative respondents to each assay.
EITB Assay
The EITB for antibodies against glycoprotein antigens was performed as described by Tsang et al [14]. In brief, 7 purified T. solium glycoprotein antigens (diagnostic bands GP50, GP42–39, GP24, GP21, GP18, GP14, and GP13) are separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes and immobilized. Antibody detection is performed by exposing antigen-loaded nitrocellulose strips to the patient serum sample and later developing the strips using 3.3′-diaminobenzidine tetrahydrochloride dihydrate as a substrate. Positive identification was based on visualization of ≥1 diagnostic bands, and EITB results were expressed as the number of reactive bands (0–7).
Ag-ELISA Assay
An mAb–based ELISA for the detection of circulating antigens was used to detect circulating parasite antigen as described by Brandt et al in 1992 [10] and later adapted by Van Kerckhoven et al and Dorny et al [20, 21]. The assay uses Nunc MaxiSorp plates sensitized with a trapping mAb (B158C11A10) in bicarbonate buffer at 5 µg/mL. After blocking, serum samples (pretreated with 5% trichloroacetic acid to break existing immune complexes) are added, followed by the second mAb (B60H8A4-BIOT), streptavidin, o-phenylenediamine (OPD/H2O2) as substrate/chromogen, and incubated in the dark for 15 minutes. The reaction is stopped with H2SO4 and plates are read at 490/650 nm. To minimize interplate variation, we used antigen ratio instead of a raw value of optic density (OD). The antigen ratio is estimated by dividing the OD of the tested sample with the mean of 8 negative samples plus 3 standard deviations.
Imaging
CT scans were performed using a 64-slice multidetector CT scanner before and after contrast injection. CT scan readings were performed by a neuroradiologist who was not a part of the study and reviewed by an experienced neurologist (E.J.P.) before enrollment to exclude patients with CT images compatible with viable intra- or extraparenchymal cysts. Study MRIs included at least T2 and fluid-attenuated inversion recovery sequences and were performed no later than 2 months from CT and serologic testing. MRIs were also read by a neuroradiologist who was not a part of the study and reviewed by several members of the study team. Degenerated cysts, lesions without discernible liquid contents (“enhancing lesions,” “cysticercal granulomas”), were not considered viable cysts and thus not included in the analysis.
Data Analysis
Receiver operating characteristic (ROC) analysis was elaborated to estimate the best cutoff values for Ag-ELISA and EITB. Using these cutoffs, the proportion of positive results for Ag-ELISA and EITB were independently compared in patients with and without viable cysts in MRI using the Fisher exact test. Sensitivity, specificity, and positive likelihood ratios were calculated for each assay. Quantitative or semiquantitative test results (antigen ratio for Ag-ELISA, number of reactive bands on EITB) were compared between patients with and those without viable cysts on MRI using the Mann-Whitney U test, and the correlation between the serologic test result and the number of viable cysts on MRI was evaluated using the Pearson correlation test.
The associations of other covariates of potential interest (type of cysts, number of calcifications, time of disease, previous antiparasitic treatment, and time since last seizure) with the serologic results (antigen ratio for Ag-ELISA and number of bands on EITB) were examined in order to rule out confounding factors. Mann-Whitney U test was used to compare the serologic results in patients with and those without previous antiparasitic treatment and the Pearson test was used to assess the correlation of serologic results with quantitative covariables.
All the data were entered in Excel XP worksheets (Microsoft) and analyzed using Stata software (version 8). The confidence intervals were calculated at the 95% confidence level and the results were considered to be statistically significant if P < .05.
RESULTS
Study participants included 20 (51.3%) men and 19 women. The mean age in our population was 35.7 years (range, 15–65 years). All the patients entered the study with a baseline CT showing a median of 2 calcifications per patient (interquartile range, 1–5; range, 1–48). Ag-ELISA, EITB, and MRI were performed in all patients with a median time between the serologic tests and the MRI of 9 days (range, 1–51)
Individuals With Viable Cysts on MRI
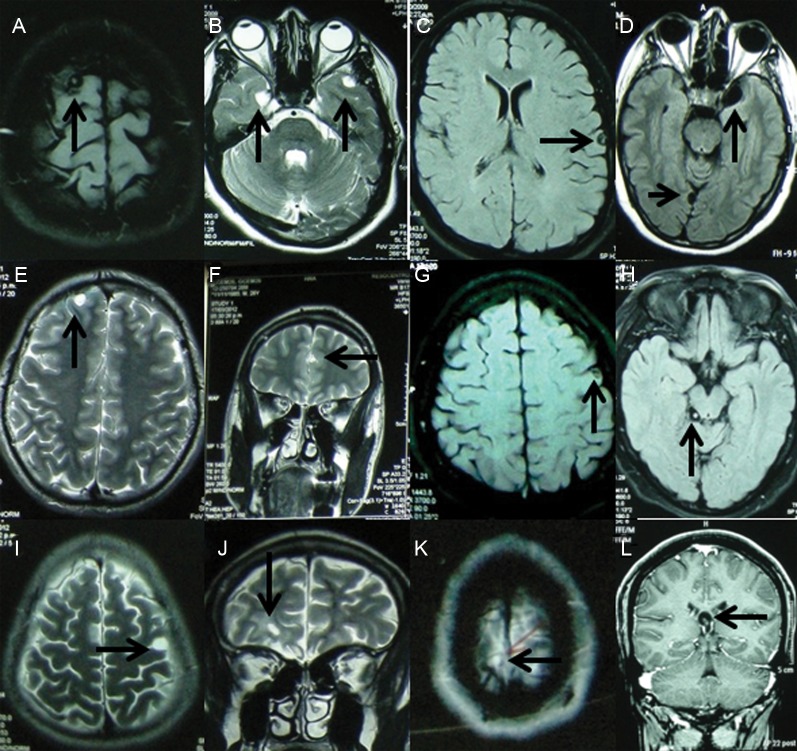
Seven of 39 (18.0%) patients had viable cysts on brain MRI (Figure 1). The median cyst number was 2 (range, 1–4). Of these 7 patients, 4 had only intraparenchymal cysts, 2 had both intraparenchymal and subarachnoid cysts, and 1 had an intraventricular cyst only. Intraparenchymal lesions were reported as cysts without inflammation in 5 patients and cysts with and without inflammation in 1 case. None of them had basal subarachnoid neurocysticercosis.
Figure 1.
Magnetic resonance imaging demonstrated viable cysticercosis in 7 patients, 1 with 4 lesions (A–C, arrows), 4 with 2 lesions each (D, E and F, G and H, I and J, respectively; arrows), and 2 with 1 lesion each (K and L, arrows).
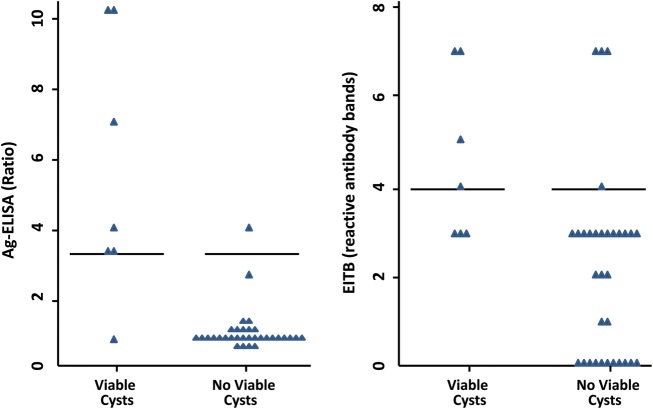
Figure 2.
Enzyme-linked immunosorbent assay antigen detection and enzyme-linked immunolectrotransfer blot assay results of individuals with only calcified neurocysticercosis on computed tomography with and without viable cysts demonstrated by magnetic resonance imaging, at different cutoff levels. Abbreviations: Ag-ELISA, enzyme-linked immunosorbent assay antigen detection; EITB, enzyme-linked immunoelectrotransfer blot assay.
Relation Between Ag-ELISA Results and Presence of Viable Cysts on MRI
Previous studies had used an ELISA ratio (OD of the sample divided by the mean of 8 known negatives run in the same plate ±3 SD) cutoff value of 1 to define a positive test. Using this cutoff, 10 patients (25.6%) were seropositive and the test had a sensitivity of 85.7% (6/7), specificity of 87.5% (28/32), and a positive likelihood ratio (LR) of 6.8. The mean antigen ratio in the patients with viable cysts was 13.1 (SD, 18.4) compared to 0.7 (SD, 0.7) in the patients without viable cysts (P = .002). ROC analysis of the antigen ELISA ratio for detecting viable cysts found an area under the curve of 0.88 (95% confidence interval [CI], .66–1) and marked an ELISA ratio of 3.3 as the best cutoff point to consider the test positive. Patients with high antigen ratios (>3.3) were significantly more likely to have viable brain lesions than patients with lower antigen ratios (6/7 [85.7%] vs 1/32 [14.3%]; odds ratio [OR], 186 [95% CI, 1–34 470.0], P < .001) (Figure 2). With this cutoff, the Ag-ELISA had a sensitivity of 85.7% (6/7), specificity of 96.9% (31/32), and positive LR of 27.4 to detect viable cysts. The antigen ELISA ratio significantly correlated with the number of viable cysts found on MRI (correlation coefficient, 0.32; P = .04).
Relation Between EITB Results and Presence of Viable Cysts on MRI
EITB is considered positive if 1 or more bands are found in the test. Using this criterion, 28 of the 39 patients were positive on EITB, with a sensitivity of 100% (7/7), a specificity of 34.4% (11/32), and a positive LR of 1.5. ROC analysis demonstrated an area under the curve of 0.82 (95% CI, .691–.95) and a preferred cutoff of 4 bands. For the purposes of this study this optimized cutoff value performed better than the standard, with a specificity of 87.5% (28/32), and a positive LR of 4.6 to detect patients with viable brain cysts, although the sensitivity dropped from 100% to 57.1% (4/7). Even after the modification of the cutoff value, the EITB had lower sensitivity, specificity, and positive LR than Ag-ELISA. The correlation between the number of EITB bands and the number of viable cysts did not reach statistical significance (correlation coefficient, 0.30; P = .057).
Relation Between Serological Tests and Total Number of Lesions Including Calcifications
There was a significant association between the total number of brain cysticercosis lesions (including calcifications and granulomas) and the number of reactive antibody bands on EITB (Pearson P = .47; P = .002). On the other hand, antigen levels did not correlate with the total number of lesions (Pearson's P = .06; P = .68).
Other variables were explored searching for possible confounding factors. The type of cysts found on MRI was not considered because of the low number of patients for each cyst type. The antigen ratio level was not associated with the number of calcifications (P = .84), length of disease (P = .77), previous antiparasitic treatment (P = .46), or time since last seizure (P = .74).
DISCUSSION
Calcifications are the most common imaging finding in NCC, accounting for >30% of symptomatic cases and ≥95% of the general population screened by CT in endemic regions. Because of its wider availability and high sensitivity for calcification, CT remains the screening imaging modality of choice [3, 7]. Our findings demonstrate that small viable cysts may be frequently missed in patients with CTs showing only calcified NCC lesions. MRI will likely detect most of these lesions, but it is highly expensive and rarely available in endemic countries [7]. Because patients with viable lesions may benefit from specific therapeutic modalities [19] and having MRIs in all patients with calcified NCC is not to be expected, immunodiagnostic assays may provide a practical way to define patients with a high likelihood of having viable brain cysts [22, 23]. Individuals with a positive Ag-ELISA (above a conservatively high cutoff level) were 27 times more likely to have undetected viable brain parasites than did antigen-negative individuals.
We applied a high cutoff value on Ag-ELISA to discriminate cases with viable lesions, determined by using a ROC curve. This assay routinely uses a cutoff value derived from known NCC-negative samples [12, 17, 18]. Negative control samples had very low OD readings and the cutoff values derived from these seem to be too low for practical use. Using an optimized, ROC-derived cutoff, the Ag-ELISA had a sensitivity of 85.7% and specificity of 96.9% to detect missed viable brain lesions. Despite the increase in the cutoff value, the sensitivity was comparable to previous reported estimations (86%) [11]. Nonetheless, it is important to consider that the sensitivity and specificity found in this work applies to the specific study population of patients with apparently calcified cysticercosis only in CT and should not be extrapolated to the general population. Although our sample was small, antigen levels were significantly correlated with the number of viable cysts on MRI. Basal subarachnoid and ventricular cysts are commonly associated with a high antigen ratio [22, 24–26]. One of our patients did have an intraventricular cyst that may explain his very high antigen ratio (51.948). A high antigen ratio (20.933) was found as well in our patient with subarachnoid and intraparenchymal cysts.
We also examined the ability of EITB to predict viable cysts missed on brain CT. The cutoff was also set at a high value (4 bands) and obtained a maximal positive LR of 5. Thus, as expected, antigen detection performed better in predicting the finding of missed viable cysts than antibody detection, as antibodies may persist well after all cysts have died [12, 22, 26]. This may also explain the fact that the correlation between the number of EITB bands and the number of viable cysts did not reach statistical significance whereas there was a significant correlation between the number of EITB bands and the total number of lesions including calcifications and granulomas.
In conclusion, we demonstrated that a positive Ag-ELISA in serum predicts the presence of undetected viable brain cysticercosis lesions, selecting a group of high-risk patients who would benefit from more advanced imaging techniques and possibly antiparasitic treatment or eventually surgery. Further studies in larger endemic populations are warranted to determine whether Ag-ELISA could be routinely recommended in patients with a diagnosis of calcified NCC on CT.
Notes
Acknowledgments. We thank the staff at the Cysticercosis Unit at the Instituto de Ciencias Neurologicas for their help in patient recruitment and data gathering. We also express our gratitude to the laboratory personnel for sample processing.
Financial support. This work was supported in part by the Fogarty International Center/National Institutes of Health (training grant D43 TW001140). H. H. G. is supported by a Wellcome Trust Senior International Research Fellowship in Public Health and Tropical Medicine.
Disclaimer. The funders had no role in study design; data collection, analysis, or interpretation; in writing the report; or in the decision to submit the article for publication.
Potential conflicts of interest. S. G. and P. D., through the Prince Leopold Institute of Tropical Medicine in Antwerp, Belgium, report discussing and coordinating marketing of an ELISA kit for cysticercosis antigen detection through a private company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Garcia HH, Herrera G, Gilman RH, et al. Discrepancies between cerebral computed tomography and Western blot in the diagnosis of neurocysticercosis. Am J Trop Med Hyg. 1994;50:152–7. doi: 10.4269/ajtmh.1994.50.152. [DOI] [PubMed] [Google Scholar]
- 3.García HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Trop. 2003;87:71–8. doi: 10.1016/s0001-706x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez AL, Lindbäck J, Schantz PM, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–58. [PubMed] [Google Scholar]
- 5.Cruz ME, Schantz PM, Cruz I, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Noval J, Moreno E, de Mata F, et al. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol. 2001;95:167–75. doi: 10.1080/00034980120050260. [DOI] [PubMed] [Google Scholar]
- 7.Suss RA, Maravilla KR, Thompson J. MR imaging of intracranial cysticercosis: comparison with CT and anatomopathologic features. AJNR Am J Neuroradiol. 1986;7:235–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathog Glob Health. 2012;106:286–98. doi: 10.1179/2047773212Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989;11:351–70. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–7. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 11.Chen JP, Zhang XY, Tan W, Liu MF, Liu GL, Hu YX. Determination of circulating antigen in cysticercosis patients using McAb-based ELISA [in Chinese] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1991;9:122–5. [PubMed] [Google Scholar]
- 12.Garcia HH, Harrison LJ, Parkhouse RM, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg. 1998;92:411–4. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proaño-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, García-Jerónimo RC, Correa D. Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol. 2002;40:2115–8. doi: 10.1128/JCM.40.6.2115-2118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–9. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 15.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. J Infect Dis. 1997;175:486–9. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguekam A, Zoli AP, Vondou L, et al. Kinetics of circulating antigens in pigs experimentally infected with Taenia solium eggs. Vet Parasitol. 2003;111:323–32. doi: 10.1016/s0304-4017(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 17.Garcia HH, Parkhouse RM, Gilman RH, et al. Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg. 2000;94:673–6. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia HH, Gonzalez AE, Gilman RH, et al. Cysticercosis Working Group in Peru. Circulating parasite antigen in patients with hydrocephalus secondary to neurocysticercosis. Am J Trop Med Hyg. 2002;66:427–30. doi: 10.4269/ajtmh.2002.66.427. [DOI] [PubMed] [Google Scholar]
- 19.García HH, Evans CA, Nash TE, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15:747–56. doi: 10.1128/CMR.15.4.747-756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol. 1998;76:269–74. doi: 10.1016/s0304-4017(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 21.Dorny P, Phiri IK, Vercruysse J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–76. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26:137–44. doi: 10.1016/j.pt.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Dorny P, Brandt J, Zoli A, Geerts S. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 2003;87:79–86. doi: 10.1016/s0001-706x(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 24.Zamora H, Castillo Y, Garcia HH, et al. Cysticercosis Working Group in Peru. Drop in antigen levels following successful treatment of subarachnoid neurocysticercosis. Am J Trop Med Hyg. 2005;73:S41. [Google Scholar]
- 25.Bobes RJ, Hernández M, Márquez C, et al. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health. 2006;11:943–50. doi: 10.1111/j.1365-3156.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez S, Dorny P, Tsang VC, et al. Cysticercosis Working Group in Peru. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199:1345–52. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]