Abstract
Cognitive event-related brain potential (ERP) studies of decision-making and attention, language, and memory impairments in Alzheimer’s disease (AD) and mild cognitive impairment (MCI) are reviewed. Circumscribed lesions of the medial temporal lobe (MTL), as may be the case in individuals with amnestic MCI, generally produce altered plasticity of the late positive P600 component, with relative sparing of earlier sensory ERP components. However, as the neuropathology of AD extends to neocortical association areas, abnormalities of the P300 and N400 (and perhaps even P50) become more common. Critically, ERP studies of individuals at risk for AD may reveal neurophysiological changes prior to clinical deficits, which could advance the early detection and diagnosis of “presymptomatic AD”.
Keywords: Alzheimer’s disease (AD), mild cognitive impairment (MCI), synaptic dysfunction, preclinical AD, event-related potentials (ERP), P300, N400, P600, Late Positive Component (LPC), EEG
INTRODUCTION/OVERVIEW
This article offers a concise overview of the scientific literature on several event-related brain potential (ERP) components with demonstrated sensitivity to Alzheimer’s disease (AD). These components include the N200, P300, N400, and P600 (or LPC for late positive component), each of which taps different aspects of perceptual and/or cognitive processing. ERPs provide a flexible and powerful technique, with superb temporal resolution, which can be used to probe subtle, sometimes ‘subclinical’, abnormalities of cognition. Despite over 30 years of ERP research since the initial P300 studies, the full potential of cognitive ERPs for diagnosing and/or treating AD patients has yet to be realized. In this era of rapidly evolving brain imaging techniques, non-invasive electrophysiological data are increasingly important in advancing our understanding of the what and where of cognition. Understanding the mechanisms by which AD causes an unraveling of many key cognitive processes, far broader than memory processes alone, is finally within the reach of cognitive neuroscientists.
COGNITIVE EVENT-RELATED POTENTIALS (ERPS)
Cognitive ERPs provide a powerful, non-invasive, tool for studying the brain’s synaptic function. ERPs are an instantaneous reflection of the summated post-synaptic excitatory (EPSPs) and inhibitory (IPSPs) membrane potentials, primarily of pyramidal cells in the neocortex [1–3]. The temporal immediacy of ERPs is especially advantageous in the study of memory, given that memory encoding and retrieval processes can be very fast, and in light of the evidence that the temporal encoding of information may be important if not essential for synaptic plasticity [4, 5]. Because ERPs (like Magnetoencephalography or MEG) reflect the precise timing and temporal patterns of neuronal activity, they are very useful in quantifying the timing and sequence of the various stages or aspects of cognitive processing, more generally. In broad stroke, evoked potential (EP) and ERP research have shown that early brain responses (e.g., the visual N1) generally reflect the sensory input characteristics, while later responses are relatively more dependent on the mental operations performed on the stimuli as well as on non-sensory factors such as predictability, higher perceptual and semantic features. It is these slower, later, so-called endogenous components that have shown particular sensitivity to Alzheimer’s disease (AD), a disease with predilection for the medial temporal lobes and higher association neocortical regions [6, 7].
AD AND SYNAPTIC DYSFUNCTION
In recent years, several investigators have suggested that AD may be primarily a disorder of the synapse and synaptic plasticity [8–10]. Several transgenic animal models of AD, for instance, have revealed prominent inhibition of long-term potentiation (LTP) and/or reduced synaptic transmission before the appearance of extensive AD pathology (amyloid plaques and neurofibrillary changes) or neuron loss [11, 12]. As a consequence, much of the current basic research focuses on the mechanisms of synaptic dysfunction in AD and its relationship to Aβ oligomers [13]. Selkoe [9], for example, made a substantive change in his model of AD pathogenesis, when he suggested that the earliest changes in synaptic function may be due to soluble forms of Aβ and precede the appearance of any extracellular amyloid deposits. Clinico-neuropathologic studies, likewise, have implicated the synapse as a primary mediator of dementia severity. Terry et al. [14], for example, found that nearly 90% of the variance in dementia severity could be accounted for by the density of pre-synaptic terminals in mid-frontal cortex. Electron microscopy of cortical biopsies in early- to mid-stage AD shows a 30% decrease in synaptic density and a 25% decrease in synapses/neuron [15]. Bertoni-Freddari et al. [16] reported a large increase in the proportion of deafferented synapses on hippocampal neurons from autopsied AD cases. In short, it is reasonable to conceive of AD as a diffuse deafferentation syndrome, in which both the neocortex and hippocampus have lost a critical proportion of their normal inputs [17]. Accordingly, cognitive ERPs may provide a highly sensitive biomarker for AD.
MIDDLE-LATENCY ERP COMPONENTS IN AD DEMENTIA (P50, N100, P200 AND N200)
In general, EP and ERP studies in AD have shown normal latency and amplitude of the early “sensory” components (e.g., visual and auditory N1 or N100). In 1987, Goodin and Aminoff [18] reported normal auditory N100 and P200 latencies in AD in response to frequent, standard (i.e., non- target) tones in a classic auditory “oddball” P300 paradigm (see also section on P300 studies of AD below). This finding was despite the fact that their AD patient group was severely impaired (6 of 22 AD patients were too severe to be tested with the mini-mental state exam (MMSE)). N100 and P200 latencies were also found to be relatively insensitive to normal aging, but sensitive to subcortical dementias such as Huntington’s or Parkinson’s disease [19].
Delayed P200 latency to pattern reversal or to flash stimuli in AD has been reported in some (e.g., [20, 21]) but not other studies (e.g., [22]). A delayed flash P200 has been suggested as useful in distinguishing AD from other dementias, particularly when normal flash P100 and pattern reversal P100 s are present [23, 24]. Martinelli et al. [20] also found delayed visual P200 in AD, using pattern visual evoked potentials. Furthermore, they reported that P200 amplitude over the right posterior scalp correlated with visuospatial abilities. However, Saitoh [25] used a visual target P300 paradigm, and found normal P100, N100, and P200 components in AD.
Most investigations of verbal stimuli – spoken [26] or written [27] also have found normal (amplitude and latency) N100 and P200 components in mild AD patients. In response to non-verbal, auditory tones, Golob and Starr [28] found robust P50 potentials (also termed the auditory P1) in mild AD, which were significantly enlarged compared to healthy elderly. One caveat for this result is that reduced slow-negative “readiness potentials” in AD also resulted in slightly more positive pre-stimulus baseline voltage in the AD group.
N200
Rather consistent abnormalities in auditory N200 latency have been reported in AD [19, 29, 30]. The N200 is the earliest ERP component which consistently differentiates target from non-target stimuli in an “oddball” task, and immediately precedes the P300 discussed below. It is also sensitive to normal aging, becoming smaller and slower with age [31] at a rate nearly as fast as the slowing of P300 latency [19] (e.g., estimated at 0.74 ms/year, in comparison to 1.15 ms/year for the P300). Duffy et al. [32, 33] have shown that visual motion-elicited ERPs may help identify a subtype of AD. AD patients with low ability to detect random dot motion also showed large decrease in their N200 amplitude response to optic flow with nearly absent N200 in response to radial motion of coherent dots. The authors interpreted this finding as implicating greater neuropathology in the extrastriate visual cortex of this subgroup.
In sum, later components, such as the N200 and P300 (discussed below) have shown better sensitivity to dementia than early sensory components, albeit poor specificity in differentiating among the various dementias.
P300: OVERVIEW
The P300 (or ‘P3b’) component is a scalp positivity elicited by low-probability task-relevant stimuli during stimulus classification tasks in auditory, visual, and somatosensory modalities. In the canonical P300-eliciting experiment, the “auditory oddball” task, participants are asked to detect (by counting or button press) a low-probability “target” (e.g., high-pitched) tone embedded in a stream of “standard” (e.g., low-pitched) tones. The target tones normally elicit a large scalp positivity which peaks ~300 ms post-stimulus onset and is maximal over midline centroparietal electrode sites (unlike the frontally distributed ‘P3a’ elicited by task-irrelevant stimuli, e.g., dog barking). The standard tones typically do not elicit a P300 (although see Squires et al. [34] for demonstration that standards may also occasionally elicit some P300 activity). This pattern of results (P300 s to target tones only) is dependent on attention. The P300 has been extensively studied and well characterized in both normal and neurologically-impaired populations. P300 latency is variable, and generally increases with the complexity of the stimulus evaluation and decisional processes demanded by the task. P300 amplitude and latency are modulated by a variety of factors-subjective probability, stimulus saliency, availability of attentional resources [35] —and it appears to be generated by a distributed network of neural regions—inferotemporal, perirhinal, prefrontal, cingulate, superior temporal and parietal cortices, as well as the hippocampus [36, 37] —suggesting that P300 may index a heterogeneous set of cognitive processes. On the other hand, studies of patients with damage to the temporo-parietal junction have found that the auditory (although not visual) P300 response is eliminated [38], suggesting that this neocortical region may be critical in generation or propagation of the scalp P300 (in the auditory modality). In general, the P3b amplitude has proven more sensitive to sensory-perceptual than response selection and execution factors, in contrast to reaction time measures which are sensitive to both, It is generally agreed that when a stimulus elicits a P300 component, it is reasonable to assume that the stimulus has been encoded into working memory. This is generally consistent with the hypotheses that P300 reflects processes involved in updating of working memory [39], or the processes of stimulus categorization [40].
P300 IN ALZHEIMER’S DISEASE
With normal aging, the latency of the auditory P300 increases ~1–2 ms/year [19, 41]. In AD, an even greater latency increase (~2 standard deviations above the mean of normal older individuals) is commonly reported, and some studies have found that P300 latency may be useful to differentiate between AD pathology and other disorders (e.g., depression, schizophrenia [42, 43]), although others have not [44, 45]. The clinical utility of P300 latency measures is generally enhanced in combination with standard neuropsychological tests. Goodin [46], for example, found that in cases of equivocal dementia (50% pretest probability), those with concurrent P300 latency delay showed a greater likelihood of having a dementing illness (estimated at 90%). One study found change in P300 latency was more sensitive to disease progression (over 1 year) than either the Cognitive Abilities Screening Instruments (CASI) or MMSE in both AD and MCI [47]. P300 amplitude also appears to be reduced in AD, although P300 amplitude reductions are also seen in several other neurological and psychiatric disorders (e.g., vascular dementia, schizophrenia). In summary, the literature indicates that auditory P300 measures show moderate correlations with mini-mental status exam (MMSE) scores [45, 48] and have greater sensitivity to more advanced stages of dementia.
Factors that appear to affect the clinical utility of P300 include the methodology used — especially with respect to attentional and memory-load demands —and the dementia severity of the patient group (see detailed review in Olichney and Hillert [49]). Abnormal P300 latencies are more likely to be reported in more complex tasks (e.g., counting) relative to simple target-detection tasks. It is interesting to note, however, that this effect is not simply due to task difficulty per se; Polich and Pitzer [50], for example, reported that increasing the difficulty of sensory discriminations actually decreased the discriminative sensitivity of P300 latency and amplitude measures. Although the P300 response has been most commonly studied in the auditory modality, studies using visual [50] and olfactory [51] stimuli have reported greater sensitivity to AD pathology. Morgan and Murphy [51] investigated olfactory event-related potentials (OERPs) and found delayed P200 and P300 latencies, which were significantly correlated with AD dementia severity and had a stronger (92%) value in differentiating AD from normal aging group than auditory P300 measures.
N400: OVERVIEW
The N400 is a scalp negativity elicited in response to potentially meaningful stimuli that peaks 400 ms post-stimulus over bilateral posterior channels. The N400 is typically larger over the right hemisphere for visual words, but sometimes shows a slight left-hemisphere bias for spoken words [52, 53]. Intracranial recordings have consistently found N400-like potentials in the anterior fusiform and parahippocampal gyri bilaterally [54, 55]; other candidate N400 generators include the superior temporal sulcus, and posterior parietal and ventral prefrontal cortices [56]. N400 amplitude is sensitive to the semantic congruity of the eliciting stimulus with the (preceding) context, being smaller in a congruous context (e.g., a coherent sentence, a single related word) than incongruous one. N400 amplitude is also reduced by stimulus repetition (reviewed in a later section). The effect of semantic congruity on N400 amplitude (the “N400 effect”) has been interpreted to reflect the reduction in processing effort needed to access the meaning of a stimulus, given a coherent predictable context [57, 58], i.e., “contextual integration” (though see Kutas & Federmeier [59] for an alternative account in terms of semantic memory activation).
N400 IN ALZHEIMER’S DISEASE
The presence or absence and amplitude of the N400 have been used to evaluate the integrity of semantic memory in Alzheimer’s disease. Language dysfunction is evident relatively early in the course of AD, patients often presenting with word-finding difficulties and poor performance on tests of letter and category fluency [60]. The latter is especially suggestive of a breakdown of semantic memory [61]. Indeed, behavioral studies (e.g., the triadic word task) have found evidence that semantic associations are progressively degraded in AD [62]. Nonetheless, it continues to be a matter of debate whether the semantic impairment in AD is primarily a deficit in retrieving information from an intact memory store, or a degradation of the representations themselves [63].
The N400 response to written words is sensitive to normal aging: N400 latency increases at ~2 ms/year and N400 amplitude decreases at ~0.07 μV/year across the adult lifespan [64, 65]. From ERP studies of semantic memory (reviewed below), it is apparent that the N400 is usually abnormal in AD, typically reduced in amplitude and delayed in latency beyond that seen in normal aging. The progressive flattening of the N400 may be a manifestation of failing N400 generators. Quantitative measures of N400 latency may provide an accurate metric of dementia stage and progression. Using multiple linear regression analyses, Iragui and colleagues [66] found that neuropsychological test scores could explain >80% of the variance (R = 0.90) in the N400 latency (fractional area latency of the difference wave contrasting incongruous and congruous endings to statements defining opposites).
N400: SEMANTIC CONGRUITY EFFECTS IN AD
Investigations of the N400 congruity effect in sentence processing have generally found abnormalities in AD. For example, Ford et al. [67] found that the N400 expectancy/congruity effect to sentence-terminal words in speech was significantly reduced (though still greater than zero) in AD relative to age-matched controls. Revonsuo et al. [26] likewise reported a reduced N400 congruity effect in speech in AD patients. In that study, no overt response was required of the participants—they were simply told and periodically reminded to attend to the sentences—so the results are unlikely to be ‘contaminated’ by the P300 component, known to be delayed in AD. In an early study by Hamberger et al. [68], the N400 to visually presented sentence-ending words was modulated by expectancy and semantic relation to virtually the same extent in AD patients as in young controls. Older controls showed a different pattern of N400 and RT effects, which the authors attributed to a response strategy, although N400 amplitudes in the older controls and AD patients were not reliably different.
Studies using minimal semantic contexts to elicit an N400 effect have generally found that the effect to be diminished in AD. In a study by Schwartz et al. [69], participants heard a category name, then saw a word, and judged whether the word belonged to the named category (e.g., “animal” – ‘cow’). The N400 effect — small negativity elicited by congruous relative to incongruous target words — was both smaller and delayed in AD patients relative to age-matched controls. Iragui et al. [66] observed similar results. In that study, participants listened to short statements that defined a category (e.g., “a type of flower”) or an antonymic relation (e.g., “the opposite of tall”), and then saw a word that was either congruous or incongruous with the preceding statement; their task was to judge the congruity of the statement and target word. The N400 effect was significantly reduced and delayed in AD patients relative to controls. Reduced N400 effects in AD also have been found with pictorial stimuli used as primes for lexical targets [27], as targets following lexical primes [70], and as both prime and target [71–73].
Despite the preponderance of evidence suggesting that the N400 response is abnormal in amplitude and/or latency in AD, some of these studies have nonetheless found evidence of normal semantic network structure in AD. Hamberger et al. [68], for example, found that AD patients’ N400 response followed the expected amplitude gradient across sentence ending types: unrelated-nonsense > unrelated-sense > related-sense > best completion. Schwartz et al. [69] compared the N400 effect for target words primed by superordinate and subordinate category labels and found that, in both AD patients and controls, the effect was larger for subordinate labels. Furthermore, two studies found evidence that anomia in AD may be independent of the integrity of the semantic system. Auchterlonie et al. [70] observed that the N400 congruity effect for pictures primed by words was similarly diminished for pictures, whether or not they were later named correctly. Ford et al. [27] also noted a dissociation between naming behavior and N400 response, albeit the opposite one: AD patients showed small but significant N400 congruity effects for word targets whether primed by named or unnamed pictures. One possible explanation for this apparent discrepancy is that the semantic information contained in pictures may provide a more powerful connection to the representations still present in the long-term memory of AD patients than do written words.
SUMMARY: N400 SEMANTIC CONGRUITY EFFECTS IN AD
Most ERP studies of semantic processing in AD have shown smaller and later N400 congruity effects. At the same time, AD patients have been found to show a normal gradient of N400 congruity effects over different levels of category hierarchy and expectancy, suggesting that the functional organization of semantic memory is relatively preserved in mild AD. Furthermore, ERP evidence has been used to argue that anomia in AD is not simply attributable to impaired semantic processing. Thus, there appear to be an independent deficit in the retrieval of semantic information. The N400 component may provide a useful biomarker for monitoring the stages of disease progression in AD.
LATE POSITIVE COMPONENT (LPC/P600): OVERVIEW
Many ERP studies in normal subjects have identified a Late Positive Component (LPC), sometimes called the P600, which appears to be important in the mediation of both memory encoding and retrieval processes. Subsequently recalled or recognized words generally have larger late positivities than non-recalled words [74, 75] and the size of this difference (often called “Dm” in the ERP literature) be reduced by “directed forgetting” instructions [76]. Intracranial studies in the human hippocampus have recapitulated the “Dm” effect of scalp ERPs [77], with larger positivities to words subsequently recalled [78]. The scalp P600, or ‘LPC’, is a late positivity with a centro-posterior maximum, which peaks 600 ms post-stimulus onset. Intracranial studies also identified putative P600 generators in the parahippocampal gyrus, many paralimbic cortical areas (e.g., temporal pole, rhinal & perirhinal cortex, posterior cingulate) and in multimodal association (e.g., ventrolateral prefrontal, lateral temporal cortex) [37, 56]. Intracranial depth recordings have shown that very large (>200mV) P600-like potentials are generated in the human hippocampus (HC) but it is unclear to what extent these potentials propagate to the scalp [37].
N400/P600: QUANTITATIVE MEASURES OF REPETITION EFFECTS IN ALZHEIMER’S DISEASE
The neuropathology of AD affects the medial temporal lobes early in the course of the disease, and deficits of episodic memory are usually the earliest presenting symptom. One might thus expect ERP word-repetition effects in AD to resemble those of medial-temporal amnesics. Studies of ERP word-repetition effects in AD, however, have produced a complex pattern of results.
Using a continuous semantic judgment task (button-press required for ‘animal’ names) with incidental repetition of non-targets (‘non-animal’ words were repeated; average lag: 30 sec) in AD, Friedman et al. [79] reported preserved late (700–1000 ms) repetition effects in most (6 of 10) mild AD patients. These authors attributed the residual repetition effects to relatively preserved implicit memory processes in AD. Rugg et al. [80] used a similar continuous task with incidental repetition at somewhat shorter lags (average: 6–21 sec) and found ERP repetition effects (300–400 and 400–700 ms) in AD that were statistically indistinguishable from those in controls, although a trend for a smaller repetition effect with longer lags was noted. In contrast, Tendolkar et al. [81] employing an explicit word-list memory task and a longer inter-item lag (~5 minutes), found very different results. In that study, controls exhibited a large repetition positivity for correctly recognized old items in both early (300–600 ms) and late (700–900 ms) latency windows. This effect was further enhanced for items for which source memory was also correctly retrieved (words had been displayed in one of two colors) from 600–900 ms, supporting a link between late positivity (P600) and conscious retrieval processes. In AD patients, no late repetition effect was present, and an effect in early latency windows (300–500 ms) showed a distinctly frontal distribution. The authors attributed this frontal old/new effect to familiarity [82]. Furthermore, patients’ source memory was at chance, suggesting that their above-chance recognition performance (62%) was due to a sense of familiarity (or another such implicit process) and not to recollection of the study event. Using a continuous lexical decision task with incidental repetition at long lags (>90 items or >7.5 minutes), Schnyer et al. [83] likewise found a repetition positivity from 300 to 650 ms in controls, but no discernible effect in AD patients.
A study in our laboratory [84] applied a word repetition paradigm with semantically congruous and incongruous words (details described in [85]) to patients with mild AD. Normal elderly demonstrated large decrement in P600 amplitude to repeated, relative to new, congruous words, and the amplitude of this change correlated strongly with verbal memory performance [85]. Thus, we believe this P600 word repetition effect is a measure of the updating of working memory with the content of long-term memory. With efficient learning of category exemplars, this updating is not necessary for repeated target stimuli. As we had observed in patients with chronic amnesia, patients with mild AD had markedly reduced P600 word-repetition effects (statistically ‘absent’ when analyzed across all scalp channels). Unlike chronic amnesia, patients with mild AD also showed significant diminution of the N400 word-repetition effect. Thus, both the P600 and N400 repetition effects were ‘lacking’ in the AD group [84]. The loss of the N400 repetition effect may correspond to abnormal semantic/conceptual priming, as has been found in several behavioral studies of mild AD [86]. Furthermore, when 10 th percentile (in normal elderly) cutoffs for the P600 and N400 word-repetition effects were applied, all 11 mild AD patients were correctly classified as abnormal on one or both measures (sensitivity: 100%; specificity: 82%), suggesting that this paradigm has promise for use in the diagnosis or early detection of AD.
FMRI STUDIES OF WORD REPETITION IN AD
The word repetition paradigm described above has been adapted for functional Magnetic Resonance Imaging (fMRI) studies, in order to identify the neural generators underlying the P600 word repetition effect. Normal elderly showed activation to New > Old congruous words in a distributed network of putative P600 generators, including bilateral cingulate and fusiform gyri, left medial temporal lobe (LMTL), and left inferior frontal gyri (IFG) [87]. Furthermore, significant correlations were present between the magnitude of New–Old activation in these regions and subsequent memory performance, implicating this neural circuit as critical for successful verbal memory encoding. In contrast, a group of mild AD patients showed weak or absent response to New-Old congruous word contrast, with only one such significant cluster (IFG) in the entire left hemisphere [88].
EEG OSCILLATORY ABNORMALITIES IN AD
Event-related dysynchronization/synchronization (ERD/ERS), the time-locked change in power of EEG frequency band (i.e., delta, theta, alpha, beta, and gamma) activities [89], also has been employed to explore cognitive and non-cognitive neural processing in AD. In a finger movement task, for example, AD was found to show increased centromedial beta ERD during movement and increased ipsilateral rolandic beta ERS in the post-movement period, with abnormal frontal preponderance of both activities [90]. Diminished ERD in 7–17 Hz frequency over temporal area has been observed in the AD group during retrieval of a Sternberg memory task [91]. Another study using a two-back working memory paradigm found reduced beta ERS at parietal sites of AD patients [92]. Event-related oscillation analysis also has been used to evaluate the outcome of cholinesterase inhibitor treatment on AD. Both treated and untreated AD patients exhibited lower delta activity, while theta response was sensitive to the treatment with a reduction after cholinesterase inhibitor therapy [93, 94].
ERPS IN MILD COGNITIVE IMPAIRMENT (MCI)
In a 5 year follow-up study, Golob and colleagues [95] demonstrated that both P50 amplitude and P300 latency, elicited in an auditory oddball task, increase with mild cognitive impairment (MCI). P50 amplitude predicted progression from MCI to AD, and differentiated amnestic MCI subtype from MCI with other cognitive impairments beyond memory deficit [96, 97]. Several studies have found N200 and P300 abnormalities in MCI, with some results suggesting that amplitude or latency of N200 may have stronger value in discriminating MCI patients from normal control [98–100]. However, Phillips et al. [101], using the Sternberg working memory task, found no difference in either the P300 or N200 in MCI compared with controls (but their mild AD group had reduced P300 amplitude). In a sample of amnestic mild cognitive impairment (aMCI), ERP components reflecting familiarity and retrieval monitoring were preserved for picture, but not for word recognition [102].
N400/P600: REPETITION EFFECTS IN MILD COGNITIVE IMPAIRMENT
Another study in our laboratory used the congruous/incongruous word repetition paradigm to evaluate N400 and P600 repetition effects in MCI [103]. In MCI, as in controls, target words that followed congruous category statements elicited a positive shift in N400 amplitude relative to incongruous pairings, but this effect (incongruous vs. congruous word voltage difference) was delayed in MCI. The N400 repetition effect—initial vs. repeated incongruous pairings—was likewise present but delayed in MCI. The P600 repetition effect—initial vs. repeated congruous pairings—was not significantly different from zero in the MCI grand average.
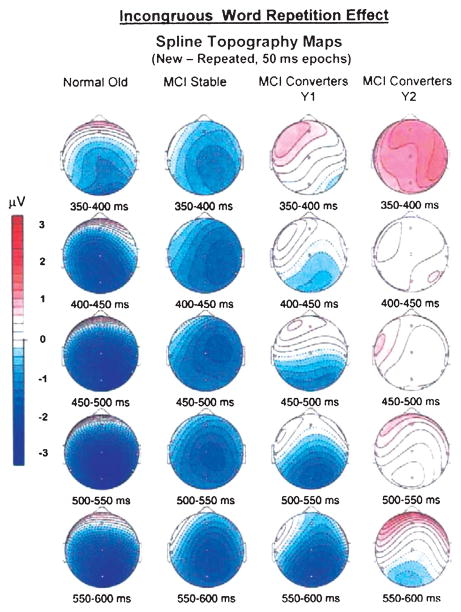
Longitudinal follow-up with annual ERP assessments [104] demonstrated that the N400 repetition effect is diminished and spatially restricted at baseline (Year 1) in MCI patients who convert to dementia within the next 3 years (“MCI converters”, see Fig. 1., 3rd column). One year later, when most of these patients were still in the MCI stage, the converter group on average showed an absence of the N400 repetition effect (right side of Fig. 1.). The P600 repetition effect also proved very sensitive to MCI converters, with no statistically significant repetition effects at either year 1 or year 2. It is noteworthy that abnormalities of either the P600 or N400 repetition effect at baseline in MCI carried a poor prognosis, with approximately 88% risk of conversion to AD within 3 years, compared to a 11–27% risk in those MCI cases with normal ERP repetition effects.
Fig. 1.
Spherical spline topographic maps illustrate the incongruous word repetition effect (ERPs to new minus old semantically incongruous words) in consecutive 50 msec epochs for normal old (left) and MCI stable (left middle) groups at year 1, and MCI converters at year 1 (right middle) and year 2 (right).
SUMMARY: N400/P600 REPETITION EFFECTS
Recent work dissociating N400 and P600 repetition effects has found that MTL amnesics, whose ability to encode events into long term memory is compromised, show intact N400 but impaired P600 effects. This pattern of findings supports an association between implicit and explicit processes and N400 and P600, respectively. In AD, both N400 and P600 repetition effects are severely diminished. In MCI, the presence of either reduced N400 or P600 repetition effects appears very promising as a potentially useful biomarker for those individuals at highest risk for subsequent conversion to AD dementia.
PRECLINICAL AD
In this era of sensitive biomarkers to amyloid deposition (e.g., PiB-PET and CSF A-beta amyloid levels), many elderly persons now have AD-related changes detected many years prior to observable cognitive symptoms, i.e. in the ‘preclinical AD’ stage. While brain amyloid is necessary for the diagnosis of AD, it may not suffice to produce cognitive decline in some elderly persons. As noted above, neuropathologic studies have implicated the synapse as the primary mediator of dementia severity in AD, with >80% of the variance in severity accounted for by the density of pre-synaptic terminals in mid-frontal cortex [14]. Transgenic animals often show inhibition of LTP and/or reduced synaptic transmission prior to the appearance of amyloid plaques and neurofibrillary changes [12]. This highlights the need for more accurate biomarkers for AD, especially biomarkers sensitive to emerging memory failure (pre-‘MCI’). Therefore, recently proposed research criteria for Preclinical AD [105] divide this entity into 3 stages, based on the presence of symptoms and evidence of synaptic dysfunction (or neurodegeneration). Elevated CSF phospho-tau is one such marker of synaptic dysfunction. To date, neither EEG nor ERP markers have been incorporated into these criteria. Non-invasive cost-effective measures of synaptic dysfunction, as can be provided by ERPs and EEG, however, could potentially be very useful for the earlier diagnosis staging of AD. In short, there is a pressing need for improved electrophysiological markers of impaired synaptic plasticity and memory. One important application for such a marker would be to aid in the differentiation of Preclinical AD, perhaps while still in the asymptomatic stage, from normal aging.
EEG/ERP ABNORMALITIES IN PRECLINICAL AD
A rich literature has shown that EEG and ERPs can both be very sensitive tools for measuring brain aging. Prichep and colleagues [106], for example, applied quantitative EEG (QEEG) to elderly persons with symptomatic memory complaints (“Reisberg FAST stage 2”) and found that certain QEEG abnormalities (e.g., increased theta power, slowed mean background frequency, changes in covariance among centro-parietal regions) were strongly predictive of subsequent cognitive decline over the next 7 years (logistic regression models achieved a predictive accuracy of 90%). ERPs likewise have shown promise in their sensitivity to preclinical stages of AD. Several ERP studies have reported various sensitivities in those at increased genetic risk for AD. Boutros and colleagues [107], for example, reported increased P50 and P300 amplitudes in a small group of normal subjects genetically at-risk for AD (first degree relatives of autopsy confirmed AD cases; mean age 53 yr old). Green and colleagues [108] reported delayed N200 and P300 latencies in a similarly-aged group of apolipoprotein E4 (the most common genetic risk factor for AD) carriers with a positive family history of AD. Murphy et al. [109] presented names of odors previously encoded (targets) or not (foils) to carriers of ApoE4, and found significantly longer P300 latencies in the ApoE4 carriers, consistent with prior reports of olfactory odor recognition memory impairment in ApoE4 carriers [110]. Golob et al. [111] examined familial AD (FAD) carriers (mean age = 34) with presenilin-1 (PSEN1) or amyloid precursor protein (APP) mutations with an auditory oddball task and obtained delayed ERP components including N100, P200, N200 and P300 in this group with familial AD while most were in the asymptomatic (CDR = 0) stage. Bobes and colleagues [74] observed that asymptomatic carriers of E280A PS-1 mutation have a parietal distribution of N400 congruity effect elicited by picture-pairs while normal elderly show a central maximum.
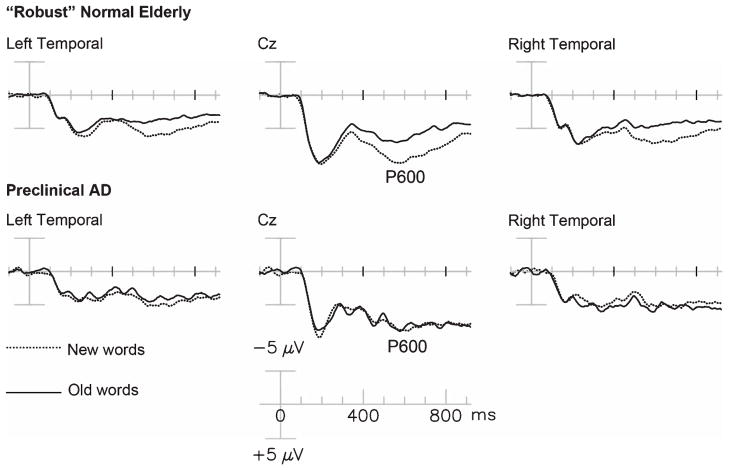
Recent retrospective review of all our “normal” elderly controls, followed longitudinally by a NIH-funded ADRC or ADC, identified 7 cases who were most probably in the early stages of Preclinical AD at the time of their ERP recordings. All entered as normal controls and continued to perform within normal limits on an annually administrated extensive neuropsychological test battery. In the years following the ERPs, however, these cases showed cognitive decline (to AD or MCI, n = 6) or had AD pathology verified at autopsy (n = 4, mean Braak stage = 3.0). Compared to 12 “robust” normal elderly (RNE) participants (top row in Fig. 2), all of whom remained cognitively normal (average follow-up = 9.1 years) with longitudinal neuropsychological testing, the Preclinical AD group had significantly smaller P600 repetition effects (mean amplitude of RNE = 3.28 μV; Pre-AD = 0.10 ± 0.89 μV) [112]. The consistently abnormal (reduced or absent) P600 effects seen in this small Preclinical AD group shows the great promise which ERP biomarkers such as the P600 have for the detection of the earliest stages of synaptic dysfunction.
Fig. 2.
Grand average ERPs to initial (dash line) and repeated (solid line) presentation of congruous words in “robust” normal elderly (RNE) (top row) and Preclinical AD patients (bottom).
CONCLUSIONS
We reviewed several abnormalities in the cognitive ERPs of AD patients. Early, sensory-evoked, obligatory potentials (e.g., N100) are typically normal in AD (though see work on P50) whereas potentials starting around 200 ms and beyond are more consistently abnormal even in the earliest stages of AD and MCI. This pattern of ERP findings is consistent with the neuropathology of AD. Predilection sites in early AD include the medial temporal lobe, other limbic areas, and multimodal association cortices with relative sparing of unimodal sensory cortex. Late endogenous components in known paradigms can be useful for assessing specific hypotheses about the change in cognitive processes in AD, MCI, and amnestic patients. A P300 paradigm, for example, can be very useful in detecting a disorder of attention or in quantifying the effects of drugs which improve attention, such as the cholinesterase inhibitors. For the early diagnosis of AD or other memory disorders, a word repetition paradigm (typically eliciting N400 and P600 modulations) with an explicit recognition task or one that fosters associative learning would be recommended. As discussed above, the N400 has potential use in tracking AD progression. Last but not least, the sensitivities of a number of ERP components have great promise in the detection and quantification of synaptic dysfunction in the presymptomatic stages of Alzheimer’s disease.
Acknowledgments
Supported by NIH grants R01 AG18442, R01 AG08313, and P30 AG010129. We would also like to thank the UC Davis ADC and Center for Mind and Brain.
References
- 1.Nunez PL. Physical principles and neurophysiological mechanisms underlying event-related potentials. In: Rohrbaugh JW, Parasuraman R, Johnson R Jr, editors. Event-related brain potentials. Oxford University Press; New York: 1990. pp. 19–36. [Google Scholar]
- 2.Nunez PL, Srinivasan R. Oxford University Press. Electric fields of the brain: the neurophysics of EEG. 2. New York: 2006. pp. 163–166. [Google Scholar]
- 3.Wood CC, Allison T. Interpretation of evoked potentials: a neurophysiological perspective. Can J Psychol. 1981;35:113–135. doi: 10.1037/h0081149. [DOI] [PubMed] [Google Scholar]
- 4.Ang CW, Carlson GC, Coulter DA. Hippocampal ca1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schack B, Weiss S. Quantification of phase synchronization phenomena and their importance for verbal memory processes. Biol Cybern. 2005;92:275–287. doi: 10.1007/s00422-005-0555-1. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Katada E, Sato K, Ojika K, Ueda R. Cognitive event-related potentials: useful clinical information in Alzheimer’s disease. Curr Alzheimer Res. 2004;1:63–69. doi: 10.2174/1567205043480609. [DOI] [PubMed] [Google Scholar]
- 8.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 9.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 10.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moechars D, Dewachter I, Lorent K, et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 12.Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos Trans R Soc Lond B Biol Sci. 2003;358:821–828. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh D, Klyubin I, Fadeeva J, Cullen W, Anwyl R, Wolfe M, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:536–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 14.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 15.Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 16.Bertoni-Freddari C, Fattoretti P, Delfino A, Solazzi M, Giorgetti B, Ulrich J, Meier-Ruge W. Deafferentative synaptopathology in physiological aging and Alzheimer’s disease. Ann N Y Acad Sci. 2002;977:322–326. doi: 10.1111/j.1749-6632.2002.tb04833.x. [DOI] [PubMed] [Google Scholar]
- 17.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 18.Goodin DS, Aminoff MJ. Electrophysiological differences between demented and nondemented patients with parkinson’s disease. Ann Neurol. 1987;21:90–94. doi: 10.1002/ana.410210116. [DOI] [PubMed] [Google Scholar]
- 19.Goodin DS, Aminoff MJ. Electrophysiological differences between subtypes of dementia. Brain. 1986;109:1103–1113. doi: 10.1093/brain/109.6.1103. [DOI] [PubMed] [Google Scholar]
- 20.Martinelli V, Locatelli T, Comi G, Lia C, Alberoni M, Bressi S, Rovaris M, Franceschi M, Canal N. Pattern visual evoked potential mapping in Alzheimer’s disease: correlations with visuospatial impairment. Dementia. 1996;7:63–68. doi: 10.1159/000106855. [DOI] [PubMed] [Google Scholar]
- 21.Swanwick GR, Rowan MJ, Coen RF, Coakley D, Lawlor BA. Prognostic value of electrophysiological markers in Alzheimer’s disease. Am J Geriatr Psychiatry. 1999;7:335–338. [PubMed] [Google Scholar]
- 22.Ruessmann K, Beneicke U. P2 latency of the flash visual evoked potential in dementia. Int J Neurosci. 1991;56:273–276. doi: 10.3109/00207459108985424. [DOI] [PubMed] [Google Scholar]
- 23.Moore NC. Visual evoked responses in Alzheimer’s disease: a review. Clin Electroencephalogr. 1997;28:137–142. doi: 10.1177/155005949702800304. [DOI] [PubMed] [Google Scholar]
- 24.Philpot MP, Amin D, Levy R. Visual evoked potentials in Alzheimer’s disease: correlations with age and severity. Electroencephalogr Clin Neurophysiol. 1990;77:323–329. doi: 10.1016/0168-5597(90)90053-g. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh E, Adachi-Usami E, Mizota A, Fujimoto N. Comparison of visual evoked potentials in patients with psychogenic visual disturbance and malingering. J Pediatr Ophthalmol Strabismus. 2001;38:21–26. doi: 10.3928/0191-3913-20010101-08. [DOI] [PubMed] [Google Scholar]
- 26.Revonsuo A, Portin R, Juottonen K, Rinne JO. Semantic processing of spoken words in Alzheimer’s disease: an electrophysiological study. J Cogn Neurosci. 1998;10:408–420. doi: 10.1162/089892998562726. [DOI] [PubMed] [Google Scholar]
- 27.Ford JM, Askari N, Mathalon DH, Menon V, Gabrieli JD, Tinklenberg JR, Yesavage J. Event-related brain potential evidence of spared knowledge in Alzheimer’s disease. Psychol Aging. 2001;16:161–176. doi: 10.1037/0882-7974.16.1.161. [DOI] [PubMed] [Google Scholar]
- 28.Golob EJ, Starr A. Effects of stimulus sequence on event-related potentials and reaction time during target detection in Alzheimer’s disease. Clin Neurophysiol. 2000;111:1438–1449. doi: 10.1016/s1388-2457(00)00332-1. [DOI] [PubMed] [Google Scholar]
- 29.Takeda M, Tachibana H, Sugita M. Multimodal evoked potentials in patients with dementia. Nippon Ronen Igakkai Zasshi. 1993;30:1058–1067. doi: 10.3143/geriatrics.30.1058. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama Y, Nakashima K, Shimoyama R, Urakami K, Takahashi K. Distribution of event-related potentials in patients with dementia. Electromyogr Clin Neurophysiol. 1995;35:431–437. [PubMed] [Google Scholar]
- 31.Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on event-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30:10–22. doi: 10.1111/j.1469-8986.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez R, Kavcic V, Duffy CJ. Neurophysiologic analyses of low- and high-level visual processing in Alzheimer disease. Neurology. 2007;68:2066–2076. doi: 10.1212/01.wnl.0000264873.62313.81. [DOI] [PubMed] [Google Scholar]
- 33.Kavcic V, Fernandez R, Logan D, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer’s disease. Brain. 2006;129:736–746. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- 34.Squires K, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- 35.Johnson R., Jr A triarchic model of p300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 36.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual odd-ball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 37.Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial erp repetition effects dissociate memory systems in the human brain. J Cogn Neurosci. 1999;11:437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi S, Knight RT. Effects of temporal-parietal lesions on the somatosensory p3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol. 1992;84:139–148. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 39.Donchin E, Coles MGH. Is the p300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- 40.Kok A. On the utility of p3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- 41.Ford JM, Pfefferbaum A. Age-related changes in event-related potentials. Advances in Psychophysiology. 1985;1:301–339. [Google Scholar]
- 42.Squires KC, Chippendale TJ, Wrege KS, Goodin DS, Starr A. Electrophysiological assessment of mental function in aging and dementia. In: Poon L, editor. Aging in the 1980s. American Psychology Association; Washington, DC: 1980. pp. 125–134. [Google Scholar]
- 43.Gordon E, Kraiuhin C, Harris A, Meares R, Howson A. The differential diagnosis of dementia using p300 latency. Biological Psychiatry. 1986;21:1123–1132. doi: 10.1016/0006-3223(86)90220-9. [DOI] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Wenegrat BG, Ford JM, Roth WT, Kopell BS. Clinical application of the p3 component of event-related potentials ii: dementia, depression and schizophrenia. Electroencephalogr Clin Neurophysiol. 1984;59:104–124. doi: 10.1016/0168-5597(84)90027-3. [DOI] [PubMed] [Google Scholar]
- 45.Patterson JV, Michalewski HJ, Starr A. Latency variability of the components of auditory event-related potentials to infrequent stimuli in aging, Alzheimer-type dementia, and depression. Electroencephalogr Clin Neurophysiol. 1988;71:450–460. doi: 10.1016/0168-5597(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 46.Goodin DS. Clinical utility of long latency ‘cognitive’ event-related potentials (p3): the pros. Electroencephalogr Clin Neurophysiol. 1990;76:2–5. doi: 10.1016/0013-4694(90)90051-k. [DOI] [PubMed] [Google Scholar]
- 47.Lai CL, Lin RT, Liou LM, Liu CK. The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin Neurophysiol. 2010;121:194–199. doi: 10.1016/j.clinph.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka F, Kachi T, Yamada T, Sobue G. Auditory and visual event-related potentials and flash visual evoked potentials in Alzheimer’s disease: correlations with mini-mental state examination and raven’s coloured progressive matrices. J Neurol Sci. 1998;156:83–88. doi: 10.1016/s0022-510x(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 49.Olichney JM, Hillert DG. Clinical applications of cognitive event-related potentials in Alzheimer’s disease. Phys Med Rehabil Clin N Am. 2004;15:205–233. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 50.Polich J, Pitzer A. P300 and Alzheimer’s disease: odd-ball task difficulty and modality effects. Electroencephalogr Clin Neurophysiol Suppl. 1999;50:281–287. [PubMed] [Google Scholar]
- 51.Morgan CD, Murphy C. Olfactory event-related potentials in Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8:753–763. doi: 10.1017/s1355617702860039. [DOI] [PubMed] [Google Scholar]
- 52.Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- 53.Kutas M, Neville HJ, Holcomb PJ. A preliminary comparison of the n400 response to semantic anomalies during reading, listening and signing. Electroencephalogr Clin Neurophysiol Suppl. 1987;39:325–330. [PubMed] [Google Scholar]
- 54.McCarthy G, Nobre AC, Bentin S, et al. Language-related field potentials in the anterior-medial temporal lobe: 1. Intracranial distribution and neural generators. J Neurosci. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- 56.Halgren E, Baudena P, Heit G, Clarke J, Marinkovic K, Clarke M. Spaciotemporal stages in face and word processing 1 . Depth Recorded Potentials in Human Occipital and Parietal Lobes. J Physiol Paris. 1994;88:1–150. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 57.Kutas M, Van Petten C. Event-related brain potential studies of language. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 3. JAI Press; Greenwich, CT: 1988. pp. 139–187. [Google Scholar]
- 58.Osterhout l, Holcomb pj. Event-related potentials and language comprehension. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-related Brain Potentials and Cognition. Oxford, UK: Oxford University Press; 1995. pp. 171–215. [Google Scholar]
- 59.Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the n400 component of the event-related brain potential (erp) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 61.Salmon DP, Butters N, Chan AS. The deterioration of semantic memory in Alzheimer’s disease. Can J Exp Psychol. 1999;53:108–117. doi: 10.1037/h0087303. [DOI] [PubMed] [Google Scholar]
- 62.Chan AS, Butters N, Salmon DP. The deterioration of semantic networks in patients with Alzheimer’s disease: a cross-sectional study. Neuropsychologia. 1997;35:241–248. doi: 10.1016/s0028-3932(96)00067-x. [DOI] [PubMed] [Google Scholar]
- 63.Ober BA, Shenaut GK. Semantic priming in Alzheimer’s disease: meta analysis and theoretical evaluation. In: Allen PA, Bashore TR, editors. Advances in Psychology: Age Differences in Word and Language Processing. Elsevier; Amsterdam: 1995. pp. 247–271. [Google Scholar]
- 64.King J, Kutas M. Do the waves begin to waver? ERP studies of language processing in the elderly. In: Allen PA, Bashore TR, editors. Advances in Psychology: Age Differences in Word and Language Processing. Amsterdam: Elsevier; 1995. pp. 314–344. [Google Scholar]
- 65.Kutas M, Iragui V. The n400 in a semantic categorization task across 6 decades. Electroencephalogr Clin Neurophysiol. 1998;108:456–471. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 66.Iragui V, Kutas M, Salmon DP. Event-related brain potentials during semantic categorization in normal aging and senile dementia of the Alzheimer’s type. Electroencephalog Clin Neurophysiol. 1996;100:392–406. [PubMed] [Google Scholar]
- 67.Ford JM, Woodward SH, Sullivan EV, Isaacks BG, Tinklenberg JR, Yesavage JA, Roth WT. N400 evidence of abnormal responses to speech in Alzheimer’s disease. Electroencephalogr Clin Neurophysiol. 1996;99:235–246. doi: 10.1016/0013-4694(96)95049-x. [DOI] [PubMed] [Google Scholar]
- 68.Hamberger MJ, Friedman D, Ritter W, Rosen J. Event-related potential and behavioral correlates of semantic processing in Alzheimer’s patients and normal controls. Brain Lang. 1995;48:33–68. doi: 10.1006/brln.1995.1002. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz TJ, Kutas M, Butters N, et al. Electrophysiological insights into the nature of the semantic deficit in Alzheimer’s disease. Neuropsychologia. 1996;34:827–841. doi: 10.1016/0028-3932(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 70.Auchterlonie S, Phillips PA, Chertkow H. Behavioral and electrical brain measures of semantic priming in patients with Alzheimer’s disease: implications for access failure versus deterioration hypotheses. Brain Cogn. 2002;48:264–267. [PubMed] [Google Scholar]
- 71.Castañeda M, Ostrosky-Solís F, Pérez M, Bobes MA, Rangel LE. Erp assessment of semantic memory in Alzheimer’s disease. Int J Psychophysiol. 1997;27:201–214. doi: 10.1016/s0167-8760(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 72.Ostrosky-Solís F, Castañeda M, Pérez M, Castillo G, Bobes MA. Cognitive brain activity in Alzheimer’s disease: electrophysiological response during picture semantic categorization. J Int Neuropsychol Soc. 1998;4:415–425. doi: 10.1017/s1355617798455012. [DOI] [PubMed] [Google Scholar]
- 73.Bobes MA, García YF, Lopera F, Quiroz YT, Galán L, Vega M, Trujillo N, Valdes-Sosa M, Valdes-Sosa P. Erp generator anomalies in presymptomatic carriers of the Alzheimer’s disease e280a ps-1 mutation. Hum Brain Mapp. 2010;31:247–265. doi: 10.1002/hbm.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mangels JA, Picton TW, Craik FIM. Neurophysiological (erp) correlates of encoding and retrieval from verbal episodic memory. (Abstract) Soc Neurosci Abs. 1996;22:1450. [Google Scholar]
- 75.Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- 76.Paller KA. Recall and stem-completion priming have different electrophysiological correlates and are modified differentially by directed forgetting. J Exp Psychol Learn Mem Cogn. 1990;16:1021–1032. doi: 10.1037//0278-7393.16.6.1021. [DOI] [PubMed] [Google Scholar]
- 77.Paller KA, McCarthy G, Wood CC. Erps predictive of subsequent recall and recognition performance. Biol Psychol. 1988;26:269–276. doi: 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- 79.Friedman D, Hamberger M, Stern Y, Marder K. Event-related potentials (erps) during repetition priming in Alzheimer’s patients and young and older controls. J Clin Exp Neuropsychol. 1992;14:448–462. doi: 10.1080/01688639208402837. [DOI] [PubMed] [Google Scholar]
- 80.Rugg MD, Pearl S, Walker P, Roberts RC, Holdstock JS. Word repetition effects on event-related potentials in healthy young and old subjects, and in patients with Alzheimer-type dementia. Neuropsychologia. 1994;32:381–398. doi: 10.1016/0028-3932(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 81.Tendolkar I, Schoenfeld A, Golz G, Fernandez G, Kuhl KP, Ferszt R, Heinze HJ. Neural correlates of recognition memory with and without recollection in patients with Alzheimer’s disease and healthy controls. Neurosci Lett. 1999;263:45–48. doi: 10.1016/s0304-3940(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 82.Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- 83.Schnyer DM, Allen JJ, Kaszniak AW, Forster KI. An event-related potential examination of masked and unmasked repetition priming in Alzheimer’s disease: implications for theories of implicit memory. Neuropsychology. 1999;13:323–337. doi: 10.1037//0894-4105.13.3.323. [DOI] [PubMed] [Google Scholar]
- 84.Olichney JM, Riggins BR, Morris SK, Salmon DP, Kutas M, Iragui VJ. Reduced effects of word repetition on the n400 and lpc event-related potentials are common in mild Alzheimer’s disease and mild cognitive impairment converters. Neurology. 2002;58:A216. [Google Scholar]
- 85.Olichney JM, Van Petten C, Paller K, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- 86.Keane M, Gabrieli J, Fennema A, et al. Evidence for a dissociation between perceptual and conceptual priming in Alzheimer’s disease. Behav Neurosci. 1991;105:326–342. doi: 10.1037//0735-7044.105.2.326. [DOI] [PubMed] [Google Scholar]
- 87.Olichney JM, Taylor JR, Hillert DG, Chan SH, Salmon DP, Gatherwright J, Iragui VJ, Kutas M. Fmri congruous word repetition effects reflect memory variability in normal elderly. Neurobiol Aging. 2010;31:1975–1990. doi: 10.1016/j.neurobiolaging.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olichney JM, Taylor JR, Chan S, Yang JC, Stringfellow A, Hillert DG, Simmons AL, Salmon DP, Iragui-Madoz V, Kutas M. Fmri responses to words repeated in a congruous semantic context are abnormal in mild Alzheimer’s disease. Neuropsychologia. 2010;48:2476–2487. doi: 10.1016/j.neuropsychologia.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfurtscheller G, Lopes da Silva FH. Event-related eeg/meg synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 90.Babiloni C, Babiloni F, Carducci F, Cincotti F, Del Percio C, De Pino G, Maestrini S, Priori A, Tisei P, Zanetti O, Rossini PM. Movement-related electroencephalographic reactivity in Alzheimer disease. Neuroimage. 2000;12:139–146. doi: 10.1006/nimg.2000.0602. [DOI] [PubMed] [Google Scholar]
- 91.Karrasch M, Laine M, Rinne JO, Rapinoja P, Sinervä E, Krause CM. Brain oscillatory responses to an auditory-verbal working memory task in mild cognitive impairment and Alzheimer’s disease. Int J Psychophysiol. 2006;59:168–178. doi: 10.1016/j.ijpsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Missonnier P, Deiber MP, Gold G, Herrmann FR, Millet P, Michon A, Fazio-Costa L, Ibañez V, Giannakopoulos P. Working memory load-related electroencephalographic parameters can differentiate progressive from stable mild cognitive impairment. Neuroscience. 2007;150:346–356. doi: 10.1016/j.neuroscience.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Yener G, Güntekin B, Başar E. Event-related delta oscillatory responses of Alzheimer patients. Eur J Neurol. 2008;15:540–547. doi: 10.1111/j.1468-1331.2008.02100.x. [DOI] [PubMed] [Google Scholar]
- 94.Yener GG, Güntekin B, Tülay E, Başar E. A comparative analysis of sensory visual evoked oscillations with visual cognitive event related oscillations in Alzheimer’s disease. Neurosci Lett. 2009;462:193–197. doi: 10.1016/j.neulet.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 95.Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- 96.Golob EJ, Johnson JK, Starr A. Auditory event-related potentials during target detection are abnormal in mild cognitive impairment. Clin Neurophysiol. 2001;113:151–161. doi: 10.1016/s1388-2457(01)00713-1. [DOI] [PubMed] [Google Scholar]
- 97.Irimajiri R, Michalewski HJ, Golob EJ, Starr A. Cholinesterase inhibitors affect brain potentials in amnestic mild cognitive impairment. Brain Res. 2007;1145:108–116. doi: 10.1016/j.brainres.2007.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papaliagkas VT, Kimiskidis VK, Tsolaki MN, Anogianakis G. Cognitive event-related potentials: Longitudinal changes in mild cognitive impairment. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2010.12.036. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 99.Papaliagkas V, Kimiskidis V, Tsolaki M, Anogianakis G. Usefulness of event-related potentials in the assessment of mild cognitive impairment. BMC Neurosci. 2008;9:107. doi: 10.1186/1471-2202-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennys K, Portet F, Touchon J, Rondouin G. Diagnostic value of event-related evoked potentials n200 and p300 subcomponents in early diagnosis of Alzheimer’s disease and mild cognitive impairment. J Clin Neurophysiol. 2007;24:405–412. doi: 10.1097/WNP.0b013e31815068d5. [DOI] [PubMed] [Google Scholar]
- 101.Phillips NA, Chertkow H, Leblanc MM, Pim H, Murtha S. Functional and anatomical memory indices in patients with or at risk for Alzheimer’s disease. J Int Neuropsychol Soc. 2004;10:200–210. doi: 10.1017/S1355617704102063. [DOI] [PubMed] [Google Scholar]
- 102.Ally BA, McKeever JD, Waring JD, Budson AE. Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. Neuropsychologia. 2009;47:2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event-related potentials in mild cognitive impairment and incipient ad. J Neurol Neurosurg Psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with mci and n400 or p600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;6:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sperling R, Beckett L, Bennett D, et al. CRITERIA FOR PRECLINICAL ALZHEIMER’S DISEASE. 2010 http://www.alz.org/research/diagnostic_criteria/preclinical_recommendations.pdf.
- 106.Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, Torossian C, Reisberg B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006;27:471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 107.Boutros N, Torello MW, Burns EM, Wu SS, Nasrallah HA. Evoked potentials in subjects at risk for Alzheimer’s disease. Psychiatry Res. 1995;57:57–63. doi: 10.1016/0165-1781(95)02597-p. [DOI] [PubMed] [Google Scholar]
- 108.Green J, Levey AI. Event-related potential changes in groups at increased risk for Alzheimer disease. Arch Neurol. 1999;56:1398–1403. doi: 10.1001/archneur.56.11.1398. [DOI] [PubMed] [Google Scholar]
- 109.Murphy C, Solomon ES, Haase L, Wang M, Morgan CD. Olfaction in aging and Alzheimer’s disease: event-related potentials to a cross-modal odor-recognition memory task discriminate apoe epsilon4 + and apoe epsilon 4- individuals. Ann N Y Acad Sci. 2009;1170:647–657. doi: 10.1111/j.1749-6632.2009.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gilbert PE, Murphy C. The effect of the apoe epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 111.Golob EJ, Ringman JM, Irimajiri R, Bright S, Schaffer B, Medina LD, Starr A. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology. 2009;73:1649–1655. doi: 10.1212/WNL.0b013e3181c1de77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olichney JM, Pak J, Salmon DP, Yang JC, Gahagan T, Nowacki R, Hansen L, Galasko D, Kutas M, Iragui-Madoz VJ. ERP studies of Preclinical Alzheimer’s Disease (AD): P600 abnormalities are common in elderly persons who later develop MCI or AD pathology. (Abstract) Neurology Suppl. 2011;76:A233. [Google Scholar]