Abstract
Rapid-onset cardiovascular disease is a major concern for many patients suffering from SLE. Cardiovascular events are more frequent and occur much earlier in SLE patients compared to healthy controls. Traditional risk factors such as altered lipid levels, older age and smoking do not fully explain the increased risk of cardiovascular disease, strongly suggesting that autoimmunity contributes to accelerated atherosclerosis. Altered immune system function is recognized as the primary contributor to both the initiation and progression of atherosclerosis. Multiple manifestations of autoimmunity, including autoantibodies, altered cytokine levels and innate immunity response, adipokines, dysfunctional lipids, and oxidative stress appear to contribute to atherosclerotic risk. In addition, multiple SLE therapeutics appear to affect the development and progression of atherosclerosis both positively and negatively. SLE-specific biomarkers for identifying patients at risk of developing accelerated atherosclerosis are starting to be identified by multiple groups, and a comprehensive, clinically testable biomarker panel could be invaluable for identifying and treating these patients.
Introduction
Atherosclerosis, once believed to be caused by passive lipid deposits into arterial walls subsequently covered by smooth muscle and endothelial cells, is now known to be a dynamic accumulation of oxidized cholesterol over time that is primarily driven by the immune system1. Not surprisingly, many diseases defined by autoimmunity and immune system dysfunction are associated with significantly increased morbidity and mortality due to cardiovascular disease (CVD), often defined by accelerated atherosclerosis. Researchers and physicians studying accelerated atherosclerosis in SLE have three major goals: understanding the biological differences in pathology that define autoimmune CVD versus non-autoimmune CVD, identifying at-risk patients before the onset of atherosclerosis, and developing therapeutic options for prevention of atherosclerosis progression. In this Review, we will discuss the epidemiology and pathogenesis of SLE-driven atherosclerosis, the essential role of a dysregulated immune system in the progression of CVD, and strategies for minimizing and treating atherosclerosis in SLE.
Epidemiology : Increased risk of atherosclerosis in systemic lupus erythematosus (SLE) and subclinical measures
Increased risk of CVD in SLE was first described 35 years ago2. Early deaths (<1 year) were due to SLE disease activity, and later deaths were primarily due to CVD2. Subsequent analysis confirmed this bimodal pattern of SLE death, although more recent data suggest that due to improvements in SLE diagnosis and treatments, CVD and infection are the leading causes of SLE mortality, regardless of time following diagnosis3.
The overall risk of myocardial infarction (MI) in SLE patients is 10-fold higher than in the general population, even after accounting for traditional Framingham risk factors4. This risk is even more pronounced in young SLE women aged 35-44-years old, who were over 50 times more likely to have a MI than age-matched women in the Framingham Offspring Study5.
Despite the increased risk of cardiovascular events in the SLE population in general, the absolute number of events per year in any given cohort is relatively small; thus, recent research into biomarkers of atherosclerosis and treatment strategies in SLE has focused on measuring multiple surrogate (subclinical) measures of atherosclerosis. In a cross-sectional study By utilizing carotid ultrasound as a surrogate measure of atherosclerosis, multiple groups, both cross-sectionally and longitudinally, found that carotid plaque increased more that two-fold in SLE patients versus controls and progressed significantly faster in this SLE cohort6–9. More than four times as many SLE patients have coronary calcification versus healthy controls, as measured by electron beam computerized tomography (11). Using dual-isotope single photon emission computed tomographic (SPECT) myocardial perfusion imaging, 38% of SLE patients had perfusion defects indicating subclinical atherosclerosis. More than half of SLE subjects examined also have endothelial dysfunction, measured by flow-mediated dilation, versus 26% of controls10. There is also evidence that in addition to abnormalities of the macrovasculature in SLE, there is abnormal coronary microvascular function as well, as abnormal Coronary Flow Reserve (CFR) (measured using positron emission tomography scanning) was seen even in SLE patients with normal coronary arteries11. It should be noted, however, that although these measures of subclinical atherosclerosis are significantly linked to coronary events in the general population12, only abnormal myocardial perfusion has been linked to cardiovascular events in SLE.
Pathogenesis of atherosclerosis in SLE
It is unclear why patients with SLE and other autoimmune diseases are at increased risk of atherosclerosis. Traditional (Framingham) cardiac risk factors13 contribute to atherosclerosis but do not fully explain the increased CVD risk in SLE4. As atherosclerosis is currently appreciated as a process intimately intertwined with the immune system, multiple aspects of autoimmunity most likely contribute to accelerated CVD in concert with traditional cardiac risk factors.
Initiation and progression of atherosclerosis and the essential role of immune cells
Monocyte and T cell recruitment to the arterial wall
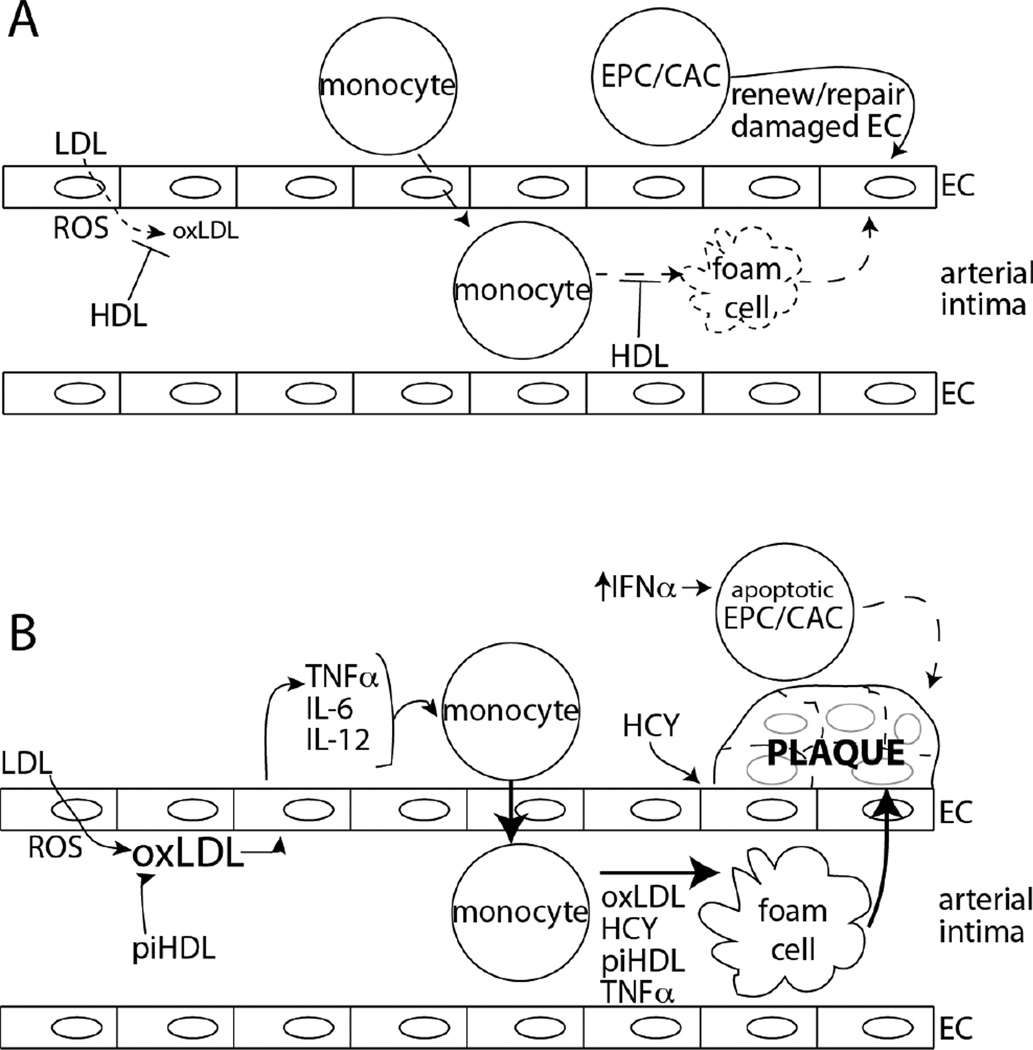
Accumulation of low density lipoproteins (LDL) in the subendothelial space, followed by the oxidation of LDL (oxLDL) by reactive oxygen species (ROS) and the activation of endothelial cells (EC) in the artery, are commonly regarded as initiating events in atherogenesis (Figure 1). Monocytes attach to EC via upregulated adhesion molecules on both cell types and upregulated cytokine release (MCP-1, IL-6, TNFα)14.
Figure 1.
Protection from and pathogenesis of atherosclerosis in SLE. A) Protective mechanisms from atherosclerosis: HDL protects LDL from oxidation by reactive oxygen species (ROS) in the arterial intima. In addition, HDL assists with reverse cholesterol transport and prevents the formation of lipid-rich foam cells, the precursor to plaque. Endothelial progenitor cells (EPC) and circulating angiogenic cells (CAC) are able to reseed and repair damaged pockets of arterial endothelial cells to minimize arterial damage. Dashed lines indicate minimized effect and influence of the cells or processes indicated. B) Initiation and progression of atherosclerosis in SLE: Pro-inflammatory HDL (piHDL), present in almost half of SLE patients, augments oxLDL production. EC release inflammatory cytokines after oxLDL stimulation, stimulating monocytes to bind the EC layer and transmigrate into the intima. Monocytes then differentiate into foam cells, assisted by increased piHDL, oxLDL, TNFα, and homocysteine (HCY) found in SLE patients. Elevated HCY also leads to increase ROS and EC damage. Defective, apoptotic and lower overall numbers of EPC/CAC diminish the EC repair system, and all of these processes lead to increased arterial plaque.
After monocytes adhere to endothelial cells, they migrate to the intima and differentiate into macrophages. Monocyte activation is globally higher in SLE, but monocyte activation does not correlate with increased coronary atherosclerosis15 (Figure 1).
T cells are also recruited to nascent plaques by similar mechanisms, although at lower numbers. These T cells are generally Th1 CD4+ cells that secrete pro-inflammatory and pro-atherogenic IFNγ.
Progression of the atherosclerotic plaque and prevention of CVD by HDL
Macrophages phagocytose abundant oxLDL and become foam cells, and form the basis of the plaque lesion. Smooth muscle cells (SMC) then grow around the expanding lesion and encroach on the vessel lumen, leading to fibrosis (Figure 1). MI can occur when a plaque ruptures or after platelet aggregation in an occluded artery14.
HDL protect against atherosclerosis in two major ways. First, reverse cholesterol transport (RCT), where cholesterol and phospholipids shuttle out of foam cells, is mediated by the interactions of lipoproteins in HDL with lipid transporters on foam cells. The lipoprotein ApoA-I, the most abundant protein component in HDL, is necessary in promoting RCT16.
The second major mechanism for the protective capacity of normal HDL is their antioxidative function. Both proteins and lipids in LDL are protected from accumulation of oxidation products by the presence of normal HDL (Figure 1).
Vascular damage and endothelial cell dysfunction in SLE
In addition to early-onset atherosclerosis, SLE patients are at risk of accelerated vascular damage versus healthy controls. Blood flow at the forearm, brachial artery, and heart is significantly impaired in SLE patients17, 18. Recent work suggests that, in SLE, vascular damage is accelerated and vascular repair mechanisms are ineffective. SLE patients have high levels of circulating apoptotic EC – indicating increased vascular damage - and lower levels of circulating endothelial progenitor cells (EPC) that repair damaged arterial tissues19, 20. Generation of reparative myelomonocytic circulating angiogenic cells (CAC) is also impaired. Secretion of IFNα, largely by plasmacytoid DC (stimulated in part by low density granulocytes undergoing NETosis, also increased in SLE patients)21 induces EPC apoptosis21 and converts CAC to dendritic cells, thus losing ability to repair vascular damage to EC20.
Immune system molecules and particles involved in atherosclerosis
Autoantibodies
Antiphospholipid antibodies (aPL)
Data on the role of aPL in promoting atherosclerosis in humans is mixed22. Patients with primary antiphospholipid syndrome have thicker carotid intima media thickening (CIMT) at multiple artery sites than controls, especially those > 40 years old23. Higher aPL levels correlate with increased MI risk in healthy men24, 25. However, even though about half of SLE patients have aPL26, there is little agreement on whether the presence of aPL correlates with accelerated atherosclerosis6, 8, 9, 27–29, Patients with aPL syndrome do not have significantly more EC dysfunction30. aPL, therefore, might contribute to a “2-hit hypothesis” where circulating aPL contribute to early EC dysfunction via interaction with beta2 glycoprotein 1 in vascular walls but other thrombotic events are probably necessary to trigger plaque and clot formation.
Anti-oxLDL antibodies
IgM antibodies that recognize oxLDL are generally considered to be protective against atherosclerosis in murine models31, although, paradoxically, the presence of anti-oxLDL antibodies increases risk for atherosclerosis in humans with SLE. A recent study suggests that anti-oxLDL antibodies develop after of anti-lipoprotein lipase autoantibodies are detected and are responsible for increased atherosclerotic risk in an SLE cohort with high disease activity32. The underlying mechanism behind why IgG anti-oxLDL antibodies are atherogenic is unclear, but decreased immune complex clearing (e.g., SLE nephritis) or the presence of aggregated oxLDL in the subendothelial space could explain the increased atherosclerotic risk.
Anti-apoA-I antibodies
Approximately 20% of non-autoimmune patients with acute coronary syndromes have circulating anti-apoA-I antibodies33, suggesting these autoantibodies might play a role in atherosclerosis development. Anti-apoA-I antibodies were noted in 32.5% of SLE patients and 22.9% of patients with primary antiphospholipid syndrome (APS)34 and presence of these antibodies correlate with increased disease activity35. As apoA-I is a major anti-inflammatory component of HDL, it is presumed (but not known) that anti-apoA-I autoantibodies render the atheroprotective capabilities of apoA-I and HDL ineffective. Future work in the general population and SLE should address this and whether the presence of anti-apoA-I antibodies correlate with atherosclerosis initiation and progression.
Cytokines
Immune cells communicate with each other and other tissues in the body by secreting small proteins called cytokines. Many cytokines are found in atherosclerotic plaques and are known to contribute, both positively and negatively, to plaque development and progression, in nonautoimmune subjects (reviewed recently in36). Much less is currently known about the role many of these cytokines play in SLE accelerated atherosclerosis – somewhat surprising, given that dysregulated immunity defines SLE - although this area of research is the focus of many groups and will certainly be better understood in the future. Th1 T cells are abundant in atherosclerotic lesions. IFNγ is the prototypical Th1 cytokine and promotes plaque instability by inhibiting growth of SMC, EC and collagen production37. IFNγ promotes foam cell formation as well as plaque rupture38, although no studies have directly examined the role of IFNγ in SLE-driven accelerated atherosclerosis.
IL-12 is expressed by macrophages, SMCs, and ECs, and is a major cytokine involved in Th1 differentiation. High levels of IL-12 have been found in atherosclerotic plaques in non-autoimmune subjects39.
TNFα and IL-1 are potent macrophage activators and can lead to arterial inflammation and EC dysfunction.40. Additionally, TNFα and IL-1 stimulate monocyte differentiation into macrophages/foam cells. High plasma TNFα levels41, 42, along with high TNF receptor levels41, have been observed in SLE patients with CVD. Conversely, lower IL-1 levels promote IFNα-driven vascular damage in SLE43, suggesting the pro-angiogenic effects of IL-1 are both beneficial (in promoting vascular repair) and deleterious.
IL-6 is an independent marker of increased mortality in CVD through C-reactive protein (CRP) production. Its role in atherosclerosis progression in SLE is unclear; high levels of IL-6 are linked to atherosclerotic risk in certain cohorts44, 45, but not in other cross-sectional6 and longitudinal46 studies.
IL-17 is secreted from a novel T cell phenotype (Th17 cells) and is believed to promote SLE disease activity47. Contradictory data exist in regard to the role of IL-17 in non-autoimmune CVD36, although two recent papers suggest that IL-17 promotes atherosclerosis in autoimmune diseases. An atherosclerosis-prone mouse model treated with the common SLE therapeutic mycophenolate mofetil (MMF) decreased IL-17 levels along with size of aortic plaques and T cell infiltrates48. In addition, impaired endothelial function correlates with high IL-17 levels in RA patients (SLE data is not currently known)49.
Although data is currently scarce, regulatory T cells are believed to play a protective role in atherosclerosis initiation50. The Treg cytokine TGFβ, in contrast to the cytokines above, is probably protective against plaque formation in the general population51. Low serum TGFβ levels have been linked to increased CIMT and LDL in SLE52. IL-10 also appears to prevent atherosclerosis in a mouse model of atherosclerosis36. However, in a study using the same mouse model transplanted with murine SLE bone marrow, reduction in plaque size by MMF treatment associated with lower IL-10 levels53. These data suggest IL-10 might have different atherogenic properties in SLE, or that MMF non-specifically targets IL-10-producing Tregs.
Innate immunity
Toll-like receptors (TLRs) are a family of receptors on multiple immune cells that mediate innate immunity. There are multiple TLR ligands, including bacterial cell wall components that have been linked to atherosclerosis development54.
Endogenous ligands, such as lipids and nucleic acids, can also trigger TLR signaling. When oxLDL binds TLR4 and CD14 on macrophages, apoptotic cell phagocytosis is inhibited, the scavenger receptor CD36 is upregulated, and oxLDL uptake is increased, leading to atherosclerosis initiation55.
The roles of TLRs and innate immunity in atherosclerosis specific to rheumatic diseases are poorly understood. Aberrant activation of TLR7 and 9, resulting in IFNα upregulation, is linked to higher SLE disease activity56.
Biomarkers: How traditional and autoimmune-specific risk factors play into risk for atherosclerosis in SLE
Framingham atherosclerosis risk factors
As defined by the Framingham heart studies, traditional risk factors for CVD are older age, male gender, smoking, high total cholesterol and LDL levels, high systolic blood pressure, diabetes, and left ventricular hypertrophy. A comprehensive review of the epidemiology of traditional cardiac risk factors to atherosclerosis in SLE was recently published in this journal57. The influence of traditional risk factors in CVD in SLE appears to be different than the nonautoimmune population, and the main focus of the clinician should be treating SLE while monitoring traditional CVD risk57.
SLE-associated risk factors for atherosclerosis
Disease activity, duration, and damage
Associations between SLE disease manifestations and atherosclerosis are not clear. One cross-sectional study suggested that higher disease activity (measured by the SLAM index) was significantly associated with less plaque, but longer disease duration positively correlated with plaque8. In another cross-sectional cohort, longer disease duration was also significantly associated with higher coronary calcium58. Similarly, longer SLE duration and higher levels of damage from lupus indepentently predicted carotid plaque in a cross-sectional6 and longitudinal study7 from a different cohort.
Pro-inflammatory HDL (piHDL)
Although HDL quantities partially determine atherosclerotic risk, HDL function is equally significant. For example, during the acute phase response HDL can be converted from their usual anti-inflammatory state to pro-inflammatory, and cause increased LDL oxidation (Figure 1). This acute phase response can also become chronic, and may be a mechanism for HDL dysfunction in rheumatic diseases. Indeed, our group has found that HDL function is proinflammatory (piHDL) in many women with SLE59. A follow-up study illustrated that 85% of SLE patients with carotid plaque have piHDL28. piHDL have also been identified as an independent risk factor in RA59, 60 and aPL syndrome61.
Oxidative stress
Oxidative stress - excess of ROS not counterbalanced by an adequate antioxidant defense system - associates with accelerated atherosclerosis in the general population. Increased oxidative stress has been identified in SLE patients, and is often elevated independent of disease activity. In one study, increased oxidative stress (F2 isoprostane excretion) was associated with patient-reported symptoms in SLE but not with inflammation or damage62.
Homocysteine
High homocysteine levels have been linked to atherosclerosis in the general population63. Homocysteine is toxic to EC64, is prothrombotic65,66, decreases nitric oxide availability67, and stimulates foam cell formation68. High homocysteine levels result from both genetic background and diet. Some studies show that elevated homocysteine levels in SLE correlated with cross sectional58, 69–72 and longitudinal progression7, 46 of subclinical atherosclerosis, but other studies showed no correlation6, 9, 73. Renal insufficiency is a known cause of elevated homocysteine levels74, but the relationship between renal function and hyperhomocysteinemia in SLE has not been fully established.
Adipokines/adipose-derived hormones
Adipokines, produced by white adipose tissue, regulate metabolism and energy homeostasis. The main function of leptin is to suppresses appetite in the hypothalamus, but leptin signaling also contributes to atherosclerosis progression75. Elevated leptin levels have been observed in adult76 and pediatric77 SLE. In addition, serum leptin levels are higher in SLE patients with carotid plaque versus patients without78.
In contrast, high serum adiponectin levels are associated with low levels of adipose tissue and risk for atherosclerosis and metabolic syndrome, and adiponectin levels are lower in patients with CVD. Data on the link between adiponectin and atherosclerosis are limited and contradictory: in one study, adiponectin levels were associated with carotid plaque79, but no correlation was found between adiponectin or leptin levels with coronary calcification in another SLE cohort80.
Strategies for minimizing risk of accelerated atherosclerosis in SLE
Minimizing Framingham risk factors
It is likely that novel “SLE-specific” risk prediction panels will be developed and validated in the future for identification of high-risk patients who should be targeted for therapeutic interventions to prevent cardiovascular complications. Currently, however, our screening and treatment strategies are extrapolated from the best available evidence for the general population. Expert panels in the US and Europe recommend that SLE patients be annually screened for traditional modifiable risk factors for cardiovascular disease, including smoking status, blood pressure, BMI, diabetes, and serum lipids (including total cholesterol, HDL, LDL, and triglycerides)81, 82. There are no randomized clinical trials for atherosclerosis prevention specifically in SLE, however, so current guidelines for modifying cardiovascular risk factors in a SLE patient population are essentially strategies for treating CVD in the general population.
Smoking
Smoking has been identified as a modifiable atherosclerosis and CVD risk factor, and smoking cessation is recommended for SLE patients82.
Diabetes Mellitus
Diabetes is considered to be a coronary artery disease equivalent by the National Cholesterol Education Panel (NCEP) guidelines83, so treatment goals for diabetic lupus patients should aim to establish and maintain glycemic control, including minimization of glucocorticoid doses.
Hypertension
The ideal blood pressure for SLE patients is 130/80, as recommended by the Joint National Committee (JNC 7), and is the same as for patients with other high-risk co-morbid conditions84, 85. Difficulty in prevention trial recruitment has prevented the establishment of an optimum atheroprotective medication regimen in SLE86. ACE inhibitors, however, should be first-line therapy in SLE patients with renal involvement87, and the EULAR guidelines also recommend them as first line therapy in hypertensive patients with inflammatory arthritis because of their potential favorable effects on inflammatory markers and EC function in RA88. Angiotensin receptor blockers (ARB) can also be considered in patients who cannot tolerate ACE inhibitor therapy. Thiazide diuretics are recommended by as first line therapy for hypertension in the general population by JNC 7, and would generally also be a safe choice in SLE subjects (although caution should be used, as thiazide diuretics also have dyslipidemic and diabetogenic effects)85. β–blockers have been shown to precipitate Raynaud’s phenomenon89, and thus should be used with caution in SLE subjects.
Treatment of Hypercholesterolemia: Statins
Statins are competitive inhibitors of HMG-CoA reductase and are widely used to reduce cardiovascular morbidity. In addition to their lipid lowering properties, statins also have a number of anti-inflammatory properties, including inhibition of inflammatory cytokines, ROS formation, T-cell activation, and upregulation of nitric oxide synthesis90. However, data for statin use in atherosclerosis prevention in SLE is inconsistent. For instance, in a short-term (8 week) trial, atorvastatin improved EC-dependent vasodilation, even after controlling for traditional cardiac risk factors91. In a longer (2 year) atorvastatin trial however, statins did not prevent progression of coronary calcium, IMT, or disease activity, and did not result in any significant improvements in measures of systemic inflammation or EC activation92. Preventive trials in the general population have utilized much larger sample sizes and longer study durations than the SLE studies, however93. Data from mouse models of atherosclerosis in lupus have also been inconsistent; although statins resulted in improvement in atherosclerosis in one SLE mouse model94, in LDLr−/− mice reconstituted with bone marrow from SLE-prone mice, statins failed to attenuate atherogenesis despite reductions in cholesterol levels53. Therefore, the effectiveness of statin treatment to prevent atherosclerosis progression in SLE is unclear and physicians prescribing statins for SLE patients should adhere to guidelines from the National Cholesterol Education Panel83.
Disease Modifying Agents in SLE: Implications for Atherosclerosis Prevention
Anti-malarial therapeutics
Hydroxychloroquine (HCQ) is believed to be cardioprotective (although there are isolated reports of HCQ cardiotoxicity95), and HCQ use has been associated with less aortic stiffness96 and less plaque on carotid ultrasound6 in SLE. Additionally, anti-malarials minimize steroid-induced hypercholesterolemia97. In addition, HCQ may be associated with reduced thrombotic events and improved survival in SLE patients98. HCQ might be cardioprotective in part because it blocks TLR 7 and 999. TLR 7 and 9 stimulation lead to increased IFNα, which, as discussed above, is implicated in EC dysfunction and abnormal vascular repair.
Glucocorticoids
Longer duration and high cumulative glucocorticoid treatment has been associated with atherosclerosis in SLE patients8, 28, 69, 70, 100. Additionally, prednisone doses >10mg/day have been shown to independently predict high cholesterol levels in SLE101. Conversely, lower predisone use and dosage correlates with more plaque in a different cohort6, suggesting that there might be an optimal window of glucocorticoid therapy where anti-inflammatory effects of steroids can be atheroprotective. Until such a threshold is determined, we recommend following the EULAR recommendations that the lowest possible dose of corticosteroids be used in individual patients88.
Mycophenolate mofetil (MMF)
MMF, an immunosuppressive agent used frequently in SLE patients, has several potential anti-atherogenic effects. MMF has been shown in animal models to inhibit NADPH-oxidase, thereby inhibiting oxidative stress102. In patients with carotid artery stenosis, 2 weeks of MMF therapy resulted in decreased plaque expression of inflammatory genes and activated T cells with increased numbers of regulatory T cells103. In LDLr−/− mice reconstituted with SLE-prone bone marrow, MMF treatment significantly reduced atherosclerotic burden and recruitment of CD4+ T cells to atherosclerotic plaques53. In addition, a retrospective study in diabetic renal transplant patients found that there was a 20% decrease in cardiovascular mortality among patients treated with MMF compared to those with immunosuppressive regimens without MMF104. A small prospective observational study from our own group suggests that treatment with MMF and hydroxychloroquine for 12 weeks, but not azathioprine, results in significant improvement of pro-inflammatory HDL function (McMahon, unpublished data). In a recently published longitudinal SLE cohort study, however, exposure of subjects to MMF was not associated with a reduction of IMT or coronary calcium progression105. Larger, prospective studies will need to be undertaken to clarify the potential role of MMF in prevention of progression of atherosclerosis in SLE.
Azathioprine
One retrospective case-control study of SLE patients with documented coronary artery disease found that patients with CAD were more likely to have been treated with azathioprine106. In multivariate analysis, azathioprine use was also associated with cardiac events in the multi-ethnic LUMINA cohort27. Azathioprine use was also associated with increased carotid IMT in the pediatric SLE APPLE cohort107. It is unclear if these associations are due to a direct effect of azathioprine, or the inability of azathioprine to overcome the inflammation that leads to atherosclerosis.
B cell directed therapies
Although not currently well understood, B cells might contribute to atherosclerosis progression. In contrast to monocytes and T cells, however, activated B cells appear to play a protective role, as the removal of B cells from atherosclerosis-prone mice lead to increased atherosclerosis108, 109. In addition, subsets of B cells produce atheroprotective molecules such as IL-10 and anti-oxLDL antibodies110. As anti-CD20 therapy depletes B cells, an unintended consequence of SLE therapy could be the risk of increasing CVD. Two studies, however, show that B cell depletion with anti-CD20 antibodies significantly reduced atherosclerosis in both the ApoE−/− and the LDLr−/− atherosclerosis-prone mouse models111, 112. Similar results have been observed in improvement of lipid profiles after anti-CD20 therapy in SLE patients113, although, as observed with piHDL and statin therapy, improved lipid levels do not always predict less atherosclerosis. With the recent introduction of anti-CD20 and anti-BLyS therapies into clinical practice, it is unclear how B cell-directed therapy will impact atherosclerosis in SLE patients.
Novel therapeutics to prevent atherosclerosis
Peptides mimicking HDL-related proteins such as apoA-I and apoJ in the prevention of atherosclerosis are currently under intense study. ApoA-I and apoJ peptides convert piHDL back to normal HDL by removing oxidation from oxLDL and HDL114. Thus, normal activity of anti-inflammatory HDL is restored and LDL are protected from oxidation. Both the apoA-I peptide 4F and an apoJ peptide ([113–122]apoJ) improve vasodilation impairment and cause plaque regression in atherosclerosis animal models114. 4F treatment, alone or with pravastatin, significantly reduced SLE-like disease in a murine lupus/accelerated atherosclerosis model115, and these therapeutics might be effective, non-toxic therapeutic options for SLE patients in the future.
Evidence for the role of IFNα at multiple points in the pathogenesis of atherosclerosis has recently emerged. Aberrant balance between apoptotic EC, lower numbers of EPC/CAC, and vasculogenesis as driven by IFNα was discussed above20, 43, 116. In addition, IFNα priming increases macrophage uptake of oxLDL117. Several IFNα inhibitors are currently in clinical trials for SLE, and future studies will be required to determine whether these medications are protective against atherosclerosis in SLE.
Should there be standardized guidelines for monitoring CVD risk in SLE patients?
EULAR has recently recommended that patients with SLE118 and RA88 be monitored for increased cardiac risk. The RA guidelines list ten unique parameters for evaluating CVD risk, including minimizing corticosteroid exposure, monitoring serum lipid levels, and traditional Framingham or Systematic Coronary Risk Evaluation (SCORE) risk. The SLE guidelines are not CVD-specific, and only one of the ten guidelines deals with CVD risk. Statin use is also recommended for RA patients, although, as discussed above, statin use might not be effective in slowing atherosclerosis in human and murine SLE. CVD-specific guidelines for SLE akin to the EULAR RA recommendations would be an effective tool for rheumatologists.
Conclusions
Atherosclerosis and CVD is a major cause of morbidity and mortality in multiple rheumatic diseases. We do not yet fully understand what causes accelerated autoimmune-specific atherosclerosis, but evidence strongly suggests that it relates to complex interplay between dysfunctional immune regulation, inflammation, traditional risk factors, aberrant endothelial cell function and repair, and therapeutics treating the underlying autoimmune disease. Recent data suggest that SLE-specific elements could contribute to increased atherosclerotic risk. A comprehensive biomarker panel incorporating these factors with more traditional CVD risk factors (homocysteine, lipid levels, etc.) could be an essential tool for identifying at-risk patients very early after rheumatic disease diagnosis. Clinical biomarker identification for some of these biomarkers remains in development (e.g., piHDL and EPC/CAC). The clinical complexity of accelerated atherosclerosis will most likely require an integrated approach for identification and treatment, and intensive study into this aspect of SLE will ultimately lead to improved cardiovascular outcomes for these patients.
Review Criteria
PubMed was searched in February, April, September and October 2011 for original and review articles in English using “lupus” along with the terms “atherosclerosis,” “vasculitis,” “cardiovascular,” “monocyte,” “therapy,” “review” and all titles of the subdivisions of this article (e.g., “antiphospholipid antibodies,” “IL-6,” etc.). Reference lists from relevant papers provided additional articles. No date restrictions were used in searches, but due to space restrictions more recent papers were sometimes cited when multiple papers had the same conclusions.
Key Points.
-
-
Cardiovascular disease is a significant contributor to morbidity and mortality in SLE
-
-
SLE-specific risk factors for accelerated atherosclerosis exist but are not well understood
-
-
Endothelial cell dysfunction plays a major role in accelerated atherosclerosis in SLE
-
-
Identification of SLE-specific mechanisms and biomarkers behind accelerated atherosclerosis should provide novel early detection screens and therapeutic targets
Acknowledgments
We apologize to the many researchers whose relevant studies were not cited here due to space constraints. Research pursuits of the authors were supported by funding from NIH/NIAMS (K01 AR-059095-01 to BJS; K23 AR-053864-01A1 to MM), the Arthritis National Research Foundation (BJS), the Arthritis Foundation, Pacific Region (BJS and MM), Rheuminations, Inc. (BHH), Alliance for Lupus Research (BHH and MM), and Lupus Research Institute (BHH and MM). BHH is the recipient of a Kirkland Scholar award.
Biographies
Dr. Skaggs is an Adjunct Assistant Professor in the Division of Rheumatology at the David Geffen School of Medicine at the University of California Los Angeles.
Dr. McMahon is an Assistant Clinical Professor in the Division of Rheumatology at UCLA. Her research interests have been focused on atherosclerosis in SLE for the past ten years.
Dr. Hahn is Professor of Medicine and Chief of Rheumatology at UCLA. Her MD and Rheumatology training are from Johns Hopkins University. She has studied murine and human lupus for many years. She is past President of the American College of Rheumatology.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Urowitz MB, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 3.Nossent J, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000--2004: relation to disease activity and damage accrual. Lupus. 2007;16:309–317. doi: 10.1177/0961203307077987. [DOI] [PubMed] [Google Scholar]
- 4.Esdaile JM, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Manzi S, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 7.Roman MJ, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412–3419. doi: 10.1002/art.22924. [DOI] [PubMed] [Google Scholar]
- 8.Manzi S, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma Y, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 10.El-Magadmi M, et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 11.Recio-Mayoral A, et al. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salel AF, et al. Accuracy of numerical coronary profile. Correlation of risk factors with arteriographically documented severity of atherosclerosis. N Engl J Med. 1977;296:1447–1450. doi: 10.1056/NEJM197706232962507. [DOI] [PubMed] [Google Scholar]
- 14.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rho YH, et al. Macrophage activation and coronary atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:535–541. doi: 10.1002/acr.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah PK, et al. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 17.Wright SA, et al. Microcirculatory hemodynamics and endothelial dysfunction in systemic lupus erythematosus. Arterioscler Thromb Vasc Biol. 2006;26:2281–2287. doi: 10.1161/01.ATV.0000238351.82900.7f. [DOI] [PubMed] [Google Scholar]
- 18.Kahlenberg JM, Kaplan MJ. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:203. doi: 10.1186/ar3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajagopalan S, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 20.Denny MF, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualtierotti R, Biggioggero M, Meroni PL. Cutting-Edge Issues in Coronary Disease and the Primary Antiphospholipid Syndrome. Clin Rev Allergy Immunol. 2011 doi: 10.1007/s12016-011-8268-9. [DOI] [PubMed] [Google Scholar]
- 23.Ames PR, Margarita A, Sokoll KB, Weston M, Brancaccio V. Premature atherosclerosis in primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis. 2005;64:315–317. doi: 10.1136/ard.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu R, et al. Antibodies against cardiolipin and oxidatively modified LDL in 50-year-old men predict myocardial infarction. Arterioscler Thromb Vasc Biol. 1997;17:3159–3163. doi: 10.1161/01.atv.17.11.3159. [DOI] [PubMed] [Google Scholar]
- 25.Vaarala O, et al. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men. Circulation. 1995;91:23–27. doi: 10.1161/01.cir.91.1.23. [DOI] [PubMed] [Google Scholar]
- 26.George J, et al. Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation. 2000;102:1822–1827. doi: 10.1161/01.cir.102.15.1822. [DOI] [PubMed] [Google Scholar]
- 27.Toloza SM, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–3957. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 28.McMahon M, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60:2428–2437. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pengo V, Bison E, Ruffatti A, Iliceto S. Antibodies to oxidized LDL/beta2-glycoprotein I in antiphospholipid syndrome patients with venous and arterial thromboembolism. Thromb Res. 2008;122:556–559. doi: 10.1016/j.thromres.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112:169–174. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 32.Fesmire J, Wolfson-Reichlin M, Reichlin M. Effects of autoimmune antibodies anti-lipoprotein lipase, anti-low density lipoprotein, and anti-oxidized low density lipoprotein on lipid metabolism and atherosclerosis in systemic lupus erythematosus. Rev Bras Reumatol. 2010;50:539–551. doi: 10.1590/s0482-50042010000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuilleumier N, et al. Presence of autoantibodies to apolipoprotein A-1 in patients with acute coronary syndrome further links autoimmunity to cardiovascular disease. J Autoimmun. 2004;23:353–360. doi: 10.1016/j.jaut.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Dinu AR, et al. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus. 1998;7:355–360. doi: 10.1191/096120398678920262. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill SG, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:845–854. doi: 10.1002/art.27286. [DOI] [PubMed] [Google Scholar]
- 36.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988;63:712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- 38.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Uyemura K, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 41.Svenungsson E, et al. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12:454–461. doi: 10.1191/0961203303lu412oa. [DOI] [PubMed] [Google Scholar]
- 42.Rho YH, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35:1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 43.Thacker SG, et al. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol. 2010;185:4457–4469. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asanuma Y, et al. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: relationship to cardiovascular risk factors. J Rheumatol. 2006;33:539–545. [PubMed] [Google Scholar]
- 45.Sabio JM, et al. Metabolic syndrome is associated with increased arterial stiffness and biomarkers of subclinical atherosclerosis in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:2204–2211. doi: 10.3899/jrheum.081253. [DOI] [PubMed] [Google Scholar]
- 46.Rua-Figueroa I, et al. Factors involved in the progress of preclinical atherosclerosis associated with systemic lupus erythematosus: a 2-year longitudinal study. Ann Rheum Dis. 2010;69:1136–1139. doi: 10.1136/ard.2008.104349. [DOI] [PubMed] [Google Scholar]
- 47.Shin MS, Lee N, Kang I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol. 2011;23:444–448. doi: 10.1097/BOR.0b013e328349a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Vietinghoff S, et al. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57:2194–2204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marder W, et al. Interleukin 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1550–1555. doi: 10.1136/ard.2010.148031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foks AC, et al. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson M, Ahmad Y, Bruce IN, Coupes B, Brenchley PE. Activation of transforming growth factor-beta1 and early atherosclerosis in systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R81. doi: 10.1186/ar1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Leuven SI, et al. Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone LDLr−/− mice. Ann Rheum Dis. 2011 doi: 10.1136/annrheumdis-2011-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell LA, et al. The acute phase reactant response to respiratory infection with Chlamydia pneumoniae: implications for the pathogenesis of atherosclerosis. Microbes Infect. 12:598–606. doi: 10.1016/j.micinf.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 56.Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43:76–83. doi: 10.3109/08916930903374618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 58.Von Feldt JM, et al. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2220–2227. doi: 10.1002/art.21967. [DOI] [PubMed] [Google Scholar]
- 59.McMahon M, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 60.Charles-Schoeman C, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60:2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charakida M, et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA. 2009;302:1210–1217. doi: 10.1001/jama.2009.1346. [DOI] [PubMed] [Google Scholar]
- 62.Avalos I, et al. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16:195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- 63.Malinow MR, Nieto FJ, Szklo M, Chambless LE, Bond G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation. 1993;87:1107–1113. doi: 10.1161/01.cir.87.4.1107. [DOI] [PubMed] [Google Scholar]
- 64.Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980;18:113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- 65.Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest. 1993;91:2873–2879. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woo KS, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542–2544. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- 67.Upchurch GR, Jr, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 68.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 69.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 70.Svenungsson E, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104:1887–1893. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 71.Refai TM, Al-Salem IH, Nkansa-Dwamena D, Al-Salem MH. Hyperhomocysteinaemia and risk of thrombosis in systemic lupus erythematosus patients. Clin Rheumatol. 2002;21:457–461. doi: 10.1007/s100670200115. [DOI] [PubMed] [Google Scholar]
- 72.Bruce IN, Urowitz MB, Gladman DD, Ibanez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159–3167. doi: 10.1002/art.11296. [DOI] [PubMed] [Google Scholar]
- 73.Manger K, et al. Factors associated with coronary artery calcification in young female patients with SLE. Ann Rheum Dis. 2003;62:846–850. doi: 10.1136/ard.62.9.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potter K, Hankey GJ, Green DJ, Eikelboom JW, Arnolda LF. Homocysteine or renal impairment: which is the real cardiovascular risk factor? Arterioscler Thromb Vasc Biol. 2008;28:1158–1164. doi: 10.1161/ATVBAHA.108.162743. [DOI] [PubMed] [Google Scholar]
- 75.Taleb S, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Gonzalez A, et al. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 77.Al M, et al. Adipokines as novel biomarkers in paediatric systemic lupus erythematosus. Rheumatology (Oxford) 2009;48:497–501. doi: 10.1093/rheumatology/kep030. [DOI] [PubMed] [Google Scholar]
- 78.McMahon M, et al. Plasma leptin levels are associated with carotid artery plaque and intima-media thickness (IMT) in women with SLE and a matched population of healthy women. Arthritis Rheum. 2007;56:S796. [Google Scholar]
- 79.Reynolds HR, et al. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis. 2010;210:569–574. doi: 10.1016/j.atherosclerosis.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung C, et al. Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus. 2009;18:799–806. doi: 10.1177/0961203309103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yazdany J, et al. Provision of preventive health care in systemic lupus erythematosus: data from a large observational cohort study. Arthritis Res Ther. 2010;12:R84. doi: 10.1186/ar3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mosca M, et al. Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice. Autoimmun Rev. 2011;10:383–388. doi: 10.1016/j.autrev.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III Update: Adjustments and Options. Am J Cardiol. 2005;96:53–59. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 85.Graham I, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194:1–45. doi: 10.1016/j.atherosclerosis.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 86.Costenbader KH, et al. Barriers to a trial of atherosclerosis prevention in systemic lupus erythematosus. Arthritis Rheum. 2005;53:718–723. doi: 10.1002/art.21441. [DOI] [PubMed] [Google Scholar]
- 87.Duran-Barragan S, McGwin G, Jr, Vila LM, Reveille JD, Alarcon GS. Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus--results from LUMINA (LIX): a multiethnic US cohort. Rheumatology (Oxford) 2008;47:1093–1096. doi: 10.1093/rheumatology/ken208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peters MJ, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 89.Coffman JD. Raynaud's phenomenon. An update. Hypertension. 1991;17:593–602. doi: 10.1161/01.hyp.17.5.593. [DOI] [PubMed] [Google Scholar]
- 90.Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007;99:732–738. doi: 10.1016/j.amjcard.2006.09.125. [DOI] [PubMed] [Google Scholar]
- 91.Ferreira GA, Navarro TP, Telles RW, Andrade LE, Sato EI. Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology (Oxford) 2007;46:1560–1565. doi: 10.1093/rheumatology/kem186. [DOI] [PubMed] [Google Scholar]
- 92.Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS) Ann Rheum Dis. 2011;70:760–765. doi: 10.1136/ard.2010.136762. [DOI] [PubMed] [Google Scholar]
- 93.Kang S, Wu Y, Li X. Effects of statin therapy on the progression of carotid atherosclerosis: a systematic review and meta-analysis. Atherosclerosis. 2004;177:433–442. doi: 10.1016/j.atherosclerosis.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Aprahamian T, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177:3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nord JE, Shah PK, Rinaldi RZ, Weisman MH. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum. 2004;33:336–351. doi: 10.1016/j.semarthrit.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Selzer F, et al. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 97.Rahman P, et al. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol. 1999;26:325–330. [PubMed] [Google Scholar]
- 98.Jung H, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62:863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 99.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6:223–235. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 100.Doria A, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:1071–1077. doi: 10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 102.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293:F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 103.van Leuven SI, et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2010;211:231–236. doi: 10.1016/j.atherosclerosis.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 104.David KM, et al. Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clin Transplant. 2005;19:279–285. doi: 10.1111/j.1399-0012.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 105.Kiani AN, Magder LS, Petri M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haque S, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. J Rheumatol. 2010;37:322–329. doi: 10.3899/jrheum.090306. [DOI] [PubMed] [Google Scholar]
- 107.Schanberg LE, et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 2009;60:1496–1507. doi: 10.1002/art.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 110.Kyaw T, Tipping P, Toh BH, Bobik A. Current understanding of the role of B cell subsets and intimal and adventitial B cells in atherosclerosis. Curr Opin Lipidol. 2011;22:373–379. doi: 10.1097/MOL.0b013e32834adaf3. [DOI] [PubMed] [Google Scholar]
- 111.Kyaw T, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 112.Ait-Oufella H, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pego-Reigosa JM, et al. Long-term improvement of lipid profile in patients with refractory systemic lupus erythematosus treated with B-cell depletion therapy: a retrospective observational study. Rheumatology (Oxford) 2010;49:691–696. doi: 10.1093/rheumatology/kep446. [DOI] [PubMed] [Google Scholar]
- 114.Van Lenten BJ, et al. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woo JM, et al. Treatment with apolipoprotein A-1 mimetic peptide reduces lupus-like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res Ther. 2010;12:R93. doi: 10.1186/ar3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaplan MJ, Salmon JE. How does interferon-alpha insult the vasculature? Let me count the ways. Arthritis Rheum. 2011;63:334–336. doi: 10.1002/art.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, et al. Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum. 2011;63:492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
- 118.Mosca M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69:1269–1274. doi: 10.1136/ard.2009.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]