Abstract
Following the induction of ischemic or toxin-mediated acute kidney injury (AKI), cellular adaptations occur that ‘re-program’ how the kidney responds to future superimposed insults. This re-programming is not simply a short-lived phenomenon; rather it can persist for many weeks, implying that a state of ‘biologic memory’ has emerged. These changes can be both adaptive and maladaptive in nature and they can co-exist in time. A beneficial adaptation is the emergence of acquired cytoresistance, whereby a number of physiologic responses develop that serve to protect the kidney against further ischemic or nephrotoxic attack. Conversely, some changes are maladaptive, such as a predisposition to Gram-negative or Gram-positive bacteremia due to a renal tubular up-regulation of toll-like receptor responses. This latter change culminates in exaggerated cytokine production, and with efflux into the systemic circulation, extra-renal tissue injury can result (so-called ‘organ cross talk’). Another maladaptive response is a persistent up-regulation of pro-inflammatory, pro-fibrotic and vasoconstrictive genes, culminating in progressive renal injury and ultimately end-stage renal failure. The mechanisms by which this biologic re-programming, or biologic memory, is imparted remain subjects for considerable debate. However, injury-induced, and stable, epigenetic remodeling at pro-inflammatory/pro-fibrotic genes seems likely to be involved. The goal of this editorial is to highlight that the so-called ‘maintenance phase’ of acute renal failure is not a static one, somewhere between injury induction and the onset of repair. Rather, this period is one in which the induction of ‘biologic memory’ can ultimately impact renal functional recovery, extra-renal injury and the possible transition of AKI into chronic, progressive renal disease.
Keywords: acute kidney injury, chronic kidney disease, epigenetics, ischemic preconditioning, sepsis syndrome
INTRODUCTION
The vast majority of studies of experimental ischemic or toxin-induced acute kidney injury (AKI) have focused on identifying basic mechanisms, which evoke proximal tubule cell necrosis and apoptotic cell death (e.g. [1–4]). The goal of these studies has been to interrogate specific injury pathways that might then be blocked by specific pharmacologic agents, with the hope of preventing the development of acute renal failure (ARF). However, as is clear to all practicing nephrologists, the vast majority of AKI patients are identified by advancing azotemia, well beyond the initial injury phase. Thus, most therapeutic interventions that are directed at mitigating acute cell injury are not likely to have utility during the so-called ARF ‘maintenance phase’.
There is a tendency to consider the AKI ‘maintenance phase’ as a non-dynamic one, only to be interrupted by the onset of tubular regeneration and renal functional recovery. However, this is an inexact view, given that dramatic ‘non-recovery’ events transpire during the AKI maintenance phase, which can potentially impact renal, extra-renal and ultimately patient outcomes. A major focus of this laboratory, over the past 30 years, has been to identify and study the biologic consequences of such delayed renal injury responses. The goal of this editorial is to present the view that a bout of AKI imprints on residual tubules a state of ‘biologic memory’, which then re-programs how residual nephrons respond to downstream events. Three examples of this re-programming include: (i) the emergence of post-AKI ‘acquired renal cytoresistance’ (‘ACR’), which impacts subsequent renal tubular injury responses; (ii) AKI-initiated renal hyper-responsiveness to sepsis syndrome and toll-like receptor (TLR) ligands, potentially influencing the severity of AKI-associated multi-organ failure and (iii) post-AKI-initiated chromatin remodeling, which can initiate progressive, chronic kidney disease (CKD).
THE PHENOMENON OF ACR
It has been recognized for over a century that in the aftermath of nephrotoxic AKI, the kidney becomes relatively resistant to subsequent nephrotoxic insults (reviewed in Honda et al. [5]). This was originally demonstrated with uranyl nitrate-induced AKI, whereby one exposure led to resistance to subsequent challenges with the same agent. Since this original description of acquired resistance to uranyl nitrate, the list of agents that can induce this response has expanded considerably, culminating in the conclusion that virtually any nephrotoxin can trigger subsequent resistance to either the original, or a different nephrotoxic agent (so-called ‘cross-resistance’, e.g. uranyl nitrate protecting against HgCl2) [5].
An early and seemingly compelling explanation for this phenomenon is that prior renal injury can lead to decreased nephrotoxin uptake upon a subsequent re-challenge. It is well known that AKI, almost irrespective of cause, produces shedding, simplification and internalization of the proximal tubule brush border [6]. Given the critical role played by the brush border in endocytosis (e.g. via the megalin–cubulin complex), decreased proximal tubule uptake of filtered nephrotoxins, e.g. myoglobin, hemoglobin, light chains, may result. Furthermore, AKI can decrease basolateral expression of organic anion (OAT) and cation transporters, thereby suppressing subsequent anionic or cationic toxin uptake [7]. Indeed, these considerations previously obfuscated the issue of whether nephrotoxin-induced ACR was either due to decreased toxin uptake upon re-challenge, or whether proximal tubular injury evokes adaptive proximal tubule responses that confer cellular resistance to further, superimposed, attack [5].
In 1984, our laboratory sought to address this issue by assessing whether non-toxin-mediated injury, i.e. a bout of renal ischemia (where toxin uptake is not a consideration), might also usher in the ACR state. To this end, rats were subjected to one bout of renal ischemia (20–40 min of renal artery occlusion), and 24–48 h later, a second ischemic insult was imposed [8]. The potential emergence of ACR was assessed by acute changes in glomerular filtration rate (GFR) in response to the second ischemic insult and the potential onset of fresh tubule necrosis, as assessed by renal histology [8]. Those studies clearly demonstrated that one bout of renal ischemia can usher in ACR against a second ischemic event, as evidenced by a relative lack of new GFR reductions or fresh morphologic damage. Furthermore, when proximal tubules were isolated from mice that had been subjected to a prior bout of renal ischemia, they manifested marked resistance to in vitro hypoxic damage [9]. In sum, these findings demonstrated that the phenomenon of ACR is expressed directly within proximal tubule cells. Since this demonstration of post-ischemic ACR in kidney, this phenomenon has been demonstrated in virtually all organs, and is now widely referred to as ‘ischemic preconditioning’ (e.g. [10]). However, the use of this term (at least, with regard to the kidney) is misleading, because injury-induced ACR encompasses far more than a post-ischemic event. For example, diverse forms of renal insults, e.g. urinary tract obstruction, heat shock and endotoxemia, can each elicit ACR, even in the absence of overt proximal tubule morphologic damage. Hence, ACR is not challenge specific, but rather, it is an integral component of an acute cellular ‘stress response’ [11].
Protein mediators of ACR
In response to ‘cellular stress’, a shift in the normal pattern of protein synthesis occurs, such that so-called ‘housekeeping’ protein production becomes relatively suppressed, with a correlate being increased ‘stress protein’ [e.g. heat shock protein (HSP)] synthesis [12]. Pioneering work by Siegel and others has given rise to the concept that HSPs, most notably HSP-70/72 can play a critical role in ACR, given their ability to stabilize a variety of pathways that are instrumental in evolving acute cell damage [13,14]. Expanding on this concept were a series of seminal studies by Nath et al. [15] who demonstrated that another HSP (HSP-32), more commonly referred to as heme oxygenase-1 (HO-1), was also up-regulated in response to diverse forms of AKI, and can play a critical role in ACR. For example, he demonstrated that HO-1 was up-regulated in response to myohemoglobinuria or endotoxemia, and that either HO-1 inhibitors (e.g. Sn or Zn protoporphyrin) or genetic HO-1 deficiency (HO-1−/− mice) markedly pre-disposed to acute renal damage [3,15]. It was originally speculated that HO-1’s dramatic cytoprotective properties resulted from its ability to catalyze pro-oxidant free heme (e.g. as released from reabsorbed myoglobin/hemoglobin; or derived from intracellular cytochromes, e.g. p450, cytochrome C). However, it soon became apparent that other mechanisms for HO-1-mediated cytoprotection must exist, given that heme cleavage, per se, results in catalytic-free iron release, which would be expected to increase, not decrease, oxidant stress. In this regard, it was recognized that in addition to porphyrin ring cleavage, HO-1 activity (and presumably free Fe release) up-regulates intracellular ferritin, which binds excess free iron, and thus, confers a potent, and adaptive cytoprotective effect. Additionally, as a result of porphyrin ring degradation, HO-1 generates bilirubin and biliverdin, each of which possesses anti-oxidant activity. Finally, it has more recently been demonstrated that HO-1 generates carbon monoxide, which in physiologic concentrations, possesses potent cytoprotective and anti-inflammatory properties [3].
Although the HSPs, including HO-1, have received the greatest attention, it is notable that AKI also up-regulates other cytoprotective proteins. For example, Safirstein and colleages [16] demonstrated that cell cycle regulatory proteins, e.g. p21, can play a critical role in renal susceptibility to AKI. Furthermore, our laboratory has recently speculated on an entirely different ‘protein stress response’ that likely impacts the cytoresistant state: the ‘renal hepatization response’. In a series of extensive experiments, we have demonstrated that the mRNAs of four proteins (albumin, alpha-fetoprotein, haptoglobin and hemopexin) that are virtually exclusively synthesized in the liver, are remarkably up-regulated in response to diverse forms of ischemic and toxic AKI [17–19]. As a result, dramatic increases in haptoglobin, hemopexin, alpha-fetoprotein and albumin levels within proximal tubule cells develop. These findings indicate that the injured kidney assumes selected features of a ‘hepatic phenotype’. Noteworthy in this regard is that each of these proteins can exert potent cytoprotective effects. For example, albumin can bind cytotoxic fatty acids, thereby limiting mitochondrial toxicity and lytic membrane damage; the heme/heme-binding proteins, hemopexin and haptoglobin, respectively, can each exert anti-oxidant effects; and alpha-fetoprotein is a potent immunomodulary molecule [17–19]. While definitive evidence is lacking that the ‘renal hepatization response’ contributes to ACR, the existing non-renal literature provides a compelling theoretical case. What can be stated definitively is that the ‘renal hepatization response’ is not simply a phenomenon confined to the experimental laboratory. For example, we demonstrated that patients with established AKI have ∼50-fold increases in haptoglobin and alpha-fetoprotein urinary concentrations. Indeed, that these increases quantitatively parallel urinary neutrophil gelatinase associated lipocalin concentrations (a well-accepted biomarker of AKI) indicates the robust nature of the ‘renal hepatization response’ in patients [17,18].
Lipid mediators of ACR
As can be inferred from the above discussion, the bulk of interest in underlying mechanisms for ACR has focused on AKI-induced up-regulation of cytoprotective proteins. However, increasing evidence indicates that stress-activated lipid pathways may also be involved. Our initial interest in this possibility stemmed from our observation that the addition of low concentrations of phospholipase A2, with the subsequent generation of lysophospholipids and release of unsaturated free fatty acids (FFAs), can confer a dramatic in vitro cytoresistant state [20,21]. Because AKI-initiated FFA increases are short lived in duration, whereas ACR is a more durable response, in subsequent studies we sought more stable lipid alterations that might help mediate the cytoresistant state. This search was complicated by the fact that AKI-induced alterations in cellular lipid homeostasis generally occur in concert with stress protein synthesis. Thus, it became necessary to dissociate the effects of AKI-induced lipid versus stress protein stress reactants. To achieve this goal, cultured human proximal tubule (HK-2) cells were incubated in the presence of cycloheximide or verrucarin A, which induce a cellular stress response by completely inhibiting protein synthesis. Despite the fact that these agents induced >99% protein synthesis inhibition, a stress response-mediated cytoresistant state emerged [22,23]. Hence, it seemed clear that while de novo stress proteins are clearly important to ACR, they are not the only factors that can induce this state.
Subsequent studies revealed that the sphingomyelinase pathway, which mediates sphingomyelin degradation to ceramide and ultimately sphingosine/sphingosine-1 phosphate, is activated during AKI, and the resultant ceramide and sphingosine increases can contribute to ACR [23]. Further evidence in this regard comes from Jo et al. [24] who demonstrated the cytoprotective effects of sphingosine-1 phosphate. Because sphingomyelin is normally tightly associated with free cholesterol within the plasma membrane, we next sought to determine whether cholesterol might also be up-regulated post-AKI and contribute to ACR. Indeed, the results of those studies have provided perhaps the most compelling evidence for changes in lipid homeostasis as a mediator of ACR, based on the following observations [25–29]: first, every form of AKI that we have tested, and which are known to induce ACR (including ischemia, diverse nephrotoxins, urinary tract obstruction, endotoxemia and heat shock) consistently induce an ∼30% increase in renal cortical cholesterol content. Secondly, the temporal pattern of cholesterol increases parallels that of the cytoresistant state (i.e. both increasing and decreasing over the same time period, ranging from days to weeks). Thirdly, statin-induced blockade of cholesterol synthesis in cultured proximal tubule cells prevents the emergence of ACR, and increases cellular susceptibility to superimposed attack. Fourthly, the in vitro extraction of excess cholesterol from the plasma membrane (e.g. with methylcyclodextrin) negates tubule cytoresistance. Fifthly, prevention of normal cellular cholesterol cycling (e.g. by p-glycoprotein inhibition) also blocks ACR and sixthly, enzymatic cholesterol modification, e.g. with cholesterol oxidase, exerts dramatic cytotoxic effects. Finally, it is noteworthy that stress-activated cholesterol synthesis can also occur in non-renal cells and mediate ACR. For example, we have demonstrated that chemotherapeutic attack of acute myelogenous leukemia (AML) cells, e.g. with daunorubricin, increases AML cell cholesterol content, and that this induces resistance to subsequent chemotherapeutic attack [30,31]. Thus, while cholesterol-mediated ACR might be beneficial for the kidney by helping to stave off further bouts of AKI, it may have adverse consequences in different clinical circumstances, e.g. rendering cancer cells relatively resistant to subsequent courses of chemotherapy. The mediators of injury-induced cholesterol accumulation following AKI can be multi-factorial, e.g. increased cholesterol synthesis, increased cholesterol uptake from the plasma via the LDL receptor or decreased cholesterol efflux. However, increased HMG CoA reductase activity, which is associated with injury-induced gene-activating histone modifications at the HMG CoA reductase gene, is typically involved [29,32,33]. While the mechanisms by which cholesterol mediates cytoprotection have been incompletely defined, one operative pathway is a cholesterol-mediated increase in plasma membrane rigidity, which prevents membrane rupture during the process of necrotic cell death [34].
Uremia as a cytoprotectant
In addition to cell injury-induced alterations in stress protein synthesis and cholesterol accumulation, our laboratory has pursued a third pathway by which AKI might induce ACR; i.e. that uremia-associated compounds, which are normally excreted in urine, but which accumulate with renal insufficiency, can exert renal cytoprotective effects. Several lines of evidence support this new hypothesis, as follows: first, if rodents are subjected to progressive surgical ablation of renal mass, the remnant renal tissues become increasingly resistant to superimposed ischemic AKI [35]. Secondly, if mice are subjected to a fixed bout of unilateral (e.g. left kidney) ischemic injury (e.g. 30 min) in the absence or presence of uremia (created by inducing stepwise increases in contralateral kidney ischemia), the greater the degree of the initial azotemia, the greater the degree of left kidney protection against evolving renal damage [36]. Thirdly, if mice are subjected to severe ARF and then subjected to acute peritoneal dialysis, the recovered peritoneal dialyzate confers cytoprotection on cultured proximal tubular cells [37,38]. Fourthly, if experimental uremia is induced in the absence of direct renal damage (created by performing bilateral ureteral transection), the structurally normal kidneys exist in an acute uremic milieu, and their tubules are resistant to in vitro attack [37] and fifthly, the addition of a low-molecular weight ultrafiltrates of normal human urine confer both cytoprotective as well as anti-inflammatory effects on cultured human proximal tubule cells [37]. The nature of these uremic cytoprotective molecules remains unknown. However, their identification could have substantial therapeutic relevance, given that their administration could potentially protect against or mitigate the course of ARF. Alternatively, one might speculate as to whether their aggressive removal by intensive renal replacement therapy could potentially have adverse consequences for renal recovery.
Does ACR exist in humans?
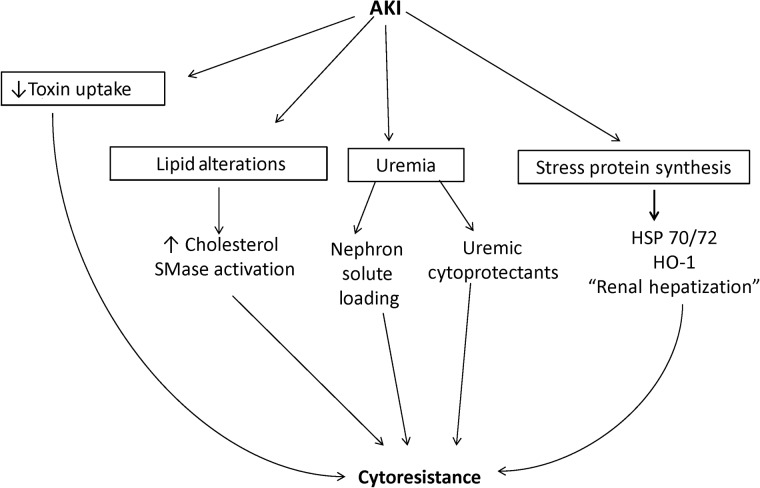
While the above data clearly indicate the existence of ACR in a laboratory setting, its development in the clinical arena has been a subject of debate. However, a recent clinical report from our group has provided positive data in this regard [39]. Within the first year following hematopoetic cell transplantation (HCT), ∼25% of patients will develop either acute or subacute renal damage, as evidenced by reductions in estimated glomerular filtration rate (eGFR), compared with that of basal values [39]. If the above hypothesis is correct, i.e. that a reduced GFR causes the accumulation of low-molecular weight cytoprotective molecules, one might reasonably speculate that the risk of HCT-mediated eGFR reductions would be inversely correlated with the baseline renal function. To test this hypothesis, the records of over 1200 patients who received HCTs at the Fred Hutchinson Cancer Center were reviewed, and support for this hypothesis emerged. In brief, it was observed that the higher the baseline eGFR, the greater was the incidence and severity of subsequent AKI, as assessed over the ensuing 1 year. Clearly, contradictory information exists vis à vis the existence of ACR in the clinical arena. For example, renal insufficiency is a known risk factor for radiocontrast-induced ARF. However, multiple potential explanations for these contradictory observations exist, and space limitations preclude a discussion of them. As just one example, a ‘normal dose’ of radiographic contrast agent administered to patients with renal insufficiency represents a significantly greater contrast dose per functional nephron, which might then overwhelm inherent cytoprotective mechanisms. Suffice it to say that it seems clear that renal injury, and the resulting azotemia, up-regulate a variety of cytoprotective pathways in both the experimental and clinical arena. Future clinical investigation will be required to more fully unravel ACR's clinical, and possible future therapeutic, implications. The interactive pathways that may give rise to ACR are presented in Figure 1.
FIGURE 1:
Proposed mechanisms by which AKI induces cytoresistance against further ischemic or toxic insults. Four pathways by which AKI can initiate renal resistance are presented. The most prominently mentioned in the literature is AKI-initiated stress protein production, particularly within the HSP family (far right). Most notable are the induction of HSP 70/72 and HSP-32 (more commonly referred to as ‘HO-1’). In addition, recent work has revealed that AKI can initiate a ‘renal hepatization’ response, leading to the activation of the albumin, alpha fetoprotein, haptoglobin and hemopexin genes within proximal tubule cells. Since these four genes are typically active only in the liver, this ‘renal hepatization’ response suggests that injured proximal tubules assume features of an hepatic phenotype (up-regulation of normally silent genes in the kidney). Because each of these four proteins can exert cytoprotective and/or anti-inflammatory effects, they may contribute to the cytoresistant state. AKI can also impact lipid, and not just ‘stress protein’ expression. For example, AKI activates the HMG CoA reductase pathway, leading to increased cholesterol content within proximal tubule cell membranes. This decreases membrane fluidity, and thus protects the membrane from rupture during the evolution of necrotic cell death. Activation of the sphingomyelinase (SMase) may also occur, leading to the production of cytoprotective sphingosine and sphingosine-1 phosphate. Additional protective pathways include a suppression of nephrotoxin uptake due to decreased brush border and OAT/cation transport pathways. Independent of cytoprotectve protein and lipid synthesis, uremia, per se, may confer cytoprotection via the retention of low-molecular ‘uremic compounds' that possess cytoprotective properties. Finally, uremia is associated with an increase in solute loads per nephron (due to a decrease in functional nephrons, thereby increasing the loads of urea, NaCl, etc. per nephron). Thus, this imposes a relative solute diuresis per functional nephron, potentially decreasing cast formation. In concert, these factors act to initiate and maintain a cytoresistant state.
AKI-INITIATED HYPER-RESPONSIVENESS TO SEPSIS AND TLR LIGANDS
A pathophysiologic link between sepsis syndrome and AKI has been firmly established in both the clinical and experimental arena. For example, experimental and presumably clinical sepsis syndrome can trigger acute renal injury, culminating in ARF [40]. Alternatively, in patients with established ARF, sepsis syndrome is a leading cause of death [41]. In a recent series of studies, we tested a new hypothesis that could yield new pathogenic insights into the interplay between sepsis syndrome and ARF; i.e. that AKI, whether induced by nephrotoxins or ischemia, ‘re-programs’ the kidney such that it hyper-responds to superimposed septic events, culminating in exaggerated renal cytokine and chemokine production. To test this hypothesis, mice were subjected to diverse forms of AKI (e.g. ischemia, nephrotoxins and urinary tract obstruction). After ARF was established, the mice were challenged with either endotoxin (a TLR4 ligand) or lipoteichoic acid (a TLR2 ligand; the biologic equivalent to endotoxin in Gram-positive bacteria). Cytokine/chemokine (e.g. TNF-α/MCP-1) protein levels were then assessed in both plasma and the renal cortex. In each instance, the presence of pre-existent AKI greatly exaggerated LPS- or LTA-driven cytokine/chemokine increases, as assessed in blood and kidney [42–47]. To prove that the kidney, per se, was indeed hyper-responding to the lipopolysachharide (LPS) or lipoteichoic acid (LTA) injections, renal cortical cytokine and chemokine production rates were assessed by measuring renal cortical cytokine/chemokine mRNAs and degrees of RNA polymerase II (Pol II) binding (a marker of gene transcription) to representative cytokine/chemokine genes (TNF-α, MCP-1). In the presence of ARF, these markers hyper-responded to LPS, compared with LPS-challenged controls. Therefore, these findings underscore that the presence of ARF can directly impact the expression of sepsis syndrome by exaggerating intra-renal cytokine and chemokine production. With renal efflux, increased systemic cytokine burdens, with the potential for so-called ‘organ cross talk’ (one organ damaging another), may result. Finally, given that cytokines and chemokines are normally cleared by the kidney due to their relatively low-molecular weights, a decrease in GFR, per se, would be expected to increase systemic cytokine burdens.
In an attempt to unravel the potential mechanism(s) for this renal hyper-responsiveness to pro-inflammatory stimuli, three hypotheses were pursued. First was the concept that AKI might lead to an up-regulation of TLR densities within the kidney, thereby exaggerating LPS or LTA binding, and hence downstream gene responses. However, western blotting and immunohistochemistry for TLR4 (the LPS receptor) revealed decreased, not increased, densities within the kidney [45]. Secondly, we tested whether the establishment of AKI/ARF leads to stabilization of cytokine mRNAs; if so, then any given level of cytokine mRNA production (and hence cytokine generation) could be exaggerated by a decrease in cytokine mRNA degradation rates. However, by measuring TNF-α mRNA degradation rates in proximal tubules harvested from kidneys with AKI, it was clearly established that this was not the case [46]. Thus, we turned to a third hypothesis: that AKI activates chromatin remodeling enzymes, which evoke ‘gene-activating’ histone modifications at pro-inflammatory genes. This gene activation occurs because selective histone remodeling (e.g. trimethylation, acetylation and histone exchange) at critical genomic sites can loosen chromatin structure, which then augments RNA Pol II recruitment to, and hence increased transcription rates of, target genes. By probing renal tissues by PCR to quantify mRNA levels, and by using chromatin immunoprecipitation assays to detect chromatin remodeling, this third hypothesis appears to be the case, based on the following pieces of information. First, in response to AKI, increased mRNA levels of two chromatin remodeling enzymes (SET-1, a methyltransferase; and BRG-1, a histone exchanger) were observed in the whole renal cortex; secondly, increased levels of these two enzymes were observed at the transcription start site of the TNF-α gene; thirdly, increased levels of histone H3 trimethylation and of the gene-activating H2 histone variant H2A.Z resulted, presumably due to SET-1 and BRG-1 activity, respectively and fourthly, when BRG-1 binding to the TNF-α gene was ‘knocked down’ with an siRNA in cultured human proximal tubule cells, decreased TNF-α gene transcription resulted [46,47]. Thus, based on these findings, we have hypothesized that AKI initiates chromatin/epigenetic remodeling, and the latter leads to a pro-inflammatory LPS/LTA hyper-responsive state. Ultimately, this evokes exaggerated intra-renal cytokine/chemokine production, and with renal cytokine efflux into the systemic circulation, this process can impact both renal and extra-renal injury. In a sense, this is a seeming paradox with the phenomenon of ACR, as discussed above. Whereas AKI can lead to ‘downstream’ renal cytoprotection against further insults, it may also contribute to extra-renal tissue injury by exaggerating renal responses to a superimposed septic state.
Finally, we sought initial clinical support for the above concept of renal hyper-responsiveness to sepsis by studying urinary samples from critically ill (intensive care unit hospitalized) septic patients with and without ARF. If AKI were a major risk factor for exaggerated intra-renal cytokine production, then increased urinary cytokine levels might be expected in the patients with sepsis syndrome + AKI, versus sepsis syndrome alone. MCP-1 was chosen as the assessed biomarker of this process, and ∼2-fold greater urinary MCP-1 levels were observed in the AKI (+) versus the AKI (–) septic group [48]. Given the fact that urinary cytokine levels may reflect filtered, and hence extra-renal, cytokine generation, we next sought more direct evidence for excess intra-renal MCP-1 generation, and possibly, underlying chromatin remodeling at the MCP-1 gene. To achieve this goal, pelleted urinary cells were extracted for chromatin and the latter were probed for increased levels of Pol II at the start exon of the MCP-1 gene [48]. Indeed, degrees of Pol II binding (as noted above, a marker of gene transcription rates) paralleled the increased urinary MCP1 protein levels. Furthermore, increased levels of histone 3 lysine 4 trimethylation were observed at the MCP-1 gene, implying that in vivo epigenetic remodeling had occurred. In sum, these clinical findings directly supported the experimental concept of gene-activating epigenetic remodeling, increased Pol II recruitment and increased MCP-1 gene transcription within the injured kidney. Whether this phenomenon of AKI-initiated exaggerated intra-renal cytokine/chemokine production during sepsis actually impacts patient outcomes remains to be defined. The above concepts are presented as a flow diagram in Figure 2.
FIGURE 2:
A proposed mechanism by which AKI initiates a state of hyper-responsiveness to sepsis syndrome and TLR ligands. AKI up-regulates selected chromatin remodeling enzymes (e.g. SET1, BRG-1) that translocate to the nucleus where they can induce gene-activating ‘epigenetic’ alterations (e.g. methylation, acetylation and histone variant exchange). By loosening DNA winding within the nucleosome (comprises a four matched pairs of histones and associated DNA), these histone modifications can increase RNA Pol II binding when the kidney is confronted with TLR ligands (e.g. LPS from Gram-negative bacteria; lipoteichoic acid from Gram-positive bacteria). This culminates in increased gene transcription, leading to increased cytokine and chemokine production (lipoteichoic acid). Not only can the excessive cytokine/chemokine levels potentially increase intra-renal inflammation, the cytokines can efflux into the systemic circulation where they can potentially contribute to multi-organ failure (so-called organ ‘cross talk’). To the degree to which AKI leads to a decrease in GFR, decreased renal cytokine clearance results, thereby increasing plasma and tissue cytokine/chemokine burdens. Thus, AKI, both via increased cytokine production and decreased cytokine clearance, can contribute to multi-organ failure.
AKI-INITIATED CHROMATIN REMODELING AND PROGRESSIVE RENAL DISEASE
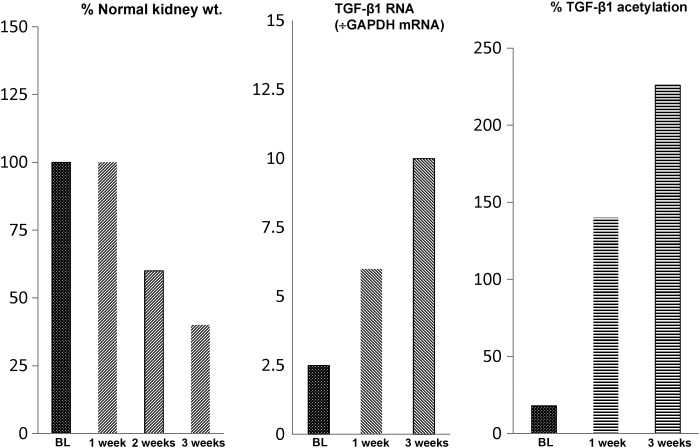
A burgeoning clinical literature implies that a bout of AKI represents a significant risk factor for progressive renal disease [49–51]. Indeed, some experimental support for this concept comes from our previous experiments with our unilateral ischemic AKI model in the mouse, whereby a single, relatively modest, ischemic insult leads to progressive renal failure [36–52], as denoted by ongoing proximal tubule necrosis, progressive intra-renal inflammation, increasing lipidosis and renal fibrosis, eventuating in a 50% loss of renal mass over 3 weeks (equivalent in time to ∼3 years of human life). A correlate of this ongoing renal damage is a progressive up-regulation of pro-inflammatory/pro-fibrotic gene activity, as denoted by stepwise increases in TNF-α, MCP-1, TGF-β1 and collagen III mRNA levels over time [52] (see Figure 3 for an example of these phenomena). Additionally, progressive activation of the endothelin 1 (ET-1) gene, and of its ETA (vasoconstriction mediating) receptor results [53]. Given its potent vasoconstrictive actions, ET-1 could evoke ongoing renal vasoconstriction and tissue hypoxia beyond the initial ischemic period. Two sets of observations indicate the functional significance of the above-described pro-inflammatory and renal vasoconstrictive states to the observed progressive renal damage. First, high-dose glucocorticoid therapy mitigated the extent of post-ischemic nephron loss [52]; and secondly, administration of a potent, ETA receptor-specific antagonist (ABT-627; ‘Atrasentan’) markedly attenuated post-ischemic tissue damage, leading to a marked preservation of post-ischemic renal mass ([53]; see Figure 3).
FIGURE 3:
Progressive renal failure can follow unilateral ischemic renal injury. Following the induction of 30 min of unilateral ischemic injury, a progressive loss of renal mass ensues, as indicated by a progressive reduction in renal weight. Thus, by 3 weeks post-ischemia in the mouse (equivalent to ∼3 years of human life), a 50% reduction in renal mass occurs, consistent with a post-AKI transition to CKD/ESRD (left-hand panel). The histologic correlate of this loss of renal weight is progressive tubule necrosis, increased inflammatory and pro-fibrotic cytokine production, and activation of pro-fibrotic genes, leading to fibrosts. These changes are associated with gene-activating histone marks (acetylation is depicted). The latter facilitates increases in Pol II binding to the start exon of pro-inflammatory/pro-fibrotic genes, leading to ongoing gene transcription (these changes at one representative gene, TGF-β1, is depicted).
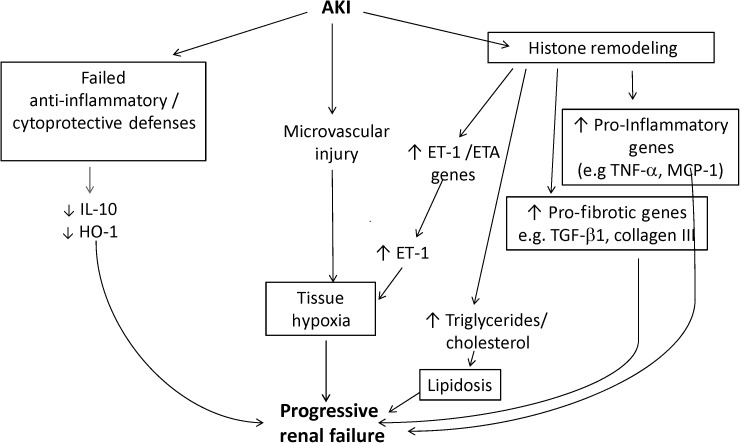
The question that emerges from these studies is what mediates this unremitting, if not intensifying, post-ischemic renal inflammation and ET-1-mediated vasoconstriction. Drawing an analogy to injury-evoked epigenetic remodeling as discussed above, we questioned whether gene-activating histone modifications might occur at target pro-inflammatory and pro-fibrotic genes as well as at the ET1 gene. To explore this possibility, chromatin immunoprecipitation assay was used to probe for gene-activating histone alterations at each of the above-referenced genes, and indeed, progressive methylation, acetylation and histone exchange were observed [52–54]. In contrast to the progressive activation of these injury-provoking genes, the expression of selected cytoprotective genes, e.g. HO-1 and IL-10, were probed and decreased, not increased, gene activities were observed. Thus, these reciprocal changes in injury-provoking versus cytoprotective genes suggested ‘a tipping the balance’ towards a pro-inflammatory/pro-fibrotic state (Figure 4). While the above results were obtained in the unilateral ischemia model, [52] it is notable that comparable results were also obtained in a nephrotoxic AKI model (glycerol-mediated rhabdomyolysis induced ARF; ref. [54]). Thus, it appears that AKI can lead to histone remodeling, this remodeling is a relatively stable, if not a progressive, event, and as such, an initial bout of tissue injury is then encoded in the genome (i.e. biologic memory is imparted), which then sets the stage for progressive renal damage. If this paradigm is correct, then it suggests the potential for new therapeutic targets to prevent disease progression. For example, a variety of pharmacologic agents are becoming available that interfere with histone remodeling enzymes. Thus, their administration could potentially prevent the establishment of ‘post-injury biologic memory’, and hence, prevent progressive renal disease. Alternatively, targeting the specific downstream products of these activated genes might also have utility. For example, as noted above, the administration of an ET1A receptor antagonist, AB-627, blunts ongoing tissue ischemia, and as a result a marked attenuation of the AKI transition to CKD results [53].
FIGURE 4:
AKI can initiate the onset of progressive renal disease. This figure depicts three potential pathways by which ischemic AKI may initiate progressive renal disease. As shown at the right-hand-side of the illustration, histone remodeling can lead to progressive activation of pro-inflammatory and pro-fibrotic genes (as depicted in Fig. 3). Additionally histone modifications, e.g. at the HMG CoA reductase gene, can lead to progressive lipid accumulation, which is known to exert pro-inflammatory effects. A second post-ischemic event is the induction of microvascular injury, setting the stage for ongoing tissue hypoxia. This pathway is intensified by a persistent up-regulation of the ET-1 gene, and that of its ETA receptor, which facilitates ongoing hypoxic/ischemic tissue damage. Finally, there is a relative failure of counter-regulatory mechanisms, most notably of IL-10 and HO-1, leaving pro-inflammatory/pro-fibrotic pathways relatively unopposed. As a consequence, progressive nephron loss, declining renal mass and ultimately end-stage kidney disease can result.
CONCLUSIONS
From the information presented above, it seems clear that the kidney that has sustained AKI is not simply in a passive state, somewhere between AKI induction and the onset of repair. Rather, dynamic changes that are both adaptive and maladaptive in nature emerge. The onset of acute cytoresistance can clearly be viewed as a beneficial adaptation, helping to stave off recurrent bouts of superimposed ischemic or nephrotoxic insults such as frequently befall critically ill AKI patients. Conversely, the state of TLR hyper-responsiveness is most likely a maladaptive one, given its potential to greatly exaggerate renal cytokine production and result in excessive cytokine efflux into the systemic circulation. Finally, AKI-initiated epigenetic remodeling, which plays important roles in the emergence of cytoresistance and the hyper-inflammatory state, may also set the stage for post-AKI disease progression to CKD. The relative degrees to which these beneficial versus adverse biologic events play out undoubtedly vary considerably depending upon a host of biologic variables. A better understanding of these processes could potentially lead to new therapeutic strategies that might help ‘tip the balance’ towards adaptive versus maladaptive responses, and thus, potentially speed renal recovery, improve patient outcomes and ultimately limit progression to CKD. Thus, identifying ways to alter AKI-initiated biologic memory represents an important therapeutic avenue for future investigation.
ACKNOWLEDGEMENTS
The author expresses his sincere thanks to Ms Ali CM Johnson who has been a critical member of our laboratory for the past 15 years. Through her dedicated support and insights, she helped make much of the above-described work possible. I also thank Dr Karol Bomstzyk for providing insights and technical assistance with our studies of epigenetic remodeling in response to renal injury. Finally, the author thanks NIDDK for helping to provide the research funding that made most of the described work possible over the past 30 years. This work was supported by research grants from the National Institutes of Health (DK38432, DK083310).
CONFLICT OF INTEREST STATEMENT
The author declares that he has no financial conflicts of interest.
REFERENCES
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. doi:10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 3.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–420. doi: 10.1681/ASN.2006080894. doi:10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW, Wang W, Poole B, et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda N, Hishida A, Ikuma K, et al. Acquired resistance to acute renal failure. Kidney Int. 1987;31:1233–1238. doi: 10.1038/ki.1987.136. doi:10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- 6.Venkatachalam MA, Bernard DB, Donohoe JF, et al. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. doi:10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Meng Q, Wang C, et al. Changes in expression of renal Oat1, Oat3 and Mrp2 in cisplatin-induced acute renal failure after treatment of JBP485 in rats. Toxicol Appl Pharmacol. 2012;264:423–430. doi: 10.1016/j.taap.2012.08.019. doi:10.1016/j.taap.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Zager RA, Baltes LA, Sharma HM, et al. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. doi:10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- 9.Zager RA, Iwata M, Burkhart K, et al. Post-ischemic acute renal failure protects proximal tubular cells from O2 deprivation injury, possibly by inducing uremia. Kidney Int. 1994;45:1760–1768. doi: 10.1038/ki.1994.229. doi:10.1038/ki.1994.229. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning and clinical scenarios. Curr Opin Neurol. 2013;26:1–7. doi: 10.1097/WCO.0b013e32835bf200. doi:10.1097/WCO.0b013e32835bf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43–48. doi: 10.1097/00041552-200201000-00007. doi:10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Koumenis C, Naczki C, Koritzinsky M, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. doi:10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Why SK, Siegel NJ. Heat shock proteins in renal injury and recovery. Curr Opin Nephrol Hypertens. 1998;7:407–412. doi: 10.1097/00041552-199807000-00010. doi:10.1097/00041552-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Gall JM, Bonegio RG, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79:861–870. doi: 10.1038/ki.2010.527. doi:10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. doi:10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76:604–613. doi: 10.1038/ki.2009.224. doi:10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol. 2011;300:F628–F638. doi: 10.1152/ajprenal.00654.2010. doi:10.1152/ajprenal.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury 'stress response'. Am J Physiol. 2012;303:F139–F148. doi: 10.1152/ajprenal.00168.2012. doi:10.1152/ajprenal.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol. 2012;303:F1460–F1472. doi: 10.1152/ajprenal.00426.2012. doi:10.1152/ajprenal.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zager RA, Schimpf BA, Gmur DJ, et al. Phospholipase A2 activity can protect renal tubules from oxygen deprivation injury. Proc Natl Acad Sci USA. 1993;90:8297–8301. doi: 10.1073/pnas.90.17.8297. doi:10.1073/pnas.90.17.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zager RA, Burkhart KM, Conrad DS, et al. Phospholipase A2 induced cytoprotection of proximal tubules: potential determinants, and specificity for ATP depletion injury. J Am Soc Nephrol. 1996;7:64–72. doi: 10.1681/ASN.V7164. [DOI] [PubMed] [Google Scholar]
- 22.Iwata M, Herrington JB, Zager RA. Protein synthesis inhibition induces cytoresistance in cultured proximal tubular (HK-2) cells. Am J Physiol. 1995;268:F1154–F1163. doi: 10.1152/ajprenal.1995.268.6.F1154. [DOI] [PubMed] [Google Scholar]
- 23.Iwata M, Herrington J, Zager RA. Sphingosine: a mediator of acute renal tubular injury and subsequent cytoresistance. Proc Natl Acad Sci USA. 1995;92:8970–8974. doi: 10.1073/pnas.92.19.8970. doi:10.1073/pnas.92.19.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo SK, Bajwa A, Awad AS, et al. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. doi:10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zager RA. Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int. 2000;58:193–205. doi: 10.1046/j.1523-1755.2000.00154.x. doi:10.1046/j.1523-1755.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- 26.Zager RA, Kalhorn T. Changes in free and esterified cholesterol: hallmarks of acute tubular injury and acquired cytoresistance. Am J Pathol. 2000;157:1007–1016. doi: 10.1016/S0002-9440(10)64613-5. doi:10.1016/S0002-9440(10)64613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zager RA, Johnson AC. Renal cortical cholesterol accumulation: an integral component part of the systemic stress response. Kidney Int. 2001;60:2229–2310. doi: 10.1046/j.1523-1755.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 28.Zager RA. P glycoprotein-mediated cholesterol cycling: a potentially important determinant of proximal tubular cell viability. Kidney Int. 2001;60:944–956. doi: 10.1046/j.1523-1755.2001.060003944.x. doi:10.1046/j.1523-1755.2001.060003944.x. [DOI] [PubMed] [Google Scholar]
- 29.Zager RA, Shah VO, Shah HV, et al. The mevalonate pathway during acute tubular injury: selected determinants and consequences. Am J Pathol. 2002;161:681–692. doi: 10.1016/S0002-9440(10)64224-1. doi:10.1016/S0002-9440(10)64224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li HY, Appelbaum FR, Willman CL, et al. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. doi:10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- 31.Banker DE, Mayer SB, Li HL, et al. Cholesterol synthesis and low density lipoprotein import contribute to protective cholesterol increments in AML cells. Blood. 2004;104:1816–1824. doi: 10.1182/blood-2004-01-0395. doi:10.1182/blood-2004-01-0395. [DOI] [PubMed] [Google Scholar]
- 32.Naito M, Bomstzyk K, Zager RA. Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol. 2009;174:54–62. doi: 10.2353/ajpath.2009.080602. doi:10.2353/ajpath.2009.080602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zager RA, Johnson ACM, Hanson SY, et al. Acute tubular injury causes dysregulation of cellular cholesterol transport proteins. Am J Pathol. 2003;163:313–320. doi: 10.1016/S0002-9440(10)63655-3. doi:10.1016/S0002-9440(10)63655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zager RA, Burkhart KM, Johnson A, et al. Increased proximal tubular cholesterol content: implications for cell injury and the emergence of ‘acquired cytoresistance. Kidney Int. 1999;56:1788–1797. doi: 10.1046/j.1523-1755.1999.00745.x. doi:10.1046/j.1523-1755.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Zager RA, Baltes LA. Progressive renal insufficiency induces increasing protection against ischemic acute renal failure. J Lab Clin Med. 1984;103:511–523. [PubMed] [Google Scholar]
- 36.Zager RA, Johnson AC, Becker K. Post ischemic azotemia as a partial ‘brake’, slowing progressive kidney disease. Nephrol Dialysis Transplant. doi: 10.1093/ndt/gft040. 2013; epub 23543590 (hard copy not yet printed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zager RA, Johnson AC, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol. 2009;297:F961–F970. doi: 10.1152/ajprenal.00381.2009. doi:10.1152/ajprenal.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zager RA. Uremia induces proximal tubular cytoresistance and heme oxygenase-1 expression in the absence of acute kidney injury. Am J Physiol. 2009;296:F362–F368. doi: 10.1152/ajprenal.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zager RA. Acquired cytoresistance in the setting of hematopoietic cell transplantation. Clin J Am Soc Nephrol. 2010;5:2150–2153. doi: 10.2215/CJN.07780910. doi:10.2215/CJN.07780910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zager RA, Prior RB. Gentamicin and Gram-negative bacteremia: a synergism for the development of experimental nephrotoxic acute renal failure. J Clin Invest. 1986;78:196–204. doi: 10.1172/JCI112552. doi:10.1172/JCI112552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodrow G, Turney JH. Cause of death in acute renal failure. Nephrol Dial Transplant. 1992;7:230–234. doi: 10.1093/oxfordjournals.ndt.a092111. [DOI] [PubMed] [Google Scholar]
- 42.Zager RA, Johnson ACM, Hanson SY, et al. Ischemic proximal tubular injury primes mice to endotoxin induced TNF-α generation and release. Am J Physiol. 2005;289:F289–F297. doi: 10.1152/ajprenal.00023.2005. doi:10.1152/ajprenal.00023.2005. [DOI] [PubMed] [Google Scholar]
- 43.Zager RA, Johnson AC, Hanson SY, et al. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin—driven cytokine/chemokine production. Kidney Int. 2006;69:1181–1188. doi: 10.1038/sj.ki.5000022. doi:10.1038/sj.ki.5000022. [DOI] [PubMed] [Google Scholar]
- 44.Zager RA, Johnson ACM, Lund S, et al. Acute renal failure: determinants and characteristics of the injury induced hyper-inflammatory response. Am J Physiol. 2006;291:F546–F556. doi: 10.1152/ajprenal.00072.2006. doi:10.1152/ajprenal.00072.2006. [DOI] [PubMed] [Google Scholar]
- 45.Zager RA, Johnson ACM, Lund S, et al. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol. 2007;292:F304–F312. doi: 10.1152/ajprenal.00237.2006. [DOI] [PubMed] [Google Scholar]
- 46.Naito M, Bomsztyk K, Zager RA. Endotoxin mediated RNA polymerase II recruitment to target genes in acute renal failure. J Am Soc Nephrol. 2008;19:1321–1330. doi: 10.1681/ASN.2007121368. doi:10.1681/ASN.2007121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naito M, Zager RA, Bomsztyk K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J Am Soc Nephrol. 2009;20:1787–1796. doi: 10.1681/ASN.2009010118. doi:10.1681/ASN.2009010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munshi R, Johnson AC, Siew E, et al. Monocyte chemoattractant protein 1 gene activation as a marker of acute kidney injury. J Am Soc Nephrol. 2011;22:165–175. doi: 10.1681/ASN.2010060641. doi:10.1681/ASN.2010060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wald R, Quinn RR, Luo J, et al. University of Toronto Acute Kidney Injury Research Group. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. doi:10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 50.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. doi:10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. doi:10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zager RA, Johnson ACM, Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and end stage kidney disease. Am J Physiol. 2011;301:F1334–F1345. doi: 10.1152/ajprenal.00431.2011. doi:10.1152/ajprenal.00431.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zager RA, Johnson ACM, Andress D, et al. Progressive activation of the endothelin 1 gene following experimental ischemic-reperfusion injury: role in the initiation of chronice/‘end stage’ kidney disease. Kidney Int. in press. [Google Scholar]
- 54.Zager RA, Johnson AC. Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury. Am J Physiol. 2009;298:F827–837. doi: 10.1152/ajprenal.00683.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]