Abstract
Background
In hemodialysis patients, higher serum creatinine (Cr) concentration represents larger muscle mass and predicts greater survival. However, this association remains uncertain in peritoneal dialysis (PD) patients.
Methods
In a cohort of 10 896 PD patients enrolled from 1 July 2001 to 30 June 2006, the association of baseline serum Cr level and change during the first 3 months after enrollment with all-cause mortality was examined.
Results
The cohort mean ± SD age was 55 ± 15 years old and included 52% women, 24% African-Americans and 48% diabetics. Compared with patients with serum Cr levels of 8.0–9.9 mg/dL, patients with serum Cr levels of <4.0 mg/dL and 4.0–5.9 mg/dL had higher risks of death {HR 1.36 [95% confidence interval (95% CI) 1.19–1.55] and 1.19 (1.08–1.31), respectively} whereas patients with serum Cr levels of 10.0–11.9 mg/dL, 12.0–13.9 mg/dL and ≥14.0 mg/dL had lower risks of death (HR 0.88 [95% CI 0.79–0.97], 0.71 [0.62–0.81] and 0.64 [0.55–0.75], respectively) in the fully adjusted model. Decrease in serum Cr level over 1.0 mg/dL during the 3 months predicted an increased risk of death additionally. The serum Cr–mortality association was robust in patients with PD treatment duration of ≥12 months, but was not observed in those with PD duration of <3 months.
Conclusions
Muscle mass reflected in serum Cr level may be associated with survival even in PD patients. However, the serum Cr–mortality association is attenuated in the early period of PD treatment, suggesting competing effect of muscle mass versus residual renal function on mortality.
Keywords: creatinine, malnutrition, mortality, muscle, peritoneal dialysis
INTRODUCTION
Serum creatinine (Cr) is typically used to estimate glomerular filtration rate (GFR) during a steady state of renal function. However, serum Cr originates from creatine, 95% of which is located in muscle [1, 2]. Although influenced by both muscle mass and GFR, serum Cr level may be used as a surrogate of muscle mass in end-stage renal disease (ESRD) patients among whom there is a balanced distribution of low GFRs [3–10]. In hemodialysis (HD) patients, increased serum Cr level has been associated with greater survival, whereas lower serum Cr level has been associated with increased mortality [10–13]. These findings suggest that low serum Cr level as a proxy of low muscle mass and protein-energy wasting (PEW) may be associated with adverse outcomes in HD patients [6, 14–17].
However, in studies of peritoneal dialysis (PD) patients, there have been variable associations between serum Cr level and mortality [18–20]. One reason for this may be that, compared with HD patients, PD patients have greater preservation of residual GFR [21–24] which is independently associated with mortality [25–27] as well as serum Cr level [28, 29]. In PD patients, total Cr clearance (peritoneal dialytic + renal Cr clearance) may be a greater determinant of serum Cr level than muscle mass and, in this scenario, higher serum Cr level (i.e. a proxy of lower Cr clearance) would relate to increased mortality. In contrast, muscle mass may have a greater influence on serum Cr level and, in this scenario, higher serum Cr level (i.e. proxy of larger muscle mass) would relate to greater survival. In a recent study of PD patients, higher estimated GFR by the Modification of Diet in Renal Disease (MDRD) equation [30, 31] was associated with increased mortality, but measured GFR was not related to mortality [32]. These data suggest that, in PD patients, serum Cr level may in fact be a surrogate of muscle mass, and that low muscle mass is associated with worse outcomes in this context.
We thus hypothesized that elevated serum Cr level as a proxy of large muscle mass may be associated with better outcomes in PD patients. In a large and contemporary cohort of PD patients, we examined the association of baseline serum Cr level and change in serum Cr level during the first 3 months after enrollment with all-cause mortality. In addition, we examined effect modification of PD treatment duration on the serum Cr–mortality association by conducting stratified analyses.
MATERIALS AND METHODS
Patients
We retrospectively examined data from all patients receiving PD treatment from 1 July 2001 to 30 June 2006 (i.e. for 20 consecutive calendar quarters) in a large US dialysis organization, DaVita, Inc. As the dialysis population is a dynamic cohort with a high turnover rate, a non-concurrent cohort was created to include all existing maintenance dialysis patients from the first quarter (q1) and all new patients from subsequent quarters (q2 through q20), which has been described in previous studies [33, 34]. The first (baseline) quarter for each patient was the calendar quarter in which the patient's dialysis duration was >90 days. Dialysis modality (PD versus HD) was ascertained according to the type of treatment patients were receiving at the time of entry into cohort. The follow-up time began on the date of entry into the cohort. Patients were censored at the time of death, renal transplantation, departure from DaVita facilities or end of the study period (30 June 2007). The study was approved by the Harbor-UCLA Medical Center Institutional Review Board with exemption of the requirement for a written consent form.
Demographic and clinical measures
Data from the DaVita database was merged with data from the US Renal Data System (USRDS). Information regarding the date of the first dialysis treatment, race/ethnicity, marital status, insurance and comorbidities was obtained from the USRDS. Race/ethnicity was reported as one of the three mutually exclusive categories: non-Hispanic whites, non-Hispanic African-Americans and Hispanics. In this report, the former two groups are referred to Caucasians and African-Americans, respectively. The following 10 baseline comorbidities were considered: diabetes mellitus, hypertension, ischemic heart disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, malignancy, non-ambulatory state and current smoking. The presence of diabetes mellitus was ascertained using data from the DaVita database. Dialysis duration was defined as the time between the first day of dialysis treatment and the first day that the patient entered the cohort. Body weight averaged over 13 weeks during the first (baseline) quarter was used to calculate body mass index (BMI).
Laboratory measures
All laboratory values were typically measured on a monthly basis by automated and standardized methods. Blood samples were transported to the central laboratory center (DaVita Laboratory, Deland, FL) within 24 h. To minimize effects of short-term variations, all repeated measures for each patient during the first quarter were averaged, and the summary estimates were used in all models. Change in serum Cr level (ΔCr) was calculated as the mean serum Cr during the second quarter minus the mean serum Cr during the first quarter.
Statistical analysis
Cox proportional hazards models were used to determine the relationship of baseline serum Cr level (mean serum Cr concentration during the first quarter) and ΔCr level with all-cause mortality. We divided serum Cr levels into seven categories (<4.0, 4.0–5.9, 6.0–7.9, 8.0–9.9, 10.0–11.9, 12.0–13.9, ≥14.0 mg/dL) a priori. ΔCr levels were divided into <−1.0, −1.0 to 1.0 and >1.0 mg/dL. For the stratified analyses, PD duration was divided into <3, 3 to <12 and ≥12 months.
For each analysis, three levels of multivariable adjustment were examined: (i) unadjusted models that included the main predictor and the entry calendar quarter (q1 through q20); (ii) case-mix models that included age, sex, diabetes mellitus, race/ethnicity, comorbidities, primary insurance and marital status; (iii) models adjusted for case-mix and malnutrition inflammation cachexia syndrome (MICS) covariates, which included all of the covariates in the case-mix model as well as the following: BMI; serum levels of albumin, calcium, phosphorus, bicarbonate, total iron-binding capacity and ferritin; peripheral white blood cell count, lymphocyte percentage; hemoglobin concentration. The models using ΔCr as the main predictor were additionally adjusted for the baseline serum Cr level. The proportional hazards assumption was assessed by log–log plots and Schoenfeld residuals after model fitting.
Data for age, sex, race, PD duration and diabetes were missing for <1% of the cohort. Data for comorbidities, insurance and marital status had 7.5, 11.5 and 22.1% missing values, respectively. Data for BMI, total iron binding capacity, serum ferritin, peripheral white blood cell count and lymphocyte percentage had 9.3, 1.4, 2.7, 3.1 and 8.8% missing values. The remaining variables had <1% missing values. The missing values were handled using the following approaches: for categorical variables, a missing indicator was created; for continuous variables, the mean or median of existing values was imputed by serum Cr categories. All analyses were carried out with SAS, version 9.3 (SAS Institute, Cary, NC) and STATA, version 12.1 (Stata Corporation, College Station, TX).
RESULTS
Patient characteristics
During the overall cohort period, 12 734 patients received PD treatment. Among them, patients with missing baseline serum Cr data (n = 1480) and incomplete follow-up information (n = 358) were excluded, resulting in 10 896 PD patients in the final study cohort. Comparison of demographics between included and excluded patients demonstrated no meaningful differences. Baseline characteristics of the 10 896 PD patients stratified by baseline serum Cr levels are listed in Table 1. Patients with higher serum Cr levels tended to be younger, male, African-American, non-diabetic, and had longer dialysis duration. In patients with lower serum Cr levels, cardiovascular comorbidities, including ischemic heart disease, congestive heart failure, cerebrovascular disease and peripheral vascular disease, were more frequent. Serum albumin and phosphorus levels were positively correlated with serum Cr levels (r = 0.31, P < 0.001 and r = 0.51, P < 0.001). Lower hemoglobin and higher ferritin levels were observed with higher serum Cr levels. Lower peripheral white blood cell counts and higher lymphocyte percentages were observed with higher serum Cr levels.
Table 1.
Baseline characteristics according to serum creatinine level (n = 10 896)
| Variables | Serum creatinine (mg/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| <4.0 | 4.0–5.9 | 6.0–7.9 | 8.0–9.9 | 10.0–11.9 | 12.0–13.9 | ≥14.0 | P-value | |
| N | 783 | 2143 | 2414 | 2103 | 1524 | 965 | 964 | |
| Age (years) | 63 ± 14 | 61 ± 14 | 58 ± 15 | 56 ± 15 | 52 ± 15 | 48 ± 14 | 43 ± 14 | <0.001 |
| Gender (% females) | 56 | 53 | 49 | 50 | 45 | 38 | 28 | <0.001 |
| Race (%) | ||||||||
| Caucasian | 65 | 64 | 59 | 52 | 46 | 35 | 21 | <0.001 |
| African-American | 10 | 13 | 18 | 23 | 30 | 39 | 54 | <0.001 |
| Hispanic | 15 | 13 | 13 | 13 | 14 | 14 | 15 | 0.30 |
| Asian | 4 | 4 | 4 | 5 | 4 | 5 | 4 | 0.25 |
| Insurance | ||||||||
| Medicare | 69 | 62 | 62 | 68 | 69 | 68 | 76 | <0.001 |
| Medicaid | 3 | 3 | 3 | 3 | 4 | 5 | 5 | <0.001 |
| Other | 28 | 35 | 35 | 29 | 27 | 26 | 19 | <0.001 |
| Marital status (%) | ||||||||
| Married | 61 | 61 | 60 | 59 | 54 | 50 | 48 | <0.001 |
| Divorced | 6 | 7 | 7 | 10 | 10 | 9 | 8 | 0.001 |
| Single | 17 | 20 | 23 | 23 | 30 | 37 | 42 | <0.001 |
| Widowed | 16 | 12 | 10 | 8 | 5 | 4 | 2 | <0.001 |
| Comorbidity (%) | ||||||||
| PD duration (months) | 2 (1–3) | 2 (1–4) | 3 (1–21) | 16 (2–48) | 23 (3–55) | 28 (8–61) | 34 (13–64) | <0.001 |
| DM | 68 | 61 | 54 | 47 | 39 | 30 | 21 | <0.001 |
| HTN | 77 | 81 | 80 | 78 | 76 | 74 | 78 | <0.001 |
| IHD | 28 | 22 | 17 | 13 | 11 | 6 | 3 | <0.001 |
| CHF | 32 | 20 | 16 | 15 | 11 | 10 | 6 | <0.001 |
| CBVD | 9 | 7 | 6 | 4 | 3 | 3 | 1 | <0.001 |
| PVD | 15 | 12 | 8 | 7 | 4 | 3 | 2 | <0.001 |
| COPD | 8 | 4 | 4 | 3 | 2 | 1 | 1 | <0.001 |
| Non-ambulatory | 3 | 1 | 1 | 0 | 0 | 0 | 0 | <0.001 |
| Malignancy | 6 | 5 | 4 | 3 | 3 | 2 | 1 | <0.001 |
| Current smoker | 6 | 6 | 5 | 6 | 5 | 5 | 4 | 0.79 |
| BMI (kg/m2) | 26.1 ± 6.1 | 26.7 ± 6.3 | 26.5 ± 6.0 | 26.3 ± 6.8 | 25.9 ± 7.1 | 25.5 ± 7.5 | 25.8 ± 7.4 | <0.001 |
| Laboratory values | ||||||||
| Hemoglobin (g/dL) | 12.5 ± 1.5 | 12.2 ± 1.5 | 12.1 ± 1.5 | 11.9 ± 1.5 | 11.9 ± 1.5 | 11.8 ± 1.5 | 11.6 ± 1.6 | 0.01 |
| Albumin (g/dL) | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.9 ± 0.4 | <0.001 |
| TIBC (mg/dL) | 243 ± 60 | 239 ± 57 | 233 ± 54 | 223 ± 52 | 222 ± 51 | 222 ± 50 | 223 ± 49 | <0.001 |
| Ferritin (ng/mL) | 188 (89–374) | 209 (101–414) | 240 (106–529) | 338 (150–691) | 355 (142–644) | 400 (178–696) | 351 (161–669) | <0.001 |
| Ca (mg/dL) | 9.1 ± 0.6 | 9.1 ± 0.7 | 9.2 ± 0.8 | 9.3 ± 0.8 | 9.3 ± 0.8 | 9.3 ± 0.9 | 9.3 ± 1.0 | <0.001 |
| Phosphorus (mg/dL) | 4.0 ± 0.8 | 4.6 ± 1.0 | 5.1 ± 1.2 | 5.6 ± 1.3 | 6.1 ± 1.6 | 6.4 ± 1.7 | 6.8 ± 1.7 | <0.001 |
| Total CO2 (mEq/L) | 26 ± 3 | 25 ± 3 | 25 ± 3 | 24 ± 3 | 23 ± 3 | 23 ± 3 | 23 ± 3 | <0.001 |
| WBC (×103/mm3) | 7.7 ± 2.7 | 7.6 ± 2.7 | 7.7 ± 2.9 | 7.6 ± 2.4 | 7.5 ± 2.4 | 7.3 ± 2.3 | 6.9 ± 2.2 | <0.001 |
| Lymphocyte (%) | 19 ± 8.3 | 19 ± 7.8 | 19 ± 7.7 | 19 ± 7.8 | 20 ± 8.0 | 21 ± 8.5 | 22 ± 8.5 | <0.001 |
Categorical variables are reported as percentages; continuous variables are reported as means ± standard deviation or medians (interquartile range). Dialysis duration was defined as the time between the first day of dialysis treatment and the first day that the patient entered the cohort. P-values were estimated by χ2 test and one-way ANOVA, according to data type. Conversion factors for units: albumin and hemoglobin in g/dL to g/L, ×10; creatinine in mg/dL to μmol/L, ×88.4; calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229. No conversion necessary for ferritin in ng/mL and μg/L, and WBC count in 103/μL and 109/L.
DM, diabetes mellitus; HTN, hypertension; IHD, ischemic heart disease; CHF, congestive heart failure; CBVD, cerebrovascular disease; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; BMI, body mass index; TIBC, total iron-binding capacity; WBC, white blood cells.
Baseline serum Cr level and all-cause mortality
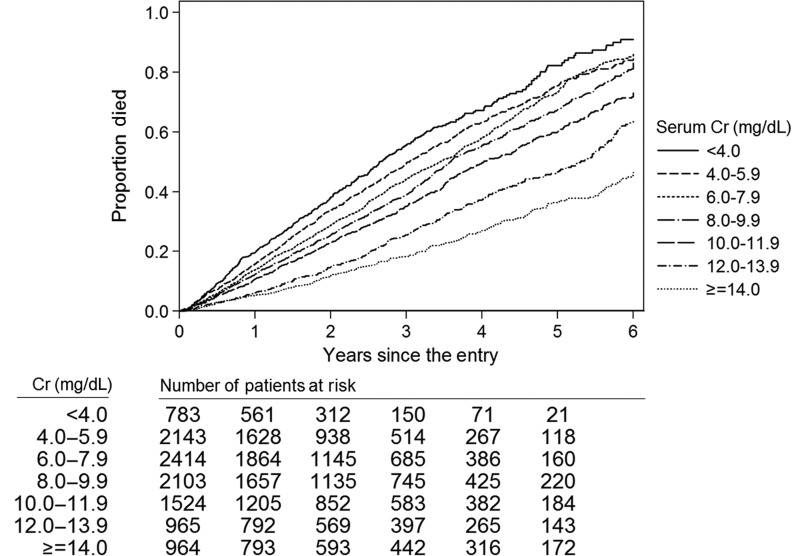
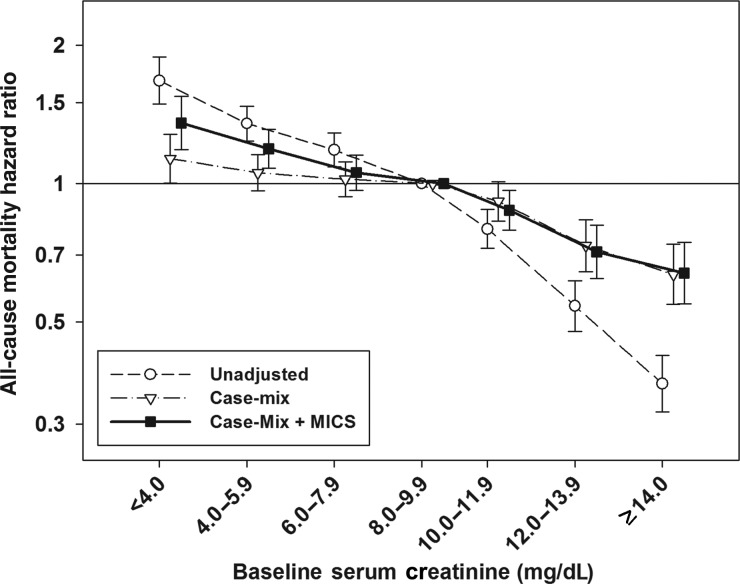
The mean ± SD duration of follow-up was 2.4 ± 1.6 years during which time a total of 4833 (44%) all-cause deaths were reported {crude mortality rate, 184 deaths [95% confidence interval (95% CI) 179–189 deaths] per 1000 person-years}. Kaplan–Meier curves showed a stepwise decline in deaths with higher serum Cr categories (Figure 1). Compared with patients with serum Cr levels of 8.0–9.9 mg/dL (reference group), decreasing serum Cr levels below this threshold were associated with incrementally higher unadjusted hazard ratios (HR) for all-cause mortality (Table 2). Adjustment for case-mix covariates attenuated the mortality predictability of lower serum Cr levels. However, in fully adjusted (case-mix and MICS) models, serum Cr levels of 4.0–5.9 and <4.0 mg/dL were associated with greater mortality: adjusted HRs 1.19 (95% CI 1.08–1.31) and 1.36 (95% CI 1.19–1.55), respectively. In contrast, higher serum Cr levels ≥10.0 mg/dL were associated with greater survival when compared with the reference group. Both case-mix and fully adjusted models demonstrated similar HRs with higher serum Cr levels. In fully adjusted models, patients with serum Cr levels of 10.0–11.9, 12.0–13.9 and ≥14.0 mg/dL showed greater survival: adjusted HRs 0.88 (95% CI 0.79–0.97), 0.71 (95% CI 0.62–0.81) and 0.64 (95% CI 0.55–0.75), respectively (Figure 2).
FIGURE 1:
Kaplan–Meier curves for all-cause mortality according to baseline serum creatinine categories in 10 896 PD patients.
Table 2.
Association between baseline serum creatinine level and all-cause mortality (n = 10 896)
| Cr (mg/dL) | N | Death (%) | Unadjusted HR (95% CI) | Case-mix adjusted HR (95% CI) | Case-mix and MICS adjusted HR (95% CI) |
|---|---|---|---|---|---|
| <4.0 | 783 | 397 (51) | 1.68 (1.49–1.89) | 1.13 (1.00–1.28) | 1.36 (1.19–1.55) |
| 4.0–5.9 | 2143 | 1043 (49) | 1.35 (1.24–1.48) | 1.06 (0.97–1.16) | 1.19 (1.08–1.31) |
| 6.0–7.9 | 2414 | 1146 (48) | 1.18 (1.09–1.29) | 1.02 (0.94–1.11) | 1.06 (0.97–1.15) |
| 8.0–9.9 | 2103 | 1029 (49) | Ref. | Ref. | Ref. |
| 10.0–11.9 | 1524 | 665 (44) | 0.80 (0.72–0.88) | 0.92 (0.83–1.01) | 0.88 (0.79–0.97) |
| 12.0–13.9 | 965 | 314 (33) | 0.54 (0.48–0.62) | 0.73 (0.64–0.83) | 0.71 (0.62–0.81) |
| ≥14.0 | 964 | 239 (25) | 0.37 (0.32–0.42) | 0.63 (0.55–0.74) | 0.64 (0.55–0.75) |
Cr, creatinine; MICS, malnutrition-inflammation cachexia syndrome; HR, hazard ratio; CI, confidence interval.
FIGURE 2:
Association between baseline serum creatinine level and all-cause mortality in 10 896 PD patients. Note: case-mix models were adjusted for age, sex, diabetes mellitus, race, comorbidities, primary insurance and marital status. Case-mix and MICS models were adjusted for all of the covariates in the case-mix model and BMI, serum albumin, total iron-binding capacity, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count, percentage of lymphocyte and hemoglobin concentration. MICS, malnutrition-inflammation cachexia syndrome.
Effect of PD duration on the association between serum Cr level and mortality
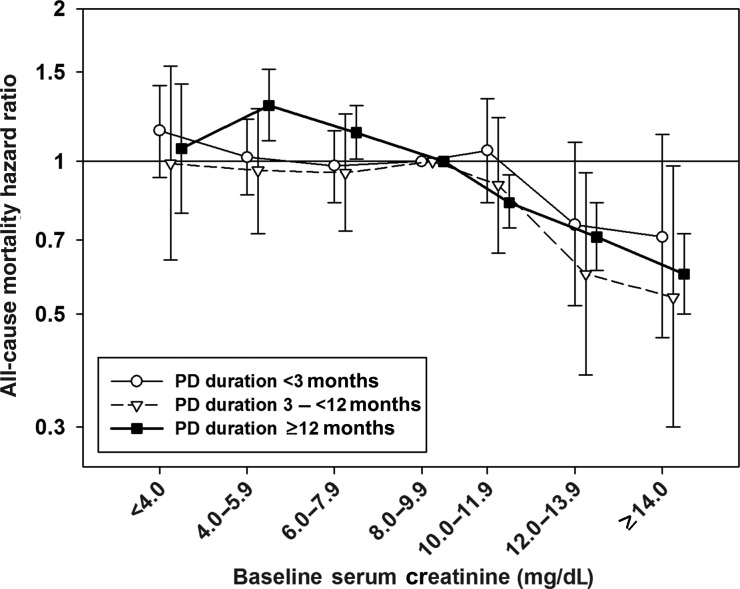
The patients were divided by PD duration of <3, 3 to <12 and ≥12 months. Adjusted HRs from fully adjusted models according to serum Cr level and PD duration are presented in Table 3. No association between serum Cr level and mortality was observed in patients with PD duration of <3 months. In patients with PD duration of 3 to <12 months, serum Cr levels of 12.0–13.9 and ≥14.0 mg/dL were associated with a lower mortality risk when compared with serum Cr levels of 8.0–9.9 mg/dL. In patients with PD duration of ≥12 months, there was an overall inverse association between serum Cr level and mortality except in the lowest serum Cr category (<4.0 mg/dL), in which serum Cr levels of 4.0–7.9 mg/dL were associated with higher mortality, whereas higher serum Cr levels >10 mg/dL were associated with a greater survival (Figure 3).
Table 3.
Fully adjusted HRs for all-cause mortality by serum creatinine level and peritoneal dialysis duration (n = 10 896)
| Cr (mg/dL) | <3 months (n = 4903) |
3-<12 months (n = 1217) |
≥12 months (n = 4776) |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| <4.0 | 1.15 | 0.93–1.41 | 0.99 | 0.64–1.54 | 1.06 | 0.79–1.42 |
| 4.0–5.9 | 1.02 | 0.86–1.21 | 0.96 | 0.72–1.27 | 1.29 | 1.10–1.52 |
| 6.0–7.9 | 0.98 | 0.83–1.15 | 0.95 | 0.73–1.24 | 1.14 | 1.01–1.29 |
| 8.0–9.9 | Ref. | Ref. | Ref. | |||
| 10.0–11.9 | 1.05 | 0.83–1.33 | 0.90 | 0.66–1.22 | 0.83 | 0.74–0.94 |
| 12.0–13.9 | 0.75 | 0.52–1.09 | 0.60 | 0.38–0.95 | 0.71 | 0.61–0.83 |
| ≥14.0 | 0.71 | 0.45–1.13 | 0.54 | 0.30–0.98 | 0.60 | 0.50–0.72 |
HR, hazard ratio; CI, confidence interval.
FIGURE 3:
Mortality predictability of serum creatinine levels according to peritoneal dialysis duration. The models were adjusted for age, sex, diabetes mellitus, race, comorbidities, primary insurance, marital status, BMI, serum albumin, total iron-binding capacity, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count, percentage of lymphocyte and hemoglobin concentration.
Change in serum Cr level and all-cause mortality
Among 10 896 PD patients, 247 died during the first quarter and 1668 additional patients did not have a serum Cr level in the second quarter, resulting in 8981 patients for this secondary analysis. During the first 3 months after the entry, serum Cr level was stable in 5835 (65%) patients [ΔCr −1.0 to1.0 mg/dL], increased in 2138 (24%) patients [ΔCr >1.0 mg/dL] and decreased in 1008 (11%) patients [ΔCr <−1.0 mg/dL]. Compared with patients with ΔCr of −1.0 to1.0 mg/dL, patients with ΔCr of <−1.0 mg/dL demonstrated higher mortality in the fully adjusted model: adjusted HR 1.16 (95% CI) 1.05–1.28. However, ΔCr >1.0 mg/dL was not associated with a survival benefit (Table 4).
Table 4.
Association between change in serum creatinine (ΔCr) during the first 3 months after study entry and mortality (n = 8981)
| ΔCr (mg/dL) | N | Death (%) | Unadjusted | Case-mix adjusted | Case-mix and MICS adjusted |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| <(−1.0) | 1008 | 496 (49) | 1.36 (1.23–1.50) | 1.24 (1.12–1.37) | 1.16 (1.05–1.28) |
| (−1.0) −1.0 | 5835 | 2612 (45) | Ref. | Ref. | Ref. |
| >1.0 | 2138 | 888 (42) | 1.03 (0.96–1.12) | 0.97 (0.90–1.05) | 0.98 (0.90–1.06) |
ΔCr was calculated as mean of serum Cr for the second quarter minus mean of serum Cr for the first quarter.
MICS, malnutrition-inflammation cahexia syndrome; HR, hazard ratio; CI, confidence interval.
DISCUSSION
In this large and contemporary cohort of 10 896 PD patients, we observed that higher baseline serum Cr levels were associated with greater overall survival. However, this association was modified by PD duration, in which a significant association was observed in patients with PD duration >12 months, but not in those with shorter PD duration. A decline in serum Cr level within the first 3 months of study entry was associated with increased mortality independent of baseline serum Cr level.
PEW is frequently observed in dialysis patients and has been associated with adverse outcomes [14–17]. Because serum Cr is derived from skeletal muscle, it may serve as a biomarker of somatic body protein particularly in ESRD patients with very low GFRs and stable daily protein intake and dialysis dose [3–10]. Several studies in HD patients have demonstrated a linear association between higher serum Cr level with survival [11–13, 35]. This suggests that serum Cr level reflects muscle mass and that low muscle mass resulting from PEW is associated with poor outcomes in HD patients. In addition, some studies evaluating a survival benefit of early start of dialysis showed that a higher estimated GFR based on serum Cr level at dialysis initiation was associated with poorer survival, which could not be fully abolished by adjusting for known confounders such as age, sex, race, comorbidities, hemoglobin and serum albumin level [36–38]. This phenomenon is also likely to be caused by patients with a low muscle mass leading to a low plasma Cr and thus a high estimated GFR [32, 39]. Our results showed that the association between higher serum Cr level with greater survival is still observed in a large PD cohort. A previous study of 140 PD patients demonstrated that age, diabetes, low serum albumin level and low serum Cr level were independent predictors of mortality [18]. Additionally, the Canada–USA Peritoneal Dialysis Study Group reported that percent lean body mass determined from Cr kinetics was associated with survival; among 680 patients divided into tertiles of percent lean body mass (>73, 63–73 and <63%), the 2-year survival probabilities were 88.3, 81.2 and 65.2%, respectively [40]. A recent post hoc analysis of data from PD patients enrolled in ADEMEX study demonstrated that every 1 mL/min/1.73 m2 increase in estimated GFR by the four-variable MDRD equation was associated with a 6% increase in risk of death, but there was no association between measured Cr clearance and survival. This study also demonstrated a negative association between estimated GFR and Cr appearance rates [32]. Our results also suggest that muscle mass plays a predominant role in the serum Cr–mortality association in PD patients, supporting findings from earlier studies. Thus, large muscle mass may be beneficial to survival not only in HD patients, but also in PD patients.
Residual GFR may be better preserved and provide greater contribution to total Cr clearance in PD patients compared with HD patients particularly in patients of shorter dialysis duration [21–24]. Given that residual GFR is an important predictor of survival [25–27], the serum Cr–mortality association may be modified by residual GFR. Because information for residual GFR was unavailable in our database, we compared the mortality predictabilities of serum Cr level among subgroups stratified by PD duration as a proxy of residual GFR. In patients with PD duration of <3 months, an association between serum Cr level and mortality was not observed, suggesting that residual GFR may attenuate this association. In the patients with PD duration of ≥12 months, serum Cr level was inversely associated with mortality except among those with serum Cr levels of <4.0 mg/dL. In patients with longer PD duration in whom there is a lower likelihood of residual renal function, there was a robust association between serum Cr level and survival. The absence of an association between low serum Cr level and higher mortality among those with Cr levels <4.0 mg/dL may be related to the small sample size in this subgroup or may have been a reflection of residual GFR preservation in some longer-term PD patients. When employing serum Cr level as a surrogate of muscle mass and prognostic indicator in PD patients, residual GFR and/or dialysis duration should also be considered.
Our results also showed that a decrease in serum Cr level over time was prognostic of increased mortality, independent of baseline serum Cr level. Patients who experienced a 1.0 mg/dL decline in serum Cr level during the first 3 months after study entry had a 16% higher risk of death compared with patients with stable serum Cr levels. Given that residual GFR typically falls with increasing dialysis duration, reductions in serum Cr level more likely reflects loss of muscle mass or poor protein intake, which are consequently associated with increased mortality. However, an increase in serum Cr level did not show a significant association with better survival. It is our opinion that an increase in serum Cr level may be observed with both loss of residual GFR as well as gaining of muscle mass, which may have competing influences on mortality outcome. The association between serum Cr level reductions over time and higher mortality further support the relationship between sarcopenia with mortality in PD patients.
Despite its strength, our study has several limitations which bear mention. First, due to data limitations, we were unable to directly examine residual renal function, peritoneal transport rate, type of PD (continuous ambulatory or automated PD) and delivered dialysis dose (weekly Kt/V or weekly Cr clearance). Although prior evidence has not shown that small-solute clearance within the range achieved in clinical practice has an effect on patient outcomes [41, 42], and a differential association between continuous ambulatory versus automated PD with mortality has not been observed [43–45], we cannot exclude the possibility of bias from lack of data on delivered dialysis dose and type of PD. Second, we did not have information regarding serum C-reactive protein levels, which is independently associated with mortality in PD patients or other inflammatory cytokines [46, 47]. Although we included several other inflammatory surrogate measures, such as total white blood cell count, lymphocyte percentage, serum albumin, total iron-binding capacity and ferritin level, residual confounding cannot be excluded. Third, data on volume status was not available and hence not included in the models. Fourth, although information regarding comorbid conditions was obtained at the time of dialysis initiation and adjusted for in multivariable models, annotation of this information spanned a period of 3–22 months before study entry. Moreover, baseline characteristic data demonstrated that patients with higher serum Cr levels had more favorable comorbid profiles and other nutritional markers than those with lower serum Cr levels, and thus, we cannot exclude residual confounding on the basis of health status. In baseline characteristic, lower hemoglobin and higher serum ferritin levels were observed in patients with higher serum Cr levels. However, it is difficult to explain a reason because data for erythropoiesis stimulating agent and iron dose were not included in this analysis. Last, the muscle mass was not assessed in this study. We did not have estimates of muscle mass such as lean body mass by dual-energy X-ray absorptiometry, bioimpedance analysis or near-infrared interactance,[17, 48] so that could not evaluate an association of the muscle mass and mortality directly. In addition, serum Cr level can be influenced by recent intake of meats. Greater appetite was also reported to associate with better survival in HD patients.[49, 50] It could be a concern that some of the survival benefit associated with higher serum Cr level may be related to the association between better appetite and greater survival in part.
In summary, our findings validate the prognostic value of serum Cr level as a surrogate of muscle mass in a large cohort of PD patients. Higher muscle mass may be associated with greater survival in PD patients as HD patients. However, using serum Cr level to estimate muscle mass should consider residual renal function or at least dialysis duration. Further studies examining efficient strategies for nutritional support are warranted to preserve muscle mass in PD patients.
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research (DCR) for providing the clinical data, analysis and review for this research project.
FUNDING
The study was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R21 DK 077341 (K.K.Z. and R.M.), a philanthropist grant from Harold Simmons (K.K.Z.) and research grants from DaVita Clinical Research (K.K.Z. and R.M.). M.Z.M. is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012). C.M.R. is supported by an NIH/NIDDK grant (F32 DK093201).
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format. R.M. has received grant support and/or honoraria from Baxter Healthcare and DaVita. A.R.N. is employee of DaVita. K.K.Z. was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA, during 2007–12. Other authors have declared that no financial conflict of interest exists.
REFERENCES
- 1.Flugel-Link RM, Salusky IB, Jones MR, et al. Enhanced muscle protein degradation and amino acid release from the hemicorpus of acutely uremic rats. Adv Exp Med Biol. 1984;167:545–555. doi: 10.1007/978-1-4615-9355-3_48. [DOI] [PubMed] [Google Scholar]
- 2.Andrews R, Greenhaff P, Curtis S, et al. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19:617–622. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- 3.Keshaviah PR, Nolph KD, Moore HL, et al. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol. 1994;4:1475–1485. doi: 10.1681/ASN.V471475. [DOI] [PubMed] [Google Scholar]
- 4.Schutte JE, Longhurst JC, Gaffney FA, et al. Total plasma creatinine: an accurate measure of total striated muscle mass. J Appl Physiol. 1981;51:762–766. doi: 10.1152/jappl.1981.51.3.762. [DOI] [PubMed] [Google Scholar]
- 5.Patel SS, Molnar MZ, Tayek JA, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2012;4:19–29. doi: 10.1007/s13539-012-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 7.Noori N, Kovesdy CP, Bross R, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:130–139. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaizu Y, Ohkawa S, Kumagai H. Muscle mass index in haemodialysis patients: a comparison of indices obtained by routine clinical examinations. Nephrol Dial Transplant. 2002;17:442–448. doi: 10.1093/ndt/17.3.442. [DOI] [PubMed] [Google Scholar]
- 9.Moreau-Gaudry X, Guebre-Egziabher F, Jean G, et al. Serum creatinine improves body mass index survival prediction in hemodialysis patients: a 1-year prospective cohort analysis from the ARNOS study. J Ren Nutr. 2011;21:369–375. doi: 10.1053/j.jrn.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Walther CP, Carter CW, Low CL, et al. Interdialytic creatinine change versus predialysis creatinine as indicators of nutritional status in maintenance hemodialysis. Nephrol Dial Transplant. 2012;27:771–776. doi: 10.1093/ndt/gfr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noori N, Kovesdy CP, Dukkipati R, et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33:157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Streja E, Molnar MZ, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175:793–803. doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Norris KC. Is the malnutrition-inflammation complex the secret behind greater survival of African-American dialysis patients? J Am Soc Nephrol. 2011;22:2150–2152. doi: 10.1681/ASN.2011101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Shinaberger CS, Kalantar-Zadeh K. Epidemiology of dietary nutrient intake in ESRD. Semin Dial. 2010;23:353–358. doi: 10.1111/j.1525-139X.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noori N, Kovesdy CP, Dukkipati R, et al. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. Am J Clin Nutr. 2010;92:1060–1070. doi: 10.3945/ajcn.2010.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avram MM, Mittman N, Bonomini L, et al. Markers for survival in dialysis: a seven-year prospective study. Am J Kidney Dis. 1995;26:209–219. doi: 10.1016/0272-6386(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 19.Avram MM, Fein PA, Bonomini L, et al. Predictors of survival in continuous ambulatory peritoneal dialysis patients: a five-year prospective study. Perit Dial Int. 1996;16(Suppl 1):S190–S194. [PubMed] [Google Scholar]
- 20.Jager KJ, Merkus MP, Dekker FW, et al. Mortality and technique failure in patients starting chronic peritoneal dialysis: results of the Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int. 1999;55:1476–1485. doi: 10.1046/j.1523-1755.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 21.Lysaght MJ, Vonesh EF, Gotch F, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37:598–604. [PubMed] [Google Scholar]
- 22.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 23.Misra M, Vonesh E, Van Stone JC, et al. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001;59:754–763. doi: 10.1046/j.1523-1755.2001.059002754.x. [DOI] [PubMed] [Google Scholar]
- 24.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 25.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 26.Merkus MP, Jager KJ, Dekker FW, et al. Predictors of poor outcome in chronic dialysis patients: the Netherlands Cooperative Study on the Adequacy of Dialysis. The NECOSAD Study Group. Am J Kidney Dis. 2000;35:69–79. doi: 10.1016/s0272-6386(00)70304-0. [DOI] [PubMed] [Google Scholar]
- 27.Szeto CC, Lai KN, Wong TY, et al. Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1999;34:1056–1064. doi: 10.1016/S0272-6386(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 28.Lameire NH. The impact of residual renal function on the adequacy of peritoneal dialysis. Contrib Nephrol. 1998;124:76–93. doi: 10.1159/000059932. discussion –. [DOI] [PubMed] [Google Scholar]
- 29.Krediet RT, Douma CE, van Olden RW, et al. Augmenting solute clearance in peritoneal dialysis. Kidney Int. 1998;54:2218–2225. doi: 10.1046/j.1523-1755.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Sloand JA, Leypoldt JK, Culleton BF, et al. Assessing creatinine clearance from modification of diet in renal disease study equations in the ADEMEX cohort: limitations and potential applications. Clin J Am Soc Nephrol. 2011;6:598–604. doi: 10.2215/CJN.04970610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–428. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 36.Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13:2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 37.Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24:3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 38.Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77:700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 39.Grootendorst DC, Michels WM, Richardson JD, et al. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26:1932–1937. doi: 10.1093/ndt/gfq667. [DOI] [PubMed] [Google Scholar]
- 40.McCusker FX, Teehan BP, Thorpe KE, et al. How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney Int Suppl. 1996;56:S56–S61. [PubMed] [Google Scholar]
- 41.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 42.Lo WK, Ho YW, Li CS, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64:649–656. doi: 10.1046/j.1523-1755.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 43.Badve SV, Hawley CM, McDonald SP, et al. Automated and continuous ambulatory peritoneal dialysis have similar outcomes. Kidney Int. 2008;73:480–488. doi: 10.1038/sj.ki.5002705. [DOI] [PubMed] [Google Scholar]
- 44.Mehrotra R, Chiu YW, Kalantar-Zadeh K, et al. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int. 2009;76:97–107. doi: 10.1038/ki.2009.94. [DOI] [PubMed] [Google Scholar]
- 45.Michels WM, Verduijn M, Boeschoten EW, et al. Similar survival on automated peritoneal dialysis and continuous ambulatory peritoneal dialysis in a large prospective cohort. Clin J Am Soc Nephrol. 2009;4:943–949. doi: 10.2215/CJN.04440908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang AY, Woo J, Lam CW, et al. Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol. 2003;14:1871–1879. doi: 10.1097/01.asn.0000070071.57901.b3. [DOI] [PubMed] [Google Scholar]
- 47.Ducloux D, Bresson-Vautrin C, Kribs M, et al. C-reactive protein and cardiovascular disease in peritoneal dialysis patients. Kidney Int. 2002;62:1417–1422. doi: 10.1111/j.1523-1755.2002.kid562.x. [DOI] [PubMed] [Google Scholar]
- 48.Bross R, Chandramohan G, Kovesdy CP, et al. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55:885–896. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 50.Bross R, Noori N, Kovesdy CP, et al. Dietary assessment of individuals with chronic kidney disease. Semin Dial. 2010;23:359–364. doi: 10.1111/j.1525-139X.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]