Abstract
Over the last decade, the number of clinical pharmacogenetic tests has steadily increased as understanding of the role of genes in drug response has grown. However, uptake of these tests has been slow, due in large part to the lack of robust evidence demonstrating clinical utility. We review the evidence behind four pharmacogenetic tests and discuss the barriers and facilitators to uptake: (1) warfarin (drug safety and efficacy); (2) clopidogrel (drug efficacy); (3) codeine (drug safety and efficacy); and (4) abacavir (drug safety). Future efforts should be directed toward addressing these issues and considering additional approaches to generating evidence basis to support clinical use of pharmacogenetic tests.
Keywords: abacavir, clopidogrel, codeine, pharmacogenetics, testing, warfarin
Introduction
It has been estimated that more than 770,000 people are injured or die each year in hospitals from adverse drug events (ADEs), costing millions of dollars in healthcare costs each year [Classen et al. 1997; Lazarou et al. 1998]. The field of genomic medicine presents one potential solution to reduce health care costs associated with ADEs and poor response to pharmacotherapy. Specifically, the field of pharmacogenetics involves using a patient’s genetic makeup in combination with other clinical information to create a personalized medication regimen with greater efficacy and safety for the individual patient. Many medications currently prescribed have pharmacogenetic data to support appropriate dosing or selection. In addition, pharmacogenetic analyses are routinely performed during drug development [Liou et al. 2012].
Although it has long been understood that genes play a role in drug response [Scott, 2011], the explosion of new discoveries from genome-wide association studies (GWASs) and large population-based cohort studies has driven pharmacogenetic testing to the forefront of the personalized medicine movement. Advances in genomic technologies, enabling more accurate, faster, and cheaper tools for data generation and clinical testing, largely account for the rapid pace of discovery and development. In fact, the substantial drop in cost from these new technologies has created somewhat of a dilemma in that it may be cheaper to perform a genome-wide analysis instead of a single gene test, generating much more information than is needed.
Despite the rapid pace of discovery and test development, the routine use of pharmacogenetic testing is stymied by the lack of data demonstrating clinical utility, or evidence that use of the test will improve health outcomes for a given patient [Lesko et al. 2010; SACGT, 2000]. While randomized controlled trials (RCTs) remain the gold standard for clinical evidence, very few have been performed in pharmacogenetics. More than 2000 papers on pharmacogenetics have been published annually over the last several years [Scott, 2011], demonstrating a huge growth in discovery. In comparison, a search of the PubMed database reveals that 212 RCTs involving pharmacogenetics have been published over the past 10 years (Figure 1). However, this number may be misleading, as it is likely that only a minority of publications are RCTs with a primary pharmacogenetic endpoint and others are likely RCTs with secondary pharmacogenetic endpoints or exploratory studies. A search of the US-based clinical trials database (ClinicalTrials.gov) for ‘pharmacogenetic OR pharmacogenomic’ returned 312 studies, 147 of which were open (defined as recruiting, not yet recruiting, or available for expanded access; as of 18 September 2012). It is highly unlikely that many RCTs will be conducted and decisions to use pharmacogenetic testing will need to be based on other types of evidence [Frueh, 2009]. Of the 147 studies found in our search of ClinicalTrials.gov, only 30 were RCTs. Several reasons may account for the relatively small number of clinical trials in pharmacogenetics: lack of funding, lack of interest in conducting clinical trials (particularly for approved drugs), failure to validate initial discoveries, and small patient populations. Indeed, current use of pharmacogenetic testing is based in large part on observational and retrospective studies.
Figure 1.
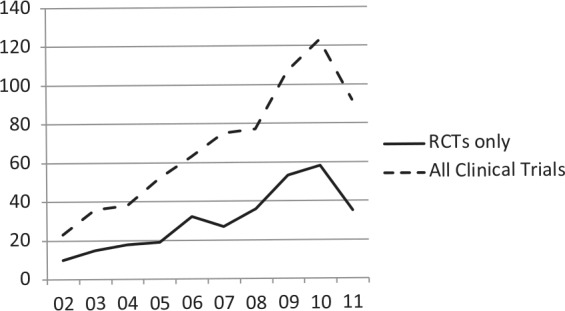
PubMed search of clinical trials for pharmacogenetic testing compared with clinical trials of drugs for heart disease and cardiovascular disease between 2002 and 2011. Search parameters: randomized controlled trial (RCTs) or clinical trials + RCTs, pharmacogenetics, between 1 January 2002 to 31 December 2011. Search conducted on 16 April 2012.
Beginning in 2003, the US Food and Drug Administration (FDA) has approved revisions to package inserts for several drugs to include information about pharmacogenetics [FDA, 2012c; Frueh et al. 2008]. Although testing is not recommended in the revised package inserts, the importance of genetic variation to risk of adverse drug reactions (ADRs) or outcome is significant enough to warrant prominent inclusion in the boxed warnings for some medications. In 2003, an estimated 1.5% of the top 200 medications included information in the package insert concerning pharmacogenomics [Zineh et al. 2006]. Repeating their analysis with the most current listing of top 200 medications sold in the US [Bartholow, 2011], we estimate that 11% of medications now include pharmacogenetic information. This 10-fold increase reflects the rapid accumulation of data about the role of genetic polymorphisms on drug safety and response.
In this review, we describe the current state of evidence of clinical utility of pharmacogenetic testing in four medications (see Table 1 for a summary). Given the many stakeholders in healthcare including the provider, patient, institution, and third-party payer, the determination of clinical utility and factors considered can vary substantially. Therefore, we also discuss other factors such as availability of clinical guidelines that may impact overall use of the test. The selected examples highlight the variability of available evidence and other factors that may impact clinical uptake in various clinical settings.
Table 1.
Summary of pharmacogenetic tests.
| Questions | Clopidogrel (CYP2C19) | Codeine (CYP2D6) | Warfarin(CYP2C9/VKORC1) | Abacavir(HLA- B*5701) |
|---|---|---|---|---|
| Is there a FDA label revision? | Yes | Yes | Yes | Yes |
| Is there an FDA-cleared test available? | Yes | Yes | Yes | No |
| Is there a published clinical guideline? | Yes CPIC[Scott et al. 2011] | No | Yes CPIC[Johnson et al. 2011] | Yes[Martin et al. 2012] |
| Have there been prospective clinical trials performed? | Yes[Epstein et al. 2010] | No | Yes[Roberts et al. 2012] | Yes[Mallal et al. 2008] |
| Are there current trials ongoing, and are they randomized?1 | Yes; 6 of 16 trials are randomized trials [ClinicalTrials.gov identifiers: NCT00891670, NCT01174693, NCT01330589, NCT01097343, NCT01452152, NCT00822666] | None listed. | Yes; 10 of 15 are randomized trials [ClinicalTrials.gov identifiers: NCT00162435, NCT0247702, NCT01633957, NCT01006733, NCT00900158, NCTO119300, NCT01119261, NCT0119274, NCT00839657, NCT00904293]. | None listed. |
We searched the ClinicalTrials.gov database using the following search parameters: (for clopidogrel) CYPC19 AND clopidogrel; (for codeine) pharmacogenomics AND codeine, CYP2D6 AND codeine; (for warfarin) CYP2C9/VKORC1 AND warfarin; (for Abacavir) pharmacogenomics AND abacavir, abacavir AND HLA-B5701. Search was limited to active or recruiting studies. The search was conducted on 15 August 2012.
Warfarin
State of evidence
Warfarin is one of the most commonly prescribed medications worldwide, used for many indications including prophylaxis and treatment of thromboembolic disorders, atrial fibrillation, or cardiac valve replacement, and systemic embolism after myocardial infarction (MI). Approved in the US in 1954, the high efficacy of warfarin is challenged by the high risk of ADRs due to its narrow therapeutic window, requiring careful monitoring and strict compliance. The international normalized ratio (INR; ratio of a patient’s prothrombin time to a normal sample) is the standard test to assess therapeutic range. New patients on warfarin require frequent monitoring of INR levels until the optimal level is attained and monthly thereafter [Guyatt et al. 2012; Johnson, 2012]. It has been reported that approximately 60% of anticoagulation clinics are able to reach desired INR levels for patients, while the remaining clinics continually need to monitor their patients until they achieve the optimal INR goals [Marin-Leblanc et al. 2012].
Treatment variability of warfarin is attributed to a combination of factors including age, race, weight, diet (specifically intake of vitamin-K-rich foods) [Rasmussen et al. 2012], alcohol consumption, and numerous drug interactions [Johnson, 2012]. Achieving the desired INR range can take several months, raising the risk of ADRs. In 2000 and 2004, polymorphisms in two genes were reported to be associated with risk of ADRs and drug response, cytochrome P450 2C9 (CYP2C9) [Taube et al. 2000] and vitamin K epoxide reductase complex subunit 1 (VKORC1), respectively [Aithal et al. 1999; Bodin et al. 2005; Li et al. 2004; Rieder et al. 2005; Rost et al. 2004; Steward et al. 1997]. CYP2C9 is involved in the metabolism of warfarin; VKORC1 is the molecular target of the drug.
In 2009, an international collaboration published a landmark paper defining appropriate warfarin doses based on a validated dosing algorithm of clinical biomarkers and VKORC1/CYP2C9 genotypes [Klein et al. 2009]. In 2010, Epstein and colleagues conducted a prospective study of the utility of warfarin and demonstrated a reduction in hospitalizations when starting doses were informed by patient genotypes for VKORC1/CYP2C9 [Epstein et al. 2010]. Several large studies are underway (Coumagen II, Clarification of Optimal Anticoagulation Through Genetics [COAG] [French et al. 2010] and Genetics Informatics Trial of Warfarin Therapy [GIFT] trial [Do et al. 2011]) to assess the benefits of pharmacogenetic testing prior to warfarin dosing [Carlquist and Anderson, 2011; ClinicalTrials.gov identifier: NCT00927862]. A handful of groups have demonstrated the utility of genotype-guided warfarin initiation therapy [Anderson et al. 2012; Caraco et al. 2008; Gong et al. 2011].
Revisions to package insert
In 2005, an FDA advisory committee reviewed the published literature on the association of genetic variants of VKORC1 and CYP2C9 and outcomes to warfarin treatment (Figure 2) [FDA, 2005]. In 2007, the package insert was revised to include information about the impact the impact of the genetic variants in CYP2C9/VKORC1 on treatment outcomes [FDA, 2007a]. In 2010, the FDA approved additional revisions to the package insert to include a table of warfarin starting doses based on patient genotypes of VKORC1/CYP2C9, but did not recommend testing prior to treatment [FDA, 2010a]. The package insert revisions in 2007 were primarily based on retrospective studies [Higashi et al. 2002; Lindh et al. 2005; Taube et al. 2000], while the second round of revisions in 2010 were informed by data from both retrospective and prospective study outcomes [Anderson et al. 2007; Caraco et al. 2008; Jneid et al. 2012; Limdi et al. 2008; Wadelius et al. 2009; Wen et al. 2008].
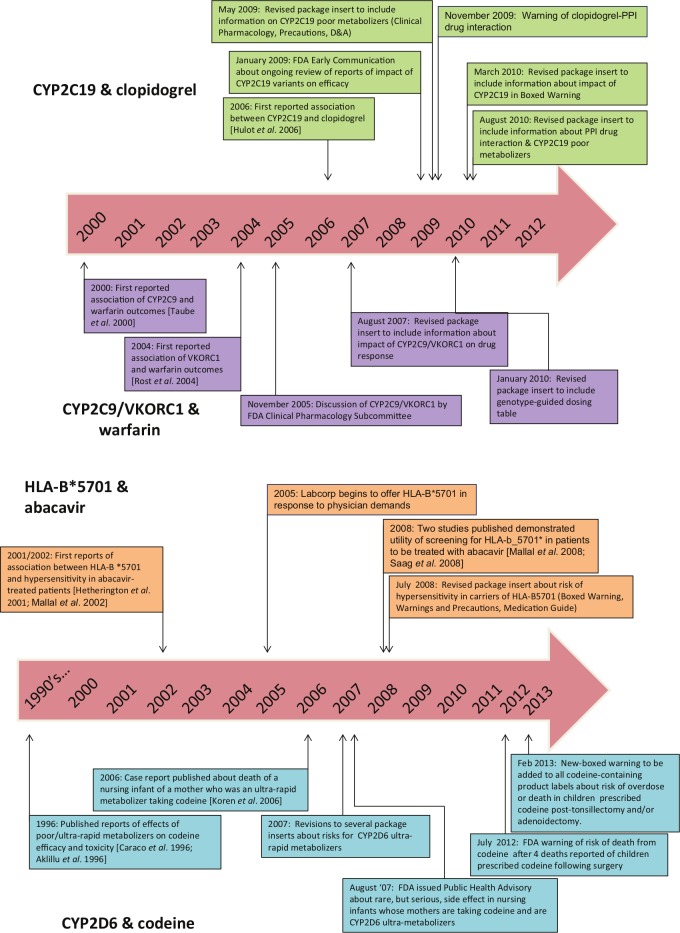
Figure 2.
Timeline of major developments of pharmacogenetic testing for the drugs clopidogrel, warfarin (top), abacavir and codeine (bottom).
CYP, cytochrome P450 enzyme; FDA, US Federal Drug Administration; PPI, proton pump inhibitor.
Clopidogrel
State of evidence
Like warfarin, clopidogrel is a commonly prescribed drug, with sales estimated to exceed US$6 billion in 2010 in the US [Alazraki, 2011]. Approved in 1997, clopidogrel is another cardiovascular medication intended to reduce the rate of atherothrombotic events in patients with recent MI or stroke or established peripheral arterial disease, unstable angina (UA) or non-ST-segment elevation (NSTEMI) managed medically or with percutaneous coronary intervention (PCI; with or without stent) or coronary artery bypass graft (CABG), and ST-segment elevation MI (STEMI) managed medically.
Clopidogrel is a prodrug that requires biotransformation to an active metabolite. Catalyzed by the enzyme CYP2C19, the active metabolite irreversibly blocks the P2Y12 component of adenosine diphosphate receptors on the platelet surface, which prevents activation of the glycoproteinIIb/IIIa receptor complex, thereby reducing platelet aggregation [Mega et al. 2009; Shuldiner et al. 2009]. In 2006, Hulot and colleagues reported that carriers of the reported loss-of-function allele CYP2C19*2 were significantly more likely to experience reduced platelet responsiveness to clopidogrel [Hulot et al. 2006]. Similarly, Simon and colleagues reported that patients found to be poor CYP2C19 metabolizers were almost four times more likely to experience a subtherapeutic antiplatelet response when treated with clopidogrel, resulting in a higher risk for cardiovascular adverse events [Simon et al. 2009]. Most poor metabolizer phenotypes are due to the CYP2C19*2 allele, where a splicing variant in exon 5 results in a truncated protein. Approximately 1–7% of Caucasians and African-Americans, and 13–23% of Asians are CYP2C19 poor metabolizers [Cavallari et al. 2011; Desta et al. 2002].
Several clinical trials have been initiated to assess the utility of testing to determine the proper dose of clopidogrel for CYP2C19-compromised patients [Simon et al. 2009]. However, the published findings are conflicting, suggesting other factors may be involved in predicting efficacy and adverse clinical outcomes. The ELEVATE-TIMI56 study demonstrated that CYP2C19*2 heterozygotes treated with threefold higher doses showed significantly reduced platelet reactivity, comparable with that seen in noncarriers on standard maintenance doses [Mega et al. 2011]. In addition, nonresponse in CYP2C19*2 heterozygotes was significantly reduced from 52% to 10% with the higher doses. CYP2C19*2 homozygotes did not respond to higher doses. Similarly, the Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism (AACEL-DOUBLE) trial reported that the use of high-maintenance dose clopidogrel in high-risk patients with PCI and carriers of CYP2C19 polymorphisms showed higher platelet measures and increased risk of high post-treatment platelet reactivity than noncarriers [Jeong et al. 2010]. In contrast, Cuisset and colleagues reported that patients with CYP2C19*2 given an increased dose of 150 mg once daily did not show significant benefit and alternative medication was suggested [Cuisset et al. 2011]. Several recent meta-analyses have reported conflicting conclusions as well regarding the impact of CYP2C19 genotype on drug efficacy and/or cardiovascular events [Bauer et al. 2011; Holmes et al. 2011; Mega et al. 2010].
Revisions to the package insert
In 2009, the FDA announced that they were conducting a safety review of reports about the impact of CYP2C19 variants on efficacy and potential drug–drug interactions [FDA, 2009]. In 2010, the package insert for clopidogrel was revised to include information about the effect of CYP2C19 genetic variants on ADRs and response. Specifically, a boxed warning was added about the reduced effectiveness of clopidogrel in patients who are CYP2C19 poor metabolizers, and to inform healthcare professionals about the availability of genetic tests for CYP2C19 [Ellis et al. 2009; FDA, 2010c].
In addition, the insert was revised a second time in 2010 to include a warning about the concomitant use of proton pump inhibitors (PPIs) and clopidogrel and increased cardiovascular risk, since the majority of PPIs are metabolized by CYP2C19 [FDA, 2010b]. However, the evidence of an association between cardiovascular risk and PPIs in patients treated with clopidogrel is conflicting. Early reports suggested that concomitant use of PPIs reduced clopidogrel’s efficacy [Gilard et al. 2006, 2008], while other reports suggested no such effect [Bhatt et al. 2010; Charlot et al. 2010; O’Donoghue et al. 2009; Ray et al. 2010; Siller-Matula et al. 2009; Small et al. 2008]. Some argue that the increased cardiovascular risk linked to PPIs may be attributed to the lack of data on baseline characteristics such as smoking status, lipid levels, and body mass index [Charlot et al. 2010] or the effect is specific to select PPIs [Juurlink et al. 2009].
Codeine
State of evidence
Codeine is a commonly used drug to treat mild to moderate pain. Like clopidogrel, codeine is a prodrug; the majority of codeine (50–70%) is converted to the active metabolite codeine-6-glucuronide by the enzyme UGT2B7and a smaller proportion (0–15%) is converted to morphine by the enzyme CYP2D6 [Thorn et al. 2009]. CYP2D6 is a highly polymorphic gene, resulting in a range of phenotypes [Bernard et al. 2006; Bradford, 2002; Cascorbi, 2003]. In 1996, two reports were published about the effects of poor and ultra-rapid metabolizers on codeine efficacy and toxicity [Aklillu et al. 1996; Caraco et al. 1996]. CYP2D6 ultra-rapid metabolism is generally due to duplication of the slightly reduced activity (*2) or wild-type (*1) alleles; allele frequencies, vary between racial/ethnic groups [Bernard et al. 2006; Bradford, 2002; Cascorbi, 2003]. Several reports have been published on codeine intoxication and/or death following codeine use in children and adults with ultra-rapid CYP2D6 activity [Ciszkowski et al. 2009; Gasche et al. 2004; Kelly et al. 2012; Madadi et al. 2010; Voronov et al. 2007]. Ultra-rapid metabolizers treated with codeine exhibit symptoms of extreme sleepiness, confusion or shallow breathing; the lowest possible dose should be prescribed to these patients. Patients that are CYP2D6 poor metabolizers will not achieve sufficient pain control due to their inability to convert the drug to its active form of morphine.
Two vulnerable populations in which codeine is highly prescribed are postpartum women and children. It is estimated that 90–99% of women take some type of pain medication in the first week postpartum [Anderson, 1991; Hale, 2004; Ito and Lee, 2003] and as many as three to four drugs during breast-feeding [Stultz et al. 2007]. Oral analgesics have been reported to be the second most commonly used drug after vitamins during the postpartum period [Schirm et al. 2004]. In 2006, a case report was published of the death of an infant caused by exposure to high levels of morphine; the mother was determined to be a CYP2D6 ultra-rapid metabolizer [Koren et al. 2006]. Another paper reported that two mothers of infants with severe toxicity were also CYP2D6 ultra-rapid metabolizers [Madadi et al. 2009]. During lactation, use of higher doses of codeine in a CYP2D6 ultra-rapid metabolizer mother would likely result in the rapid transfer of drug metabolites to the milk and infant exposure. The slow metabolism and elimination by the infant during the newborn period can compound the toxic effect [Bouwmeester et al. 2003, 2004].
More recently, reports have been published of children under the age of 5 years experiencing a life-threatening event or death following use of codeine post-surgery. Specifically, four children have died from codeine toxicity; three of which were tested to be CYP2D6 ultra-rapid metabolizers and the fourth was an extensive metabolizer and all exhibited toxicity that led to these adverse events [Ciszkowski et al. 2009; Kelly et al. 2012].
Revisions to the package insert
In 2007, package inserts for approved drugs with codeine as a major component were revised to include information about ultra-rapid metabolizers of CYP2D6 in the package insert [FDA, 2007c]. Newly approved versions of codeine drugs, such as codeine sulfate, also included information about ultra-rapid metabolizers of CYP2D6 [FDA, 2012a]. In addition, two statements have been released regarding codeine use and the impact of CYP2D6 variants in children. In August 2007, the FDA recommended that physicians prescribe the lowest possible doses of codeine to nursing mothers [FDA, 2007b]. In August 2012, the FDA issued a statement warning of the danger of codeine use in children post-surgery [FDA, 2012b]. It is recommended that the ‘lowest effective dose be used for the shortest period of time’ or use of other analgesics for children undergoing tonsillectomy and/or adenoidectomy for obstructive sleep apnea syndrome. In 2013, the FDA announced it was revising all codeine-containing product labels to include a new boxed warning about the risk of codeine in pain management in children post-tonsillectomy and/or adenoidectomy. The FDA is recommending use of alternative pain relievers for pediatric patients [FDA, 2013]. Of the four drugs described here, codeine is the only one for which no evidence from prospective studies exist.
Abacavir
State of evidence
Approved in 1998, abacavir is a potent nucleoside reverse transcriptase inhibitor used to reduce viral load in HIV patients. It is indicated as a first-line therapy in combination with other HIV antivirals including lamivudine and/or zidovudine. Approximately 5–8% of patients will develop hypersensitivity, usually during the first 6 weeks after initiation of therapy [Hetherington et al. 2001]. Patients may experience a range of symptoms including fever, rash, gastrointestinal tract symptoms (abdominal pain, diarrhea, nausea, vomiting), respiratory symptoms (pharyngitis, dyspnea, cough), and potentially life-threatening hypotension. Careful monitoring was required during the initial phase of therapy as no clinical tests were available to identify patients at risk for developing hypersensitivity.
In 2002, Mallal and colleagues reported that three alleles involved in major histocompatibility complex-I antigen presentation were associated with abacavir-induced hypersensitivity [Mallal et al. 2002]. The presence of HLA-B*5701 can lead to restricted CD8+ cytotoxic T-cell activation which results in the secretion of the inflammatory mediators of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gammas [Chessman et al. 2008]. This cascade of inflammatory mediators leads to a delayed type of hypersensitivity reaction [Chessman et al. 2008; Mallal et al. 2002].
The clinical utility of pharmacogenetic screening for HLA- B*5701 was demonstrated in two major studies. The PREDICT-1 study was a large randomized double-blind trial that demonstrated a significantly reduced risk of hypersensitivity in the prescreened arm [Mallal et al. 2008]. The incidence of clinically diagnosed hypersensitivity reaction and immunologically confirmed hypersensitivity reaction was reduced by 56% and 100%, respectively, through HLA-B*5701 screening compared with no screening [Mallal et al. 2008]. The SHAPE study was a retrospective, case-control study, which also demonstrated the sensitivity of the HLA-B*-5701 marker in Black and White populations [Saag et al. 2008].
Revisions to the package insert
In July 2008, based on the outcomes of the PREDICT-1 study [Mallal et al. 2008] and the SHAPE study [Saag et al. 2008], the FDA announced that the package insert for abacavir would be revised to include information about the increased risk of hypersensitivity in patients who are carriers of the HLA-B*5701 allele [FDA, 2008a]. Specifically, it was recommended that HLA-B*5701 testing be performed prior to initiation of abacavir [FDA, 2008b].
Clinical facilitators and barriers: gradual movement toward clinical integration
Reports suggest that the use of pharmacogenetic testing is gradually increasing [Fargher et al. 2007; Faruki et al. 2007; Faruki and Lai-Goldman, 2010; Higgs et al. 2010; Hoop et al. 2010]. In particular, the use of HLA-B*5701 substantially increased following announcement of evidence of clinical utility [Lai, 2008]. However, as with any new clinical application, particularly during this challenging economy, the utility of pharmacogenetic tests has been debated considerably. As demonstrated with the four examples, the number and type of studies conducted vary substantially between the drugs. Warfarin appears to be the most well-studied of the four drugs. As the utility of pharmacogenetic testing for warfarin continues to be debated, larger studies are ongoing. The package insert for warfarin contains specific genotype-based dosing recommendations not included or required for the other drugs. Thus, RCTs are likely to be conducted for drugs for which consensus has not been reached about use of testing, particularly for very common drugs. In contrast, these expensive and lengthy studies are unlikely to be performed for drugs that have more robust data (e.g. abacavir), their side effects more severe (e.g. codeine), or comparable treatment alternatives are available (e.g. codeine or clopidogrel).
Despite the increasing scientific understanding of pharmacogenetics, anticipated benefit and patient interest, which may all drive eventual clinical uptake, two major hurdles for physicians and insurers are the absence of robust data and education/knowledge of pharmacogenetics [Lesko and Johnson, 2012; Schnoll and Shields, 2011]. In addition, other issues pose practical challenges to routine clinical use of pharmacogenetic testing such as test turnaround time (resulting in potential delay of treatment), lack of reimbursement, and lack of clinical guidelines [Lunshof and Gurwitz, 2012; Mrazek and Lerman, 2011; Schnoll and Shields, 2011; Shah, 2004; van Schie et al. 2011]. We discuss in further detail here these and other issues critical to the widespread use of pharmacogenetic testing.
Physicians may choose to avoid the use of drugs that warrant pharmacogenetic testing for various reasons. If alternative drugs are available, such as prasugrel and ticagrelor instead of clopidogrel, which have no known evidence of pharmacogenetics impacting therapy, physicians may simply switch their prescribing practices. However, since clopidogrel is the only generic medication currently available, many physicians may be compelled to prescribe it due to robust data and cost savings. In the case of warfarin, physicians are likely to be very comfortable using the time-tested INR monitoring to achieve the optimal warfarin dose compared with pharmacogenetic testing, despite the expense and inconvenience to patients due to frequent testing during the initial treatment period. Although the data have been conflicting, a recent meta-analysis reported that self-testing reduces the incidence of thromboembolic events, but not hemorrhagic events or death [Heneghan et al. 2012]. For codeine, due to the complex metabolism of the drug and availability of alternative pain medications, it is uncertain whether testing will be routinely used. Without testing, failed attempts at pain management may necessitate treatment with non-CYP2D6 analgesics [Brousseau et al. 2007].
An alternative approach to ordering pharmacogenetic testing per drug at the point of care is the use of preemptive testing, perhaps as part of an annual exam in young adults or even children that require multiple treatments. As a result of the increasing number of drugs with pharmacogenetic data, the preemptive use of testing could significantly optimize drug outcomes [Schildcrout et al. 2012]. Regardless of when ordered (at time of treatment or prior), due to the continuing decline of costs of genomic testing technologies, a broad-based pharmacogenetic screen may yield the greatest cost savings.
One of the primary drivers of test use is insurance coverage. A recent review by one of the authors [Hresko and Haga, 2012] of coverage determinations of top US insurers shows that coverage is generally low, although some insurers are covering pharmacogenetic testing for warfarin and clopidogrel. Large pharmacy benefit managers in the US (Caremark and Medco) offer testing for patients prescribed warfarin [Caremark, 2010; Medco, 2012], further evidence of the perceived importance of testing [SACGHS, 2006].
For the four tests described here, cost-effectiveness studies have not been overly convincing regarding their routine use of some pharmacogenetic tests with the exception of abacavir [Eckman et al. 2009; Hughes et al. 2004; Meckley et al. 2010; Patrick et al. 2009; Perlis et al. 2009; Rosove and Grody, 2009; Wolf et al. 2010]. One important part of cost-effectiveness analysis is the prevalence of the phenotypes with increased risk of poor or no response or adverse effects [Flowers and Veenstra, 2004]. As noted, the prevalence of phenotypes for a given allele can substantially vary between populations. As a result, the risks posed by testing will be weighed against the odds that a given patient is likely to carry an allele associated with poor response or increased risk of adverse events. As most of the alleles follow Hardy–Weinberg distributions, the proportion of patients with intermediate phenotypes will be far more prevalent than the extreme phenotypes (ultra-rapid or poor). However, the evidence basis for patients with the extreme phenotypes is greater, resulting in uncertainty for the larger proportion of patients with the intermediate phenotype.
Several professional medical organizations, federal committees, and experts have released position statements, technology assessments, or guidelines for and against the use of pharmacogenetic testing [De Leon et al. 2006a; Relling and Klein, 2011; Scott, 2011; Zineh et al. 2011]. Other groups including the Clinical Pharmacogenetics Implementation Consortium (CPIC; http://www.pharmgkb.org/page/cpic) and the Dutch Pharmacogenomics Working Group (see http://www.pharmgkb.org/page/dpwg) have developed several documents describing how pharmacogenetic test results should be used [Relling and Klein, 2011]. Of the four tests discussed, CYP2C9/VKORC1 appears to be the most controversial amongst medical organizations [CTAF, 2008; Flockhart et al. 2008; Holbrook et al. 2012]. In general, the statements concluded that while the evidence for analytical and clinical validity of these tests has been met, there is insufficient evidence at this time to recommend for or against routine CYP2C9 and VKORC1 testing in warfarin-naive patients [CMS, 2009; Hirsh et al. 2008]. For clopidogrel, one recent guideline recognized the potential of reduced efficacy of patients with CYP2C19 variants, but concluded that use of testing is uncertain [Jneid et al. 2012]. In contrast, due to its delayed response of the adverse event and strong evidence of clinical validity and utility, there is professional consensus to test all abacavir-naive patients prior to initiation [Aberg et al. 2009; Martin et al. 2012; PAGAA, 2012].
In the early days of pharmacogenetics, there was some lag between the time that package inserts were revised to include pharmacogenetic information following publication of evidence on clinical validity and utility (Figure 2). However, the FDA has responded much more quickly in disseminating information about the impact of genetic polymorphisms on drug safety and response through announcements and revisions to package inserts, likely due to increased familiarity with the science and availability of expert staff to review the data. Of the four examples, warfarin was the first to undergo review and have its package insert revised, 8 years after the initial publication of the association between CYP2C9 and risk of over-anticoagulation. Even though the most studies have been published on the pharmacogenetics of warfarin, the benefits of the test remain highly debatable. In contrast, for clopidogrel, the package insert was changed 4 years after the initial report of an association between CYP2C19 and drug response. For abacavir, the revised package insert happened even more rapidly, the same year the two major studies of clinical utility were published. Likewise, the warnings on codeine were issued relatively quickly following the reported deaths and the in the absence of any clinical trial data.
Other potential factors that may impact physician uptake are availability of FDA-approved tests and test turnaround time. Clinicians may be reluctant to use nonapproved tests (although most genetic tests in the US are laboratory-developed tests and do not currently require FDA approval). The first pharmacogenetic test, the Amplichip CYP450, was approved by the FDA in 2004, testing for 30 common mutations in CYP2D6 and CYP2C19 [de Leon et al. 2006b]. With the increasing number of companion diagnostic tests (developed and approved concurrently with the drug), however, we anticipate that as many tests will be developed in this manner going forward as those developed following drug approval. For the tests described here, many of the major national laboratories in the US (e.g. Labcorp, Quest, ARUP, and Mayo) offer testing. The turnaround time of testing is another important consideration depending on the urgency of treatment and decisions regarding dosing and initiation of therapy must be balanced with risk of adverse response in the absence of testing.
Conclusion
The incorporation of genetic information obtained from pharmacogenetic testing holds substantial promise to improve therapeutic decision making through improved efficacy and reduced adverse events [Liou et al. 2012]. As discussed here, many of the factors essential to the uptake of testing are gradually being addressed, but a major obstacle remains the absence of data demonstrating clinical utility. In addition to ongoing randomized clinical trials to address this data gap, other modes of assessment will need to be employed to more rapidly determine whether testing is useful, particularly for commonly used drugs. Although the routine use of these tests will likely remain slow for some time, this may be beneficial to allow health professionals and patients alike to increase their knowledge of testing and become more comfortable. The use of preemptive testing may address some of the practical barriers in the integration of pharmacogenetic information to avoid delays in treatment. In addition, innovative delivery models may need to be developed to insure the safe and appropriate use of pharmacogenetic testing.
Acknowledgments
We thank Ms. Rachel Mills for her assistance in preparing the manuscript.
Footnotes
Funding: This work was supported by the US National Institutes of Health (grant number5R01-GM081416-05).
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Jivan Moaddeb, Clinical Pharmacist, Duke Institute for Genome Sciences and Policy, 304 Research Drive, Box 90141, Durham, NC 27708, USA.
Susanne B. Haga, Associate Research Professor, Institute for Genome Sciences and Policy (IGSP) and Sanford School of Public Policy; Director, IGSP Education and Training, Duke University, Durham, NC, USA
References
- Aberg J., Kaplan J., Libman H., Emmanuel P., Anderson J., Stone V., et al. (2009) Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 49: 651–681 [DOI] [PubMed] [Google Scholar]
- Aithal G., Day C., Kesteven P., Daly A. (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353:717–719 [DOI] [PubMed] [Google Scholar]
- Aklillu E., Persson I., Bertilsson L., Johansson I., Rodrigues F., IngelmanSundberg M. (1996) Frequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther 278: 441–446 [PubMed] [Google Scholar]
- Alazraki M. (2011) The 10 Biggest-Selling Drugs That Are About to Lose Their Patent. Available at: http://www.dailyfinance.com/2011/02/27/top-selling-drugs-are-about-to-lose-patent-protection-ready/
- Anderson J., Horne B., Stevens S., Grove A., Barton S., Nicholas Z., et al. (2007) Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116: 2563–2570 [DOI] [PubMed] [Google Scholar]
- Anderson J., Horne B., Stevens S., Woller S., Samuelson K., Mansfield J., et al. (2012) A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation 125: 1997–2005 [DOI] [PubMed] [Google Scholar]
- Anderson P. (1991) Drug-use during breast-feeding. Clin Pharm 10: 594–624 [PubMed] [Google Scholar]
- Bartholow M. (2011) Top 200 drugs of 2010. Available at: http://www.pharmacytimes.com/publications/issue/2011/May2011/Top-200-Drugs-of-2010
- Bauer T., Bouman H., van Werkum J., Ford N., ten Berg J., Taubert D. (2011) Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ 343: d4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard S., Neville K., Nguyen A., Flockhart D. (2006) Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist 11: 126–135 [DOI] [PubMed] [Google Scholar]
- Bhatt D., Cryer B., Contant C., Cohen M., Lanas A., Schnitzer T., et al. (2010) Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 363: 1909–1917 [DOI] [PubMed] [Google Scholar]
- Bodin L., Verstuyft C., Tregouet D., Robert A., Dubert L., Funck-Brentano C., et al. (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106: 135–140 [DOI] [PubMed] [Google Scholar]
- Bouwmeester N., Anderson B., Tibboel D., Holford N. (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92: 208–217 [DOI] [PubMed] [Google Scholar]
- Bouwmeester N., Hop W., van Dijk M., Anand K., van den Anker J., Tibboel D. (2003) Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med 29: 2009–2015 [DOI] [PubMed] [Google Scholar]
- Bradford L. (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3: 229–243 [DOI] [PubMed] [Google Scholar]
- Brousseau D., McCarver D., Drendel A., Divakaran K., Panepinto J. (2007) The effect of CYP2D6 polymorphisms on the response to pain treatment for pediatric sickle cell pain crisis. J Pediatr 150: 623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraco Y., Blotnick S., Muszkat M. (2008) CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin Pharmacol Ther 83: 460–470 [DOI] [PubMed] [Google Scholar]
- Caraco Y., Sheller J., Wood A. (1996) Pharmacogenetic determination of the effects of codeine and prediction of drug interactions.J Pharmacol Exp Ther 278: 1165–1174 [PubMed] [Google Scholar]
- Caremark (2010) CVS Caremark and Generation Health Online Target Medications That Will Be the Focus of New Pharmacogenomics Partnership. Available at: http://info.cvscaremark.com/newsroom/press-releases/cvs-caremark-and-generation-health-outline-target-medications-will-be-focus-
- Carlquist J., Anderson J. (2011) Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation 124:2554–2559 [DOI] [PubMed] [Google Scholar]
- Cascorbi I. (2003) Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Investig 33: 17–22 [DOI] [PubMed] [Google Scholar]
- Cavallari L., Jeong H., Bress A. (2011) Role of cytochrome P450 genotype in the steps toward personalized drug therapy. Pharmacogenom Personalized Med 4: 123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlot M., Ahlehoff O., Norgaard M., Jorgensen C., Sorensen R., Abildstrom S., et al. (2010) Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med 153: 378–386 [DOI] [PubMed] [Google Scholar]
- Chessman D., Kostenko L., Lethborg T., Purcell A., Williamson N., Chen Z., et al. (2008) Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 28: 822–832 [DOI] [PubMed] [Google Scholar]
- Ciszkowski C., Madadi P., Phillips M., Lauwers A., Koren G. (2009) Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med 361: 827–828 [DOI] [PubMed] [Google Scholar]
- Classen D., Pestotnik S., Evans R., Lloyd J., Burke J. (1997) Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 277: 301–306 [PubMed] [Google Scholar]
- CMS (2009) CMS Manual System: Pub 100–03 Medicare National Coverage Determinations. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R111NCD.pdf
- CTAF (2008) Use of Genetic Testing to Guide the Initiation of Warfarin Therapy: A Technology Assessment. Available at: http://www.ctaf.org/sites/default/files/assessments/814_file_WarfarinGenetics_final_W.pdf
- Cuisset T., Quilici J., Cohen W., Fourcade L., Saut N., Pankert M., et al. (2011) Usefulness of high clopidogrel maintenance dose according to CYP2C19 genotypes in clopidogrel low responders undergoing coronary stenting for non ST elevation acute coronary syndrome. Amer J Cardiol 108: 760–765 [DOI] [PubMed] [Google Scholar]
- De Leon J., Armstrong S., Cozza K. (2006a) Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP4502C19. Psychosomatics 47: 75–85 [DOI] [PubMed] [Google Scholar]
- de Leon J., Susce M., Murray-Carmichael E. (2006b) The AmpliChip (TM) CYP450 genotyping test - integrating a new clinical tool. Mol Diagn Ther 10: 135–151 [DOI] [PubMed] [Google Scholar]
- Desta Z., Zhao X., Shin J., Flockhart D. (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41: 913–958 [DOI] [PubMed] [Google Scholar]
- Do E., Lenzini P., Eby C., Bass A., McMillin G., Stevens S., et al. (2011) Genetics informatics trial (GIFT) of warfarin to prevent deep vein thrombosis (DVT): rationale and study design. Pharmacogenomics J 12: 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman M., Rosand J., Greenberg S., Gage B. (2009) Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 150: 73–83 [DOI] [PubMed] [Google Scholar]
- Ellis K., Stouffer G., McLeod H., Lee C. (2009) Clopidogrel pharmacogenomics and risk of inadequate platelet inhibition: US FDA recommendations. Pharmacogenomics 10:1799–1817 [DOI] [PubMed] [Google Scholar]
- Epstein R., Moyer T., Aubert R., Dj O., Xia F., Verbrugge R., et al. (2010) Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 55: 2804–2812 [DOI] [PubMed] [Google Scholar]
- Fargher E., Tricker K., Newman W., Elliott R., Roberts S., Shaffer J., et al. (2007) Current use of pharmacogenetic testing: a national survey of thiopurine methyltransferase testing prior to azathioprine prescription. J Clin Pharm Ther 32: 187–195 [DOI] [PubMed] [Google Scholar]
- Faruki H., Heine U., Brown T., Koester R., Lai-Goldman M. (2007) HLA-B*5701 clinical testing: early experience in the United States. Pharmacogenet Genom 17: 857–860 [DOI] [PubMed] [Google Scholar]
- Faruki H., Lai-Goldman M. (2010) Application of a pharmacogenetic test adoption model to six oncology biomarkers. Personalized Med 7: 441–450 [DOI] [PubMed] [Google Scholar]
- FDA (2005) Summary Minutes of the Clinical Pharmacology Subcommittee Meeting of the Advisory Committee for Pharmaceutical Science. Available at: http://www.fda.gov/OHRMS/DOCKETS/AC/05/minutes/2005-4194M1.pdf
- FDA (2007a) Coumadin Package Insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009218s105lblv2.pdf
- FDA (2007b) FDA News Release: FDA Warning on Codeine Use by Nursing Mothers, May Increase Chance of Serious Side Effects in Infants. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108968.htm
- FDA (2007c) Fiorinal with Codeine Package Insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019429s023lbl.pdf
- FDA (2008a) Information for Healthcare Professionals: Abacavir (marketed as Ziagen) and Abacavir-Containing Medications. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm123927.htm
- FDA (2008b) ZIAGEN (abacavir sulfate) Package Insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020977s017,020978s020lbl.pdf
- FDA (2009) Early Communication about an Ongoing Safety Review of clopidogrel bisulfate (marketed as Plavix). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm079520.htm
- FDA (2010a) Coumadin Package Insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/009218s108lbl.pdf
- FDA (2010b) PLAVIX (clopidogrel bisulfate) Package Insert (revised August 2010) Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020839s048lbl.pdf
- FDA (2010c) PLAVIX (clopidogrel bisulfate) Package Insert (revised March 2010). Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020839s042lbl.pdf
- FDA (2012a) Codeine Package Insert (revised April 2012). Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022402s003lbl.pdf
- FDA (2012b) FDA Drug Safety Communication: Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm313631.htm
- FDA (2012c) Table of Pharmacogenomic Biomarkers in Drug Labels. Available at: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm
- Flockhart D., O’Kane D., Williams M., Watson M., Flockhart D., Gage B., et al. (2008) Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med 10: 139–150 [DOI] [PubMed] [Google Scholar]
- Flowers C., Veenstra D. (2004) The role of cost-effectiveness analysis in the era of pharmacogenomics. PharmacoEconomics 22: 481–493 [DOI] [PubMed] [Google Scholar]
- French B., Joo J., Geller N., Kimmel S., Rosenberg Y., Anderson J., et al. (2010) Statistical design of personalized medicine interventions: The Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Trials 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh F. (2009) Back to the future: why randomized controlled trials cannot be the answer to pharmacogenomics and personalized medicine. Pharmacogenomics 10: 1077–1081 [DOI] [PubMed] [Google Scholar]
- Frueh F., Amur S., Mummaneni P., Epstein R., Aubert R., DeLuca T., et al. (2008) Pharmacogenomic biomarker information in drug labels approved by the United States food and drug administration: prevalence of related drug use. Pharmacotherapy 28: 992–998 [DOI] [PubMed] [Google Scholar]
- Gasche Y., Daali Y., Fathi M., Chiappe A., Cottini S., Dayer P., et al. (2004) Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med 351(27): 2827–2831 [DOI] [PubMed] [Google Scholar]
- Gilard M., Arnaud B., Cornily J., Le Gal G., Lacut K., Le Calvez G., et al. (2008) Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 51: 256–260 [DOI] [PubMed] [Google Scholar]
- Gilard M., Arnaud B., Le Gal G., Abgrall J., Boschat J. (2006) Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J Thrombosis Haemostasis 4: 2508–2509 [DOI] [PubMed] [Google Scholar]
- Gong I., Tirona R., Schwarz U., Crown N., Dresser G., Larue S., et al. (2011) Prospective evaluation of a pharmacogenetics-guided warfarin loading and maintenance dose regimen for initiation of therapy. Blood 118: 3163–3171 [DOI] [PubMed] [Google Scholar]
- Guyatt G., Akl E., Crowther M., Schunemann H., Gutterman D., Zelman Lewis S., et al. (2012) Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl.): 48S–52S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. (2004) Maternal medications during breastfeeding. Clin Obstet Gynecol 47: 696–711 [DOI] [PubMed] [Google Scholar]
- Heneghan C., Ward A., Perera R., Bankhead C., Fuller A., Stevens R., et al. (2012) Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet 379: 322–334 [DOI] [PubMed] [Google Scholar]
- Hetherington S., McGuirk S., Powell G., Cutrell A., Naderer O., Spreen B., et al. (2001) Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Therapeut 23: 1603–1614 [DOI] [PubMed] [Google Scholar]
- Higashi M., Veenstra D., Kondo L., Wittkowsky A., Srinouanprachanh S., Farin F., et al. (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287: 1690–1698 [DOI] [PubMed] [Google Scholar]
- Higgs J., Gambhir N., Ramsden S., Poulton K., Newman W. (2010) Pharmacogenetic testing in the United Kingdom genetics and immunogenetics laboratories. Genet Test Mol Biomarkers 14: 121–125 [DOI] [PubMed] [Google Scholar]
- Hirsh J., Guyatt G., Albers G., Harrington R., Schunemann H. (2008) Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(6 Suppl.): 71S–109S [DOI] [PubMed] [Google Scholar]
- Holbrook A., Schulman S., Witt D., Vandvik P., Fish J., Kovacs M., et al. (2012) Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl.): e152S–e184S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M., Perel P., Shah T., Hingorani A., Casas J. (2011) CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA 306: 2704–2714 [DOI] [PubMed] [Google Scholar]
- Hoop J., Lapid M., Paulson R., Roberts L. (2010) Clinical and ethical considerations in pharmacogenetic testing: views of physicians in 3 “early adopting” departments of psychiatry. J Clin Psychiatry 71: 745–753 [DOI] [PubMed] [Google Scholar]
- Hresko A., Haga S. (2012) Insurance coverage policies for personalized medicine. J Personalized Med 2: 201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Vilar F., Ward C., Alfirevic A., Park B., Pirmohamed M. (2004) Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics 14: 335–342 [DOI] [PubMed] [Google Scholar]
- Hulot J., Bura A., Villard E., Azizi M., Remones V., Goyenvalle C., et al. (2006) Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108: 2244–2247 [DOI] [PubMed] [Google Scholar]
- Ito S., Lee A. (2003) Drug excretion into breast milk - Overview. Adv Drug Delivery Rev 55: 617–627 [DOI] [PubMed] [Google Scholar]
- Jeong Y., Kim I., Park Y., Kang M., Koh J., Hwang S., et al. (2010) Carriage of cytochrome 2C19 polymorphism is associated with risk of highpost-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study. JACC - Cardiovasc Intervent 3: 731–741 [DOI] [PubMed] [Google Scholar]
- Jneid H., Anderson J., Wright R., Adams C., Bridges C., Casey D., Jr, et al. (2012) 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 60:645–681 [DOI] [PubMed] [Google Scholar]
- Johnson J.A. (2012) Warfarin pharmacogenetics: a rising tide for its clinical value. Circulation 1259: 1964–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Gong L., Whirl-Carrillo M., Gage B., Scott S., Stein C., et al. (2011) Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 90:625–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D., Gomes T., Ko D., Szmitko P., Austin P., Tu J., et al. (2009) A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. Can Med Assoc J 180: 713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L., Rieder M., van den Anker J., Malkin B., Ross C., Neely M., et al. (2012) More codeine fatalities after tonsillectomy in North American children. Pediatrics 129: e1343–e1347 [DOI] [PubMed] [Google Scholar]
- Klein T., Altman R., Eriksson N., Gage B., Kimmel S., Lee M., et al. (2009) Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 360: 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Cairns J., Chitayat D., Gaedigk A., Leeder S. (2006) Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 368: 704. [DOI] [PubMed] [Google Scholar]
- Lai E. (2008) Presentation to FDA Advisory Committee for Pharmaceutical Science and Clinical Pharmacology: Incorporation of Pharmacogenetic in Healthcare, www.fda.gov/ohrms/dockets/ac/08/slides/2008-4351s1-01-GuestSpeakers-corepresentation.ppt
- Lazarou J., Pomeranz B., Corey P. (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279: 1200–1205 [DOI] [PubMed] [Google Scholar]
- Lesko L., Johnson J. (2012) Academia at the crossroads: education and training in pharmacogenomics. Personalized Med 9(5): 10. [DOI] [PubMed] [Google Scholar]
- Lesko L., Zineh I., Huang S. (2010) What is clinical utility and why should we care? Clin Pharmacol Ther 88: 729–733 [DOI] [PubMed] [Google Scholar]
- Li T., Chang C., Jin D., Lin P., Khvorova A., Stafford D. (2004) Identification of the gene for vitamin K epoxide reductase. Nature 427: 541–544 [DOI] [PubMed] [Google Scholar]
- Limdi N., McGwin G., Goldstein J., Beasley T., Arnett D., Adler B., et al. (2008) Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 83: 312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh J., Lundgren S., Holm L., Alfredsson L., Rane A. (2005) Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin Pharmacol Ther 78: 540–550 [DOI] [PubMed] [Google Scholar]
- Liou S., Stringer F., Hirayama M. (2012) The impact of pharmacogenomics research on drug development. Drug Metab Pharmacokinet 27: 2–8 [DOI] [PubMed] [Google Scholar]
- Lunshof J., Gurwitz D. (2012) Pharmacogenomic testing: knowing more, doing better. Clin Pharmacol Ther 91: 387–389 [DOI] [PubMed] [Google Scholar]
- Madadi P., Hildebrandt D., Gong I., Schwarz U., Ciszkowski C., Ross C., et al. (2010) Fatal hydrocodone overdose in a child: pharmacogenetics and drug interactions. Pediatrics 126: e986–e989 [DOI] [PubMed] [Google Scholar]
- Madadi P., Ross C., Hayden M., Carleton B., Gaedigk A., Leeder J., et al. (2009) Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther 85: 31–35 [DOI] [PubMed] [Google Scholar]
- Mallal S., Nolan D., Witt C., Masel G., Martin A., Moore C., et al. (2002) Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359: 727–732 [DOI] [PubMed] [Google Scholar]
- Mallal S., Phillips E., Carosi G., Molina J., Workman C., Tomazic J., et al. (2008) HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358(6): 568–579, http://www.ncbi.nlm.nih.gov/pubmed/18256392 [DOI] [PubMed] [Google Scholar]
- Marin-Leblanc M., Perreault S., Bahroun I., Lapointe M., Mongrain I., Provost S., et al. (2012) Validation of warfarin pharmacogenetic algorithms in clinical practice. Pharmacogenomics 13: 21–29 [DOI] [PubMed] [Google Scholar]
- Martin M., Klein T., Dong B., Pirmohamed M., Haas D., Kroetz D. (2012) Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther 91: 734–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckley L., Gudgeon J., Anderson J., Williams M., Veenstra D. (2010) A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. PharmacoEconomics 28: 61–74 [DOI] [PubMed] [Google Scholar]
- Medco (2012) Express Scripts: Our programs. Available at: http://www.medcohealth.com/medco/corporate/home.jsp?BV_SessionID=@@@@1584666560.1347849007-mm387541470449@@@@&BV_EngineID=ccicadfhklgjmjhcfklcgffdghfdffn.0&articleID=CorpPM_PersonalizedMedicine
- Mega J., Close S., Wiviott S., Shen L., Hockett R., Brandt J., et al. (2009) Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 360: 354–362 [DOI] [PubMed] [Google Scholar]
- Mega J., Hochholzer W., Frelinger A., Kluk M., Angiolillo D., Kereiakes D., et al. (2011) Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 306: 2221–2228 [DOI] [PubMed] [Google Scholar]
- Mega J., Simon T., Collet J., Anderson J., Antman E., Bliden K., et al. (2010) Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304: 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek D., Lerman C. (2011) Facilitating clinical implementation of pharmacogenomics. JAMA 306: 304–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue M., Braunwald E., Antman E., Murphy S., Bates E., Rozenman Y., et al. (2009) Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 374: 989–997 [DOI] [PubMed] [Google Scholar]
- PAGAA (2012) Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- Patrick A., Avorn J., Choudhry N. (2009) Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circulation Cardiovasc Qual Outcomes 2: U429–U451 [DOI] [PubMed] [Google Scholar]
- Perlis R., Patrick A., Smoller J., Wang P. (2009) When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology 34: 2227–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M., Skov J., Bladbjerg E., Sidelmann J., Vamosi M., Jespersen J. (2012) Multivariate analysis of the relation between diet and warfarin dose. Eur J Clin Pharmacol 68: 321–328 [DOI] [PubMed] [Google Scholar]
- Ray W., Murray K., Griffin M., Chung C., Smalley W., Hall K., et al. (2010) Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 152: 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling M., Klein T. (2011) CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther 89: 464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder M., Reiner A., Gage B., Nickerson D., Eby C., McLeod H., et al. (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352: 2285–2293 [DOI] [PubMed] [Google Scholar]
- Roberts J., Wells G., Le May M., Labinaz M., Glover C., Froeschl M., et al. (2012) Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 379: 1705–1711 [DOI] [PubMed] [Google Scholar]
- Rosove M., Grody W. (2009) Should we be applying warfarin pharmacogenetics to clinical practice? No, not now. Ann Intern Med 151:270–273, W295. [DOI] [PubMed] [Google Scholar]
- Rost S., Fregin A., Ivaskevicius V., Conzelmann E., Hortnagel K., Pelz H., et al. (2004) Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427: 537–541 [DOI] [PubMed] [Google Scholar]
- Saag M., Balu R., Phillips E., Brachman P., Martorell C., Burman W., et al. (2008) High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis 46: 1111–1118 [DOI] [PubMed] [Google Scholar]
- SACGHS (2006) Coverage and Reimbursement of Genetic Tests and Services. Available at: http://oba.od.nih.gov/oba/sacghs/reports/CR_report.pdf
- SACGT (2000) Enhancing the oversight of genetic tests: Recommendations of the SACGT. Available at: http://oba.od.nih.gov/oba/sacgt/reports/oversight_report.pdf
- Schildcrout J., Denny J., Bowton E., Gregg W., Pulley J., Basford M., et al. (2012) Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther 92: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirm E., Schwagermann M., Tobi H., de Jong-van den Berg L. (2004) Drug use during breastfeeding. A survey from the Netherlands. Eur J Clin Nutrit 58: 386–390 [DOI] [PubMed] [Google Scholar]
- Schnoll R., Shields A. (2011) Physician barriers to incorporating pharmacogenetic treatment strategies for nicotine dependence into clinical practice. Clin Pharmacol Ther 89: 345–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. (2011) Personalizing medicine with clinical pharmacogenetics. Genet Med 13: 987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S., Sangkuhl K., Gardner E., Stein C., Hulot J., Johnson J., et al. (2011) Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 90: 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. (2004) Criteria influencing the clinical uptake of pharmacogenomic strategies. BMJ 328: 1482–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner A., O’Connell J., Bliden K., Gandhi A., Ryan K., Horenstein R., et al. (2009) Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302: 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-Matula J., Spiel A., Lang I., Kreiner G., Christ G., Jilma B. (2009) Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Amer Heart J 157: 148, e141–e145 [DOI] [PubMed] [Google Scholar]
- Simon T., Verstuyft C., Mary-Krause M., Quteineh L., Drouet E., Meneveau N., et al. (2009) Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 360: 363–375 [DOI] [PubMed] [Google Scholar]
- Small D., Farid N., Payne C., Weerakkody G., Li Y., Brandt J., et al. (2008) Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 48: 475–484 [DOI] [PubMed] [Google Scholar]
- Steward D., Haining R., Henne K., Davis G., Rushmore T., Trager W., et al. (1997) Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics 7: 361–367 [DOI] [PubMed] [Google Scholar]
- Stultz E., Stokes J., Shaffer M., Paul I., Berlin C. (2007) Extent of medication use in breastfeeding women. Breastfeed Med 2: 145–151 [DOI] [PubMed] [Google Scholar]
- Taube J., Halsall D., Baglin T. (2000) Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood 96:1816–1819 [PubMed] [Google Scholar]
- Thorn C., Klein T., Altman R. (2009) Codeine and morphine pathway. Pharmacogenet Genom 19: 556–558 [DOI] [PubMed] [Google Scholar]
- van Schie R., de Boer A., Maitland-van der Zee A. (2011) Implementation of pharmacogenetics in clinical practice is challenging. Pharmacogenomics 12: 1231–1233 [DOI] [PubMed] [Google Scholar]
- Voronov P., Przybylo H., Jagannathan N. (2007) Apnea in a child after oral codeine: a genetic variant - an ultra-rapid metabolizer. Paediatr Anaesth 17: 684–687 [DOI] [PubMed] [Google Scholar]
- Wadelius M., Chen L., Lindh J., Eriksson N., Ghori M., Bumpstead S., et al. (2009) The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 113: 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M., Lee M., Chen J., Chuang H., Lu L., Chen C., et al. (2008) Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther 84: 83–89 [DOI] [PubMed] [Google Scholar]
- Wolf E., Blankenburg M., Bogner J., Becker W., Gorriahn D., Mueller M., et al. (2010) Cost impact of prospective HLA-B*5701-screening prior to abacavir/lamivudine fixed dose combination use in Germany. Eur J Med Res 15: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zineh I., Mummaneni P., Lyndly J., Amur S., La Grenade L., Chang S., et al. (2011) Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics 12: 1741–1749 [DOI] [PubMed] [Google Scholar]
- Zineh I., Pebanco G., Aquilante C., Gerhard T., Beitelshees A., Beasley B., et al. (2006) Discordance between availability of pharmacogenetics studies and pharmacogenetics-based prescribing information for the top 200 drugs. Ann Pharmacother 40: 639–644 [DOI] [PubMed] [Google Scholar]