Abstract
Sodium channel blocker insecticides (SCBIs) are a relatively new class of insecticides, with a mechanism of action different from those of other classes of insecticides that target voltage-gated sodium channels. These compounds have no effect at hyperpolarized membrane potentials, but cause a voltage-dependent, nearly irreversible block as the membrane potential is depolarized. The mechanism of action of SCBIs is similar to that of local anesthetics (LAs), class I anticonvulsants and class I antiarrhythmics. In this article, we review the physiological actions of these compounds on the whole animal, the nervous system and sodium channels, and also present the results from recent studies that elucidate the receptor site of SCBIs.
Introduction
Voltage-gated sodium channels are responsible for the initiation and propagation of action potentials in almost all excitable cells. They are well known as the primary target of DDT, naturally occurring pyrethrins found in extracts of the flowers of Chrysanthemum species, and modern synthetic pyrethroids, which are structural derivatives of pyrethrins [1]. Furthermore, this large, functionally complex channel protein is known to possess at least nine independent target sites for a variety of neurotoxins produced by plants and animals for defense or predation [2, 3], such as tetrodotoxin, α and ß-scorpion toxins, and batrachotoxin. While it has long been known that many therapeutically useful local anesthetic, antiarrhythmic and anticonvulsant drugs block the sodium channel by occluding its pore, no compounds acting by that mechanism showed insecticidal properties, until the discovery of pyrazolines (also known as dihydropyrazoles) [4, 5]. These insecticides, along with the more recently derived indoxacarb and metaflumizone, are collectively called sodium channel blocker insecticides (SCBIs) [6]. Benzhydrolpiperidines, discovered at FMC corporation and chemically unrelated to the pyrazolines, cause similar symptoms to pyrazolines and appear to have the same mode of action [7].
Chemical evolution of SCBIs
The history and development of the SCBIs leading to indoxacarb has recently been reviewed [6, 8, 9]. The first insecticidal pyrazoline sodium channel blockers, represented by PH 60-41, were highly active against coleopteran and lepidopteran pests [10], and the addition of a phenyl ring at the 4-position of the pyrazoline ring, as in PH-60-42, led to even greater activity (Fig. 1) [11], but the compounds had problems with environmental persistence, long-term toxicity and bioaccumulation [12]. The 4-methyl, 4-carbomethoxy pyrazolines, such as RH3421, had high insecticidal efficacy and more rapid environmental degradation [13, 14], but still had a problem with long-term mammalian toxicity [5]. Modifications to the pyrazoline nucleus by chemists at DuPont resulted in the discovery of several classes of related structures, all with similarly high levels of insecticidal activity. Expanding the pyrazoline ring by one oxygen atom and bridging the C-4 position with the C-3 aryl substituent gave highly-active oxadiazine compounds that were optimized to obtain indoxacarb, which controls a wide spectrum of insect pests, including many lepidoptera as well as plant bugs, leafhoppers, fleahoppers, weevils, beetles, flies, cockroaches and ants. Indoxacarb has excellent safety to mammals and other non-target organisms, and obtained a reduced-risk registration in 2000 [6, 8].
Figure 1.
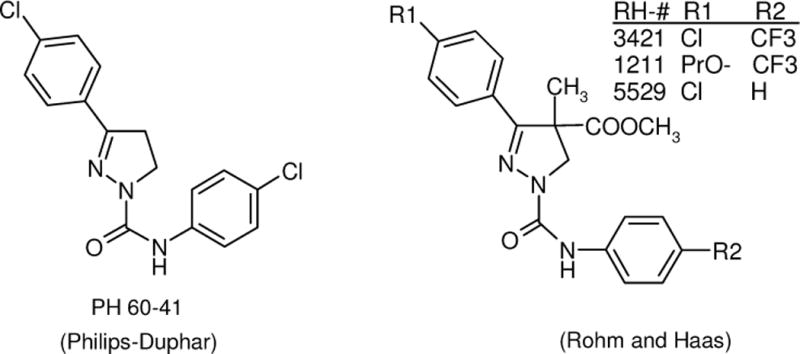
Structures of sodium channel blocker insecticides referred to in this article.
The semicarbazones are ring-opened pyrazolines discovered at Nihon Nohyaku Co., Ltd, by removing the carbon at the 5-position of the 3,4-diphenyl pyrazoline of the type of PH 60-42 [15]. Optimization of the semicarbazones led to metaflumizone, which is being co-developed globally by BASF SE, Fort Dodge Animal Health, a Division of Wyeth Corporation and Nihon Nohyaku Co., Ltd. Metaflumizone provides good to excellent control of most economically important lepidopterous pests and certain pests in the orders Coleoptera, Hemiptera, Hymenoptera, Diptera, Isoptera and Siphonaptera [16]. Metaflumizone provides long-lasting control of fleas on companion animals with a single spot-on application and is being marketed for this use under the trade name Promeris by Fort Dodge Animal Health, a Division of Wyeth. Indoxacarb and metaflumizone are the only SCBIs that have been commercialized to date.
Indoxacarb is a prodrug that requires metabolic activation by insects before it acts as a strong sodium channel blocker. An esterase or amidase cleaves the carbomethoxy group from the urea linkage, liberating the free urea (N-decarbomethoxyllated DPX-MP062 or DCMP) which then acts as the voltage-dependent sodium channel blocker. This was first demonstrated for the racemic compound DPX-JW062 (50:50 mixture of active S and inactive R enantiomers) being converted to the corresponding N-decarbomethoxyllated compound DCJW [17]. However it appears that all of the insecticidal activity resides in the S-enantiomer of DCJW [17], as has been observed for dihydropyrazoles [18].
Block of spontaneous activity in the nervous system
Pyrazolines, indoxacarb and metaflumizone produce identical acute neurotoxic symptoms in insects, characterized by a distinctive pseudoparalysis, so named because poisoned insects appear to be paralyzed, but can move, sometimes violently, when disturbed [4, 6, 19]. Pseudoparalyzed cockroaches produce violent convulsions when disturbed, whereas lepidopteran larvae squirm and produce tremors of legs and mandibles that are evident upon microscopic examination. Higher doses of pyrazolines, especially in lepidopterous larvae, lead within a few hours to flaccid paralysis [4].
Pseudoparalysis is associated with a complete absence of spontaneous activity in the nervous system of insects poisoned by pyrazolines [4], indoxacarb or DCJW [17] or metaflumizone [19]. The complete absence of neural activity in poisoned insects indicates that SCBIs block not only tonic sensory receptors, but also pacemaker activity in the central nervous system. Both of these effects involve action potential generation in regions of neurons that are able to generate action potentials repetitively in response to constant stimuli. The ability of phasic receptors to respond long after paralysis at a high dose suggests that phasic receptors are not as sensitive as the tonic ones [4].
Intracellular recordings from the abdominal slowly adapting stretch receptor neuron of the crayfish showed that pyrazolines increased the threshold for spike generation in response to depolarizing pulses, indicating that voltage-dependent sodium channels, whose activation determines the threshold and initiates the action potential, were blocked by pyrazolines [4]. Similar experiments showed an identical action of metaflumizone on neurons isolated from the CNS of Manduca sexta larvae [19].
State-dependent block of sodium channels by SCBIs
Because axonal action potential conduction appeared to be relatively insensitive to pyrazolines, it was postulated that the compounds block sodium channels in a voltage-dependent manner, and are therefore selective for sodium channels in the spike initiation zone, where the resting potential is near the threshold for action potential generation, in the range of −70 to −50 mV. In contrast, axons rest near −90 mV, where sodium channels spend most of their time in the resting state. When depolarized to the range where sodium channels begin to undergo conformational changes, axons become sensitive to pyrazolines [4]. Similarly, the extracellularly recorded compound action potential from motor nerves of M. sexta abdominal ganglia, which was likewise resistant to DCJW, was rendered sensitive by depolarization of the nerves with a high-K+ saline [17]. Furthermore, Wing et al. (1998) showed that block of M. sexta compound action potentials by DCJW was stereospecific for the S-enantiomer, which is also the only enantiomer toxic to insects [17].
Voltage-clamp studies have confirmed that sodium channels are resistant to pyrazolines at highly negative potentials, but become sensitive when the cell is depolarized into the range where activation and inactivation occur [5, 20–22]. The transient natures of both open and activated states imply that the inactivated states of sodium channels are most likely to be sensitive to SCBIs. There are two partially independent inactivation processes, known as fast and slow inactivation. Fast inactivation occurs on a millisecond time scale and serves to terminate the action potential, whereas slow inactivation occurs on a much slower time scale, over hundreds of milliseconds, and performs a slow, modulatory function. Slow inactivation occurs at more negative potentials than fast inactivation, so that in the steady-state, channels are either resting or slow-inactivated. It appears that pyrazolines bind to the slow-inactivated state and effectively shift the slow inactivation curve to the left (Fig. 2) [5]. Whereas fast inactivation occurs with a time course of hundreds of microseconds to a few milliseconds, and slow inactivation on the order of tens to hundreds of milliseconds, the changes in peak sodium current in the presence of SCBIs occur on a much slower time scale, on the order of 15 minutes. This slow suppression of peak current in response to voltage change can therefore be attributed to binding and unbinding of SCBIs to sodium channels.
Figure 2.
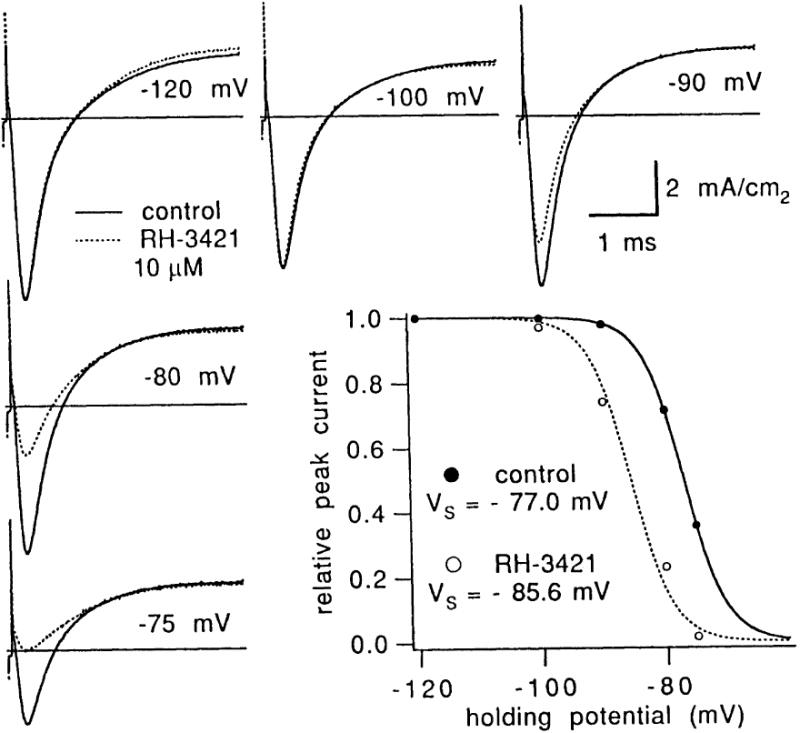
Pyrazoline RH-3421 shifts the steady state slow inactivation curve in the direction of hyperpolarization. Ionic current traces were scaled by a common factor so that the peak at −120 mV matched the peak before treatment. Peak INa was depressed most at depolarized potentials, whereas outward current, Ik, was not affected by the treatment. The graph shows plots of peak current normalized to the value at −120 mV. RH-3421 (10 μM) appears to shift the steady-state inactivation relation to the left by 8.6 mV [5].
When the slow inactivation gate of sodium channels was removed by pretreatment of the internal face of the axon membrane with trypsin, block by the pyrazoline RH-1211 at −90 mV was comparable to that in intact axons, indicating that RH-1211 can also block fast-inactivated channels. Pretreatment of axons with both trypsin to remove slow inactivation and the protein-modifying reagent N-bromoacetamide (NBA) yields sodium channels that do not inactivate. RH-1211 was just as potent at blocking these non-inactivating channels as intact channels, showing that it can also block open channels [5].
Although the SCBIs appear to enhance slow inactivation, it is important to realize that slow inactivation itself is not affected. Instead, slow inactivation appears to be enhanced because of the addition to the system of the highly stable SCBI-bound slow-inactivated state. In the presence of the SCBI, time for this very slow equilibration of the drug with the receptors must be allowed in order to measure slow inactivation properly. It is not enough to simply measure slow inactivation with pulses long enough to attain a steady state in the absence of SCBI. While pulses on the order of 1 second are long enough to attain steady-state slow inactivation in control axons, 15 minutes are required in the presence of insecticide [5].
Recent studies provide evidence for action of SCBIs on more than one sodium channel subtype. The action of DCJW on sodium channels in dorsal unpaired median (DUM) neurons isolated from the CNS of P. americana has been studied with the patch clamp technique [23]. Action potentials in these pacemaking neurons were blocked by 100 nM DCJW, and this action was shown to be due to block of voltage-dependent sodium channels. The block was very potent, with an IC50 of 28 nM at −90 mV, in good agreement with the IC50 of racemic DCJW of 40 nM in blocking the compound action potential in Manduca sexta CNS determined by Wing et al. (1998) [17]. An additional effect on the P. americana DUM neurons observed by Lapied et al. (2001) [23] was a strong hyperpolarization of the resting potential associated with an increase in membrane resistance, indicating block of a depolarizing conductance thought to be the background sodium channels involved in the maintenance of the resting potential [24, 25]. This finding is interesting and should be investigated in more detail, because it demonstrates the existence of sodium channel variants that may have different sensitivities to insecticides. Consistent with this hypothesis, Zhao et al. (2005) identified two types of TTX-sensitive sodium currents in cockroach neurons that exhibited distinct voltage dependencies of inactivation and differential sensitivities to indoxacarb and DCJW [26]. Furthermore, two cockroach sodium channel variants, BgNav1-1 and BgNav1-4, also exhibited different gating properties and differential sensitivities to DCJW [27]. A lysine to glutamic acid change (K1689E) in IVS4 of BgNav1-4 was identified as being responsible for both the enhanced sensitivity of BgNav1-4 to DCJW and large negative shifts in the voltage-dependencies of both the fast and slow inactivation gating of this channel variant.
SCBIs bind within the sodium channel pore
The blocking action of pyrazolines on voltage-dependent sodium channels was recognized as being similar to that of local anesthetics (LAs), class I anticonvulsants and class I antiarrhythmics [5], a structurally broad range of drugs [28, 29] all known to act at a common blocker site within the sodium channel pore. Like the SCBIs, drugs acting at the local anesthetic site all exhibit voltage dependence of block deriving from selective binding to open and inactivated channel states. Biochemical and pharmacological evidence indicates possible overlapping binding sites between these two classes of sodium channel blockers. Local anesthetics displace [3H]-Batrachotoxin-B (BTX) from its binding site in the sodium channel [30, 31]. The interaction is allosteric, because local anesthetics can block BTX-modified open channels without displacing the toxin, [32, 33], although they speed the dissociation of BTX from its binding site. Pyrazolines were also shown to potently displace specific binding of [3H]-BTX-B [5, 34]. Payne et al. (1998) further examined the interaction between the pyrazoline RH-3421 and the local anesthetic dibucaine in BTX binding studies [35]. Each of these compounds decreased the potency of the other as an inhibitor of BTX binding, approximately as much as expected from the assumption that they share a common binding site. Furthermore, like local anesthetics, RH-3421 increased the dissociation rate of [3H]-BTX-B from its binding site [34]. Finally, in voltage-clamp studies in oocytes, phenytoin, an anticonvulsant, decreased the inhibitory activity of DCJW when applied in tandem [20].
Lapied et al. (2001) examined the interactions of DCJW with the local anesthetic lidocaine and the guanidinium blocker tetrodotoxin (TTX), using dorsal unpaired median (DUM) neurons isolated from the CNS of P. Americana [23]. TTX is known to block the pore at a binding site that is distinct from the binding site of the local anesthetics [2, 3, 36]. The IC50 for DCJW was not affected by the presence of an IC50 concentration of TTX in the external solution, consistent with independent action of the two compounds at distinct binding sites. In the presence of an IC50 concentration of lidocaine, however, the IC50 for DCJW was increased about 30-fold. A 2-fold shift in equilibrium binding would be expected from the hypothesis that both compounds act at the same site [37], so the mechanism of the observed 30-fold shift is not fully understood. Nevertheless, available evidence is consistent with the action of the SCBIs at or near the local anesthetic site.
Molecular basis of the binding and action of SCBIs
Recent studies using insect and mammalian sodium channels expressed in the Xenopus oocyte expression system have begun to reveal the sodium channel interactions of SCBIs at the molecular level [38, 39]. Sodium channels consist of four homologous domains (I–IV), each containing six transmembrane segments (S1-S6) connected by extra- and intracellular loops [40, 41]. Site-directed mutagenesis of mammalian sodium channels identified a number of residues in the S6 transmembrane segments of domains I, III, and IV that are critical for the binding and action of a variety of therapeutic sodium channel blockers [42–49]. In particular, two residues in IVS6, F1579 and Y1586 (numbered according to their location in the rat Nav1.4 sodium channel) are key residues in the binding and action of these therapeutic drugs including LAs (Fig. 3) [42, 50]. The notion of potentially overlapping binding sites for LAs and SCBIs on sodium channels prompted an evaluation of the involvement of these two residues in the action of RH3421, indoxacarb, and DCJW in a recent study by Silver and Soderlund (2007) using the Nav1.4 sodium channel expressed in Xenopus oocytes [38]. Alanine substitution at F1579 in Nav1.4 resulted in a significant reduction in the ability of DCJW and RH3421 to inhibit Nav1.4 sodium channels expressed in Xenopus oocytes [38]. In contrast, alanine substitution of the tyrosine residue at position 1586 (Y1586A) in Nav1.4 channels resulted in a significant increase in the potency of indoxacarb, DCJW, and RH3421 [38]. It appears that the phenylalanine residue in IVS6 provides a crucial aromatic-aromatic interaction with the drug/insecticide molecule in mammalian sodium channels, whereas the tyrosine residue, while providing important pi-electron interactions for local anesthetics, appears to interfere with SCBI activity since its removal enhances insecticide activity [38, 51].
Figure 3.
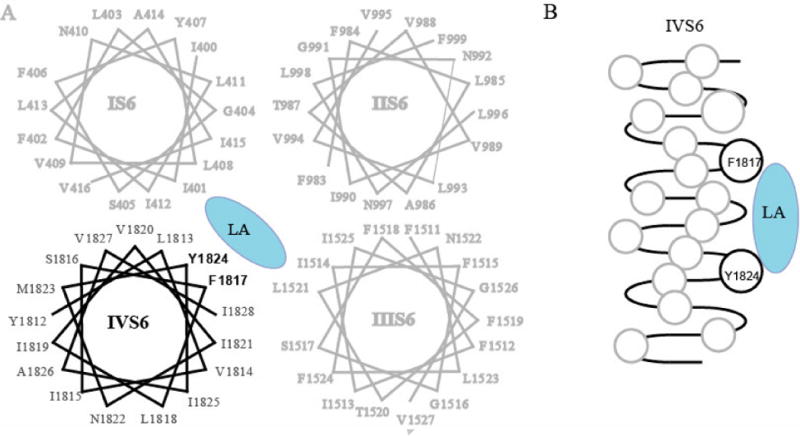
A. Helical wheel diagram showing all four homologous domains of BgNav1-1a highlighting the relationship between the residues of IVS6 and a generic local anesthetic molecule. B. A side view of IVS6 showing the vertical orientation of a local anesthetic molecule to the F1817 and Y1824 residues of IVS6.
To examine the roles of F1817 and Y1824 in IVS6 (homologous to F1579 and Y1586 in rat Nav1.4) in the interactions of insect sodium channels with SCBIs, alanine substitutions of F1817 and Y1824 were introduced into a DCJW-sensitive cockroach sodium channel variant, BgNav1-1A [39]. The effects of alanine substitutions on the action of indoxacarb, DCJW and metaflumizone were examined. The blocking activities of two SCBIs on the wildtype and mutant cockroach sodium channels were determined using two different recording protocols, one assessing the onset of block at a depolarizing potential (close to V0.5 of slow inactivation) and the other determining block of fully-inactivated Na channels. The F1817A substitution had no effect on inhibition by indoxacarb or DCJW using either protocol. Unlike its role in the interaction of Nav1.4 channels with DCJW, the phenylalanine residue (F1817, corresponding to F1579 in Nav1.4) is not important for the action of DCJW on BgNav1-1A sodium channels. On the other hand, the F1817A substitution enhanced the inhibition by metaflumizone, as measured by the onset of block at half inactivation, and accelerated the recovery of F1817A channels from metaflumizone block at hyperpolarized potentials. Therefore, although the F1817 residue is not an SCBI-binding residue in BgNav1-1A channels, the replacement of its aromatic side chain with a smaller side chain appears to allow metaflumizone (but not DCJW) to get in and out of its receptor site more readily.
As mentioned above, in rat Nav1.4 sodium channels, alanine substitution of the tyrosine residue caused a 58-fold increase in inhibition by DCJW of Nav1.4 channels that were completely inactivated [38]. However, alanine substitution of the corresponding residue, Y1824, in IVS6 of BgNav1-1A did not have a significant effect on inhibition by DCJW or metaflumizone in conditions that did not permit complete equilibration between insecticide and channel [39]. In contrast, results from the recovery from slow inactivation assay, in which inactivated channels were able to completely equilibrate with insecticide, show that inactivated BgNav1-1AY1824A channels have a higher affinity to both DCJW and metaflumizone than wildtype channels. Thus, the Y1824 residue does not play a role in SCBI activity. Furthermore, the SCBI receptor in insect sodium channels appears to be formed by residues distinct from those in mammalian sodium channels.
Mechanism of action of lidocaine on an insect sodium channel
In contrast to mammalian sodium channels, the molecular determinants of local anesthetic action on insect sodium channels have not been identified. Song et al. evaluated the effects of lidocaine on wildtype (BgNav1-1A) sodium channels and the effects of the mutations F1817A and Y1824A of IVS6 on the activity of lidocaine on BgNav1-1A sodium channels (unpublished data, manuscript in preparation). Lidocaine blocked BgNav1-1A sodium channels and rat Nav1.2 sodium channels in the resting state (i.e., tonic block) with similar potency [52]. However, a significant difference in the effects of lidocaine on mammalian and insect sodium channels was observed. Use-dependent and frequency-dependent block of mammalian sodium channels are key features of local anesthetic block and are vital for the clinical use of LA drugs. This effect is explained by a modulated drug receptor that has a low affinity for channels in resting states, but a higher affinity for channels in open or inactivated states [53, 54]. However, lidocaine caused very little use-dependent and frequency-dependent inhibition of BgNav1-1A sodium channels (unpublished data). This difference could be due to faster recovery from inactivation of BgNav1-1A sodium channels consequently reducing the availability of inactivated states for the action of lidocaine [23].
Nevertheless, residues in the cockroach sodium channel corresponding to the LA-interacting residues in IVS6 also contribute to the action of lidocaine on BgNav1-1A channels, albeit to a minor extent. Specifically, BgNav1-1AF1817A and BgNav1-1AY1824A sodium channels exhibited smaller lidocaine-induced hyperpolarizing shifts in the voltage dependence of slow inactivation than BgNav1-1A channels (unpublished data). However, the tonic block was not affected by these substitutions, in contrast to previous studies with mammalian sodium channels, for which the F1710A mutation (in Nav1.3) also reduced the resting-state affinity [42, 43, 50, 55]. Taken together, these results suggest that F1817A and Y1824A substitutions attenuate the activity of lidocaine in the cockroach BgNav1-1A channel, possibly by reducing the binding affinity of lidocaine to the slow-inactivated state.
In conclusion, SCBIs are hypothesized to bind at or near the LA-binding site on mammalian and insect sodium channels. This hypothesis predicts that LA-binding residues, particularly a phenylalanine (F1579) and a tyrosine (Y1586) in IVS6 of the mammalian Nav1.4 channel, would be required for the action of metaflumizone, indoxacarb, and DCJW. In support of this hypothesis, alanine substitution of the LA-binding residue F1579 results in reduced sensitivity of the mammalian sodium channel Nav1.4 to DCJW, [38]. However, results from the analysis of an insect sodium channel, as presented above, revealed that the corresponding residues are not of primary importance to the action of SCBIs in insect sodium channels [39]. Our results raise the intriguing possibility that the receptor for SCBIs, and possibly local anesthetics, is determined by a different subset of amino acid residues in insect sodium channels than in mammalian sodium channels. Future site-directed mutagenesis of other regions of insect sodium channels is necessary to identify key molecular determinants of the SCBI binding site on insect sodium channels.
Mechanism of insect selectivity of SCBIs
Insect selectivity and mammalian safety of modern SCBIs is achieved through a combination of selective metabolism and selective action at the target site. The pyrazoline RH-3421 had an IC50 of 270 nM for rat brain Na channels, as measured by displacement of radiolabeled batrachotoxin B. In crayfish giant axons, the IC50 for binding to the slow-inactivated state, measured by voltage clamp was 680 nM [5]. The potency in blocking CNS and sensory activity in various insect preparations was also in this range [4]. This lack of insect selectivity of the pyrazolines at the target site and their potential for bioaccumulation contributed to the toxicological problems that prevented their development [5].
In contrast to the pyrazolines, indoxacarb, the first SCBI to be commercialized, has an excellent toxicological profile and received a reduced-risk registration [56]. Indoxacarb was designed as a prodrug, and is selectively metabolized by hydrolases in insects to DCJW [17], which is only a minor pathway in higher animals, where inactivation via P450-mediated attack of the indanone and oxadiazine rings is favored [56]. Furthermore, DCJW is significantly more potent against insect than mammalian sodium channels: whereas it blocks the rat Nav1.4 channel with an IC50 of 1000 nM [38], it is 40-fold more potent against the Bg Nav1-1a channel, with an IC50 of 25.5 nM [39]. Metaflumizone likewise offers low risk to mammals, beneficial insects and other non-target organisms [57], but the mechanisms of insect selectivity have not been investigated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narahashi T. Molecular and cellular approaches to neurotoxicology: past, present, and future. In: Lunt G, editor. Neurotoxicology 1988: Molecular Basis of Drug and Pesticide Action. Elsevier; New York: 1988. pp. 563–582. [Google Scholar]
- 2.Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 4.Salgado VL. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic Sci. 1990;28:389–411. [Google Scholar]
- 5.Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992;41:120–126. [PubMed] [Google Scholar]
- 6.Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology in insects. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; New York: 2005. pp. 30–53. [Google Scholar]
- 7.Bloomquist JR, Payne GT, Kinne L, Lyga J, Leong D, Nicholson RA. Toxicity and mode of action of benzhydrolpiperidines and related compounds in insects. Pestic Biochem Physiol. 2002;73:18–26. [Google Scholar]
- 8.McCann SF, Cordova D, Andaloro JT, Lahm GP. Sodium channel blocking insecticides. In: Kraemer W, Schirmer U, editors. Modern Crop Protection Compounds. Vol. 3. Wiley-VCH; Weinheim: 2007. pp. 1031–1048. [Google Scholar]
- 9.Silver K, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol. 2005;81:136–143. [Google Scholar]
- 10.Mulder R, Wellinga K, van Daalen JJ. A new class of insecticides. Naturwissenschaften. 1975;62:531–532. doi: 10.1007/BF00609070. [DOI] [PubMed] [Google Scholar]
- 11.Grosscurt AC, van Hes R, Wellinga K. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J Agric Food Chem. 1979;27:406–409. [Google Scholar]
- 12.Meier GA, Silverman IR, Ray PS, Cullen TG, Ali SF, Marek FL, Webster CA. Insecticidal dihydropyrazoles with reduced lipophilicity. In: Baker DR, Fenyes JG, Steffens JJ, editors. Synthesis and Chemistry of Agrochemicals III. American Chemical Society; Washington D.C.: 1992. pp. 313–326. [Google Scholar]
- 13.Jacobson RM. A new class of insecticidal dihydropyrazoles. In: Crombie L, editor. Recent Advances in the Chemistry of Insect Control II. Royal Society of Chemistry; London: 1989. pp. 206–211. [Google Scholar]
- 14.Jacobson RM. N-aryl 3-aryl-4,5-dihydro-1H-pyrazole-2-pyrazolines. 5 798 311. US Patent. 1985
- 15.Takagi K, Hamaguchi H, Nishimatsu T, Konno T. Discovery of metaflumizone, a novel semicarbazone insecticide. Veterinary Parasitology. 2007;150:177–181. doi: 10.1016/j.vetpar.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Metaflumizone Worldwide Technical Brochure. 2007. BASF Agricultural Products. [Google Scholar]
- 17.Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch Insect Biochem Physiol. 1998;37:91–103. [Google Scholar]
- 18.Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T, Taga T. Stereochemical basis for the insecticidal activity of carbamoylated and acylated pyrazolines. Pestic Sci. 1996;46:105–112. [Google Scholar]
- 19.Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Veterinary Parasitology. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Silver K, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 22.Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee ME. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protect. 2000;19:537–545. [Google Scholar]
- 23.Lapied B, Grolleau F, Sattelle DB. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Br J Pharmacol. 2001;132:587–595. doi: 10.1038/sj.bjp.0703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapied B, Malecot CO, Pelhate M. Ionic species involved in the electrical activity of single adult aminergic neurons isolated from the sixth abdominal ganglion of the cockroach, Periplaneta americana. J Exp Biol. 1989;144:535–550. [Google Scholar]
- 25.Lapied B, Stankiewicz M, Grolleau F, Rochat H, Zlotkin E, Pelhate M. Biophysical properties of scorpion alpha-toxin-sensitive background sodium channel contributing to the pacemaker activity in insect neurosecretory cells (DUM neurons) Eur J Neurosci. 1999;11:1449–1460. doi: 10.1046/j.1460-9568.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26:455–465. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Song WZ, Liu ZQ, Dong K. Molecular basis of differential sensitivity of insect sodium channels to DCJW, a bioactive metabolite of the oxadiazine insecticide indoxacarb. Neurotoxicology. 2006;27:237–244. doi: 10.1016/j.neuro.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clare JJ, Tate SN, Nobbs M, Romanos MA. Voltage-gated sodium channels as therapeutic targets. Drug Discov Today. 2000;5:506–520. doi: 10.1016/s1359-6446(00)01570-1. [DOI] [PubMed] [Google Scholar]
- 29.Anger T, Madge DJ, Mulla M, Riddall D. Medicinal Chemistry of neuronal voltage-gated sodium channel blockers. J Med Chem. 2001;44:115–137. doi: 10.1021/jm000155h. [DOI] [PubMed] [Google Scholar]
- 30.Postma W, Catterall WA. Inhibition of [3H]batrachotoxinin A 20-α-benzoate binding to sodium channels by local anesthetics. Mol Pharmacol. 1984;25:219–227. [PubMed] [Google Scholar]
- 31.Catterall WA. Common modes of drug action of Na+ channels: local anesthetics, antiarrhythmics, and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- 32.Wasserstrom JA, Liberty K, Kelly J, Santucci P, Myers M. Modification of cardiac sodium channels by batrachotoxin: effects on gating, kinetics, and local anesthetic binding. Biophys J. 1993;65:386–395. doi: 10.1016/S0006-3495(93)81046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamponi GW, Doyle DD, French RJ. Fast lidocaine block of cardiac and skeletal muscle sodium channels: one site with two routes of access. Biophys J. 1993;65:80–90. doi: 10.1016/S0006-3495(93)81042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deecher DC, Payne GT, Soderlund DM. Inhibition of [3H]batrachotoxinin A 20-α-benzoate binding to mouse brain sodium channels by the dihydropyrazole insecticide RH 3421. Pestic Biochem Physiol. 1991;41:265–273. [Google Scholar]
- 35.Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pestic Biochem Physiol. 1998;60:177–185. [Google Scholar]
- 36.Soderlund DM. Comprehensive Molecular Insect Science. Vol. 5. Elsevier; New York: 2005. Sodium Channels; pp. 1–24. [Google Scholar]
- 37.Cheng YC, Prusoff WH. Relationship between the inhibition constant and the concentration of inhibitor which causes 50 percent inhibition of an enzymatic reaction. Biochemical Pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 38.Silver K, Soderlund DM. Point Mutations at the Local Anesthetic Receptor Site Modulate the State-Dependent Block of Rat Nav1.4 Sodium Channels by Pyrazoline-type Insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Silver K, Song W, Nomura Y, Salgado VL, Dong K. The role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of lidocaine and sodium channel blocker insecticides. Neurotoxicology. 2009;30:613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 41.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 42.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 43.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarov-Yarovoy V, Brown J, Sharp E, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- 45.Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- 46.Nau C, Wang SY, Strichartz GR, Wang GK. Point mutations at N434 in D1-S6 of μ1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- 47.Wang GK, Quan C, Wang SY. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated μ1 Na+ channels. Eur J Physiol. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- 48.Wang SY, Nau C, Wang GK. Residues in Na+ channel D3-S6 segment modulate batrachotoxin and local anesthetic affinities. Biophys J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang GK, Russell C, Wang SY. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain. Pain. 2004;110:166–174. doi: 10.1016/j.pain.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA. Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology. 2003;44:413–422. doi: 10.1016/s0028-3908(02)00400-8. [DOI] [PubMed] [Google Scholar]
- 51.Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 52.Pugsley MK, Goldin AL. Effects of bisaramil, a novel class I antiarrhythmic agent, on heart, skeletal muscle and brain Na channels. Eur J Pharmacol. 1998;342:93–104. doi: 10.1016/s0014-2999(97)01420-9. [DOI] [PubMed] [Google Scholar]
- 53.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hondeghem LM, Katzung BG. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annual Review of Pharmacology and Toxicology. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- 55.Li HL, Galue A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol Pharmacol. 1999;55:134–141. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- 56.EPA. Fact Sheet on Indoxacarb, Issued Oct 30, 2000. Environmental Protection Agency; Washington D.C.: 2000. [Google Scholar]
- 57.Hempel K, Hess FG, Boegi C, Fabian E, Hellwig J, Fegert I. Toxicological properties of metaflumizone. Veterinary Parasitology. 2007;150:190–195. doi: 10.1016/j.vetpar.2007.08.033. [DOI] [PubMed] [Google Scholar]


