Abstract
Melanoma is highly metastatic and resistant to chemotherapeutic drugs. Our previous studies have demonstrated that caffeic acid phenethyl ester (CAPE) suppresses the growth of melanoma cells and induces reactive oxygen species generation. However, the exact mechanism of the growth suppressive effects of CAPE was not clear. Here, we determined the potential mechanism of CAPE against melanoma in vivo and in vitro. Administration of 10 mg/kg/day CAPE substantially suppressed the growth of B16F0 tumor xenografts in C57BL/6 mice. Tumors from CAPE-treated mice showed reduced phosphorylation of phosphoinositide 3-kinase, AKT, mammalian target of rapamycin and protein level of X-linked inhibitor of apoptosis protein (XIAP) and enhanced the cleavage of caspase-3 and poly (ADP ribose) polymerase. In order to confirm the in vivo observations, melanoma cells were treated with CAPE. CAPE treatment suppressed the activating phosphorylation of phosphoinositide 3-kinase at Tyr 458, phosphoinositide-dependent kinase-1 at Ser 241, mammalian target of rapamycin at Ser 2448 and AKT at Ser 473 in B16F0 and SK-MEL-28 cells in a concentration and time-dependent study. Furthermore, the expression of XIAP, survivin and BCL-2 was downregulated by CAPE treatment in both cell lines. Significant apoptosis was observed by CAPE treatment as indicated by cleavage of caspase-3 and poly (ADP ribose) polymerase. AKT kinase activity was inhibited by CAPE in a concentration-dependent manner. CAPE treatment increased the nuclear translocation of XIAP, indicating increased apoptosis in melanoma cells. To confirm the involvement of reactive oxygen species in the inhibition of AKT/XIAP pathway, cells were treated with antioxidant N-acetyl-cysteine (NAC) prior to CAPE treatment. Our results indicate that NAC blocked CAPE-mediated AKT/XIAP inhibition and protected the cells from apoptosis. Because AKT regulates XIAP, their interaction was examined by immunoprecipitation studies. Our results show that CAPE treatment decreased the interaction of AKT with XIAP. To establish the involvement of AKT in the apoptosis-inducing effects of CAPE, cells were transfected with AKT. Our results revealed that AKT overexpression attenuated the decrease in XIAP and significantly blocked CAPE-mediated apoptosis. Similarly, overexpression of XIAP further decreased CAPE-induced apoptosis. Taken together, our results suggest that CAPE suppresses phosphoinositide 3-kinase/AKT/XIAP pathway leading to apoptosis in melanoma tumor cells in vitro and in vivo.
Introduction
Melanoma is a neoplasm of melanocytes that are predominantly found in skin (1,2). According to epidemiological studies, the incidence of malignant melanoma has increased more rapidly in Western population (2,3). The World Health Organization reported that 48 000 melanoma patients die worldwide every year, of which 8000 of the deaths occur in the USA alone (1,4). It is predicted that 1 in 58 Americans will develop invasive melanoma in their life time. Incidence and mortality rate of melanoma are highest among adults of age 75 years and older (5). Ultraviolet rays are one of the most preventable known risk factors for melanoma (6). Genetic factors are also associated with melanoma. For example, people with lighter skin, blue eyes and red hairs are at increased risk of developing melanoma (7). Current chemotherapy for melanoma has marginal impact on survival (2,3). Therefore, there is an urgent need to identify novel agents that can prevent or treat melanoma.
Phosphoinositide 3-kinase (PI3K)/AKT is a survival pathway that facilitates resistance to the apoptotic effect of chemotherapy in a variety of cancer types including melanoma (8). AKT promotes cell survival and suppresses apoptotic death in a number of cancer cells by numerous stimuli such as growth factor withdrawal, cell-cycle discordance, loss of cell adhesion and induction of inhibitor of apoptosis proteins, including X-linked inhibitor of apoptosis protein (XIAP) (9,10). Several studies reported that PI3K and AKT are constitutively activated in melanoma (8,11). The mechanism behind AKT activation in melanoma cells remains unclear.
Caffeic acid phenethyl ester (CAPE), an ester derivative of caffeic acid, a bioactive component extracted from honeybee hive propolis, possesses antioxidant, anti-inflammatory and immunomodulatory capabilities (12–16). Previous studies suggested that CAPE has significant cytotoxic effect against various cancer cells (17,18). CAPE was reported to be effective against breast, prostate and leukemic cancers by nuclear factor-kappaB inhibition (19–21), induction of Bax (19,22), activation of c-jun N-terminal kinase and p38 (19). CAPE induces cell-cycle arrest through suppression of cyclin D1 (23,24), cyclin E (24), and c-Myc expression (24) in colon and glioma cancer cells. It has been shown that topical application of CAPE suppresses 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin (25,26). In this study, we evaluated the mechanism of anticancer effects of CAPE in an in vitro and in vivo models of melanoma.
Materials and methods
Chemicals and antibodies
CAPE (purity > 99%), antiactin antibody and N-acetyl-cysteine (NAC) were obtained from Sigma (St. Louis, MO). The antibodies against cleaved caspase-3, cleaved poly (ADP ribose) polymerase (PARP), survivin, PI3K, p-PI3K (Tyr 458), AKT, p-AKT (Ser 473), phosphoinositide-dependent kinase-1 (PDK1), p-PDK1 (Ser 241), p-mammalian target of rapamycin (mTOR) (Ser 2448), mTOR, XIAP and BCL-2 were purchased from Cell Signaling (Danvers, MA). Lamin B was purchased from Santa Cruz Biotechnology (CA). Apoptosis detection kit Annexin V–fluorescein isothiocyanate (FITC) was procured from BD Bio-Sciences (La Jolla, CA). Alexa Fluor 488 (green) and Alexa Fluor 594 (red) conjugated goat-antirabbit secondary antibody and 4′,6-diamidino-2-phenylindole were obtained from Invitrogen (Eugene, OR). AKT kinase activity kit was purchased from Assay Designs (Plymouth Meeting, PA). LY294002, a specific inhibitor of PI3K, was procured from Cell Signaling.
Cell culture
Human melanoma cell line SK-MEL-28 was a generous gift from Dr K.M.McMasters, Division of Surgical Oncology University of Louisville, KY. Murine melanoma cell line B16F0 was obtained from ATCC (Manassas, VA). Monolayer cultures of SK-MEL-28 were maintained in modified Eagle’s medium and B16F0 was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin–streptomycin–neomycin antibiotic mixture (10ml/l), 2mM l-glutamine, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1mM sodium pyruvate and 20% glucose. All the cultures were maintained at 37°C in a humidified chamber of 95% air and 5% CO2.
Tumor therapy model
Tumor therapy experiment was performed as we have described previously (1) with minor modifications. About 6- to 8-week-old male C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA). The use of C57BL/6 mice and their treatment was approved by the Institutional Animal Care and Use Committee, Texas Tech University of Health Science Center. All the experiments were carried out in strict compliance with their regulations. Briefly, exponentially growing B16F0 (1 × 106) cells were injected subcutaneously into the right flanks of 24 mice. After 7 days when the tumor reached a palpable size of 70mm3, mice were randomly segregated into two groups with 12 mice in each group. Test group of mice received 10 mg/kg/day CAPE in 150 µl of 50% dimethyl sulfoxide/saline by intraperitoneal injection for seven subsequent days, whereas control mice received vehicle alone (1). The animals were weighed twice a week. All the mice were killed 2 h after drug injection on day 14. Subcutaneous tumors were excised and tumor volume was measured as described by us previously (27).
Transient transfection
B16F0 cells were transiently transfected with wild-type AKT and wild-type XIAP plasmid by electroporation using nucleofection transfection reagent (Lonza, Walkersville, MD), according to the manufacturer’s instruction. Briefly 1 × 106 B16F0 cells/sample were collected and suspended in 100 µl nucleofection solution and then combined with 2 µg of respective DNA. Once the electroporation was completed, cuvettes were taken out and 500 µl of preincubated respective medium was added immediately to the cuvettes and samples were transferred into the six-well plate. Transfected cells were treated with 20 µM CAPE for indicated time period.
Immunoprecipitation assay
Immunoprecipitation assay was performed to examine the effect of CAPE on the interaction of AKT with XIAP or vice versa as we have described previously (28). Briefly, B16F0 or SK-MEL-28 cells were treated with dimethyl sulfoxide or 20 µM CAPE for 48 h and whole-cell lysates were lysed using RIPA buffer and immunoprecipitated with AKT or XIAP antibodies. The samples were immunoblotted with p-AKT (Ser 473) and XIAP antibodies.
Immunofluorescence assay
Immunofluorescence assay was performed as we have described previously (28). Briefly, SK-MEL-28 cells were plated on coverslips and allowed to attach overnight and then treated with 20 µM of CAPE for 48 h. Treated and untreated cells were fixed with acetone:methanol (1:1) mixture, blocked with goat serum for 1 h and incubated with XIAP or p-AKT (Ser 473) antibodies overnight at 4°C. Immunofluorescence was detected with antirabbit immunoglobulin G conjugated with Alexa Fluor 594 (red), 4′,6-diamidino-2-phenylindole (blue). After four washings, coverslips were mounted with antifade mounting reagents. Nuclei were stained with 4′,6-diamidino-2-phenylindole and the immunofluorescence was observed by a fluorescence microscope using oil immersion at ×60 magnification.
Annexin V/FITC apoptosis assay
Apoptosis induction by CAPE was assessed by Annexin V/FITC by flow cytometry as we described previously (29). About 0.3 × 106 B16F0 or SK-MEL-28 cells were seeded in a six-well plate and treated with 20 µM CAPE for 48 h after 1 h pretreatment with NAC. In another experiment, apoptosis was determined after AKT or XIAP transfection followed by CAPE treatment for 48 h. Apoptosis was determined using APOPTEST™-FITC kit according to manufacturer’s instructions and analyzed by Accuri C6 flow cytometer.
AKT kinase assay
AKT kinase activity was determined as described by us previously (28). Briefly, SK-MEL-28 or B16F0 cells were treated with various concentration of CAPE for 48 h. Cell lysates were prepared and AKT kinase activity was determined using a kit (Assay Designs) according to the manufacturer’s instruction.
Western blot analysis
Cells were exposed to various concentrations or 20 µM CAPE for 48 h and lysed on ice as described by us previously (27,30). Whole-cell extracts were prepared as mentioned above. The tumors from control and CAPE-treated mice were minced and lysed by the procedure described by us previously (27). The cell lysate was cleared by centrifugation at 14 000g for 30min. Cell lysate containing 10–80 µg protein was resolved by 6–12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the proteins were transferred onto polyvinylidene fluoride membrane. After blocking with 5% non-fat dry milk in Tris buffered saline, pH 7.4, membrane was incubated with the desired primary antibody (1:1000 dilutions) overnight. Subsequently, the membrane was incubated with appropriate secondary antibody (1:2000 dilutions) and the antibody binding was detected using enhanced chemiluminescence kit according to the manufacturer’s instructions. Each membrane was stripped and re-probed with antibody against actin (1:20 000 dilutions) or lamin B to ensure equal protein loading.
Statistical analysis
All statistical calculations were performed using Graph Pad Prism 5.0. Analysis of variance was used to test the statistical significance of difference between control and treated groups followed by Bonferroni’s post hoc analysis for multiple comparisons. P-values less than 0.05 were considered statistically significant.
Results
CAPE suppresses tumor growth in C57BL/6 mice
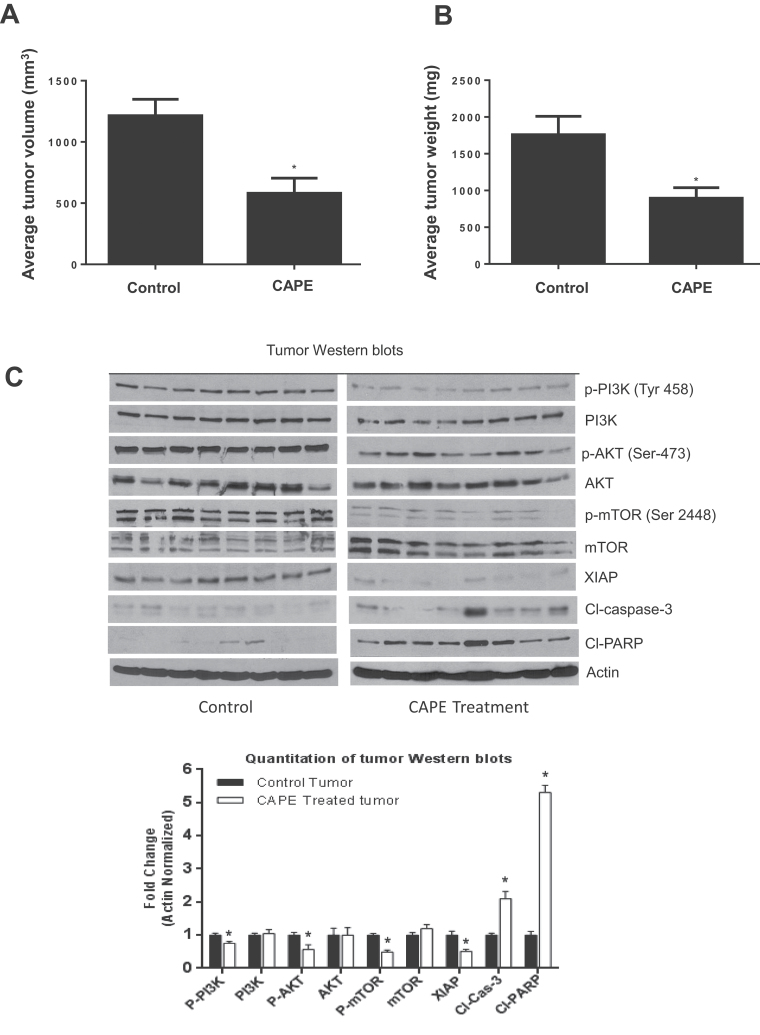
In order to test the in vivo efficacy of CAPE, B16F0 cells were injected subcutaneously into the right flanks of C57BL/6 mice. After 7 days when the size of the tumors was about 70mm3, mice were divided into two groups. CAPE was administered intraperitoneally at a dose of 10 mg/kg body wt every day to the treatment group, whereas control group received vehicle only. Our results demonstrate that CAPE treatment significantly reduced the growth of melanoma tumors (Figure 1A). At day 13 of the treatment, tumor volume in the treated group was reduced by 59% (100 ± 35mm3 versus 41±32, n = 12 per group) compared with control group (Figure 1A). The average wet weight of the tumor dissected from treated mice was decreased by 43% compared with the control tumors (Figure 1B). The weight of the mice did not change significantly, suggesting that there was no noticeable toxicity induced by CAPE in treated mice (data not shown).
Fig. 1.
CAPE suppresses tumor growth in C57BL/6 mice by inhibiting PI3K/AKT/XIAP pathway. C57BL/6 mice were kept on antioxidant-free diet for a week. Exponentially growing B16F0 (1 × 106) cells were injected subcutaneously into the right flanks of 24 mice. When the tumor reached 70 mm3 in size, mice were randomly segregated into two groups with 12 mice in each group. Treated group of mice received 10 mg/kg/day CAPE in 150 µl of 50% dimethyl sulfoxide/saline by intraperitoneal injection for subsequent 7 days, whereas control mice received vehicle alone. The animals were weighed twice a week. Tumors were measured using vernier calipers. Effect of CAPE on (A) % tumor volume and (B) % tumor weight was evaluated. In order to determine the mechanism of tumor growth suppression, tumors were homogenized, lysed and subjected to western blot. (C) Representative immunnoblots showed the effect CAPE treatment on thephosphorylation of PI3K at Tyr 458, AKT at Ser 473, mTOR at Ser 2448 and protein levels of PI3K, AKT, mTOR, XIAP, cleaved caspase-3 and cleaved PARP. Each lane represents tumor data from eight control and eight treated mice, respectively. The blots were stripped and re-probed for actin to ensure equal protein loading. Values are mean ± SD of 12 samples. *P < 0.05, statistically significant compared with control.
Inhibition of tumor growth by CAPE was associated with the inhibition of PI3K/AKT/XIAP pathway
We hypothesized that melanoma tumor growth suppression by CAPE treatment was associated with inhibition of PI3K/AKT/XIAP pathway, as this pathway is known to be aberrantly activated in melanoma. In order to test our hypothesis in vivo, CAPE-treated and untreated tumors were lysed and levels of PI3K, AKT and XIAP were examined by western blotting. Our results show that CAPE treatment significantly reduced the phosphorylation of PI3K at Tyr 458, AKT at Ser 473, mTOR at Ser 2448 and protein levels of XIAP, compared with control tumors (Figure 1C). On the other hand, CAPE treatment increased the cleavage of caspase-3 and PARP indicating apoptosis (Figure 1C). Expression of PI3K, AKT and mTOR did not change significantly. Overall our results suggested that CAPE-mediated suppression of melanoma tumor growth was associated with inhibition of PI3K/AKT/XIAP pathway, leading to apoptosis in melanoma cells.
CAPE inhibits PI3K/AKT/XIAP pathways in melanoma cells
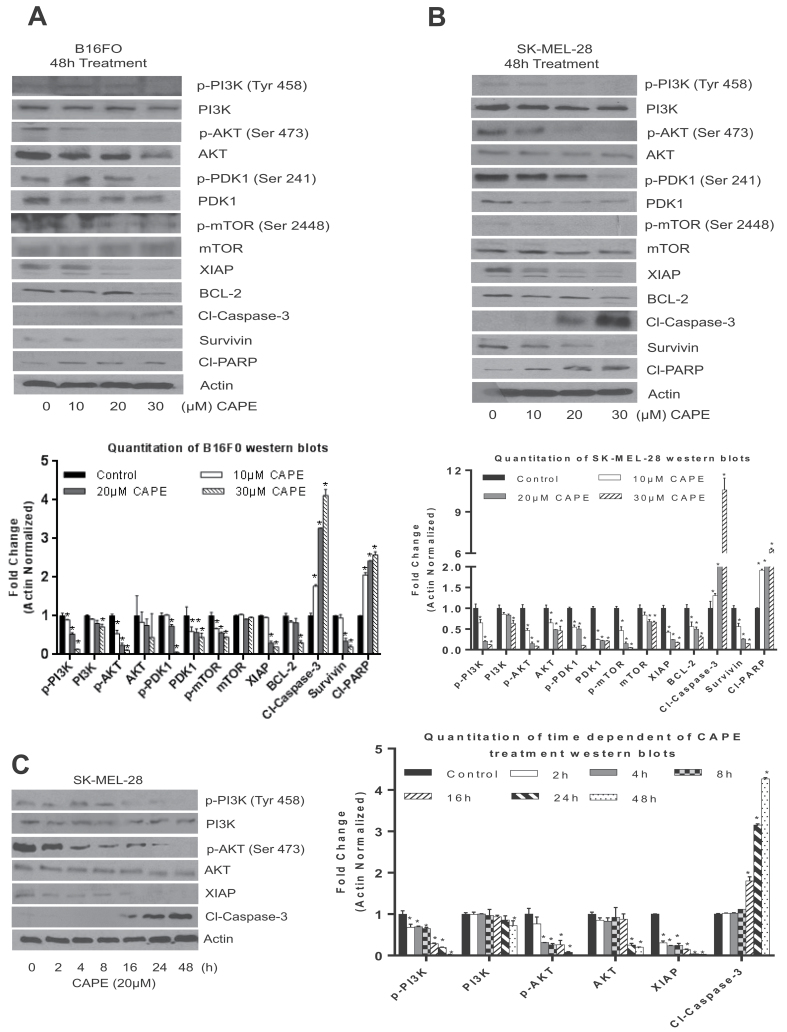
To confirm the observation made in vivo, SK-MEL-28 and B16F0 melanoma cells were treated with various concentrations of CAPE for 48 h or with 20 µM CAPE for various time intervals. Our results demonstrate that CAPE treatment significantly suppressed the phosphorylation of PI3K at Tyr 458, AKT at Ser 473, PDK1 at Ser 241 and mTOR at Ser 2448 in a concentration-dependent manner in both of the melanoma cell lines (Figure 2A and B). Furthermore, CAPE treatment also reduced the protein levels of AKT, PDK1, XIAP, BCL-2 and survivin. Cleavage of caspase-3 and PARP by CAPE treatment indicated apoptosis in melanoma cells (Figure 2A and B). In a time-dependent study, CAPE treatment significantly decreased the phosphorylation of PI3K and AKT and protein levels of XIAP. These changes were as early as 2 h, whereas cleavage of caspase-3 was observed at 16 h of CAPE treatment, indicating that CAPE-induced apoptosis was associated with the inhibition of PI3K and AKT phosphorylation and reduced protein levels of XIAP (Figure 2C). These results suggest that CAPE induced apoptosis by inhibiting PI3K/AKT/XIAP signaling.
Fig. 2.
CAPE inhibits the PI3K/AKT/XIAP pathways in melanoma cells. (A) B16F0, (B) and (C) SK-MEL-28 melanoma cells were treated with various concentrations or various time points with 20 µM of CAPE for 48 h. Cells were lysed and subjected to western blot. Immunoblots were probed for p-PI3K (Tyr 458), p-AKT (Ser 473), p-PDK1 (Ser 241) and p-m-TOR (Ser 2448), PI3K, AKT, PDK1, mTOR, XIAP, BCL-2, survivin, cleaved caspase-3 and cleaved PARP. Same blots were stripped and re-probed for actin to ensure equal protein loading. The experiments were repeated three times with similar results obtained. *P < 0.05, statistically significant compared with control.
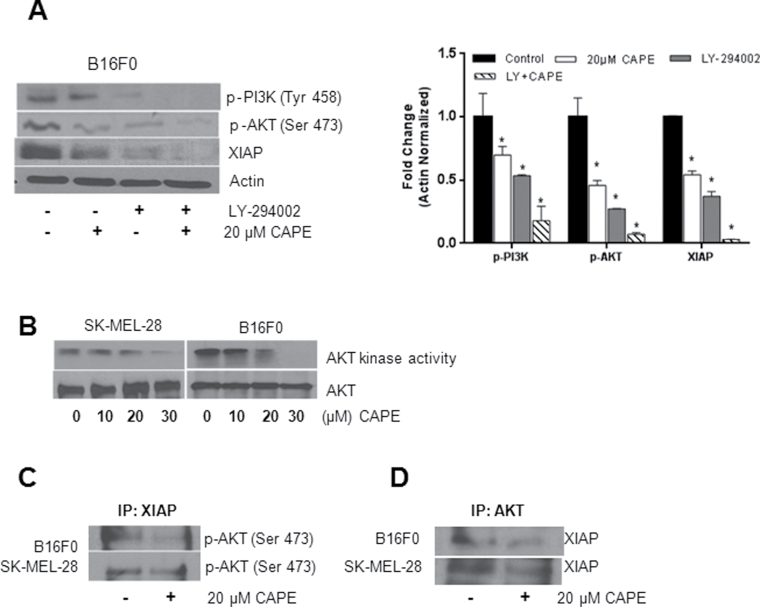
Effect of CAPE on AKT kinase activity
To confirm PI3K/AKT as a target of CAPE, B16F0 cells were pretreated with 10 µM of PI3K inhibitor (LY294002) for 1 h and then treated with 20 µM CAPE for 48 h. Our results show that PI3K inhibitor increased CAPE-mediated effects on p-PI3K (Tyr 458), p-AKT (Ser 473) and XIAP, indicating that PI3K/AKT/XIAP pathway plays an important role in CAPE-induced apoptosis in melanoma cells (Figure 3A). Because we observed a decrease in AKT phosphorylation by CAPE treatment, we next planned to investigate whether CAPE treatment would decrease the functionality of AKT. Our results show that CAPE treatment drastically decreased AKT kinase activity in a concentration-dependent manner in both cell lines (Figure 3B), indicating AKT as a target of CAPE in melanoma cells.
Fig. 3.
Effect of CAPE on AKT kinase activity and the interaction of AKT/XIAP in melanoma cells. (A) B16F0 cells were pretreated for 1 h with LY294002 followed by 20 µM CAPE treatment for 48 h. Cell lysates were immunoblotted with p-PI3K (Tyr 458), p-AKT (Ser 473) and XIAP. Same blots were stripped and re-probed for actin to ensure equal protein loading. *P < 0.05, statistically significant compared with control. (B) SK-MEL-28 or B16F0 cells were treated with various concentrations of CAPE for 48 h. Cell lysates were prepared and AKT kinase activity was determined using a kit according to the manufacturer’s instruction. B16F0 and SK-MEL-28 cells were treated with 20 µM CAPE for 48 h and immunoprecipitated (C) with XIAP and (D) with AKT antibodies and immunoblotted with p-AKT (Ser 473) and XIAP antibodies, respectively. The experiments were repeated three times with similar results obtained.
CAPE treatment decreases interaction of AKT/XIAP in melanoma cells
Because AKT is well known to regulate XIAP, we aimed to investigate if CAPE treatment disrupts the AKT/XIAP interaction. In order to test this hypothesis, B16F0 and SK-MEL-28 cells were treated with 20 µM CAPE for 48 h. Control and CAPE-treated cells were immunoprecipitated with XIAP and AKT antibodies and immunoblotted with p-AKT (Ser 473) and XIAP antibodies. Our co-immunoprecipitation results show that CAPE treatment suppressed the phosphorylation of AKT (Ser 473) when immunoprecipitated with XIAP (Figure 3C) and vice versa (Figure 3D) in both B16F0 and SK-MEL-28 cell lines, indicating that CAPE treatment reduced the AKT/XIAP interaction and thereby leading melanoma cells into apoptosis.
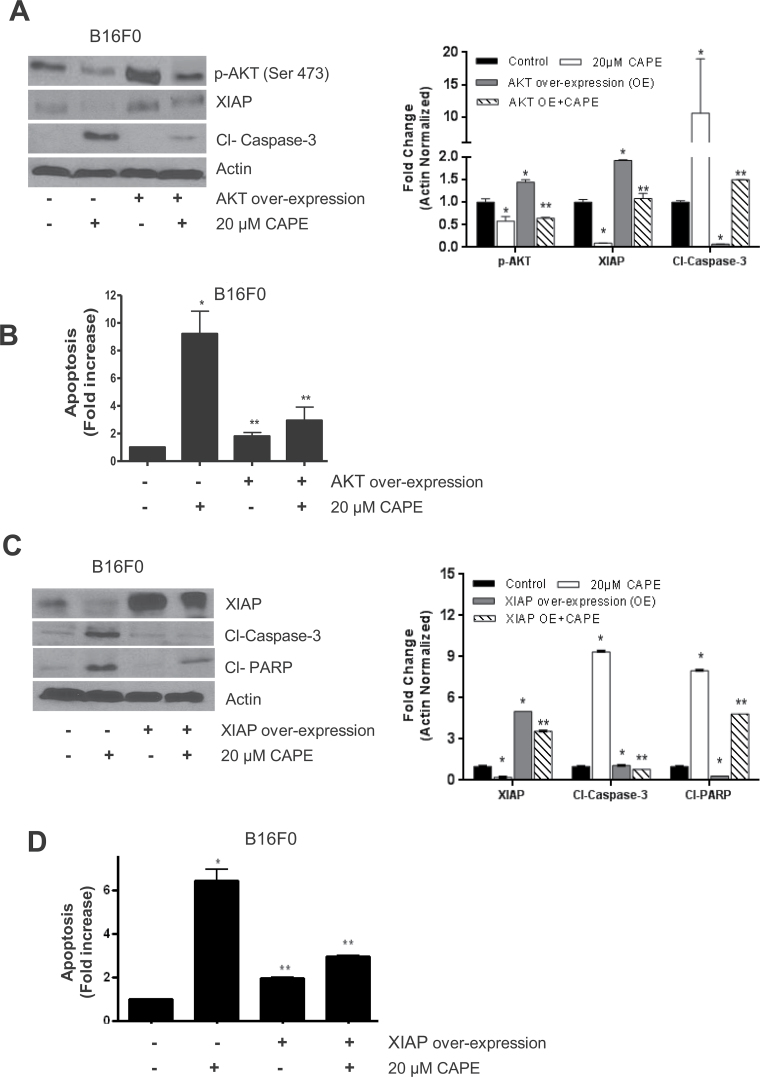
CAPE-induced apoptosis is blocked by AKT and XIAP overexpression
Next, we investigated if CAPE-induced apoptosis can be inhibited by AKT and XIAP overexpression. B16F0 and SK-MEL-28 cells were transiently transfected with AKT or XIAP plasmid. Our results show that ectopic expression of AKT by transient transfection significantly increased XIAP levels and decreased CAPE-induced apoptosis in B16F0 cells, suggesting that AKT regulates XIAP in our model (Figure 4A). CAPE-induced apoptosis in AKT overexpressing cells was further confirmed by Annexin V/FITC assay (Figure 4B). Annnexin-V/FITC assay showed that overexpression of AKT significantly decreased CAPE-induced apoptosis. On the other hand, as expected, ectopic expression of XIAP by transient transfection substantially suppressed cleavage of caspase-3 and PARP, indicating that overexpression of XIAP inhibits CAPE-induced apoptosis (Figure 4C). CAPE-induced apoptosis was confirmed by Annexin V/FITC assay in XIAP overexpressing cells (Figure 4D).
Fig. 4.
Effect of CAPE in cells overexpressing with AKT and XIAP. B16F0 melanoma cells were transiently transfected with 2 µg AKT or 2 µg of XIAP plasmid by electroporation followed by 20 µM CAPE treatment for 48 h. (A and C) Cell lysates were subjected to western blots and immunoblotted with p-AKT (ser 473), XIAP, cleaved caspase-3 and cleaved PARP. Same blots were stripped and re-probed for actin to ensure equal protein loading. (B and D) Apoptosis wasdetermined using Annexin V/FITC and propidium iodide and analyzed by flow cytometry as described in Materials and methods section. Results are expressed as mean ± SD of three independent experiments. *P < 0.05, statistically significant compared with control. **P < 0.05, statistically significant compared with CAPE treatment.
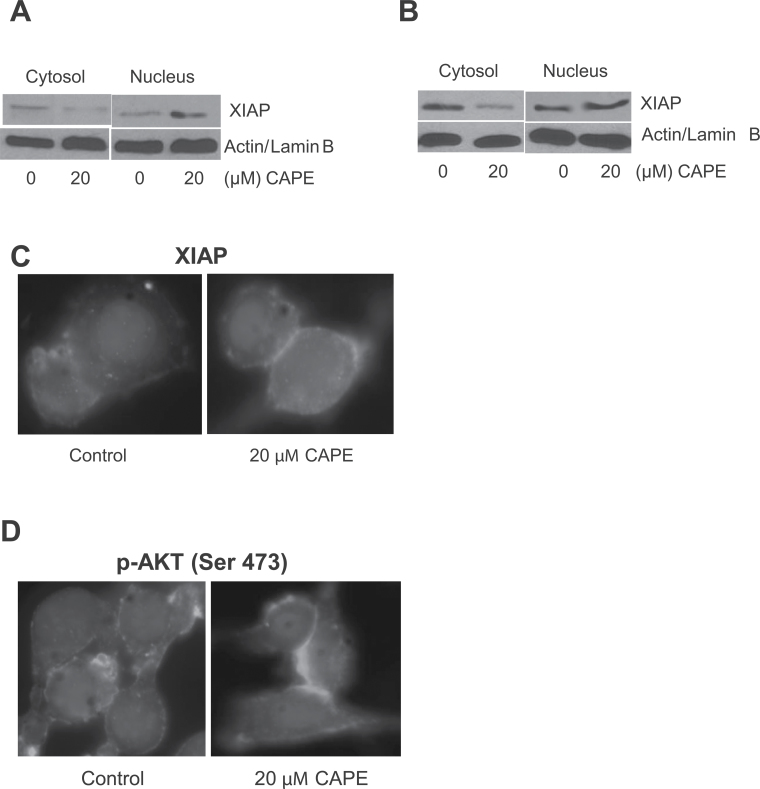
CAPE regulates nuclear shuttling of XIAP
Previous studies have shown that nuclear localization of XIAP increased cell death (31,32). In order to observe the nuclear localization of XIAP, SK-MEL-28 and B16F0 cells were treated with 20 µM CAPE for 48 h and then cytosolic and nuclear proteins were isolated using nuclear fractionation kit (Figure 5A and B). Our results showed that XIAP levels steadily increased in the nuclear fraction of the cells treated by CAPE (Figure 5A and B). The nuclear retention of XIAP was further confirmed by immunofluorescence study. Our results show intensified staining of XIAP in the nucleus in response to the CAPE treatment (Figure 5C). We also confirmed the modulation of AKT phosphorylation by immunofluorescence. As expected, CAPE treatment drastically decreased cytosolic p-AKT (Ser 473) as indicated by reduced staining (Figure 5D).
Fig. 5.
CAPE increases nuclear translocation of XIAP. (A) SK-MEL-28 and (B) B16F0 melanoma cells were treated with 20 µM CAPE for 48 h and nuclear fraction were isolated using nuclear fractionation kit. Represented western blots were immunoblotted with XIAP antibody. Same blots were stripped and re-probed with actin and lamin B to ensure equal protein loading. (C and D) B16F0 cells were treated with dimethyl sulfoxide or 20 µM CAPE, immunostained with XIAP and p-AKT (Ser 473), and actin antibodies and visualized under fluorescence microscope (Olympus Inc). The experiments were repeated three times with similar results obtained.
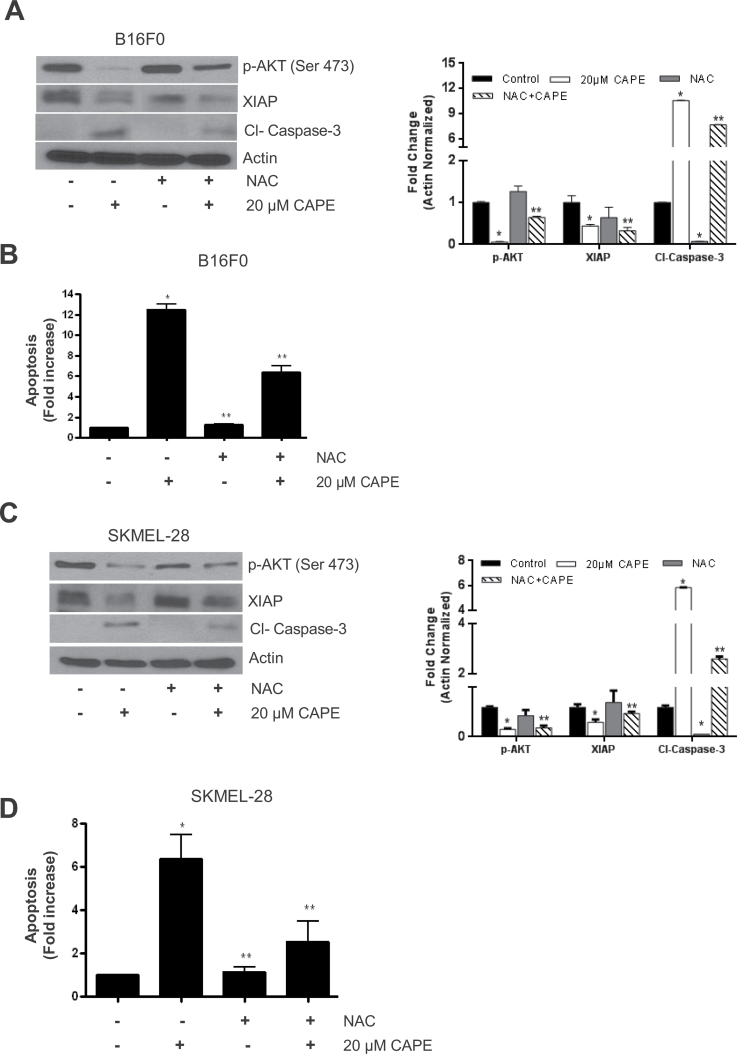
CAPE-mediated reactive oxygen species generation inhibits AKT/XIAP pathway
In our previous study, we have shown that CAPE treatment caused reactive oxygen species (ROS) generation in melanoma cells (1). In this study, we investigated the involvement of ROS in the cytotoxic effects of CAPE in melanoma cells. B16F0 and SK-MEL-28 cells were pretreated with 10 mM NAC (an antioxidant) for 1 h, followed by 20 µM CAPE treatment for 48 h. Our results demonstrated that CAPE treatment failed to suppress the phosphorylation of AKT, and the protein levels of XIAP, in the cells pretreated with NAC (Figure 6A and B). Further, NAC treatment blocked CAPE-mediated cleavage of caspase-3, indicating that CAPE-mediated ROS was involved in modulating AKT/XIAP pathway, leading to apoptosis in melanoma cells. These observations were further confirmed by Annexin V/FITC assay (Figure 6C and D).
Fig. 6.
CAPE-mediated ROS generation inhibits AKT/XIAP pathway. B16F0 and SK-Mel-28 melanoma cells were pretreated with 5 mM NAC followed by 20 µM CAPE treatment for 48 h. (A and B) whole-cell lysates were immunoblotted with p-AKT (Ser 473), XIAP and cleaved caspase-3. Same blots were stripped and re-probed for actin to ensure equal protein loading. (C and D) Apoptosis assay was determined using Annexin V/FITC and propidium iodide and analyzed by flow cytometry as described in Materials and methods section. Results are expressed as mean ± SD of three independent experiments. *P < 0.05, statistically significant compared with control. **P < 0.05, statistically significant compared with CAPE treatment.
Discussion
In our previous studies, we have demonstrated that CAPE treatment suppressed the growth of melanoma cells by inducing apoptosis (1). However, the exact mechanism by which CAPE-induced apoptosis was not clear. Our current study demonstrated that administration of 10 mg/kg CAPE substantially suppressed the growth of melanoma tumors in C57BL/6 mice. Moreover, tumor growth suppression by CAPE treatment was associated with inhibition of PI3K/AKT/XIAP pathway and increased apoptosis. Our results showed that CAPE treatment inhibited the phosphorylation of PI3K at Tyr 458, AKT at Ser 473 and mTOR at Ser 2448 in CAPE-treated tumors and in melanoma cells. On the other hand, CAPE treatment increased the cleavage of caspase-3 and PARP, indicating apoptosis. Overexpression of AKT or XIAP significantly blocked the apoptosis-inducing effects of CAPE, indicating that CAPE suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway.
Constitutive activation of PI3K/AKT pathway was associated with poor clinical outcome in various types of cancers including melanoma. Previous report has shown that more than 55% of the cancers have hyperactivation of AKT, making it as an attractive molecular target (33). A previous study has shown that CAPE induces G1-phase cell-cycle arrest but not apoptosis in prostate cancer cells via Akt inhibition (34). In contrast, our previous study demonstrated S-phase cell-cycle arrest by CAPE treatment in melanoma cells (1). The discrepancy in these observations could be due to the difference in the cancer model and cell lines used. Our in vitro results showed that CAPE treatment drastically suppressed the phosphorylation of PI3K at Tyr 458, AKT at Ser 473, PDK1 at Ser 241 and mTOR at Ser 2448 in concentration- and time-dependent manner in SK-MEL-28 and B16F0 melanoma cells. CAPE treatment also drastically reduced the XIAP protein levels. XIAP belonging to the family of inhibitors of apoptosis proteins is regulated through PI3K pathway (10). Our results are in agreement with other study, which reported that CAPE can suppress the proliferation of PC-3 cells (8). PI3K inhibitor LY294002 significantly increased the effects of CAPE in melanoma cells confirming the involvement of PI3K in CAPE-induced apoptosis.
Because PI3K activates a number of signaling proteins such as AKT, we investigated here whether the regulation of XIAP by the PI3K pathway is mediated through AKT. Our immunoprecipitation study demonstrated that CAPE treatment drastically decreased the interaction of AKT with XIAP, as well as AKT kinase activity in SK-MEL-28 and B16F0 melanoma cells, suggesting that AKT/XIAP is the target of CAPE. On the other hand, ectopic expression of AKT substantially increased the levels of XIAP that was primarily inhibited by CAPE and suppressed apoptosis in melanoma cells, suggesting that XIAP expression was regulated by AKT. Our results also revealed that CAPE treatment increased the nuclear translocation of XIAP in melanoma cells. These results are in agreement with other studies where nuclear translocation of XIAP increased apoptosis (31,32). In addition, CAPE treatment decreased XIAP expression and increased apoptosis in melanoma cells. We reported previously that CAPE treatment causes ROS generation in melanoma cells (1). We hypothesized that CAPE-mediated ROS were involved with the inhibition of PI3K/AKT/XIAP pathways. In agreement with our hypothesis, our results showed that antioxidant NAC blocked CAPE-mediated inhibition of PI3K/AKT/XIAP, indicating that involvement of pro-oxidants in the inhibition of PI3K/AKT/XIAP pathway.
Apoptosis is a genetically controlled process, regulated by multiple anti- and pro-apoptotic proteins. XIAP is a known caspase inhibitor and suppresses apoptosis induced by various stimuli (35–37). Previous study has shown that XIAP downregulation induces apoptosis in chemoresistance human ovarian cancer cells (38). In agreement with this, our results revealed that XIAP was inhibited by CAPE treatment, and XIAP overexpression suppressed CAPE-induced apoptosis in melanoma cells. Therefore, it is likely that downregulation of XIAP expression by CAPE treatment will decrease the threshold of cell death and may increase the efficacy of chemotherapy-induced cell death in melanoma cancer cells. A proposed molecular mechanism of CAPE in melanoma cells has been described in Supplementary Figure 1, available at Carcinogenesis Online. In summary, our in vivo and in vitro results showed that CAPE-mediated melanoma tumor growth suppression was associated with the inhibition of PI3K/AKT/XIAP pathway.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (R01 grants CA106953, CA129038) to S.K.S.
Supplementary Material
Acknowledgement
Kind gift of AKT expression plasmid by Dr D.Altschuler (University of Pittsburgh) is greatly appreciated.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CAPE
caffeic acid phenethyl ester
- FITC
fluorescein isothiocyanate
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-cysteine
- PARP
poly (ADP ribose) polymerase
- PDK1
phosphoinositide-dependent kinase-1
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- XIAP
X-linked inhibitor of apoptosis protein.
References
- 1. Kudugunti S.K., et al. (2011). Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Invest. New Drugs 29, 52–62 [DOI] [PubMed] [Google Scholar]
- 2. Cartee T.V., et al. (2011). Melanoma reporting to central cancer registries by US dermatologists: an analysis of the persistent knowledge and practice gap. J. Am. Acad. Dermatol., 65(5 suppl. 1),S124–S132 [DOI] [PubMed] [Google Scholar]
- 3. Kudugunti S.K., et al. (2010). Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines. Chem. Biol. Interact., 188, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doan P.S. (2008). Primary cutaneous malignant melanoma. Aviat. Space. Environ. Med., 79, 919–921 [DOI] [PubMed] [Google Scholar]
- 5. Watson M., et al. (2011). Melanoma surveillance in the United States: overview of methods. J. Am. Acad. Dermatol., 65(5 suppl. 1), S6–16 [DOI] [PubMed] [Google Scholar]
- 6. Whiteman D., et al. (1999). The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med., 341, 766–767 [PubMed] [Google Scholar]
- 7. Bataille V., et al. (2008). Melanoma–Part 1: epidemiology, risk factors, and prevention. BMJ., 337, a2249 [DOI] [PubMed] [Google Scholar]
- 8. Lin H.P., et al. (2012). Caffeic acid phenethyl ester causes p21 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS One., 7, e31286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dan H.C., et al. (2004). Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem., 279, 5405–5412 [DOI] [PubMed] [Google Scholar]
- 10. Carter B.Z., et al. (2003). Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia., 17, 2081–2089 [DOI] [PubMed] [Google Scholar]
- 11. Vidwans S.J., et al. (2011). A melanoma molecular disease model. PLoS One., 6, e18257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang C., et al. (2005). Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology., 213, 81–96 [DOI] [PubMed] [Google Scholar]
- 13. Jung W.K., et al. (2008). Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int. J. Biochem. Cell Biol., 40, 2572–2582 [DOI] [PubMed] [Google Scholar]
- 14. Demestre M., et al. (2009). CAPE (caffeic acid phenethyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice. Phytother. Res., 23, 226–230 [DOI] [PubMed] [Google Scholar]
- 15. Bhimani R.S., et al. (1993). Inhibition of oxidative stress in HeLa cells by chemopreventive agents. Cancer Res., 53, 4528–4533 [PubMed] [Google Scholar]
- 16. Bhimani R.S., et al. (1995). Human promyelocytic leukemia cells (HL-60): a new model to study the effects of chemopreventive agents on H2O2 production. Cancer Detect. Prev., 19, 292–298 [PubMed] [Google Scholar]
- 17. Su Z.Z., et al. (1991). Suppression of adenovirus type 5 E1A-mediated transformation and expression of the transformed phenotype by caffeic acid phenethyl ester (CAPE). Mol. Carcinog., 4, 231–242 [DOI] [PubMed] [Google Scholar]
- 18. Grunberger D., et al. (1988). Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia., 44, 230–232 [DOI] [PubMed] [Google Scholar]
- 19. Watabe M., et al. (2004). Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J. Biol. Chem., 279, 6017–6026 [DOI] [PubMed] [Google Scholar]
- 20. McEleny K., et al. (2004). Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int., 94, 402–406 [DOI] [PubMed] [Google Scholar]
- 21. Wang L.C., et al. (2010). Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol., 160, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y.J., et al. (2001). Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J. Agric. Food Chem., 49, 5615–5619 [DOI] [PubMed] [Google Scholar]
- 23. He Y.J., et al. (2006). Inhibitory effect of caffeic acid phenethyl ester on the growth of SW480 colorectal tumor cells involves beta-catenin associated signaling pathway down-regulation. World J. Gastroenterol., 12, 4981–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuo H.C., et al. (2006). Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett., 234, 199–208 [DOI] [PubMed] [Google Scholar]
- 25. Frenkel K., et al. (1993). Inhibition of tumor promoter-mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester. Cancer Res., 53, 1255–1261 [PubMed] [Google Scholar]
- 26. Huang M.T., et al. (1996). Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis., 17, 761–765 [DOI] [PubMed] [Google Scholar]
- 27. Pramanik K.C., et al. (2012). Apoptosis signal-regulating kinase 1-thioredoxin complex dissociation by capsaicin causes pancreatic tumor growth suppression by inducing apoptosis. Antioxid. Redox Signal., 17, 1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boreddy S.R., et al. (2011). Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin. Cancer Res., 17, 1784–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pramanik K.C., et al. (2011). Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One., 6, e20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahu R.P., et al. (2009). The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J. Natl Cancer Inst., 101, 176–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell J.C., et al. (2008). Nuclear translocation of X-linked inhibitor of apoptosis (XIAP) determines cell fate after hypoxia ischemia in neonatal brain. J. Neurochem., 106, 1357–1370 [DOI] [PubMed] [Google Scholar]
- 32. Nowak D., et al. (2004). Upon drug-induced apoptosis in lymphoma cells X-linked inhibitor of apoptosis (XIAP) translocates from the cytosol to the nucleus. Leuk. Lymphoma., 45, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 33. Schlieman M.G., et al. (2003). Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br. J. Cancer., 89, 2110–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chuu C.P., et al. (2012). Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. (Phila)., 5, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deveraux Q.L., et al. (1997). X-linked IAP is a direct inhibitor of cell-death proteases. Nature., 388, 300–304 [DOI] [PubMed] [Google Scholar]
- 36. Deveraux Q.L., et al. (1998). IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J., 17, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Datta R., et al. (2000). XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J. Biol. Chem., 275, 31733–31738 [DOI] [PubMed] [Google Scholar]
- 38. Sasaki H., et al. (2000). Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res., 60, 5659–5666 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.