Abstract
Angiopoietins 1 and 2, ligands for the receptor kinase Tie-2, have been proposed to play critical but opposing roles in vascular development. Since signaling by Tie-2 is likely affected by other endothelial cell receptors such as Flk-1, the receptor for VEGF, and cell-cell adhesion receptors PECAM1 and VE-cad, we explored their interactions in a 3D model of vasculogenesis. When murine embryoid bodies (EBs) were treated with VEGF in Matrigel in the presence or absence of Ang-1 or Ang-2 for eight days, Ang-1 abrogated vascular sprouting for treatments started at days 0 or 3. In contrast, Ang-2 greatly accelerated vascular sprouting compared to untreated EBs. These results were confirmed in a second model system where VEGF treated HUVECs were grown in Matrigel in the presence or absence of Ang-1 or Ang-2. Since vascular sprouting must be precisely controlled in the developing embryo, it is likely that cell-cell adhesion molecules play a role in sensing the density of vascular sprouts. In this respect, we have shown that PECAM1 and CEACAM1 play essential roles in vascular sprouting. We now show that PECAM1 is associated with Tie-2, becomes phosphorylated on its ITIMs, and recruits the inhibitory phosphatases SHP-1 and SHP-2. In addition, PECAM1 is associated with VE-cad and may similarly regulate its signaling via recruitment of SHP-1/2.
Keywords: Ang-1, Ang-2, PECAM1, CEACAM1, Tie-2. VE-cad, vasculogenesis, angiogenesis
Introduction
Vasculogenesis, the generation of the vascular system in the developing embryo, involves coordinated signaling among cell surface receptors that respond to environmental signals. While VEGF and its receptor Flk-1 are among the most studied [1], other ligands such as Angiopoietins-1 and -2 (Ang-1 and Ang-2) and their receptor Tie-2, also play critical roles [2, 3]. In addition, the extent of vascular sprouting is controlled by cell-cell adhesion molecules such as PECAM1 [4], CEACAM1 [4] and VE-cad [5, 6]. In an effort to determine the possible interactions among these receptors, we developed a 3D model of vasculogenesis in which VEGF treated murine embryonic stem cells differentiated into embryoid bodies (EBs) undergo vascular sprouting when transferred to 3D cultures containing Matrigel, a source of extracellular matrix. The sprouting was inhibited and the vessel architecture was abnormal when EBs were treated with either anti-PECAM1 or anti-CEACAM1 monoclonal antibodies prior to embedding in Matrigel, while identical treatment with an antibody isotype control had no effect [4]. These results suggest that inhibition of cell-cell signaling affected the function of many of the critical receptors for endothelial cells. In an effort to understand these interactions, we tested the response of EBs to Ang-1 and Ang-2 and the possible association of Tie-2 with PECAM1 and CEACAM-1.
Tie-2 (endothelial cell-specific tyrosine kinases 2) is a receptor tyrosine kinase highly expressed on endothelial cells with wide-ranging effects that include angiogenesis, inflammation, and vascular extravasation [2, 3]. Genetic ablation of Tie-2 is embryonic lethal due to specific defects in the formation of primary capillary plexus and higher order branching vessels [7]. Ang-1 is mainly produced by vascular mural cells, whereas endothelial cells are the main producers of Ang-2 [3]. However, the regulatory roles of Ang-1 and Ang-2 in angiogenesis are controversial with Ang-1 behaving as both an agonist and antagonist [8]. Nonetheless, the absence of Ang-1 causes severe vascular abnormalities in the developing mouse embryo [9], and over-expression of Ang-2 in transgenic mouse embryos leads to a major disruption of the developing vessels [10]. Since Tie-2 was down regulated in our model system, it is a good candidate for negative or positive regulation by Ang-1/2 and negative regulation by either PECAM1 or CEACAM1, both of which possess ITIMs.
PECAM1 is expressed on both immature and mature endothelial cells mediating cell-cell adhesion through either homotypic or heterophilic interactions [11, 12]. PECAM1 is a member of the Ig-gene super family, comprising 6 extracellular Ig-like domains with a molecular weight of 110-130kDa [13]. The cytoplasmic domain contains two ITIM motifs that when phosphorylated by a Src kinase in endothelial cells associate with β-catenin and recruit SHP-2, an inhibitory phosphatase that has been shown to dephosphorylate tyrosine phosphates on β-catenin [14, 15]. Due to its association with β-catenin, a key component of adherent junctions [14, 16] and its recruitment of SHP-2 [17], PECAM1 may play a role in the inhibition of the formation of adherent junctions in endothelial cells. An inhibitory function may be important in the early stages of vascular sprouting when new sprouts invade the extracellular matrix. Given this possibility, it was important to include β-catenin in the list of targets for PECAM1 regulation. In support of this idea, PECAM1 was up-regulated in our model system [4] and is a well-known effecter of both vasculogenesis and angiogenesis [18].
Like PECAM1, CECAM1 has a variable number of Ig-like extracellular domains (2 or 4 in the case of murine CEACAM1) that participate in homotypic and heterotypic cell-cell adhesion [19] and has a cytoplasmic domain containing two ITIMs. Its ITIMs may be phosphorylated by a Src kinase and recruit SHP-1/2 tyrosine phosphatases [20]. CEACAM1 can also associate with β-catenin [21], in addition to a number of cell surface receptors on leukocytes [22, 23-25]. Although CEACAM1 is expressed on newly developing endothelial cells, it is not expressed on mature endothelial cells [26, 27]. Thus, although CEACAM1 and PECAM1 share many features in common, they differ in their temporal expression on endothelial cells.
Vascular endothelial-cadherin (VE-cad) is a third critically important receptor on endothelial cells. Genetic ablation of VE-cad leads to embryonic lethality due to failure of development of a mature vascular system [5, 6]. VE-cad is a type I transmembrane protein expressed throughout the vascular endothelium and has been reported to be involved in both vasculogenesis and angiogenesis [28]. Since VE-cad negatively regulates Flk-1 [29] and its expression follows that of Flk-1 in our model system, it is likely that VE-cad is held in check during the early stages of vascular sprouting when proliferation predominates. Thus, VE-cad is another possible candidate for regulation by inhibitory co-receptors such as PECAM1 and/or CEACAM1.
The possible association of PECAM1 and CEACAM1 with β-catenin, Tie-2, and VE-cad, as well as their ITIM phosphorylation and recruitment of SHP1/2 phosphatases was assessed by immunoprecipitation with antibodies to each of these molecules. We demonstrated that both PECAM1 and CEACAM1 were tyrosine phosphorylated during vascular sprouting and that CEACAM1 recruits SHP-1, while PECAM1 recruits both SHP-1 and SHP-2. Furthermore, Tie-2 is associated with PECAM1, but not CEACAM1, suggesting that their functions do not overlap. We demonstrated that VE-cad is associated with PECAM1, but not CEACAM1. The novel interaction of PECAM1 with VE-cad was further confirmed in human HUVEC cells, suggesting that this is a general interaction between PECAM1 and VE-cad. The association of PECAM1 with Tie-2 was assessed by treatment of ES cells with Ang-1 or Ang-2, presumptive antagonist or agonist, respectively, to Tie-2. Ang-1 completely inhibited while Ang-2 greatly accelerated vasculogenesis. Furthermore, Ang-2 promoted while Ang-1 inhibited Tie-2 phosphorylation. Thus, we conclude that the inhibitory role of PECAM1 is essential to control the degree of vasculogenesis at the level of both Tie-2 and VE-Cad, and that Ang-1 and Ang-2 oppose each other via the endothelial cell receptor Tie-2. Therefore, PECAM1 may operate by inhibition of both the Tie-2 and VE-cad signaling pathways in vasculogenesis. The pathway(s) regulated by CEACAM1 in this model system require further study.
Material and Methods
Antibodies
Goat IgG1, Mouse IgG1, Rat IgG2a and Rabbit IgG1 purchased from eBioscience (San Diego, CA) used as isotype controls. Antibodies used for IPs and/or western blots were: anti-murine CEACAM1, CC1 (mouse IgG1), a kind of gift of Dr. Holmes (University of Colorado Health Sciences, CO); anti-human CEACAM1, T84.1 (mouse IgG1) developed at City of Hope; anti-mouse PECAM1 (Rat IgG2a), anti-mouse β-catenin, anti-phospho-tyrosine (4G10), anti-mouse Tie-2, and anti-mouse Flk-1 from Millipore (Billerica, MA); anti-human PECAM1, anti-human Flk-1, anti-human VE-cad, anti-mouse VE-cad, and anti-human Tie-2 from Santa Cruz Biotechnology (Santa Cruz, CA); anti-mouse SHP-1 and SHP-2 obtained from BD Bioscience (Rockville, MD).
Embryoid body formation
Mouse embryonic stem cells derived from strain 129S1-SVImj were cultured on amitotically inactivated STO-Neo/LIF feeder layer [from EJ Robertson] in DMEM (Mediatech Inc, Herndon, VA) containing 16% fetal bovine serum, non-essential amino acids (GIBCO, stock solution diluted 1:100, final concentration 100 nM), GlutaMax (Invitrogen), penicillin/streptomycin, and 30 nM 2-mercaptoethanol (ES medium). The ES cells were digested at 37 °C with Trypsin/EDTA, and reduced to a single-cell suspension by repeated pipetting. The STO feeder cells were depleted by incubating the cell suspension in a plastic tissue-culture dish in ES medium in a 5% CO2 incubator at 37 °C for 45min. The non-adherent cells were transferred to a fresh plastic tissue-culture dish, and incubated in a 5% CO2 incubator at 37 °C for an additional 45 min. The resulting non-adherent cells contained ca 90% ES cells, and the enriched ES cells were frozen in 20% FBS/10% DMSO/70% DPBS without Ca2+/Mg2+.
To form Embryoid bodies, ES cells were cultured up to 8 days on 1% agar coated Petri-dishes in DMEM (Mediatech Inc, Herndon, VA) with 14% heat inactivated fetal bovine serum (Omega Scientific Inc., Tarzana, CA), nonessential amino acids (GIBCO, stock solution diluted 1:100, final concentration 100 nM), 2-mercaptoethanol (GIBCO, final concentration 30 nM) and 5% of Antibiotic–Antimycotic (GIBCO, stock solution diluted 1:100, final concentration 1000 U of penicillin, 1000 μg of streptomycin), 50 ng/ml recombinant murine VEGF165 (PEPRO TECH) and 10 μg/ml bovine insulin (Invitrogen) were added into medium.
In vitro tube formation assay of Embryoid bodies in Matrigel
Matrigel (500μl, BD Biosciences, Bedford, MA) was added to 6-well plates and allowed to solidify for 20 min at 37°C. After the Matrigel solidified, an additional 500μl of Matrigel mixed with embryoid bodies was then plated on the top of previous Matrigel layer and allowed to solidify for 20 min at 37°c. Complete medium including VEGF and insulin was then added and the plates were then incubated at 37°c with 5% CO2. of Ang-1(100ng/ml, R&D system, Cat# 923-AN) or Ang-2 (R&D system, Cat# 623-AN) was added to the medium. Media were changed every other day. Cells were cultured for 8 days in order to form embryoid bodies. After mouse embryoid bodies were moved into Matrigel, 100ng/ml of Ang-1 or Ang-2 was added to the culture medium either at day 0 or at day 3. Media were changed every other day. EBs were cultured for 8 days in Matrigel plus Ang-1 and Ang-2.
In vitro tube formation assay of Human HUVECs in Matrigel
The human HUVEC cell line was purchased from ATCC (CRL-1730). Cells were grown in the complete growth medium F-12K (ATCC 30-2004) with 10% fetal bovine serum (ATCC 30-2020), 5% pen-strep (ATCC 30-2300), 0.1mg/ml heparin (Invitrogen), 0.05 mg/ml endothelial cell growth supplement (ECGS) (BD biosciences, lot # 63988). Medium was changed every other day. Cells were maintained in 5% CO2 incubator at 37°C.
Matrigel (500μl, BD Biosciences, Bedford, MA) was added to 6-well plates and allowed to solidify for 20 min at 37°C. After the Matrigel solidified, 1×105 HUVEC cells in the complete medium plus 10 ng/ml of recombinant VEGF165 (PEPRO TECH) were seeded on the top of Matrigel for up to 24 hours. Plates were incubated at 37°C. Ang-1 (200 ng/ml, R&D system, Cat# 923-AN) or Ang-2 (R&D system, Cat# 623-AN) were then added to the wells after one hour incubation.
Immunoprecipitation
Day 0 embryoid bodies and HUVEC were lysed in RIPA lysis buffer (Sigma, St. Louis, USA) with protease inhibitor cocktail (Roche, USA) and phosphatase inhibitor cocktail (Thermo Scientific, USA). Day 7 embryoid bodies were recovered from 100% matrigel using matrisperse (BD, USA) at 4°C for 4 hours with shaking, then lysed with complete RIPA lysis buffer. Recombinant protein G agarose (100μL, Invitrogen, Oregon) was mixed with 4 μg of isotype control antibodies (e.g. mouse IgG, rat IgG2a, rabbit IgG), incubated at 4°C with rotation for 1 hour, followed by washing twice with PBS. Protein G beads (50μL) were added to the cell lysate (total protein was 500μg) and incubated at 4°C for 30 min, centrifuged, and the supernatant incubated with 50μL of antibody coated beads at 4°C for another 30 min. After centrifugation, the beads were gently removed and the IP antibodies were added into the clear supernatant according to the manufacture’s recommendation. Mixtures were then incubated at 4°C over night on a rocker platform. On the second day, 50μL of fresh beads were added to the mixtures and incubated at 4°C for four hours. The agarose beads were collected by centrifugation and the supernatant removed. Beads were washed 3 times with ice-cold cell lysis buffer with 15 min incubation each time. The beads were re-suspended in 30 μl of 4X running buffer (Invitrogen) and boiled for 5 min. For each of the IPs, the non-reactive serum control was performed for each of the appropriate antibodies (data not shown, available upon request).
Confocal microscopy
EB were grown in Matrigel and stained with Alexa Fluor 488 conjugated anti-mouse PECAM1 antibody (1μg/ml, BioLegend) or Alexa Fluor 488 rat IgG2a antibody (1μg/ml, BioLegend) for 10h at 37°C, then wells were washed with DMEM medium containing 12% FBS and growth factors for 15 min 3 times at 37°C. Confocal microscopy was performed on a Zeiss Model 510 (Oberkochen, Germany) confocal microscope.
Western blot analysis
Proteins from immunoprecipitation or from cell lysates were separated by sodium dodecyl sulfate–gel electrophoresis, transferred to nitrocellulose membranes and probed with antibodies described above. Signals were detected on the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Results
PECAM1, CEACAM1, Flk-1, VE-cad and Tie-2 are phosphorylated in day 0 EB
We previously demonstrated that PECAM1 and CEACAM1 are both expressed in EB in a 3D model of vasculogenesis [4]. Since both PECAM1 and CEACAM1 have cytoplasmic ITIMs that can be phosphorylated after cell-cell adhesion [20], it was important to measure their phosphorylation status in our model system. In addition, it was necessary to examine the tyrosine phosphorylation status of their potential receptor targets, since their inhibitory activities depend on the recruitment of SHP-1/2, which in turn, can dephosphorylate activated receptors. Total cell lysates from VEGF treated ES cells that had been differentiated into EBs (prior to transfer to Matrigel, designated as day 0 EBs) were immunoprecipitated with antibodies to CEACAM1, PECAM1, Tie-2, Flk-1, VE-cad and β-catenin, proteins separated by SDS gel electrophoresis and subjected to western blot analysis with 4G10, an antibody which targets tyrosine phosphorylation (Fig1A; IP control in FigS1). The choice of day 0 EBs was based on the real-time PCR results performed in the previous study, in which endothelial marker genes either began to be expressed or were already highly expressed at this time point. For example, Tie-2, a key receptor involved in vasculogenesis and angiogenesis [30, 31], reached its peak expression in day 0 EB and then decreased during vascular sprouting in Matrigel. These results suggested that Tie-2 was negatively regulated during vasculogenesis, making it a viable candidate for regulation by either PECAM1 or CEACAM1. In contrast, Flk-1, the main receptor for VEGF stimulated vasculogenesis, was an unlikely candidate since its expression continued even after transfer of EBs to matrigel and sprouts occurred in the absence or presence of VEGF. In addition, we assessed the tyrosine phosphorylation status of β-catenin, since a previous study from our lab demonstrated that CEACAM1 interacts with β-catenin [21] and, according to Madri and coworkers [32], VEGF binding to Flk-1 in endothelial cells causes tyrosine phosphorylation of β-catenin, followed by recruitment of PECAM-1. ECM stimulation of phosphorylation of PECAM1 recruits SHP-2, which in turn, can dephosphorylate β-catenin thereby weakening cell-cell interactions. Thus, PECAM1 may function as an inhibitor of β-catenin modulated cell-cell adhesion.
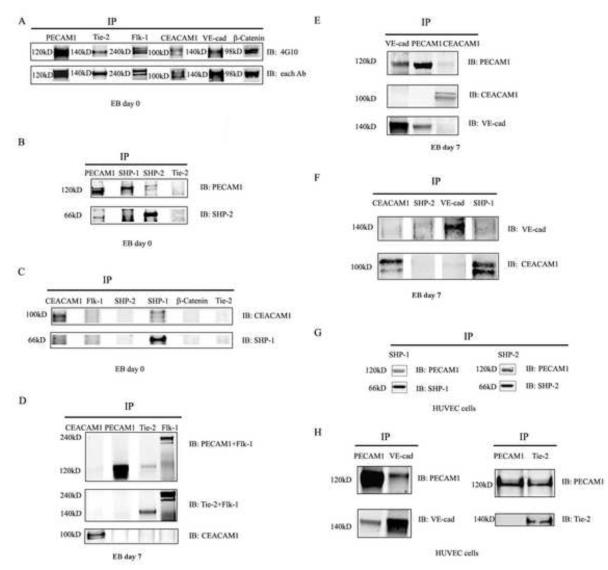
Fig 1. Tyrosine phosphorylation of PECAM1, Tie-2, Flk-1 and CEACAM1 in day 0 EBs.
A. PECAM1, CEACAM1, Tie-2 and Flk-1 were immunoprecipitated from the day 0 EB cell lysates, proteins separated by SDS gel electrophoresis, and western blotted with anti-tyrosine phosphate 4G10 antibody. B. PECAM1 is associated with SHP-1/2 in day 0 EBs. PECAM1, SHP-2 and SHP-1 were immunoprecipitated from the day 0 EB cell lysates, proteins separated by SDS gel electrophoresis and western blotted with anti-PECAM1, anti-SHP1 and anti-SHP-2 antibodies. C. CEACAM1 is associated with SHP-1in day 0 EBs. CEACAM1, Flk-1, SHP-2, SHP-1 and β-Catenin were immunoprecipitated from day 0 EB cell lysates, proteins separated by SDS gel electrophoresis, and western blotted with anti-CEACAM1, anti-SHP-1 and anti-SHP-2 antibodies. D. PECAM1, but not CEACAM1, is associated with Tie-2 in day 7 EBs. PECAM1, Tie-2, Flk-1 and CEACAM1 were immunoprecipitated from day 7 EB cell lysates, proteins separated by SDS gel electrophoresis and immunoblotted with anti-PECAM1, anti-Flk-1 antibodies, anti-Tie-2, anti-Flk-1 antibodies, and anti-CEACAM1 antibody. E. PECAM1 is associated with VE-cadherin in day 7 EBs. CEACAM1, PECAM1, and VE-cad were immunoprecipitated from day 7 EB lysates and immunoblotted with anti-VE-cadherin, anti-CEACAM1, and anti-PECAM1 antibodies. F. CEACAM1 is not associated with VE-cadherin in day 7 EBs. CEACAM1, VE-cad, SHP-1 and SHP-2 were immunoprecipitated from day 7 EB lysates and immunoblotted with anti-VE-cadherin and anti-CEACAM1 antibodies. G. PECAM1 is associated with SHP-1 and SHP-2 in HUVECs. SHP-1 and SHP-2 from HUVEC were immunoprecipitated from total HUVEC total cell lysates, proteins separated by SDS gel electrophoresis and immunoblotted with anti-PECAM1 antibody. H. PECAM1 is associated with VE-cadherin and Tie-2 in HUVECs. PECAM1, Tie-2 and VE-cadherin were immunoprecipitated from HUVEC total cell lysates and immunoblotted with anti-PECAM1, anti-Tie-2 or anti-VE-cadherin antibodies.
Phosphotyrosine blots of PECAM1, CEACAM1, Tie-2, VE-cad and β-catenin IPs showed that PECAM1, CEACAM1, Flk-1, Tie-2, VE-cad and β-catenin are all tyrosine phosphorylated in day 0 EBs (Fig 1A; IP control in FigS1). We conclude that each of the potential target receptors are activated by tyrosine phosphorylation in day 0 EBs and therefore are candidates for regulation by PECAM1 and/or CEACAM1, both of which are also tyrosine phosphorylated.
PECAM1 associates with both SHP-1 and SHP-2 while CEACAM1 associates with SHP-1 only in the 3D model of vasculogenesis
Since both PECAM1 and CEACAM1 were tyrosine phosphorylated in day 0 EBs, it was necessary to determine if they also recruited SHP-1/2 phosphatases to their phosphorylated ITIMs. PECAM1, CEACAM1, Flk-1, SHP-1, SHP-2 and β-catenin were immunoprecipitated as above and the blots probed with antibodies to PECAM1, CEACAM1, SHP-1 and SHP-2. As shown in Fig 1B (IP control in Fig S1), PECAM1 is associated with both SHP-1 and SHP-2. Since we observed two bands for PECAM1 on the SHP1/2 IPs, we conclude that the upper band is the phosphorylated form and the lower band is the non-phosphorylated form. We found that PECAM1 associated with SHP-2 only when the SHP-2 IP blot was probed with the anti-PECAM1 antibody, and not the reverse, a result that is likely due to the relative affinity of each antibody in a given assay.
As shown in Fig 1C, SHP-1 but not SHP-2 is associated with CEACAM1 (SHP-1/2 IP, western blot with anti-CEACAM1). Notably, the CEACAM1 band in the SHP-1 IP lane migrated slower than in the CEACAM1 IP lane. The change in migration may be due to phosphorylation, a modification which is known to retard the migration of proteins on SDS gel electrophoresis. We also found that CEACAM1 associated with SHP-1 only when probed in the SHP-1 IP blot with anti-CEACAM1 antibody. This result may also be due to a higher antibody affinity for CEACAM1 than the anti-SHP-1 antibody. In addition, there was no evidence to show SHP-1 and SHP-2 associated with Tie-2 (Fig 1B and 1C)
PECAM1 but not CEACAM1 associates with Tie-2 in EBs grown in Matrigel for 7 days
The Tie-2 receptor is highly expressed on the cell surface of endothelial cells [2]. As previously mentioned, Tie-2 has two ligands, Ang-1 and Ang-2 for which the regulatory roles in angiogenesis are controversial [8, 10]. The fact that Tie-2 was down-regulated while PECAM1 and CEACAM1 were up-regulated at the mRNA level in our previous study [4], suggests that Tie-2 is a good candidate for negative regulation by either PECAM1 or CEACAM1. Since Flk-1 is the main VEGF receptor and plays a central role in vasculogenesis and angiogenesis, we also included Flk-1 in our analysis. Therefore, we immunoprecipitated PECAM1, CEACAM1, Flk-1 and Tie-2 from day 7 EB cell lysates and probed western blots with anti-PECAM1, anti-CEACAM1, anti-Flk-1, and anti-Tie-2 antibodies. We chose the 7 day point for analysis, a time point at which sprouting had reach its half maximum value [4]. The results demonstrated that PECAM1, but not CEACAM1 is associated with Tie-2 in day 7 EB (Fig 1D). However, there was no evidence that Flk-1 was associated with either CEACAM1 or PECAM1 (Fig 1D).
PECAM1 but not CEACAM1 associates with VE-cad in EBs grown in Matrigel for 7 days
VE-cad is widely expressed in the vascular endothelium, has been reported to be involved in vasculogenesis and angiogenesis [28], and shown to positively regulate TGF-β signaling [29] and negatively regulate Flk-1 signaling [29]. Since its expression follows that of Flk-1 in the 3D model of vasculogenesis, it is likely that VE-cad must be held in check during the early stages of vascular sprouting. In order to test the possible association of VE-cad with either PECAM1 or CEACAM1, we immunoprecipitated PECAM1, CEACAM1, VE-cad, SHP-1 and SHP-2 from day 7 EB cell lysates and western blots were probed with anti-PECAM1, anti-CEACAM1, anti-VE-cad antibodies. The results showed that PECAM1, but not CEACAM1 is associated with VE-cad in day 7 EBs (Fig 1E, and 1F).
PECAM1 but not CEACAM1 associates with Tie-2 and VE-cad in HUVECs
The association of PECAM1 with VE-Cad is a novel finding that may partly explain previous observations in human HUVECs in which the activation of VE-cad has opposite effects on Flk-1 and TGFβ signaling pathways [33]. HUVECs, vascular endothelium cells isolated from the human umbilical vein, are known to express Tie-2, Flk-1, PECAM1 and VE-cad [34]. HUVECs have also been reported to express CEACAM1 after TNF-α stimulation [35]; however, since we observed no CEACAM1 expression after TNF-α treatment, the results of that treatment is not shown here. To test the possibility that PECAM1 could negatively regulate VE-cad in HUVECs grown in 3D culture, the cells were analyzed by IP analysis. The results showed that PECAM1 associated with SHP-1 and SHP-2 (Fig 1G), and associated with Tie-2 and VE-cad in HUVEC (Fig 1H). These results support the idea that PECAM1 and VE-cad are constitutively associated with each other in both early murine vascular sprouts (the 3D model system) and in mature human endothelial cells. We also examined the phosphorylation status of PECAM1, VE-Cad, Tie-2, and Flk-1 in the absence of VEGF. All four receptors were phosphorylated (Fig S2), indicating that culturing in 3D was sufficient to activate the receptors. Since the addition of VEGF accelerated sprouting, it was included in the final tube formation assay.
Opposite effects of Ang-1 and Ang-2 on vascular sprouting
Since PECAM1 was associated with Tie-2 in our model system, the critical role of Tie-2 was further explored by treating ES cells or HUVEC cells with either Ang-1 or Ang-2, ligands of Tie-2. When ES cells were treated with Ang-1 or Ang-2 for 8 days, the diameter of embryoid bodies in Ang-2 treatment were greater than controls (P<0.05), while after Ang-1 treatment, the diameter of embryoid bodies were smaller than controls (P<0.05); (Fig 2A-C). When embryoid bodies were grown in Matrigel for 8 days in the presence of Ang-1, vascular sprouting was completely blocked, while Ang-2 treatment greatly accelerated vasculogenesis (Fig 3A-C). In order to determine if Ang-1 and Ang-2 treatment affects existing vascular sprouts, we first cultured EBs in Matrigel for 3 days in the absence of Ang-1 or Ang-2 to allow EBs develop vascular tubes, then began treatment with Ang-1 or Ang-2. Further vascular tube sprouting was accelerated by Ang-2 treatment, while Ang-1 slowed further vascular sprouting in EBs (Fig 4). As expected, PECAM1 staining coincides with the vascular sprouts (Fig 5). In order to determine if Ang-1 or Ang-2 treatment of EBs affected the phosphorlation status of Tie-2, we analyzed EBs grown in Matrigel at days 5 and 8 treated with Ang-1 or Ang-2. In the presence of Ang-2, phosphorylated of Tie-2 was increased compared to untreated controls (Fig 6). Ang-1 treatment suppressed Tie-2 phosphorylation on day 5 (Fig 6). As a cell surface receptor control, we examined the expression of CEACAM1 at these time points and found no changes (Fig 6). These results suggest that Ang-1 is an antagonist of Tie-2, while Ang-2 is an agonist of vascular sprouting. Furthermore, it is likely that PECAM1 inhibits the pro-vasculogenic effect of Ang-2, since in the absence of Ang-2, vasculogenesis is moderate and PECAM1 is associated with Tie-2, the receptor for Ang-2.
Fig 2. Ang-2 promotes while Ang-1 inhibits vasculogenesis in ES cells.
Ang-1 or Ang-2 (100ng/ml) were added into day -8 ES cells for 8 days. A. Phase contrast photographs were taken at day -3 and day 0. Ang-1 treated ES cells form smaller diameter EBs than controls, but Ang-2 treated ES cells form larger diameter EBs than controls. Mag 50X. EBs were divided into three different diameter size groups and were counted at day -3 (B) and day 0 (C). The experiments were repeated twice. Values were analyzed by two-way ANOVA (Ang-1 or Ang-2 compared to control* P<0.05, ** P<0.01, *** P<0.001. Ang-1 compared to Ang-2 #P<0.05, ##P<0.01, ### P<0.001).
Fig 3. Ang-2 promotes while Ang-1 inhibits vasculogenesis in EBs grown in Matrigel.
Ang-1 or Ang-2 (100ng/ml) was added to day 0 EBs for 8 days in Matrigel. A. Phase contrast photographs were taken at day 5 and day 8 (Mag 50X). EBs were divided into three different diameter size groups and were counted at day 5 (B) and day 8 (C). The experiments were repeated twice. Values were analyzed by two-way ANOVA (Ang-1 or Ang-2 compared to controls, * P<0.05, ** P<0.01, *** P<0.001. Ang-1 compared to Ang-2 #P<0.05, ##P<0.01, ### P<0.001).
Fig 4. Ang-2 promotes while Ang-1 inhibits vasculogenesis in EBs grown in Matrigel after established tube formation for 3 days.
Ang-1 or Ang-2 (100 ng/ml) was added to day 3 EBs and continued until day 8 in Matrigel. Phase contrast photographs were taken at day 5 and day 8 (Mag 50X). The experiments were repeated three times.
Fig 5. Confocol analysis of PECAM1 protein expressed on EB vascular sprouts grown in 3D culture Ang-1 or Ang-2 treatment.
Imaged by phase contrast at 24 hours and 48 hours Ang-1 and Ang-2 treatment (Mag 100X).
Fig 6. Ang-2 treatment promotes Tie-2 phosphorylation while Ang-1 treatment suppresses Tie-2 phosphorylation in EBs grown in Matrigel.
Day 5 and day 8 EBs with Ang-1 or Ang-2 treatments were recovered from Matrigels and were lysed. Cell lysates were separated by SDS page and membranes were probed by anti-Tie-2, 4G10, anti –mouse CEACAM1 and beta-actin antibodies.
Since the roles of Ang-1 and Ang-2 in vasculogenesis are controversial, we tested their functions in a second model of vasculogenesis using human endothelial cells. HUVECs grown on the top of Matrigel were treated with Ang-1 or Ang-2 for up to 24 hours in the presence of VEGF. We found that as early as 6 hours, Ang-2 promoted tube formation, while Ang-1 inhibited tube formation (Fig 7). These results confirm that in a second 3D model of vasculogenesis, Ang-1 is indeed an antagonist of Tie-2 while Ang-2 is an agonist of Tie-2 mediated sprouting.
Fig 7. Ang-2 treatment promotes while Ang-1 treatment inhibits tube formation in HUVECs grown in Matrigel.
Ang-1 or Ang-2 (200 ng/ml) were added to HUVECs grown on the top of Matrigel for 24 hours in the presence of 10 ng/ml VEGF (Mag 50X).
Discussion
Vasculogenesis is an important developmental program that is required for growth of the embryo beyond an initial size of a few millimeters. In the embryo, the endothelial cells that initiate the formation of the vascular system are found in mesodermal cell masses called the “blood island” [36]. Thus, one of the earliest functions of the developing embryo is to develop a vascular system. It is of interest to study early markers of endothelial cells that regulate this process. In this respect, the roles of ligands such as VEGF, Ang-1 and Ang-2 as well as their receptors Flk-1 and Tie-2, have been intensively studied. Since the extent of vascular sprouting must be under the control of cell-cell adhesion molecules, we were particularly interested in the potential roles of the cell-cell adhesion molecules PECAM1 and CEACAM1 because of their previous implication in regulation of the highly related process, angiogenesis [37]. In this regard, both PECAM1 [38] and CEACAM1 [4] are expressed as early as murine embryonic day 7.5, at which point the blood island has just started to form. Since it is difficult to study this process in intact embryos, a model system of vasculogenesis was required. In our in vitro model of vascular development we have observed vascular sprouting from murine EBs embedded in 3D culture allowing us to study the process in a temporal manner. We found that both PECAM1 and CEACAM1, molecules that have striking structural and functional similarities, were expressed even before transfer of EBs to 3D culture, and that treatment of the EBs with antibodies to either cell-cell adhesion molecule resulted in greatly attenuated vascular sprouting [4].
Although these studies suggested that both PECAM1 and CEACAM1 were essential regulators of vasculogenesis, it is noteworthy that vasculogenesis is unimpaired in either Pecam1-/- or Ceacam1-/- mice. Explanations include the possibility that other related molecules may substitute for their functions. In this regard, both PECAM1 and CEACAM1 share the inhibitory function related to phosphorylation of their ITIMs and recruitment of SHP-1/2, which in turn, can dephosphorylate other critical receptors activated during vasculogenesis. In this respect, several candidate receptors emerged from our previous study [4], including Tie-2 and VE-cad. Thus, further studies were required to determine their mechanism of regulation in the in vitro model.
Since both PECAM1 and CEACAM1 were expressed in EBs even before transfer to 3D culture, we first asked if they were phosphorylated on the tyrosine residues of their ITIMs and if they recruited either SHP-1/2. Indeed, this was the case, suggesting that both PECAM1 and CEACAM1 were activated by their primary cell-cell adhesion function. Interestingly, CEACAM1 recruited SHP-1 (albeit weakly), while PECAM1 recruited both SHP-1and SHP-2 in day 0 EBs. Further analysis demonstrated that this association continued on EBs that were maintained in 3D culture for 7 days. At this time point, extensive vascular sprouting had occurred and the association of CEACAM1 with SHP-1 was increased. Thus, even though CEACAM1 is expressed early in VEGF treated EBs, its effect on vascular sprouting may be pronounced at later times, perhaps by termination of sprouting.
Having demonstrated that both PECAM1 and CEACAM1 were phosphorylated and had recruited a tyrosine phosphatase, we next attempted to identify putative co-receptors for which they could exert their inhibitory effect. As mentioned earlier, we selected likely candidates as those that were down-regulated during vascular sprouting. Thus, we chose the 7 day point for analysis, a time point at which sprouting had reach its half maximum value [4] . Tie-2 was chosen since it was tyrosine phosphorylated in day 0 and day 7 EBs and down-regulated over the entire course of vascular sprouting. IP and western blot analysis demonstrated that Tie-2 only associated with PECAM1, but not CEACAM1. This indicates that although CEACAM1 and PECAM1 have similar negative regulatory functions, their co-receptor associations must be different. In agreement with this observation, PECAM1 is expressed in both the early and later steps of angiogenesis [18, 39], while CEACAM1 is expressed in only the early steps [40].
Since Tie-2 is the main receptor for Ang-1/2 and the regulation of endothelial cell proliferation is Ang-1/2 dependent [3, 8], PECAM1 may negatively regulate the Tie-2 pathway. To further explore the role of the Tie-2 pathway in this system, ES cells were treated with either Ang-1 or Ang-2. The dramatic results indicate that Ang-1 inhibits while Ang-2 greatly accelerates vascular sprouting even after vascular sprouts have been allowed to develop for 3 days. Since out results showed that Ang-2 is an agonist for Tie-2, it was necessary to show that Ang-2 promoted Tie-2 phosphorylation. As expected, we found that Tie-2 is highly phosphorylated after Ang-2, but not Ang-1 treatment. Since vascular sprouting is more moderate in the absence of exogenous Ang-2, we conclude that PECAM1 inhibits Tie-2 signaling unless overcome by the addition of exogenous Ang-2. This would make sense, since Ang-2 but not Ang-1 is produced by endothelial cells. In one respect, these results need to be reconciled with previous studies suggesting that Ang-1 is an agonist, while Ang-2 is an antagonist of the Tie-2 receptor [10]. However, a more recent study demonstrated that Tie-1/2 heterodimers signal via Ang-1 stabilized sprouting, while Ang-2 signals increased sprouting [41]. Since the ES cells express both Tie-1 and Tie-2 in our model system [4], it is reasonable to conclude that Ang-1 is an antagonist and Ang-2 an agonist. Furthermore, a monoclonal antibody to Ang-2 was shown to possess potent anti-tumor activity by virtue of its ability to inhibit Tie-2 phosphorylation, further supporting the mounting evidence that Ang-2 is pro-angiogenic [42].
Our finding that the inhibitory co-receptor PECAM1 associates with Tie-2 in the ES/EB model system, suggests that it moderates the activity of Tie-1/2 positive signaling. Thus, the addition of the antagonist Ang-1 would be predicted to further inhibit sprouting, as was the case. On the other hand, the addition of the agonist Ang-2 was able to over come PECAM1 inhibition and increase sprouting. However the effect of PECAM1 on sprouting is further complicated by the finding that PECAM1 also associates with VE-cad, which in turn, can negatively regulate Flk-1 in endothelial cells [17].
Since it could be argued that our findings on Ang-1/2 could be model specific, we tested the effects of Ang-1 and Ang-2 in a second model of vascular sprouting using VEGF treated HUVECs in 3D culture. Since exactly the same results were obtained, namely inhibition of sprouting by Ang-1 and accelerated sprouting by Ang-2, we conclude that Ang-1 acts as an antagonist and Ang-2 as an agonist of Tie-2 mediated sprouting. One can ask if both of our models of vasculogenesis are subject to common confounding factors. Indeed, this is possible since both of our models utilize VEGF as the main growth factor and employ a 3D ECM environment. However, the requirements for VEGF and a 3D environment of ECM appear to be the most physiological constraints available outside the living organism that initiate and sustain sprouting. Perhaps the controversy surrounding Ang-1 and Ang-2 is due not only to the cell type context but also the environment in which the cells were tested? Perhaps additional model systems are required to answer this question.
As a potential negative control, we studied the potential association of PECAM1 or CEACAM1 with Flk-1, the main receptor for VEGF signaling. Since Vegfr2–/– mice die at embryonic days 8.5 to 9.5 due to a defect in the development of hematopieotic and endothelial cells resulting in impaired vasculogenesis [43], this receptor is expected to be free of negative regulation during vascular sprouting. In contrast to Tie-2, Flk-1 was up-regulated during the process of vascular sprouting in our model system [4]. Although we observed tyrosine phosphorylation of Flk-1 in day 0, there was no evidence of its association with either PECAM1 or CEACAM1. Thus, this control, plus the lack of association of CEACAM1, suggests that the association of PECAM1 with Tie-2 is specific and significant.
VE-cad was a second candidate for negative regulation because it is a negative regulator of vasculogenesis, it was tyrosine phosphorylated on day 7 EBs, and it was up-regulated over the time course of vascular sprouting in our model system [4]. Again using IP plus western blot analysis, we found that PECAM1, but not CEACAM1, was associated with VE-cad. Since VE-cad has been shown to negatively regulate Flk-1 signaling in endothelial cells [29], it makes sense that this receptor’s signaling requires negative regulation until vascular sprouting has terminated.
In summary, in an in vitro model of vasculogenesis, we have shown associations between PECAM1 and Tie-2 and VE-cad, two endothelial receptors that are known to play critical roles in vasculogenesis. Since PECAM1, as well as its two targets, were tyrosine phosphorylated, and PECAM1 recruited SHP-1/2, it is reasonable to assume that it negatively regulates Tie-2 and VE-cad signaling. Thus, the net effect of negative signaling from PECAM1 is likely to hold in check anti-proliferative signaling from VE-cad, and positive signaling from Tie-2. If true, VE-cad signaling acts as a brake on vascular sprouting and PECAM1 opposes that brake during the early stages. Conversely, PECAM1 acts as a brake on the positive signaling induced by Ang-2 on Tie-2, thus reducing the rate and degree of vascular sprouting. This would explain why PECAM1 is expressed early, even before EBs are transferred to 3D culture. A second prediction of the model is that PECAM1 would not be activated by tyrosine phosphorylation and recruitment of SHP-1/2 in mature endothelial cells until activated by external stimuli such as wound healing.
The function for CEACAM1 remains unsolved in this model system. Although it appears just as essential as PECAM1, appearing early during the process and required according to the antibody inhibition data [4], we have not been able to identify even one of the essential endothelial cell receptors as its co-receptor. This suggests that the association was either missed due to technical reasons (eg, low affinity of interaction) or there is another critical receptor not analyzed. Thus, each is essential for different reasons, and although absolutely required for vasculogenesis in this model system, both are dispensable in murine knock out models. In this regard, it should be noted that when endothelial cells are tested from either Pecam1-/- or Ceacam1-/- mice, they are defective [44, 45] . Further study in both knock-out models is required to unravel the precise reasons for these observations.
Supplementary Material
Supplemental Figure S1. Isotype controls for immunoprecipitation experiments. Rat antibodies that were specific for PECAM1 and Tie-2 or their isotype controls, rabbit antibodies specific for Flk-1 and beta-catenin or their isotype controls, goat antibody specific for VE-Cad or their isotype controls, and mouse antibodies specific for CEACAM1 and SHP-2 or their isotype controls were used to IP lysates from day 0 EBs and western blotted for each of the receptors. in each case the isotype controls were negative when western blotted.
Supplemental Figure S2. Phosphorylation status of PECAM1, Tie-2, Flk-1, and VE-cad in VEGF untreated control HUVECs. Lysates from HUVECs grown in 3D culture with no VEGF were immunoprecipitated with antibodies specific for each receptor and western blotted with anti-phosphotyrosine antibody 4G10.
Acknowledgements
This research was supported by NIH grants CA84202 (JES). We thank Dr. Walter Tsark for providing ES cells and Sara Brokaw (R & D Systems, Inc.) for providing samples of Ang-1 and Ang-2 and Ang-1/2 specific primers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Meissner M, Reichenbach G, Stein M, Hrgovic I, Kaufmann R, Gille J. Downregulation of vascular endothelial growth factor receptor 2 is a major molecular determinant of proteasome inhibitor-mediated antiangiogenic action in endothelial cells. Cancer Res. 2009;69:1976–1984. doi: 10.1158/0008-5472.CAN-08-3150. [DOI] [PubMed] [Google Scholar]
- [2].Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- [3].Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 2007;5:655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- [4].Gu A, Tsark W, Holmes KV, Shively JE. Role of Ceacam1 in VEGF induced vasculogenesis of murine embryonic stem cell-derived embryoid bodies in 3D culture. Exp Cell Res. 2009;315:1668–1682. doi: 10.1016/j.yexcr.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- [6].Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- [7].Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & development. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- [8].Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–1184. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- [9].Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- [10].Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- [11].Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- [12].Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- [13].Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- [14].Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- [15].Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 2003;83:337–376. doi: 10.1152/physrev.00024.2002. [DOI] [PubMed] [Google Scholar]
- [16].Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [17].Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- [19].Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Homotypic and heterotypic Ca(++)-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- [20].Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- [21].Jin L, Li Y, Chen CJ, Sherman MA, Le K, Shively JE. Direct interaction of tumor suppressor CEACAM1 with beta catenin: identification of key residues in the long cytoplasmic domain. Exp Biol Med (Maywood) 2008;233:849–859. doi: 10.3181/0712-RM-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, Blau DM, Russell S, Holmes KV, Blumberg RS. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol. 2002;168:1028–1035. doi: 10.4049/jimmunol.168.3.1028. [DOI] [PubMed] [Google Scholar]
- [23].Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–3674. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [24].Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- [25].Chen CJ, Shively JE. The cell-cell adhesion molecule carcinoembryonic antigenrelated cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J Immunol. 2004;172:3544–3552. doi: 10.4049/jimmunol.172.6.3544. [DOI] [PubMed] [Google Scholar]
- [26].Wagener C, Ergun S. Angiogenic properties of the carcinoembryonic antigen-related cell adhesion molecule 1. Exp Cell Res. 2000;261:19–24. doi: 10.1006/excr.2000.5038. [DOI] [PubMed] [Google Scholar]
- [27].Kilic N, Oliveira-Ferrer L, Wurmbach JH, Loges S, Chalajour F, Neshat-Vahid S, Weil J, Fernando M, Ergun S. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem. 2005;280:2361–2369. doi: 10.1074/jbc.M409407200. [DOI] [PubMed] [Google Scholar]
- [28].Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thrombosis and haemostasis. 2001;86:308–315. [PubMed] [Google Scholar]
- [29].Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garre M, Liebner S, Letarte M, ten Dijke P, Dejana E. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. The EMBO journal. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- [31].Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- [32].Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci. 1999;112(Pt 18):3005–3014. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- [33].Lampugnani M. Grazia, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, Newman PJ. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–1707. doi: 10.4161/cbt.5.12.3467. [DOI] [PubMed] [Google Scholar]
- [35].Muenzner P, Dehio C, Fujiwara T, Achtman M, Meyer TF, Gray-Owen SD. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect Immun. 2000;68:3601–3607. doi: 10.1128/iai.68.6.3601-3607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- [37].Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, Kilic E, Huland H, Friedrich M, Ergun S. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res. 2004;64:8932–8938. doi: 10.1158/0008-5472.CAN-04-0505. [DOI] [PubMed] [Google Scholar]
- [38].Pinter E, Barreuther M, Lu T, Imhof BA, Madri JA. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am J Pathol. 1997;150:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- [39].Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Molecular cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- [41].Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, Barton WA. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Molecular cell. 2010;37:643–655. doi: 10.1016/j.molcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, Zhou JQ, Tabrizi M, Emery S, McDermott B, Pablo L, McCoon P, Bedian V, Blakey DC. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Molecular cancer therapeutics. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- [43].Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- [44].Horst AK, Ito WD, Dabelstein J, Schumacher U, Sander H, Turbide C, Brummer J, Meinertz T, Beauchemin N, Wagener C. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest. 2006;116:1596–1605. doi: 10.1172/JCI24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, de la Pompa J. Luis, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Isotype controls for immunoprecipitation experiments. Rat antibodies that were specific for PECAM1 and Tie-2 or their isotype controls, rabbit antibodies specific for Flk-1 and beta-catenin or their isotype controls, goat antibody specific for VE-Cad or their isotype controls, and mouse antibodies specific for CEACAM1 and SHP-2 or their isotype controls were used to IP lysates from day 0 EBs and western blotted for each of the receptors. in each case the isotype controls were negative when western blotted.
Supplemental Figure S2. Phosphorylation status of PECAM1, Tie-2, Flk-1, and VE-cad in VEGF untreated control HUVECs. Lysates from HUVECs grown in 3D culture with no VEGF were immunoprecipitated with antibodies specific for each receptor and western blotted with anti-phosphotyrosine antibody 4G10.