Abstract
Objectives
To examine the reliability and prognostic importance of an in-hospital diagnosis of MetS in the setting of AMI.
Background
As the factors that comprise the metabolic syndrome (MetS) are believed to be altered in the setting of an acute myocardial infarction (AMI), the diagnosis of MetS during AMI hospitalization and its prognostic significance have not been studied.
Methods
We assessed patients within a multicenter registry for metabolic factors at baseline and 1-month post-AMI and followed them for mortality and rehospitalizations. The accuracy of an inpatient diagnosis of MetS was calculated, using a 1-month follow-up as the gold standard. Patients were categorized based on MetS diagnosis at baseline and 1 month, and the combined endpoint of death or rehospitalization over 12 months was compared between groups.
Results
Of 1129 patients hospitalized for AMI, diagnostic criteria for MetS were met by 69% during AMI hospitalization and 63% at 1 month. Inpatient MetS diagnosis had a sensitivity and specificity for outpatient diagnosis of 87% and 61%, respectively, and was associated with an 11 times increased odds of an outpatient diagnosis (c-index=0.74). Compared with patients without MetS during hospitalization and follow-up, patients classified as MetS during AMI but not follow-up had worse outcomes; while those classified MetS at follow-up had the worst outcomes (rates for combined endpoint: 27% vs. 37% vs. 38%; log-rank p=0.01).
Conclusions
In a large cohort of AMI patients, the diagnosis of MetS is common and can be made with reasonable accuracy during AMI. It is associated with poor outcomes, regardless of whether the diagnosis is confirmed during subsequent outpatient visit, and identifies a high-risk cohort of patients that may benefit from more aggressive risk factor modification.
Keywords: metabolic syndrome, myocardial infarction, long-term outcomes
Although typically thought of as a risk factor for developing incident diabetes and cardiovascular disease, metabolic syndrome (MetS) has been shown to be associated with increased mortality and recurrent ischemic events among patients with stable coronary artery disease, independent of its associations with diabetes and obesity.(1) However, it is not known whether MetS carries the same prognostic importance in the setting of an acute ischemic event. Furthermore, it is unclear if the same diagnostic criteria used in the outpatient setting can be used during AMI. The adrenergic surge that occurs with an AMI is thought to be associated with substantial variability in many of the factors that comprise the MetS, particularly blood pressure, glucose and lipid values. However, an earlier diagnosis of MetS may allow for better risk stratification and initiation of aggressive risk factor modification prior to discharge, when changes have the greatest likelihood of being implemented by the patient.(2,3)
METHODS
Study population and protocol
Details of the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) prospective cohort study have been previously published.(4) Briefly, 4340 patients from 24 U.S. hospitals were enrolled into the TRIUMPH registry (2005–08). All patients had biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of AMI. Baseline data were obtained through chart abstraction and detailed interview. Consenting patients had their waist circumference measured and fasting blood specimens collected prior to discharge. Blood was analyzed at a core laboratory (Clinical Reference Laboratory, Lenexa, KS) for glucose and lipid levels. Laboratory values drawn for clinical purposes were recorded and used if core data were unavailable. Patients could opt for 1-month follow-up by telephone or in home visit, which allowed for collection of additional clinical and laboratory data. The final blood pressure recorded in the chart was used for baseline assessment.
MetS was determined using Adult Treatment Panel III criteria (Appendix, eExhibit A).(5) Although anti-hypertensive medications are typically included in this definition, we excluded beta blockers (all patients) and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (patients with ventricular dysfunction), as these may have been used for purposes other than blood pressure. Only patients with baseline and 1-month assessments sufficient to determine the presence or absence of MetS were included (Figure 1). Patients were interviewed 6 and 12 months post-AMI, and charts from patients reporting interim rehospitalizations were requested and adjudicated.(4) Mortality was assessed by the Social Security Death Masterfile. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent.
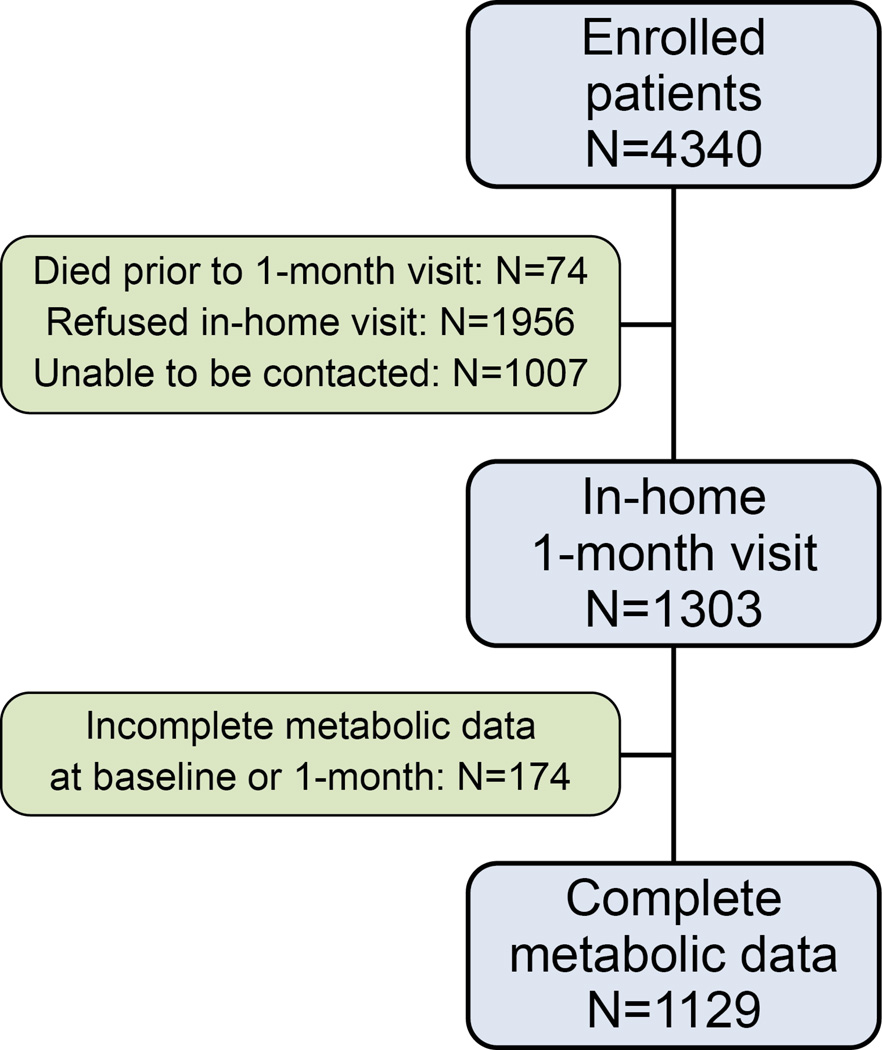
Figure 1. Flowchart of Patients in Study Cohort.
Patients could opt for 1-month follow-up by telephone or in-home visit, which allowed for collection of additional clinical and laboratory data. Only patients with baseline and 1-month assessments sufficient to determine the presence or absence of MetS were included.
Statistical analysis
Patients were categorized into 4 groups: 1) no MetS baseline and 1- month (MetS−/MetS−); 2) MetS baseline and no MetS 1-month (MetS+/MetS−); 3) no MetS baseline and MetS 1-month (MetS−/MetS+); and 4) MetS baseline and 1-month (MetS+/MetS+). Sensitivity, specificity, and positive- (PPV) and negative-predictive values (NPV) were calculated for MetS diagnosis at baseline as a predictor of outpatient MetS and for the 5 individual MetS components. Logistic regression evaluated the ability of baseline MetS diagnosis to predict follow-up MetS diagnosis.
Kaplan-Meier (KM) curves compared time to all-cause death or rehospitalization from 1 to 12 months post-AMI across the MetS groups, and Cox proportional hazards estimated hazard ratios, adjusted for the Global Registry of Acute Coronary Events (GRACE) discharge score.(6) As the outpatient diagnosis of MetS is the gold standard, we combined MetS−/MetS+ and MetS+/MetS+ groups as “true MetS” for the outcomes analysis (for 4-group sensitivity analysis see Appendix, eFigure 1). Finally, we explored whether the association between MetS and prognosis was attributable solely to the presence or absence of diabetes.
All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
Patient Population
Of 4266 patients enrolled in TRIUMPH who survived 1 month after their AMI, 1303 agreed to an in-home assessment with blood draw. Among those, 1129 patients (87%) had sufficient metabolic data at baseline and 1 month to be able to determine the presence or absence of MetS (Figure 1; for generalizability analysis, see Appendix, eExhibit B). Overall, participants had an average age of 59.7 years; 34% were women; and the average BMI was 29.9 kg/m2. Prevalent diabetes was present in 29.4%, and 20.4% had a prior AMI.
Sensitivity, Specificity, PPV, and NPV
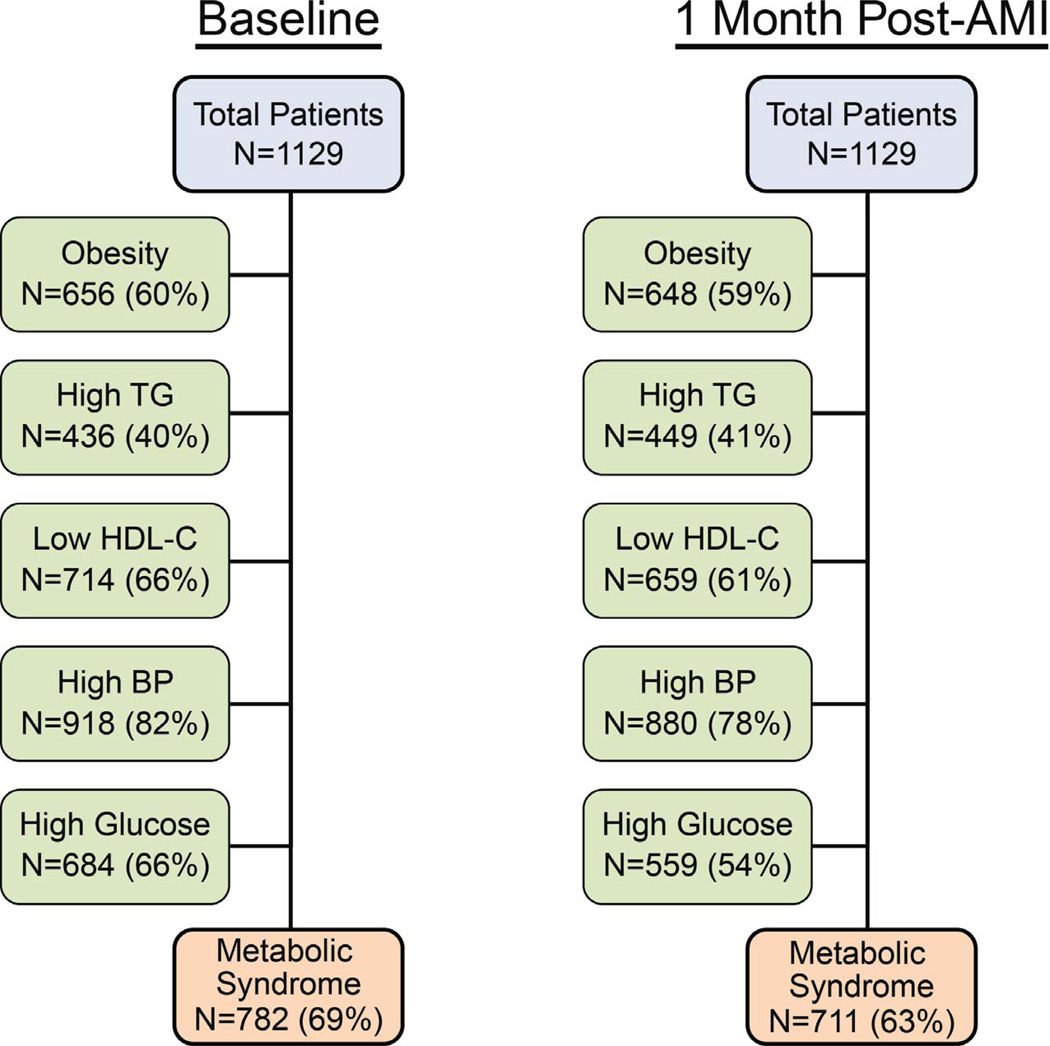
Diagnostic criteria for MetS were met by 69% of patients during AMI hospitalization and 63% at 1-month follow-up (Figure 2). The percentage meeting each criterion of MetS was similar between assessments (±5%) with the exception of impaired FBG (66% at baseline vs. 54% at 1-month). In terms of individual patients, 22% changed MetS classification from baseline to 1 month. The sensitivity and specificity of MetS diagnosis at baseline for the diagnosis at 1 month were 87% and 61%, respectively (Table 1). The most stable individual component was abdominal obesity, while the 3 laboratory assessments were more unstable from baseline to 1-month. MetS diagnosis during hospitalization was associated with a 10.8 times increased odds (95% confidence interval 8.0–14.5) of outpatient MetS diagnosis (c-index 0.742).
Figure 2. Prevalence of the Components of Metabolic Syndrome During and After AMI Hospitalization.
Number of patients who met diagnostic criteria for MetS and each of the individual components during AMI hospitalization and at 1-month follow-up.
Table 1.
Accuracy of Baseline Assessment of MetS and Its Components for 1-Month Post-AMI Assessment
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Metabolic Syndrome | 87.2% | 61.2% | 79.3% | 73.8% |
| Abdominal obesity | 92.7% | 87.8% | 91.6% | 89.3% |
| High triglycerides | 64.4% | 77.0% | 66.3% | 75.5% |
| Low HDL-C | 82.4% | 60.2% | 76.1% | 69.1% |
| High blood pressure | 93.5% | 61.1% | 89.7% | 72.3% |
| Impaired fasting glucose/diabetes | 85.7% | 57.6% | 70.0% | 77.7% |
Patient Characteristics of Different Metabolic Groups
The demographics and clinical characteristics of the 4 metabolic groups, based on the diagnosis of MetS during index hospitalization and at 1 month post-AMI, are shown in Table 2. Patients categorized as MetS+ during index hospitalization were more likely to have diabetes and to have multi-vessel disease on angiogram. Patients identified as MetS+ during index hospitalization had worse metabolic values across the spectrum of measured factors compared with patients categorized as MetS-, including higher BMIs, higher triglycerides, lower HDL-C levels, and higher glucose and insulin levels. (Appendix, eTable 1).
Table 2.
Baseline Characteristics of Four Groups Based on Baseline and 1-Month Diagnoses of MetS
| No MetS | MetS | ||||
| MetS−/MetS− n= 256 |
MetS+/MetS− n= 162 |
MetS−/MetS+ n=91 |
MetS+/MetS+ n=620 |
P-Value | |
| Age | 61.6±12.0 | 58.2±11.4 | 61.8±13.0 | 58.9±11.1 | 0.002 |
| Female | 27.0% | 29.0% | 30.8% | 38.7% | 0.003 |
| White | 74.5% | 79.0% | 75.8% | 71.5% | 0.491 |
| Current Smokers | 40.9% | 44.7% | 34.1% | 35.9% | 0.131 |
| Prior Myocardial Infarction | 15.2% | 15.4% | 19.8% | 23.9% | 0.010 |
| Prior Angioplasty | 11.7% | 18.5% | 20.9% | 23.9% | <0.001 |
| Prior Bypass Surgery | 8.6% | 7.4% | 9.9% | 15.2% | 0.007 |
| Hypertension | 51.6% | 52.5% | 67.0% | 76.3% | <0.001 |
| Diabetes | 5.9% | 13.0% | 15.4% | 45.5% | <0.001 |
| ST-Elevations | 54.3% | 47.5% | 46.2% | 44.2% | 0.058 |
| Multi-vessel disease (≥2 vessels) | 42.5% | 48.4% | 36.7% | 54.3% | 0.001 |
| LV dysfunction | 46.7% | 40.7% | 34.1% | 32.0% | <0.001 |
| GRACE score | 102.4±28.2 | 94.8±26.3 | 104.3±28.5 | 99.3±27.8 | 0.019 |
Outcomes of Different Metabolic Groups
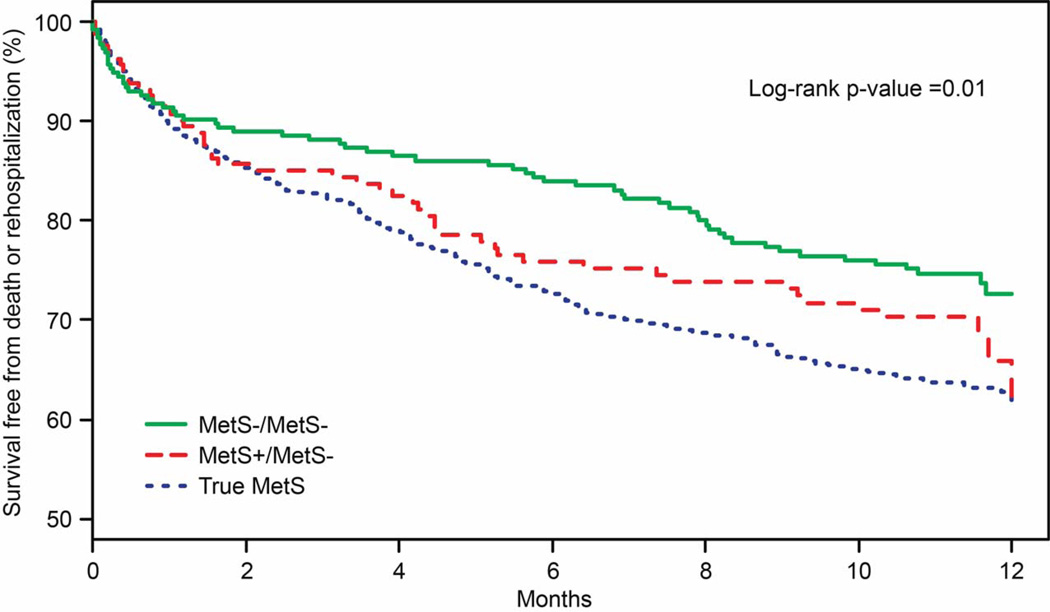
Patients with no MetS at baseline and follow-up had the best outcomes over the year following AMI (mortality=2.0%, rehospitalization=25.6%). Patients classified as MetS+ during the AMI but not follow-up had worse outcomes (mortality=2.5%, rehospitalization=33.7%). True MetS patients (MetS−/MetS+ and MetS+/MetS+; see Appendix for 4-group analysis) had the worst outcomes (mortality =4.1%, rehospitalizations=36.2%) , with rates of combined endpoints: 27% vs. 37% vs. 38%, respectively(log-rank p=0.01; Figure 3). In analyses adjusted for GRACE score (a measure of AMI severity), MetS+/MetS- was associated with a non-significant trend toward increased hazard of death or rehospitalization (HR 1.39, 95% CI 0.96–2.01, p=0.082), and true MetS was associated with a significant increased hazard (HR 1.56, 95% CI 1.19–2.06, p=0.002), (Reference: MetS−/MetS−).
Figure 3. Survival Free from Death or Rehospitalization Across Metabolic Groups.
MetS−/MetS−=No MetS at baseline and 1-month (n=256). MetS+/MetS−=MetS at baseline but not at 1 month (n=162). MetS+/MetS+ and MetS−/MetS+=true MetS patients (i.e., MetS diagnosed as an outpatient; n=711).
To examine whether the association between MetS and prognosis was driven by a concurrent diagnosis of diabetes, we additionally adjusted for diabetes and the interaction of diabetes*MetS. The interaction terms were not statistically significant (p>0.1), indicating that the association of MetS group with poor outcomes did not vary according to diabetes status. Furthermore, among patients without diabetes, the association between MetS group and poor outcome remained consistent (Appendix, eFigure 2).
DISCUSSION
In this large, multicenter prospective AMI cohort study, the diagnosis of MetS was exceedingly common both at the time of AMI at 1-month after the acute event. While many of the individual components may be altered during the AMI, the diagnosis of MetS—as a constellation of factors—has reasonable accuracy at the time of AMI hospitalization. Furthermore, patients identified as MetS during the AMI were at high-risk for poor outcomes, regardless of the MetS diagnosis at the 1-month follow-up. Therefore, identifying these patients at the time of an AMI—when therapeutic and lifestyle changes are most likely to occur(2,3)—is not only feasible but may be optimal from a patient-care perspective. Importantly, the components of MetS are all measures routinely collected as part of clinical care; therefore, these patients can be easily “flagged” as MetS (and high-risk) to the treating physician. These patients could then be targeted for more intensive lifestyle changes, closer follow-up, and reassessment during the subsequent outpatient physician visit.
Prior Studies
MetS is generally considered a constellation of factors that increases an individual’s risk for developing diabetes or a primary cardiac event, and its prognostic importance has been demonstrated most often in this capacity.(7,8) Therefore, it is not surprising that the prevalence of MetS among AMI patients in our study was much higher than in the general American adult population (~25%(9)) or among patients with stable CAD (~40–50%(10)). Importantly, MetS in our study was associated with adverse prognosis after an AMI, even after adjusting for GRACE score. Although AMI patients are already considered high-risk for recurrent ischemic events, the diagnosis of MetS had additional prognostic value even in this high-risk population. While there is some evidence of an increased risk in the stable coronary artery disease population,(1) this was mostly observed in patients with concomitant diabetes.(10) Our study shows that the diagnosis of MetS made in the setting of an AMI also confers a poor prognosis, independent of diabetes.
Limitations
First, many patients declined participation in the metabolic sub-study of TRIUMPH. However, as the baseline characteristics of non-participants were similar to participants, the analytic population remains fairly representative of a general AMI population who survives to 1 month after AMI. Second, due to a low number of events, we were limited in our ability to adjust for a large number of covariates in our outcomes models. Nevertheless, we adjusted for the GRACE score, which integrates many prognostically important variables and has excellent predictive ability for long-term post-AMI mortality.(6) Finally, while we demonstrated an association of MetS with poor outcomes and thus provided an additional tool for risk stratification after AMI, we do not yet know whether early recognition of these patients as MetS will mitigate this excessive risk. We also do not know if more aggressive lifestyle interventions beyond those routinely prescribed to AMI patients provide additional benefit in these high-risk patients. Future studies are needed to determine whether indentifying patients as MetS during their AMI will improve outcomes.
Conclusions
In this multicenter registry of AMI patients, we found that MetS was exceedingly common, could be diagnosed with reasonable accuracy during hospitalization, and was associated with increased risk of death or rehospitalization over the 12-months following AMI. Patients who were classified as MetS during the acute event but did not qualify at followup still represented a high-risk group, underscoring the importance of identifying these patients during their initial hospitalization. Further work that seeks to identify MetS patients prospectively during the AMI and institute more aggressive lifestyle changes may help reduce the excess risk for poor long-term outcomes in this population.
Supplementary Material
Acknowledgments
Sources of Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): Washington University School of Medicine SCCOR Grant #P50HL077113-01. This study was sponsored by Genentech, South San Francisco, CA. The funding organizations did not play a role in the design and conduct of the study or in the collection, management, analysis, and interpretation of the data.
ABBREVIATIONS
- AMI
acute myocardial infarction
- FBG
fasting blood glucose
- GRACE
Global Registry of Acute Coronary Events
- HDL-C
high-density lipoprotein cholesterol
- KM
Kaplan-Meier
- LDL-C
low-density lipoprotein cholesterol
- MetS
metabolic syndrome
- NPV
negative predictive value
- PPV
positive predictive valve
- TG
triglycerides
- TRIUMPH
Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: SVA: Research grants: Significant-Genentech, Eli Lilly, Sanofi-Aventis, Gilead; DKM: Consultant honoraria: Significant-Genentech; Modest-F Hoffmann LaRoche, Pfizer, Daiichi Sankyo, NovoNordisk, Sanofi Aventis, Regeneron, Tethys Bioscience. Clinical trial leadership honoraria: Boehringer Ingelheim, Takeda, Orexigen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Daiichi Sankyo, Merck Schering Plough. MK: Research grants: Significant- American Heart Association, Genetech, Sanofi-Aventis, Gilead, Medtronic Minimed, Glumetrics; Consultant honoraria: Significant-Medtronic Minimed, Modest-Genentech, Gilead, F Hoffmann LaRoche, Boehringer-Ingleheim. The other authors report no conflicts of interest.
References
- 1.Daly CA, Hildebrandt P, Bertrand M, et al. Adverse prognosis associated with the metabolic syndrome in established coronary artery disease: data from the EUROPA trial. Heart. 2007;93:1406–1411. doi: 10.1136/hrt.2006.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow GC, Gawlinski A, Moughrabi S, Tillisch JH. Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP) Am J Cardiol. 2001;87:819–822. doi: 10.1016/s0002-9149(00)01519-8. [DOI] [PubMed] [Google Scholar]
- 3.Lappe JM, Muhlestein JB, Lappe DL, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141:446–453. doi: 10.7326/0003-4819-141-6-200409210-00010. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 6.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 7.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among us Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 10.Petersen JL, Yow E, AlJaroudi W, et al. Metabolic syndrome is not associated with increased mortality or cardiovascular risk in nondiabetic patients with a new diagnosis of coronary artery disease. Circ Cardiovasc Qual Outcomes. 2010;3:165–172. doi: 10.1161/CIRCOUTCOMES.109.864447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.