Abstract
Background/Objective
Parental obesity influences infant body size. To fully characterize their relative effects on infant adiposity, associations between maternal and paternal body mass index (BMI) category (normal: ≤25 kg/m2, overweight: 25–<30 kg/m2, obese: ≥30 kg/m2) and infant BMI were compared in Fels Longitudinal Study participants.
Methods
A median of 9 serial weight and length measures from birth-3.5 years were obtained from 912 European American children born in 1928–2008. Using multivariable mixed effects regression, contributions of maternal versus paternal BMI status to infant BMI growth curves were evaluated. Cubic spline models also included parental covariates, infant sex, age, and birth variables, and interactions with child’s age.
Results
Infant BMI curves were significantly different across the three maternal BMI categories (POverall<0.0001), and offspring of obese mothers had greater mean BMI at birth and between 1.5–3.5 years than those of over- and normal weight mothers (P≤0.02). Average differences between offspring of obese and normal weight mothers were similar at birth (0.8 kg/m2, P=0.0009) and between 2–3.5 years (0.7–0.8 kg/m2, P<0.0001). Infants of obese fathers also had BMI growth curves distinct from those of normal weight fathers (P=0.02). Infant BMI was more strongly associated with maternal than paternal obesity overall (P<0.0001); significant differences were observed at birth (1.11 kg/m2, P=0.006) and from 2–3 years (0.62 kg/m2, P3years=0.02).
Conclusion
At birth and in later infancy, maternal BMI has a stronger influence on BMI growth than paternal BMI, suggesting weight control in reproductive age women may be of particular benefit for preventing excess infant BMI.
MeSH keywords: Body mass index, growth and development, infants, obesity, parent-child relationships, parents
Excessive early childhood adiposity is a prevalent and increasing concern in many parts of the world,1 particularly given the difficulty in reversing overweight/obesity once it is established2 and the subsequent associated morbidity and mortality.3–5
Parental obesity is one of several factors previously associated with infant body size. Prior studies have consistently shown that maternal body mass index (BMI) is positively associated with offspring birth weight and/or BMI6–11 and less consistently correlated with birth length,6,8 implying the maternal environment plays a substantial role in determining birth anthropometrics. Many studies also reported correlations between maternal BMI and offspring weight, BMI, and/or adiposity from birth-3 years,7,8,11–16 although some did not.6,17–19
Conversely, paternal BMI has generally not been associated with infant birth size.6,8,10 While less frequently examined, associations between paternal BMI and offspring weight and/or BMI tend to emerge later; specifically, associations were first observed at ≤3 years in most studies,6–8,11,13,14 were not observed until 712 years in another, and were nonsignificant in the remainder.17,19
Fewer studies have formally compared parental obesity effects. A handful of studies reported significantly stronger correlations for offspring birth anthropometrics with maternal versus paternal BMI,6,8–11 but only one found a stronger maternal BMI association in later infancy.14 Large cohort studies examining offspring BMI in middle- and later-childhood have not substantiated this greater maternal BMI effect, however,20,21 suggesting maternal effects are only stronger in the early postnatal period.
A minority of published studies performed longitudinal analyses,6,7,18 while most have presented correlations between parental BMI and offspring anthropometrics at selected time points.8–17,19 The relative effects of different determinants of infant BMI may vary during this period of very rapid growth, however, rendering a growth modeling approach more valuable.
Establishing a better understanding of the differential effects of parental BMI on infant growth, including timing of these effects, is an important step toward disentangling how obesity risk is transmitted from parents to children. For example, stronger maternal contributions would suggest that genetic predisposition to obesity is not solely responsible and aspects of the maternal environment, including pre- and postnatal nutrition, should be examined more closely. This study aims to evaluate relationships between maternal and paternal BMI and offspring growth from birth-3.5 years in a well-characterized cohort with extensive serial BMI measurements and measured parental BMI.
METHODS
Study population
Details regarding the Fels Longitudinal Study have been published previously.22 Briefly, individuals were enrolled in utero to track their growth at regular intervals. Family members of initial enrollees were also followed. The current analysis included 912 infants born in 1928–2008. Infancy is variously defined as 0–1, 0–2, or 0–3 years; here we examined the entire infant/early childhood period from 0–3.5 years and refer to this as “infancy” hereafter. Subjects were eligible if their weight was measured at birth and ≥3 additional ages and/or their length was measured at ≥3 ages. Using available genome-wide genotype data, nonpaternity was found to affect only 0.1% of infants. All subjects were of European American ancestry.
Data collection
Outcomes
Infant measurements were collected up to 11 times over the first 3.5 years. Weight (to nearest 0.1 kg without shoes or heavy clothing) and length (to nearest 0.1 cm without shoes) were measured at birth (hospital) and at ages 1, 3, 6, and 9 months, and 1, 1.5, 2, 2.5, 3, and 3.5 years (study center) per standard methods.23 BMI was calculated at each age as weight (kg)/length2 (m2). Exact ages for all measurements were analyzed.
Exposures
Serial maternal and paternal weight (kg), height (cm), and BMI (kg/m2) measurements were also collected. For the current analysis, maternal (non-pregnant) and paternal measurements obtained on the date closest to the infant’s birth were selected. To account for the fact that parental anthropometric measurements (and smoking histories) were not exclusively collected pre-pregnancy (mothers) or at birth (fathers), we adjusted for differences in parental ages between child’s birth date and measurement date.
Covariates
Covariate data were collected via birth records (child’s sex, birth date) and parental questionnaires (child’s birth order, maternal and paternal ages, smoking histories). Gestational age was calculated by differencing child’s birth date and mother’s self-reported date of last menstrual period. Because birth year showed non-linear associations with infant measures, birth year tertile was included in all models.
Wright State University and University of Minnesota Institutional Review Boards approved study materials. Parents provided informed consent at each visit.
Statistical analysis
Statistical analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC) except as noted. Maternal and paternal BMIs categorized as normal (≤25 kg/m2), overweight (25–<30 kg/m2), or obese (≥30 kg/m2) were the primary exposures of interest. The primary outcome was offspring BMI; secondary outcomes, offspring weight and length, were also examined to determine whether any parental effects on BMI were due to differences in either BMI component. To characterize the study population, differences across maternal BMI categories in continuous and categorical variables were tested by ANOVA and Chi-square tests, respectively. In this descriptive analysis, infants’ birth anthropometrics were expressed as Z-scores.24
Missing exposure (11% maternal and 26% paternal BMIs) and covariate data were assumed to be missing at random (due to missed study appointments) and were therefore imputed using multiple imputation25,26 to produce 10 (imputed) datasets (PROC MI); outcomes were not imputed.
To examine the effects of maternal and paternal BMI category on infant BMI growth curves, a two-step modeling approach was used. Mixed effects cubic splines27 were first fitted to the serial infant BMI (and weight and length) data to produce individual and population average growth curves. In the second step, maternal and paternal BMI category were each added to mixed effects models (PROC MIXED) as main effects (i.e., producing a vertical shift in the entire curve relative to referent group) and as interactions with all infant age terms (i.e., changing the shape of the curve relative to referent group). This modeling approach, described further in Appendix S1, allowed truly unique growth curves for each BMI category. Three models were developed for each outcome, including independent models where maternal and paternal BMI category associations were tested separately, and joint models where maternal and paternal effects were tested together. All models were adjusted for the covariates in Table 1. In addition, interactions between maternal (or paternal) BMI category and sex, birth year tertile, and maternal (or paternal) age, and maternal BMI-paternal BMI interactions, were tested but not retained in final models because they were nonsignificant (P>0.05). Models were fit over the 10 imputed datasets and results were averaged to produce single parameter estimates (PROC MIANALYZE).
Table 1.
Fels Longitudinal Study subject characteristics by maternal BMI category
| Maternal BMI category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (<25kg/m2) | Overweight (25–<30kg/m2) | Obese (>=30kg/m2) | |||||||||||
| N | (%) | Mean | SD | N | (%) | Mean | SD | N | (%) | Mean | SD | P-value* | |
| Infants | |||||||||||||
| Birth anthropometrics | |||||||||||||
| Weight-for-age Z-score† | 589 | 0.05 | 1.05 | 136 | 0.34 | 1.37 | 57 | 0.71 | 1.13 | <0.0001 | |||
| Length-for-age Z-score† | 203 | 0.70 | 1.36 | 67 | 1.24 | 1.51 | 33 | 1.30 | 1.45 | 0.005 | |||
| BMI-for-age Z-score† | 203 | −0.35 | 1.05 | 67 | −0.42 | 1.50 | 33 | 0.09 | 1.26 | 0.11 | |||
| Gestational age (weeks) | 454 | 39.6 | 1.7 | 99 | 39.5 | 2.5 | 44 | 39.7 | 2.2 | 0.73 | |||
| Male | 310 | 50.7 | 75 | 53.2 | 32 | 52.5 | 0.86 | ||||||
| Birth year tertile | |||||||||||||
| 1928–1949 | 211 | 34.5 | 33 | 23.4 | 17 | 27.9 | <0.0001 | ||||||
| 1950–1969 | 233 | 38.1 | 47 | 33.3 | 9 | 14.8 | |||||||
| 1970–2008 | 167 | 27.3 | 61 | 43.3 | 35 | 57.4 | |||||||
| Firstborn | 219 | 36.3 | 50 | 35.5 | 21 | 34.4 | 0.95 | ||||||
| Mothers | |||||||||||||
| Age at birth (years) | 611 | 28.0 | 5.5 | 141 | 29.8 | 5.5 | 61 | 28.5 | 6.1 | 0.002 | |||
| Age at measurement (years) | 611 | 29.8 | 6.4 | 141 | 32.8 | 6.3 | 61 | 31.7 | 8.9 | <0.0001 | |||
| Age difference (years) | 611 | 1.8 | 4.1 | 141 | 3.0 | 4.4 | 61 | 3.1 | 6.7 | 0.003 | |||
| Stature (cm) | 611 | 164.1 | 5.9 | 141 | 164.9 | 5.5 | 61 | 165.4 | 6.6 | 0.10 | |||
| Ever smoked at measurement | 146 | 44.6 | 31 | 38.8 | 18 | 48.6 | 0.53 | ||||||
| Fathers | |||||||||||||
| BMI category (kg/m2) | |||||||||||||
| Normal (<25) | 255 | 53.5 | 42 | 38.5 | 24 | 51.1 | <0.0001 | ||||||
| Overweight (25–<30) | 185 | 38.8 | 53 | 48.6 | 10 | 21.3 | |||||||
| Obese (>=30) | 37 | 7.8 | 14 | 12.8 | 13 | 27.7 | |||||||
| Age (years) | 602 | 30.6 | 5.9 | 140 | 32.4 | 6.7 | 60 | 32.0 | 8.8 | 0.003 | |||
| Age at measurement (years) | 477 | 34.2 | 9.0 | 109 | 35.7 | 11.7 | 47 | 35.9 | 11.3 | 0.21 | |||
| Age difference (years) | 477 | 3.7 | 7.5 | 109 | 3.5 | 10.1 | 47 | 3.5 | 7.0 | 0.97 | |||
| Stature (cm) | 477 | 176.9 | 6.5 | 109 | 178.3 | 8.7 | 47 | 178.7 | 10.3 | 0.08 | |||
| Ever smoked at measurement | 156 | 64.7 | 33 | 49.3 | 16 | 57.1 | 0.06 | ||||||
BMI=body mass index; SD=standard deviation.
P-values calculated via ANOVA (continuous variables) and Pearson χ2 test (categorical variables).
Z-scores calculated via WHO Child Growth Standards.48
Global F-tests (encompassing parameter estimates for main effects of parental BMI categories and their interactions with child’s age terms) were used to test null hypotheses: (1) growth curves for infants of normal weight, overweight, and obese mothers (and fathers) were not statistically significantly different and (2) the effect of mothers’ BMI category on infant growth curves did not differ from that of fathers. In addition, parameter estimates were used to calculate estimated differences in infant anthropometrics at each measured age between those with normal weight, overweight, and obese mothers and fathers. F- and t-tests were employed to evaluate whether differences were statistically significantly different than 0. All tests were two-sided at level of significance α=0.05.
RESULTS
Cohort characteristics
The current analysis included a median of 9 serial weight, length, and BMI measurements in 872, 890, and 850 subjects, respectively; 68–100% had measurements at each age except for length and BMI at birth (38–40%) (Table S1). Infant weight and length Z-scores at birth differed across maternal BMI categories (P<0.0001 and P=0.005, respectively; Table 1). The majority (57%) of infants of obese women were born in the 1970–2008 cohort. Parental BMIs were associated, such that children of obese mothers were more likely to have obese fathers (28%) than those of overweight (13%) and normal weight (8%) mothers.
Infant BMI
Parameter estimates from independent and joint mixed effects growth models are provided in Table S2, and global P-values comparing average growth curves are shown in Table 2. Overall, maternal BMI category was associated with infant BMI in both independent (P<0.0001) and joint models (P<0.0001, Table 2), and comparisons across the three maternal BMI groups indicated the infant BMI trajectories for each group remained distinct after adjustment for paternal BMI in the joint model (POverweight versus normal=0.04, PObese versus normal<0.0001, PObese versus overweight<0.0001). As shown in Figure 1A, growth curves for the three groups were generally similar until the maxima at 9–12 months, after which the curve for offspring of obese mothers declined more slowly than those for offspring of overweight and normal weight mothers.
Table 2.
P-values for the global associations between maternal or paternal BMI category and infant BMI growth curves (birth-3.5 years) from independent and joint mixed effects models
| Independent models (maternal or paternal BMI) | Joint models (maternal and paternal BMI) | |||
|---|---|---|---|---|
| Maternal BMI‡ | Paternal BMI§ | Maternal BMI|| | Paternal BMI|| | |
| Overweight vs. normal* | 0.02 | 0.28 | 0.04 | 0.39 |
| Obese vs. normal* | <0.0001 | 0.005 | <0.0001 | 0.02 |
| Obese vs. overweight* | <0.0001 | 0.03 | <0.0001 | 0.19 |
| Overweight/obese vs. normal† | <0.0001 | 0.01 | <0.0001 | 0.08 |
BMI=body mass index
Overall P-values from global F-tests with 8 numerator degrees of freedom.
Overall P-values from global F-tests with 16 numerator degrees of freedom.
Independent maternal models included fixed effects covariates: maternal overweight and obese indicators, sex, gestational age, birth year tertile, firstborn indicator, maternal age at time of child’s birth, difference in maternal age at child’s birth and at measurement, maternal stature, maternal smoking status at measurement, child’s age, age2, age3, cubic spline terms 1–4, child’s sex*age terms (age, age2, age3, cubic spline terms 1–4), maternal overweight indicator*child’s age terms, maternal obese indicator*child’s age terms.
Independent paternal models included fixed effects covariates: paternal overweight and obese indicators, sex, gestational age, birth year tertile, firstborn indicator, paternal age, paternal age difference, paternal stature, paternal smoking status, child’s age, age2, age3, cubic spline terms 1–4, child’s sex*age terms, paternal overweight indicator*child’s age terms, paternal obese indicator*child’s age terms.
Joint models included fixed effects covariates listed above for independent maternal and paternal models.
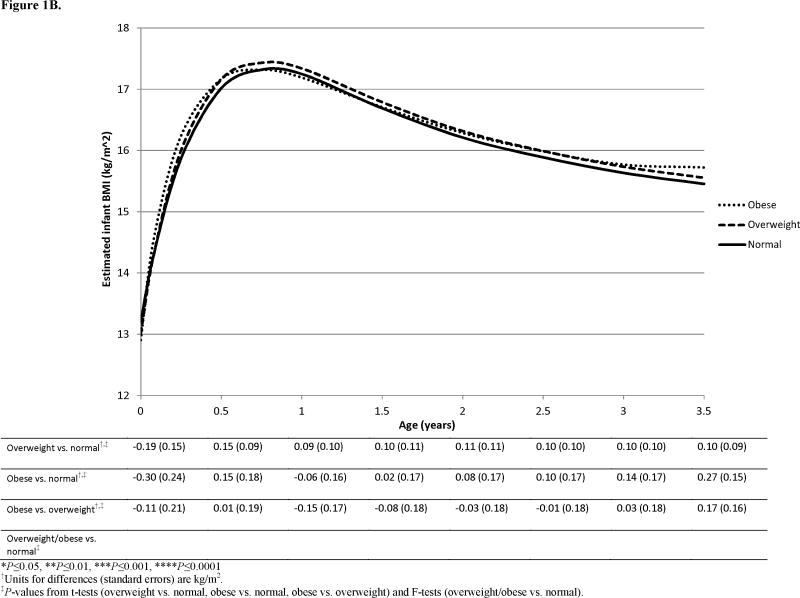
Figure 1.
Estimated infant BMI growth curves from joint mixed effects models for offspring of overweight and obese versus normal weight (A) mothers and (B) fathers. Estimated differences between curves at measured ages are shown beneath the plots.
Due to the non-linear interaction between maternal BMI category and offspring age terms, the association was examined at specific ages in the joint model. Infants of obese mothers had greater BMI at birth than those of overweight and normal weight mothers (Figure 1A, PObese versus overweight=0.004, PObese versus normal=0.0009). Children of obese mothers also had significantly greater mean BMI starting at 1.5 years (PObese versus overweight=0.02, PObese versus normal=0.005) and these differences were significant at every subsequent age up to 3.5 years. This equates to average differences between infants of obese and normal weight mothers of 0.81 kg/m2 at birth and 0.71–0.81 kg/m2 between 2–3.5 years.
Paternal BMI category was associated with infant BMI growth in the independent model (P=0.01, Table 2), where curves for infants of obese fathers were significantly different from those of overweight (P=0.03) and normal weight fathers (P=0.005), however, only the difference between infants of obese and normal weight fathers (P=0.02) persisted in the joint model. No age-specific differences were detected in the joint model (Figure 1B).
A significantly greater effect of maternal BMI than paternal BMI was observed overall (P<0.0001, Table 3) and this was attributable to the greater influence of maternal versus paternal obesity (P<0.0001). In examining individual ages, significant differences were found both at birth (1.11 kg/m2, P=0.006) and again in late infancy; specifically, by 2 years, the effect of maternal obesity was greater than that of paternal obesity (0.69 kg/m2, P=0.01) and this continued through 3 years (0.62 kg/m2, P=0.02).
Table 3.
Global P-values and estimated infant BMI differences (and P-values) at measured ages from joint mixed effects models comparing the effects of maternal and paternal BMI categories on offspring BMI
| Overall | 0mos | 6mos | 1y | 1.5y | 2y | 2.5y | 3y | 3.5y | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal vs. Paternal BMI | ||||||||||
| Overweight vs. overweight | Estimate (SE)† | 0.22 (0.23) | −0.18 (0.16) | −0.13 (0.17) | −0.06 (0.17) | 0.00 (0.17) | 0.04 (0.17) | 0.03 (0.16) | −0.04 (0.15) | |
| P-value‡,§,|| | 0.12 | |||||||||
| Obese vs. obese | Estimate (SE)† | 1.11 (0.39) | −0.24 (0.28) | 0.15 (0.25) | 0.50 (0.27) | 0.69 (0.27) | 0.71 (0.28) | 0.62 (0.27) | 0.44 (0.25) | |
| P-value‡,§,|| | <0.0001 | ** | ** | ** | * | |||||
| Overweight/obese vs. overweight/obese | P-value‡,¶,†† | <0.0001 | ** | * | * | |||||
BMI=body mass index; mos=months; y=year(s); SE=standard error
P≤0.05,
P≤0.01,
P≤0.001,
P≤0.0001
Units are kg/m2.
P-values not adjusted for multiple comparisons.
Overall P-values from global F-tests with 8 numerator degrees of freedom.
Age-specific P-values from t-tests.
Overall P-values from global F-test with 16 numerator degrees of freedom.
Age-specific P-values from F-tests with 2 numerator degrees of freedom.
In ad hoc analyses, similar results were observed for continuous parental BMIs, for infant ponderal index (kg/m3), and for parental BMIs measured ≤3.5 years after the infant’s birth (data not shown). Associations were also similar in independent models after accounting for familial relationships among subjects (data not shown).
The parental BMI-infant birth year interactions were nonsignificant, except for a significant maternal BMI category-birth year tertile interaction in the infant BMI model (Pinteraction=0.04), suggesting relative stability of the relationships between parental BMI and infant growth over time. Figure S2 illustrates that the maternal effects on infant BMI may be driven by the most recent tertile, since a majority of obese parents were observed in this group and since estimated growth curves for this group were generally similar to the overall trend (Figure 1A).
Infant weight and length
Maternal BMI was associated with infant weight (P<0.0001) and length (P<0.0001) trajectories in independent and joint models (Table S3, Figures S1A&S1C). For infant weight, each of the three curves was significantly different (Pjoint≤0.003), whereas for infant length, growth curves for offspring of obese and overweight mothers were indistinguishable (Pjoint=0.66). Paternal BMI did not have a significant overall effect on infant weight (P=0.08) or length (P=0.08) curves in independent models (Table S3, Figures S1B&S1D). After adjusting for maternal BMI in the joint model, the paternal BMI-length association was significant (P=0.05), owing to a difference between curves for offspring of overweight and normal weight fathers (P=0.03).
DISCUSSION
Utilizing serial infant measurements and growth curve modeling, we have fully characterized and formally compared associations between parental BMI and offspring growth throughout infancy. Maternal BMI category was significantly associated with infant BMI, such that offspring of obese mothers were heavier and had greater BMI than offspring of overweight and normal weight mothers at birth and from 1.5 years onward. Although maxima were not formally analyzed, visual inspection of Figure 1A reveals BMIs of infants of obese mothers were slightly higher in early infancy, but peaked at a nearly identical value as infants of normal and overweight mothers, and subsequently experienced a more gradual decline in later infancy. This suggests they may encounter the BMI rebound earlier and at a higher value, placing them at greater risk for later obesity.28 The observations of increased length, as well as increased weight, in offspring of obese mothers indicates the BMI association is not attributable to reduced length in infants of heavier mothers.
Paternal BMI was associated with offspring BMI in independent models, and the paternal obesity effect remained in the joint model. An association was also noted for infant length after adjustment for maternal BMI. Notably, in plotting results from the independent paternal model (not shown), the BMI curve for offspring of obese fathers plateaued between 3–3.5 years (P3.5years,Obese versus Normal=0.01), suggesting paternal effects emerge later, in early childhood.
In comparing our results across mothers and fathers, maternal obesity was found to be a stronger determinant of offspring BMI at birth and again at 2–3 years. Although maternal and paternal BMI were significantly correlated (r=0.23, P<0.0001), the maternal BMI associations with weight, length, and BMI persisted in the joint models, in which the effect of each parent was adjusted for the effect of the other, indicating distinct associations. Conversely, the paternal BMI-infant BMI association observed in the independent model was attenuated in the joint model, suggesting non-independence of paternal effects on infant BMI growth.
Our results regarding the individual effects of maternal and paternal BMI on infant BMI are largely consistent with those from previous studies,9,11–16 indicating BMIs from both parents are associated with infant adiposity in the infancy period. To our knowledge, this study is among the first to use longitudinal growth modeling to show that maternal obesity is a stronger determinant of postnatal infant BMI growth, a marker of later obesity risk, whereas paternal effects may emerge later, possibly between 3–4 years.
Unlike our results, in the one comparable prior longitudinal study, Botton et al also observed a maternal BMI-offspring BMI association at birth, but found similar associations at 9 months and 2 years for both parents.7 Parental BMIs were measured ≥8 years after the child’s birth in that study, however. Similarly, Knight et al reported that a maternal BMI-infant BMI association persisted at each measured time point from birth through 2 years, while a paternal BMI effect was observed at 1 and 2 years.8 Although the authors did not indicate whether either contribution was stronger, they did suggest the maternal and paternal effects were both independent and additive. Average parental BMIs were greater in the study by Knight et al than those in the current study and infants were slightly heavier and shorter at birth. Importantly, Knight et al did not model growth curves, but instead examined time points between birth and 2 years.8 These distinctions may account for the observed differences in paternal BMI effects. In addition, although some prior studies found different parental effects by offspring sex,7,13,16,29 we did not.
Parental BMI may impact offspring body size through genetic, epigenetic and/or shared environmental pathways and parental obesity represents a surrogate marker of the complex interplay between them. Our previous work demonstrated strong additive genetic effects on infant growth,30 which has also been shown by others.31–33 However, as an infant’s genetic endowment is equally derived from each parent, our finding of differential parental effects demonstrates genetic transmission of obesity risk is not the full story. In addition to genetic contributions, parental obesity may modify epigenetic patterns,34 potentially altering offspring gene expression and impacting growth.
The shared environment is a particularly important consideration as it is modifiable. Parents wield considerable influence over their child’s early environment, including the initial provision of breast milk versus formula and subsequent feeding practices,35 and parental eating behaviors shape offspring dietary patterns at an early age.36,37 In addition, obese parents are more likely to create a more obesogenic lifestyle/environment, leading to excess offspring body mass.38 Synergistic gene-by-environment interactions may also be involved, as offspring of obese individuals may have genetic predisposition to excess weight gain within obesogenic home environments.38 Data on these behavioral factors were not available here, but existing studies suggest that compared to fathers, mothers perform more early childhood care,39 have greater influence in food purchasing and preparation,39,40 and exhibit stronger dietary associations with their children.41 Our findings support the rationale for early prevention/intervention efforts, including those aimed at preventing excess maternal BMI and modifying early postnatal environmental factors common among overweight/obese mothers that lead to altered infant BMI growth trajectories.
Our study features some key strengths. Extensive serial measurements allowed growth curves to be modeled in >900 infants. Our approach provides several advantages over cross-sectional designs, such as the ability to measure individual-level change, better precision since each individual serves as his/her own control, and more flexibility because growth curve parameters can vary between individuals.42,43 Additionally, the use of random effects provides smoothing at particular time points due to individual-level variability. Parental anthropometric data were measured within, on average, a few years of the child’s birth, thereby eliminating potential systematic errors resulting from self- or proxy reporting. Finally, nonpaternity, an important confounder, was confirmed absent.
There are also limitations. Parental BMI was not consistently collected pre-pregnancy (mothers) or at birth (fathers); however, BMI is known to track strongly over the 3.5-year period examined herein44 and we adjusted for parental age differences due to the potential for BMI changes. A sensitivity analysis was conducted using parental measurements occurring before or within 3.5 years of the child’s birth. Although less precise, both maternal and paternal effects were similar to overall estimates, indicating the time lapse between birth and parental measurements does not materially impact results.
The cohort is comprised of predominantly middle-class European Americans originally from one area of Ohio and therefore results may be generalizable only to similar populations. In terms of internal validity, however, there are advantages to using homogeneous populations, including less variability in growth.45 Statistical power to detect differences between infants of obese and overweight women was reduced due to smaller sample sizes in these groups. We did not adjust for multiple comparisons in order to detect differences, however we acknowledge that differences with p-values near the 0.05 significance level may be spurious. Lastly, residual confounding may persist. For example, infant births spanned eight decades and although we adjusted for birth year tertile, residual cohort effects may remain.46 Also, socio-economic status was not collected consistently and was therefore not included, but may introduce confounding because it has been associated with adult obesity47 and child growth.48
Conclusions
This is the largest study to describe parental BMI effects on offspring growth over the entire infancy period using longitudinal modeling. Our results provide strong evidence of the importance of maternal BMI to infant BMI growth. The finding that maternal effects re-emerge and remain constant during late infancy suggests prevention efforts focused particularly on maternal BMI may be important in normalizing childhood BMI growth.
Supplementary Material
What is already known about this subject
Excessive early childhood adiposity is a prevalent and increasing concern in many parts of the world.
Parental obesity is one of several factors previously associated with infant and early childhood weight, length, and adiposity. Parental obesity represents a surrogate marker of the complex interplay between genetic, epigenetic, and shared environmental factors and is potentially modifiable.
The relative contributions of maternal and paternal BMI to infant and early childhood growth, as well as the timing of such effects, have not been firmly established.
What this study adds
Utilizing serial infant measurements and growth curve modeling, this is the largest study to fully characterize and formally compare associations between maternal and paternal BMI and offspring growth across the entire infancy and early childhood period.
Maternal obesity is a stronger determinant of offspring BMI than paternal obesity at birth and from 2 to 3 years of age, suggesting that prevention efforts focused particularly on maternal lifestyle and BMI may be important in reducing excess infant BMI.
The observation that maternal BMI effects are not constant, but rather present at birth, wane, and re-emerge during late infancy, suggests that there is a window of opportunity in early infancy when targeted interventions on children of obese mothers may be most effective.
Acknowledgments
Research supported by NIH Grants R01 HD53685, R01 HD012252, and T32 CA99936.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Author contributions: Amy M. Linabery performed the analysis and was primary author of all sections of the manuscript. Ramzi W. Nahhas contributed substantial guidance on the statistical analysis due to his expertise in statistical modeling of growth, and contributed greatly to the final manuscript. William Johnson conducted the cubic spline analysis and contributed to the final manuscript. Audrey C. Choh provided input on the study design and statistical analysis, and contributed to the final manuscript. Bradford Towne provided input on the study design and contributed to the final manuscript. Andrew O. Odegaard provided input on the statistical analysis and contributed to the final manuscript. Stefan A. Czerwinski serves as PI of the Fels Longitudinal Study and contributed to the final manuscript. Ellen W. Demerath originally formulated the idea for the analysis and paper and actively served as a consultant regarding the analysis and manuscript preparation due to her expertise in childhood growth; she contributed substantially to the final manuscript.
References
- 1.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009:CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 4.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 6.Regnault N, Botton J, Forhan A, et al. Determinants of early ponderal and statural growth in full-term infants in the EDEN mother-child cohort study. Am J Clin Nutr. 2010;92:594–602. doi: 10.3945/ajcn.2010.29292. [DOI] [PubMed] [Google Scholar]
- 7.Botton J, Heude B, Maccario J, et al. Parental body size and early weight and height growth velocities in their offspring. Early Hum Dev. 2010;86:445–450. doi: 10.1016/j.earlhumdev.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Knight B, Shields BM, Hill A, Powell RJ, Wright D, Hattersley AT. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care. 2007;30:777–783. doi: 10.2337/dc06-1849. [DOI] [PubMed] [Google Scholar]
- 9.Kivimaki M, Lawlor DA, Smith GD, et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2007;86:1509–1514. doi: 10.1093/ajcn/86.5.1509. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor DA, Smith GD, O’Callaghan M, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 11.Heude B, Kettaneh A, Rakotovao R, et al. Anthropometric relationships between parents and children throughout childhood: the Fleurbaix-Laventie Ville Sante Study. Int J Obes. 2005;29:1222–1229. doi: 10.1038/sj.ijo.0802920. [DOI] [PubMed] [Google Scholar]
- 12.Safer DL, Agras WS, Bryson S, Hammer LD. Early body mass index and other anthropometric relationships between parents and children. Int J Obes. 2001;25:1532–1536. doi: 10.1038/sj.ijo.0801786. [DOI] [PubMed] [Google Scholar]
- 13.Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes. 2003;27:505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto N, Kawasaki T, Kikuchi T, Takahashi H, Uchiyama M. Influence of parental obesity on the physical constitution of preschool children in Japan. Acta Paediatr Jpn. 1995;37:150–153. doi: 10.1111/j.1442-200x.1995.tb03287.x. [DOI] [PubMed] [Google Scholar]
- 15.Sekine M, Yamagami T, Hamanishi S, et al. Parental obesity, lifestyle factors and obesity in preschool children: results of the Toyama Birth Cohort study. J Epidemiol. 2002;12:33–39. doi: 10.2188/jea.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker RC, Deeks CM, Baughcum AE, Specker BL. The relationship of childhood adiposity to parent body mass index and eating behavior. Obes Res. 2000;8:234–240. doi: 10.1038/oby.2000.27. [DOI] [PubMed] [Google Scholar]
- 17.Ay L, Hokken-Koelega AC, Mook-Kanamori DO, et al. Tracking and determinants of subcutaneous fat mass in early childhood: the Generation R Study. Int J Obes. 2008;32:1050–1059. doi: 10.1038/ijo.2008.76. [DOI] [PubMed] [Google Scholar]
- 18.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes. 2004;28:503–513. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- 19.Stunkard AJ, Berkowitz RI, Stallings VA, Cater JR. Weights of parents and infants: is there a relationship? Int J Obes. 1999;23:159–162. doi: 10.1038/sj.ijo.0800785. [DOI] [PubMed] [Google Scholar]
- 20.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92:876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel R, Martin RM, Kramer MS, et al. Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS One. 2011;6:e14607. doi: 10.1371/journal.pone.0014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche AF. Growth, maturation, and body composition: the Fels Longitudinal Study 1929–1991. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 23.Lohman TG, Roche AF, Martorell R. In: Anthropometric standardization reference manual. Abridged, editor. Champaign, IL: Human Kinetics Books; 1991. [Google Scholar]
- 24.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.Rubin DB. Multiple Imputations in Sample Surveys - A Phenomenological Bayesian Approach to Nonresponse. Proceedings of the Survey Research Methods Section, American Statistical Association. 1978:20–34. [Google Scholar]
- 26.Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken, N.J: Wiley; 2002. [Google Scholar]
- 27.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge ; New York: Cambridge University Press; 2003. [Google Scholar]
- 28.Williams SM, Goulding A. Early adiposity rebound is an important predictor of later obesity. Obesity (Silver Spring) 2009;17:1310. doi: 10.1038/oby.2009.104. [DOI] [PubMed] [Google Scholar]
- 29.Klesges RC, Klesges LM, Eck LH, Shelton ML. A longitudinal analysis of accelerated weight gain in preschool children. Pediatrics. 1995;95:126–130. [PubMed] [Google Scholar]
- 30.Demerath EW, Choh AC, Czerwinski SA, et al. Genetic and environmental influences on infant weight and weight change: the Fels Longitudinal Study. Am J Hum Biol. 2007;19:692–702. doi: 10.1002/ajhb.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dommelen P, de Gunst MC, van der Vaart AW, Boomsma DI. Genetic study of the height and weight process during infancy. Twin Res. 2004;7:607–616. doi: 10.1375/1369052042663805. [DOI] [PubMed] [Google Scholar]
- 32.Dubois L, Girard M, Girard A, Tremblay R, Boivin M, Perusse D. Genetic and environmental influences on body size in early childhood: a twin birth-cohort study. Twin Res Hum Genet. 2007;10:479–485. doi: 10.1375/twin.10.3.479. [DOI] [PubMed] [Google Scholar]
- 33.Beardsall K, Ong KK, Murphy N, et al. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J Clin Endocrinol Metab. 2009;94:3708–3713. doi: 10.1210/jc.2009-0757. [DOI] [PubMed] [Google Scholar]
- 34.Waterland RA. Epigenetic epidemiology of obesity: application of epigenomic technology. Nutr Rev. 2008;66 (Suppl 1):S21–23. doi: 10.1111/j.1753-4887.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 35.Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes. 2010;34:1116–1124. doi: 10.1038/ijo.2010.43. [DOI] [PubMed] [Google Scholar]
- 36.Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin North Am. 2001;48:893–907. doi: 10.1016/s0031-3955(05)70347-3. [DOI] [PubMed] [Google Scholar]
- 37.Kral TV, Rauh EM. Eating behaviors of children in the context of their family environment. Physiol Behav. 2010;100:567–573. doi: 10.1016/j.physbeh.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davison KK, Birch LL. Child and parent characteristics as predictors of change in girls’ body mass index. Int J Obes. 2001;25:1834–1842. doi: 10.1038/sj.ijo.0801835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Bureau of Labor Statistics. American Time Use Survey -- 2009 Results (USDL-10-0855) June 22, 2010. News Release. Available at: http://www.bls.gov/news.release/pdf/atus.pdf.
- 40.Belch MA, Willis LA. Family decision at the turn of the century: Has the changing structure of households impacted the family decision-making process? J Cons Behav. 2001;2:111–124. [Google Scholar]
- 41.Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent-child relationships in nutrient intake: the Framingham Children’s Study. Am J Clin Nutr. 1992;56:593–598. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- 42.Berkey CS. Bayesian approach for a nonlinear growth model. Biometrics. 1982;38:953–961. [PubMed] [Google Scholar]
- 43.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, N.J: Wiley-Interscience; 2004. [Google Scholar]
- 44.Guo SS, Huang C, Maynard LM, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes. 2000;24:1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 45.Overpeck MD, Hediger ML, Ruan WJ, et al. Stature, weight, and body mass among young US children born at term with appropriate birth weights. J Pediatr. 2000;137:205–213. doi: 10.1067/mpd.2000.107163. [DOI] [PubMed] [Google Scholar]
- 46.Johnson W, Choh AC, Soloway LE, Czerwinski SA, Towne B, Demerath EW. Eighty-year trends in infant weight and length growth: the Fels Longitudinal Study. J Pediatr. 2012;160:762–768. doi: 10.1016/j.jpeds.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 48.Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity. 2008;16:275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.