Abstract
Currently, smokeless tobacco products are being proposed as an alternative mode of tobacco use associated with less harm. All of these products contain the tobacco-specific carcinogen N′-nitrosonornicotine (NNN). The major form of NNN in tobacco products is (S)-NNN, shown in this study to induce a total of 89 benign and malignant oral cavity tumors in a group of 20 male F-344 rats treated chronically with 14 p.p.m. in the drinking water. The opposite enantiomer (R)-NNN was weakly active, but synergistically enhanced the carcinogenicity of (S)-NNN. Thus, (S)-NNN is identified for the first time as a strong oral cavity carcinogen in smokeless tobacco products and should be significantly reduced or removed from these products without delay in order to prevent debilitating and deadly oral cavity cancer in people who use them.
Introduction
Smokeless tobacco use, heavily promoted by the tobacco industry with a half billion dollars per year in advertising, is increasing in the USA and is firmly entrenched in other areas of the world including parts of Scandinavia, Southeastern Asia and Africa (1). The most common products in the USA, where nearly one in five white adolescent males (18–25 years old) and 7% of all men use smokeless tobacco, are pinches or teabag-like sachets of moist snuff placed between the cheek and gum, whereas in other parts of the world a wide variety of products are used in different ways by hundreds of millions of people (1–3). As new products are regularly introduced by the tobacco industry, it seems inevitable that smokeless tobacco use will continue to increase in the USA.
Smokeless tobacco is an accepted cause of oral cavity cancer (1,4), with thousands of the estimated quarter million new cases each year worldwide attributed to its use (5,6). In USA, the current 5 year survival rate from oral cavity cancer, a disease that can severely diminish quality of life by impairing vital functions such as speech, taste and swallowing, is 61% (7). Prevention of this dreadful, debilitating and often fatal disease is crucial. Until now, no strong oral cavity carcinogens have been identified in smokeless tobacco.
All smokeless tobacco products contain the carcinogen N′-nitrosonornicotine (NNN), frequently in amounts 100- to1000-fold higher than carcinogenic nitrosamines in other products designed for ingestion such as food or beer (1,8–12). Current levels of NNN in the most popular smokeless tobacco products in the USA range from 1 to 10 µg/g dry weight, whereas in other areas of the world there is great variation from generally low levels in Swedish products to very high amounts in products from Sudan (1,9,11,12). NNN, with a chiral center at its 2′-position, exists in enantiomeric forms (Figure 1). (S)-NNN is the predominant enantiomer in currently marketed smokeless tobacco products, comprising 57–66% of total NNN in these products (13,14). The carcinogenicity of racemic NNN [a 50:50 mixture of (R)- and (S)-NNN] has been demonstrated in rats, mice, hamsters and mink (1,15). Tumors were observed at various sites, most commonly in the esophagus of rats, with only a few oral cavity tumors recorded. Carcinogenicity assays of NNN enantiomers have not been reported.
Fig. 1.
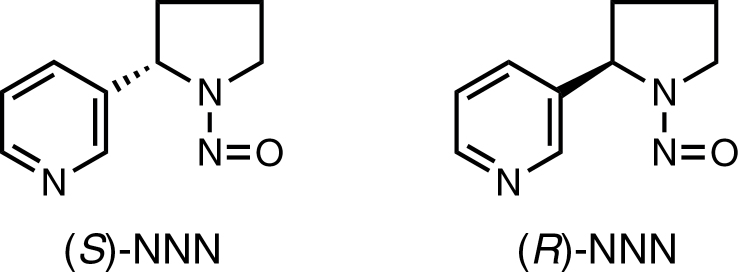
Structures of (S)-NNN and (R)-NNN.
We have previously carried out comparative studies of the metabolism and DNA binding of (S)-NNN and (R)-NNN in rats (16–18). These studies demonstrated that (S)-NNN was metabolized by 2′-hydroxylation, a known pathway leading to pyridyloxobutyl (POB)–DNA adduct formation, to a significantly greater extent than (R)-NNN in the esophagus. Further studies demonstrated that DNA adduct formation from (S)-NNN significantly exceeded that from (R)-NNN in the esophagus, oral cavity and liver of F-344 rats treated with the compounds in the drinking water for 20 weeks (16,17). These studies strongly suggested that (S)-NNN would be more carcinogenic than (R)-NNN to the rat esophagus and, intriguingly, further indicated that the oral cavity might also be a target tissue for (S)-NNN (16,17). Therefore, we carried out a comparative carcinogenicity study in F-344 rats of (S)-NNN, (R)-NNN and racemic NNN, the results of which are reported in this article.
In this study, we demonstrate the powerful oral cavity carcinogenicity of (S)-NNN in the rat, firmly establishing it as the only identified strong oral cavity carcinogen in smokeless tobacco, and a clear target for immediate removal from these products as a concrete step toward prevention of oral cavity cancer.
Materials and methods
Synthesis and characterization of NNN enantiomers
Nornicotine enantiomers were prepared as described (19), and nitrosated and purified by column chromatography on silica gel as described, providing the pure NNN enantiomers (13). Racemic NNN was similarly synthesized from racemic nornicotine. The nuclear magnetic resonance and mass spectra of (S)-NNN and (R)-NNN were identical to those of racemic NNN. Specific rotations of the enantiomers were as described previously (13). Purity as assessed by high-performance liquid chromatography (HPLC) with UV detection was 96.5% for (S)-NNN and >99% for (R)-NNN and racemic NNN.
Bioassay of NNN enantiomers in male F-344 rats
This study was approved by the University of Minnesota Institutional Animal Care and Use Committee. Male F-344 rats, age 6 weeks, were obtained from Charles River Laboratories (Kingston, NY), housed 2 per cage in 98 cages with Harlan-irradiated Corncob bedding (Harlan, Indianapolis, IN) and allowed to acclimate to the Research Animal Resources Facility, University of Minnesota, for 1 week. The rats were maintained under standard conditions (20–24°C, 29–32% relative humidity and 14/10 light/dark cycle). They were fed Harlan Teklad 2018 diet for the first 8 months of the study and then switched to Harlan Teklad 7022 diet (NIH-07) for the remainder; the compositions of these diets are virtually identical. The rats were divided into groups as follows: (S)-NNN, 24 rats; (R)-NNN, 24 rats; racemic NNN, 15 rats and control (tap water), 24 rats. Test compounds were added to the drinking water as follows: (S)-NNN or (R)-NNN, 14 p.p.m. and racemic NNN, 28 p.p.m. Aqueous stock solutions of the test compounds were prepared weekly and stored at 4°C. Stock solutions were analyzed by HPLC using an Agilent 1100 capillary flow HPLC with a diode array UV detector set at 254nm (Agilent Technologies, Palo Alto, CA). A 4.6mm × 25cm Luna 5 µm C18 column (Phenomenex, Torrance, CA) was used with a gradient from 5 to 40% CH3OH in H2O over the course of 35min at a flow rate of 10 µl/min. Based on the concentration of the stock solutions, the appropriate dilutions were performed to prepare the drinking water solutions, and the concentrations of the drinking water solutions were confirmed by HPLC. Drinking water was also analyzed after administration to the rats. The average concentrations measured for the three solutions over the course of the study duration were 14±2 p.p.m. for (S)-NNN and (R)-NNN and 28±2 p.p.m. for racemic NNN. Drinking water was changed three times weekly and consumption was measured. Rats were inspected daily and weighed monthly. Doses were calculated from the amount of water consumed in each rat cage and the average weights of the rats in that cage.
Moribund rats were humanely euthanized. Four rats from the (S)-NNN group, three rats from the racemic NNN group and two rats from the control group died unexpectedly and were not subjected to full necropsy. At termination, all animals were evaluated for number of tumors and a complete necropsy was performed. Major organs and gross lesions encountered during necropsy were fixed in 10% formalin. The head, tongue and larynx were decalcified using a commercially prepared decalcifying solution consisting of dilute HCl and ethylenediaminetetraacetic acid (Newcomer Supply, Middleton, WI). Fixed tissue specimens were processed into paraffin blocks using standard histology techniques, sectioned to 4 µm thickness and stained with hematoxylin and eosin. Histology slides were evaluated using light microscopy, and diagnoses were verified by two A.C.V.P.-certified pathologists (C.S.J. and M.G.O’S.). The tongue, larynx, pharynx, oral mucosa, soft and hard palate, and esophagus were methodically evaluated for tumors ≥0.5mm using a Nikon SMZ 1000 stereomicroscope. Tumor counts were performed blinded as to treatment group.
Statistical analysis
The number of esophageal and oral cavity tumors per rat for each of the groups was assessed using a one-way analysis of variance. We tested (i) the comparison of each of the three treated groups to the control group, (ii) the pairwise differences among the three groups and (iii) the difference between the additive effect of (R)-NNN and (S)-NNN versus racemic NNN. All tests were conducted at the 5% level of significance, and the P values obtained from the pairwise t-tests in (i) and (ii) were Bonferroni adjusted. The additive effect in (iii) was assessed using an F test. Fisher’s exact tests at 5% level of significance adjusted for multiple comparisons were used to assess the difference in the proportion of rats with papilloma or carcinoma of the oral cavity (Table II) for each of the three treatment groups and the control group.
Table II.
Results of histopathological analysis of rats treated with NNN enantiomers or racemic NNN in drinking water for 17–19 months
| Addition to drinking water | ||||
|---|---|---|---|---|
| None | (S)-NNN 14 p.p.m. | (R)-NNN 14 p.p.m. | Racemic NNN 28 p.p.m. | |
| Number of rats analyzed | 22 | 20 | 24 | 11 or 12a |
| Number of rats with each type of lesion | ||||
| Tongue | ||||
| Atypical hyperplasiab | 0 | 15 | 1 | 10 |
| Squamous cell papilloma | 0 | 13 | 3 | 11 |
| Squamous cell carcinoma | 0 | 2 | 0 | 1 |
| Oral mucosa | ||||
| Atypical hyperplasia | 0 | 16 | 0 | 9 |
| Squamous cell papilloma | 0 | 11 | 1 | 8 |
| Squamous cell carcinoma | 0 | 1 | 0 | 0 |
| Soft palate | ||||
| Atypical hyperplasia | 0 | 13 | 0 | 4 |
| Squamous cell papilloma | 0 | 10 | 4 | 3 |
| Squamous cell carcinomac | 0 | 1 | 0 | 0 |
| Epiglottis | ||||
| Atypical hyperplasia | 0 | 16 | 1 | 9 |
| Squamous cell papilloma | 0 | 11 | 0 | 6 |
| Squamous cell carcinoma | 0 | 1 | 0 | 2 |
| Pharynx | ||||
| Atypical hyperplasia | 0 | 18 | 0 | 12 |
| Squamous cell papilloma | 0 | 11 | 0 | 9 |
| Squamous cell carcinoma | 0 | 1 | 0 | 3 |
| Number of rats with papilloma or carcinoma of the oral cavity | 0 | 20 | 7 | 12 |
| Esophagus | ||||
| Atypical hyperplasia | 0 | 18 | 0 | 12 |
| Squamous cell papilloma | 0 | 19 | 1 | 12 |
| Squamous cell carcinoma | 0 | 2 | 0 | 4 |
aBecause of autolysis, a limited set of tissues (i.e. tongue, pharynx and esophagus) was evaluated from a 12th animal.
bAtypical hyperplasia refers to hyperplastic lesions involving the epithelium that are characterized by downward irregular, often finger-like, projections of the basal layers of the epithelium into the underlying tissue; increased mitoses and dysplasia reminiscent of squamous cell carcinoma in situ may also be observed in the most florid representation.
cSoft palate was secondarily involved in two control animals and one (R)-NNN-treated animal due to extension from squamous cell carcinoma involving the hard palate and/or nasal cavity.
Results
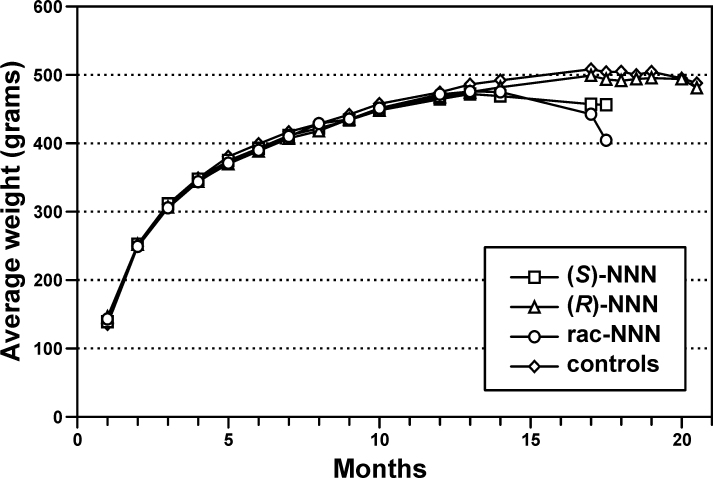
The rats in the groups treated with (S)-NNN or racemic NNN began losing weight after 1 year (Figure 2) and had died or were humanely euthanized by 17 months because of decreasing body weights or morbidity. The rats treated with (R)-NNN and the control rats were terminated at 20 months, based on decreasing weights. Total doses were as follows: (S)-NNN, 123mg/rat, 336mg/kg body wt; (R)-NNN, 143mg/rat, 362mg/kg body wt and racemic NNN, 234mg/rat, 652mg/kg body wt.
Fig. 2.
Weight curves for the groups of rats treated with (S)-NNN, (R)-NNN, racemic NNN or water only. The average weight of each group at monthly intervals is shown.
At necropsy, all rats treated with (S)-NNN had oral cavity tumors. A total of 89 oral cavity tumors were observed in the 20 rats that were subjected to necropsy, including tumors of the tongue, buccal and gingival oral mucosa, soft palate, epiglottis and pharynx. Some of the oral cavity tumors were >4mm in diameter: tongue (nine tumors); oral (buccal or gingival) mucosa (two tumors); soft palate (four tumors) and pharynx (five tumors). The rats treated with (S)-NNN also had 122 esophageal tumors. The results are summarized in Table I.
Table I.
Number of tumors in the oral cavity and esophagus observed upon necropsy of rats treated with NNN enantiomers or racemic NNN in drinking water for 17–20 months
| Addition to drinking water | ||||
|---|---|---|---|---|
| None | (S)-NNN 14 p.p.m. | (R)-NNN 14 p.p.m. | Racemic NNN 28 p.p.m. | |
| Number of rats analyzed | 22 | 20 | 24 | 12 |
| Number of tumors in target tissues | ||||
| Tongue | 0 | 30 | 4 | 28 |
| Oral mucosaa | 0 | 20 | 1 | 18 |
| Soft palate | 0 | 10 | 1 | 14 |
| Epiglottis | 0 | 14 | 0 | 16 |
| Pharynx | 0 | 15 | 0 | 20 |
| Total oral cavity | 0 | 89 (4.5)b | 6 (0.25) | 96 (8.0) |
| Esophagus | 0 | 122 (6.1) | 3 (0.13) | 153 (13) |
aBuccal or gingival mucosa.
bNumber of tumors per rat.
In contrast with the results obtained in the rats treated with (S)-NNN, only six oral cavity tumors and three esophageal tumors were observed in the 24 rats that were subjected to necropsy after treatment with (R)-NNN. Comparisons of numbers of oral cavity or esophageal tumors between the (S)-NNN and the (R)-NNN groups or the (S)-NNN and control groups were highly significant (P < 0.0001), whereas the number of these tumors induced by (R)-NNN was not significantly different from controls.
Racemic NNN was also highly carcinogenic to the rat oral cavity and esophagus. All rats treated with racemic NNN had tumors in these tissues. A total of 96 oral cavity tumors and 153 esophageal tumors were observed in the 12 rats subjected to necropsy. The carcinogenicity of racemic NNN in the oral cavity and esophagus was significantly greater (P < 0.0001) than that of (S)-NNN and than the additive effects of (S)-NNN and (R)-NNN. Thus, (R)-NNN acted as a classic co-carcinogen, synergistically enhancing the carcinogenicity of (S)-NNN while itself showing insignificant activity with respect to number of tumors per animal.
The results of the histopathological analysis are summarized in Table II. Oral cavity tissues (but not hard palate) and esophagus were identified as target tissues in this study. Squamous cell carcinoma (Figure 3) and papilloma of the oral cavity and esophagus were confirmed, and atypical hyperplasia was commonly observed in the rats treated with (S)-NNN or racemic NNN, but much less frequently in the rats treated with (R)-NNN. The proportion of rats with papilloma or carcinoma of the oral cavity was significantly greater in the (S)-NNN- or racemic NNN-treated groups compared with the rats treated with (R)-NNN or untreated (P < 0.01), and was greater in the (R)-NNN group than in controls (P < 0.01).
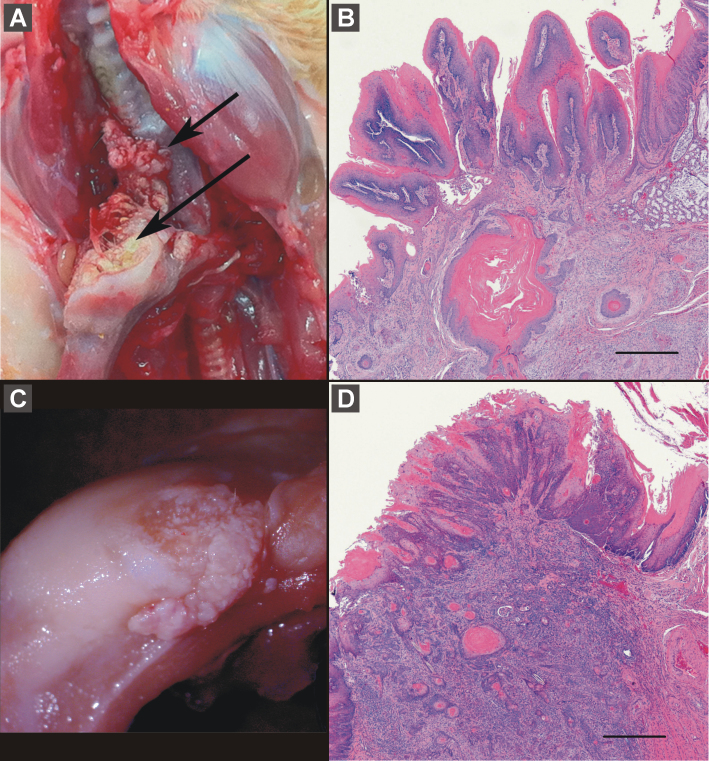
Fig. 3.
Squamous cell carcinoma in animals treated with (S)-NNN. Plate A shows squamous cell carcinomas involving both the soft palate (short arrow) and tongue (long arrow); corresponding histology showing invasion of soft palate is shown in plate B (bar = 500 µm). Plate C shows another squamous cell carcinoma involving the tongue, and corresponding histology showing invasion of underlying skeletal muscle is shown in plate D (bar = 500 µm).
We also observed squamous cell carcinoma of the hard palate and adjacent tissues in each of two control rats and one (R)-NNN-treated rat. These tumors were considered to be incidental or spontaneous tumors in aged animals.
Adenomas involving the respiratory epithelium of the nasal cavity were observed in 6 of the 20 rats treated with (S)-NNN; one of these rats also had a small olfactory neuroblastoma involving the vomeronasal organ. Respiratory epithelium nasal cavity adenomas were also seen in 4 of the 24 rats treated with (R)-NNN and 3 of the 11 rats treated with racemic NNN, but not in controls. Adenomas of the respiratory epithelium of the larynx were observed in 3 of the 20 rats treated with (S)-NNN, 5 of the 24 rats treated with (R)-NNN and 1 rat treated with racemic NNN, but not in controls. Thus, the significant differences in response to (S)-NNN and (R)-NNN, which were seen in the oral cavity and esophagus, were not observed in the nasal cavity and larynx.
Discussion
The results of this study clearly demonstrate that (S)-NNN and racemic NNN are powerful oral cavity carcinogens in the male F-344 rat. (S)-NNN is the predominant form of NNN present in popular smokeless tobacco products marketed in the USA. In view of these results, significant reduction of NNN in all smokeless tobacco products is urgently needed.
It is well established that NNN requires metabolic activation in target tissues to exert its carcinogenic activity, and the resultant POB–DNA adducts formed upon cytochrome P450-mediated 2′-hydroxylation have been extensively characterized (16). We recently compared POB–DNA adduct formation in tissues of rats chronically treated with 10 p.p.m. (S)-NNN or (R)-NNN in the drinking water (16,17). The results predicted the outcome of this study in that levels of POB–DNA adducts in both the esophagus and oral cavity were significantly higher in the rats treated with (S)-NNN than with (R)-NNN, consistent with earlier metabolism studies (18,20). These results demonstrate the critical importance of DNA adduct formation in carcinogenesis by NNN and support their use as predictive biomarkers for oral cancer. Detection of POB–DNA adducts in oral tissues of smokeless tobacco users might be a path to cancer prevention, as intensive cessation efforts and surveillance could be initiated. We have recently developed the technology for analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human exfoliated oral mucosa cells (21).
Several previous carcinogenicity studies of racemic NNN administered in the drinking water or in a liquid diet to male F-344 rats have been reported. All of those studies resulted in high incidences of esophageal tumors but none produced a significant yield of oral cavity tumors. The doses used in those studies were either so high that survival was significantly shortened by lethal esophageal tumors, preventing observation of oral cavity tumors or were too low to observe oral cavity tumors (22–25). In addition, the metabolic activation of NNN in the oral cavity and esophagus is dose dependent with higher efficiency at lower doses (18,20). The dose used in this study may have been optimal compared with previous studies for oral mucosal metabolic activation of NNN. In another carcinogenicity study, we applied a mixture of racemic NNN and the related tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) repeatedly to the rat oral cavity by swabbing (26). The total racemic NNN dose was about 40% of that reported here. Although the incidence of oral tumors was significant (9 papillomas in 8 of 30 rats), the results were not nearly as convincing as those presented in this article. NNK did not induce oral cavity tumors when swabbed repeatedly in the rat oral cavity (27).
This is the first study to report the strong oral cavity carcinogenicity of any constituent of smokeless tobacco. The most commonly used animal models for induction of oral cavity cancer by carcinogens involve treatment of hamsters with 7,12-dimethylbenz[a]anthracene or either mice or rats with 4-nitroquinoline-N-oxide (28–31). Neither of these compounds is present in tobacco. Another approach uses mice treated with dibenzo[a,l]pyrene (32) or its diol epoxide metabolite. Dibenzo[a,l]pyrene has been detected in trace quantities in tobacco smoke but not in tobacco. Benzo[a]pyrene and other polycyclic aromatic hydrocarbons in smokeless tobacco (33) do not induce oral cancer in animal models. It should be noted that carcinogenicity tests of smokeless tobacco itself are exceedingly difficult to perform in a reproducible manner because laboratory animals will under no circumstances use smokeless tobacco voluntarily and repetitively, as it is used by humans. Although some studies using specialized surgical techniques have given positive results, there is no widely accepted model for induction of oral cancer by smokeless tobacco itself (1). Our results provide a new and relevant model of oral cavity cancer induction by tobacco products.
Based on consumption of a half-tin per day (17g) of a popular smokeless tobacco product (34) containing about 3 µg NNN per gram tobacco (35) and an extraction efficiency of 60% (36), human exposure to NNN would be about 31 µg/day, compared with the daily dose in rats of about 280 µg/day. The approximate total dose of NNN in 30 years of use would be about 340mg (5mg/kg) in a smokeless tobacco user. This compares with a total dose of 123mg (336mg/kg) (S)-NNN in this study. It is unclear whether a body weight correction is relevant considering that smokeless tobacco is concentrated in the oral cavity and frequently held at one site.
Cigarette smoking is an established cause of oral cavity cancer (37). All cigarettes deliver NNN in their smoke, in amounts which range from 5 to 270 ng/cigarette (37,38). Levels of NNN in smoke are highly correlated with those in tobacco, and NNN in smoke is predominantly (S)-NNN (14,39). About 11% of NNN in smoke is transferred from tobacco during cigarette smoking (39). The results presented in this study are also likely to be highly relevant to oral cancer etiology in smokers.
It is recognized that target tissues of nitrosamines in rats may not predict those in humans. However, we have observed considerable coherence between NNN and NNK rat target tissues and cancer risk among smokers in the prospective Shanghai Cohort Study. Smokers with the highest levels of urinary NNN or NNAL (a metabolite of the lung carcinogen NNK) had significantly increased risks for esophageal and lung cancer, respectively, supporting the relevance of F-344 rat target tissues of tobacco-specific nitrosamines to those in humans (40,41).
In conclusion, the results of this study provide a previously unrecognized and critical link between a tobacco constituent and oral cavity cancer in tobacco users, thus clarifying new approaches to oral cancer prevention. Our results also establish a new and relevant rat model for oral cavity cancer induction. Although the most effective pathway to prevention is avoidance of all tobacco products, it is not always possible due to their addictive properties. The risk:benefit ratio for (S)-NNN in tobacco is infinite. The existing technology to substantially reduce its levels should be applied without delay.
Funding
United States National Cancer Institute (CA-81301).
Conflict of Interest Statement: None declared.
Acknowledgements
We thank B.Carlson for editorial assistance and J.Parker (University of Minnesota, Masonic Cancer Center, Comparative Pathology Shared Resource) for technical assistance including image preparation.
Glossary
Abbreviations:
- HPLC
high-performance liquid chromatography
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN, N′-nitrosonornicotine; POB
pyridyloxobutyl.
References
- 1. IARC (2007). Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 89 International Agency for Research on Cancer, Lyon: [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention Smokeless Tobacco Facts. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/smokeless_facts/ (31. January 2012, date last accessed).
- 3. United States Department of Health and Human Services (2012). Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. U.S. Department of Health and Human Services, Washington DC: [Google Scholar]
- 4. United States Surgeon General (1986). The Health Consequences of Using Smokeless Tobacco. U.S. Department of Health and Human Services, WashingtonDC [Google Scholar]
- 5. Boffetta P., et al. (2008). Smokeless tobacco and cancer. Lancet Oncol., 9, 667–675 [DOI] [PubMed] [Google Scholar]
- 6. Jemal A., et al. (2011). Global cancer statistics. CA. Cancer J. Clin., 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 7. Rethman M.P, et al. (2012) Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. Tex. Dent. J., 129, 491–507 [PubMed] [Google Scholar]
- 8. Hoffmann D., et al. (1974). Nʹ-nitrosonornicotine in tobacco. Science, 186, 265–267 [DOI] [PubMed] [Google Scholar]
- 9. Stepanov I., et al. (2012). Increased pouch sizes and resulting changes in the amounts of nicotine and tobacco-specific N-nitrosamines in single pouches of Camel Snus and Marlboro Snus. Nicotine Tob. Res., 14, 1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stepanov I., et al. (2012). Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from Round 1 of the New Product Watch. Nicotine Tob. Res., 14, 274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stanfill S.B., et al. (2011) Global surveillance of oral tobacco products: total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob. Control, 20, e2. [DOI] [PubMed] [Google Scholar]
- 12. Richter P., et al. (2008). Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob. Res., 10, 1645–1652 [DOI] [PubMed] [Google Scholar]
- 13. Carmella S.G., et al. (2000). Enantiomeric composition of Nʹ-nitrosonor nicotine and Nʹ-nitrosoanatabine in tobacco. Carcinogenesis, 21, 839–843 [DOI] [PubMed] [Google Scholar]
- 14. Stepanov I., et al. (2013). Levels of (S)-Nʹ-nitrosonornicotine in U.S. tobacco products. Nicotine Tob. Res., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecht S.S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol., 11, 559–603 [DOI] [PubMed] [Google Scholar]
- 16. Lao Y., et al. (2007). Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-Nʹ-nitrosonornicotine. Chem. Res. Toxicol., 20, 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S., et al. (2009). Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol., 22, 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIntee E.J., et al. (2000). Metabolism of Nʹ-nitrosonornicotine enantiomers by cultured rat esophagus and in vivo in rats. Chem. Res. Toxicol., 13, 192–199 [DOI] [PubMed] [Google Scholar]
- 19. Seeman J.I., et al. (1985). Synthesis of the enantiomers of nornicotine. J. Org. Chem., 50, 5419–5421 [Google Scholar]
- 20. Murphy S.E., et al. (1990). Comparative metabolism of Nʹ-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by cultured F344 rat oral tissue and esophagus. Cancer Res., 50, 4685–4691 [PubMed] [Google Scholar]
- 21. Stepanov I., et al. (2013). Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann D., et al. (1975) A study of tobacco carcinogenesis. XIV. Effects of Nʹ-nitrosonornicotine and Nʹ-nitrosonanabasine in rats. J. Natl Cancer Inst., 55, 977–981 [DOI] [PubMed] [Google Scholar]
- 23. Hecht S.S., et al. (1983). Comparative carcinogenicity in F344 rats and Syrian golden hamsters of Nʹ-nitrosonornicotine and Nʹ-nitrosonornicotine-1-N-oxide. Cancer Lett., 20, 333–340 [DOI] [PubMed] [Google Scholar]
- 24. Castonguay A., et al. (1984). Effects of chronic ethanol consumption on the metabolism and carcinogenicity of Nʹ-nitrosonornicotine in F344 rats. Cancer Res., 44, 2285–2290 [PubMed] [Google Scholar]
- 25. Stoner G.D., et al. (1998). Inhibition of Nʹ-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis, 19, 2139–2143 [DOI] [PubMed] [Google Scholar]
- 26. Hecht S.S., et al. (1986). Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res., 46, 4162–4166 [PubMed] [Google Scholar]
- 27. Prokopczyk B., et al. (1991) A study of betel quid carcinogenesis. IX. Comparative carcinogenicity of 3-(methylnitrosamino)propionitrile and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone upon local application to mouse skin and rat oral mucosa. Cancer Lett., 60, 153–157 [DOI] [PubMed] [Google Scholar]
- 28. Guo Y., et al. (2011). Ethanol promotes chemically induced oral cancer in mice through activation of the 5-lipoxygenase pathway of arachidonic acid metabolism. Cancer Prev. Res. (Phila), 4, 1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fong L.Y., et al. (2011). Zinc supplementation suppresses 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis. Carcinogenesis, 32, 554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanojia D., et al. (2006). 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol., 42, 655–667 [DOI] [PubMed] [Google Scholar]
- 31. Gimenez-Conti I.B., et al. (1992). The hamster cheek pouch model of carcinogenesis and chemoprevention. Adv. Exp. Med. Biol., 320, 63–67 [DOI] [PubMed] [Google Scholar]
- 32. Guttenplan J.B., et al. (2012). Mutagenesis and carcinogenesis induced by dibenzo[a,l]pyrene in the mouse oral cavity: a potential new model for oral cancer. Int. J. Cancer, 130, 2783–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stepanov I., et al. (2010). Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem. Res. Toxicol., 23, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hecht S.S., et al. (2008). Exposure to nicotine and a tobacco-specific carcinogen increase with duration of use of smokeless tobacco. Tob. Control, 17, 128–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hecht S.S., et al. (2011). Major tobacco companies have technology to reduce carcinogen levels but do not apply it to popular smokeless tobacco products. Tob. Control, 20, 443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hecht S.S., et al. (2008). Metabolism of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to its biomarker total NNAL in smokeless tobacco users. Cancer Epidemiol. Biomarkers Prev., 17, 732–735 [DOI] [PubMed] [Google Scholar]
- 37. IARC (2004). Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans., Vol. 83 International Agency for Research on Cancer, Lyon, pp. 53–1187 [PMC free article] [PubMed] [Google Scholar]
- 38. Hecht S.S. (2012). Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res., 14, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stepanov I., et al. (2012). Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob. Control, 21, 44–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuan J.M., et al. (2011). Urinary levels of the tobacco-specific carcinogen Nʹ-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis, 32, 1366–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan J.M., et al. (2011). Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res., 71, 6749–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]