Abstract
Mitochondria represent approximately one-third of the mass of the heart and play a critical role in maintaining cellular function—however, they are also a potent source of free radicals and pro-apoptotic factors. As such, maintaining mitochondrial homeostasis is essential to cell survival. As the dominant source of ATP, continuous quality control is mandatory to ensure their ongoing optimal function. Mitochondrial quality control is accomplished by the dynamic interplay of fusion, fission, autophagy, and mitochondrial biogenesis. This review examines these processes in the heart and considers their role in the context of ischemia-reperfusion injury. Interventions that modulate mitochondrial turnover, including pharmacologic agents, exercise, and caloric restriction are discussed as a means to improve mitochondrial quality control, ameliorate cardiovascular dysfunction, and enhance longevity.
Keywords: Autophagy, caloric restriction, cardioprotection, exercise, ischemia-reperfusion, mitochondria, mitochondrial turnover, preconditioning
1. Overview
The heart is an organ with high energetic demand and mitochondria comprise about 35% of the volume of adult cardiomyocytes [1]. Mitochondria perform essential functions such as ATP production via oxidative phosphorylation, heme biosynthesis, calcium signaling, ionic homeostasis, regulation of cell proliferation and cell death. However, dysfunctional mitochondria can have severe consequences to the cell by releasing cytochrome c, triggering caspase activation and apoptosis. Deficient mitochondria can also release reactive oxygen species (ROS) and calcium leading to the activation of proteases and lipases, and ultimately necrotic cell death [2]. Given the severe consequences of mitochondrial dysfunction to the cell, organisms have evolved multiple mechanisms to prevent or repair damage to mitochondria, and when that is not possible, to eliminate and replace them (Table 1). Preventive mechanisms include survival kinase signaling to activate mild depolarization to limit calcium overload, and to close ATP/ADP channels to prevent ATP hydrolysis; and stabilization of anti-apoptotic Bcl-2 family members on the outer membrane. Repair mechanisms include fusion and fission events, and selective intramitochondrial protein repair via chaperone proteins and AAA proteases. Elimination proceeds via autophagic removal and replacement via mitochondrial biogenesis, processes which also depend upon membrane depolarization, fusion and fission, and protein import via mitochondrial translocases (TOM and TIM complexes). While many reviews have addressed the various repair mechanisms, the concept of mitochondrial elimination and replacement (turnover) as a cytoprotective and homeostatic mechanism important to cardioprotection is relatively novel, and will be the primary focus of this review.
Table.
Therapeutic targets for mitochondrial protection.
| Stimulus | Target | Effect | Pathway |
|---|---|---|---|
| CsA | Cyclophilin D |
MPTP inhibition | Inner Membrane Stabilization |
| Bcl-2, Bcl-XL | Bax, Bak | Bax neutralization | Outer Membrane Stabilization |
| Diazoxide | Mito KATP | Mild depolarization | Mitophagy |
| IPC | ?? | Mild depolarization | Mitophagy |
| CCPA | AdoA1 R | ? | Autophagy |
2. Ischemia-reperfusion injury
Oxygen deprivation during ischemia causes a halt to oxidative phosphorylation, decreasing intracellular ATP and creatine phosphate levels and compromising cardiac contractility [3]. The accumulation of lactic acid and decrease in pH during ischemia inhibits ATP generation from glycolysis. The Na+/H+ antiporter is activated in an attempt to restore the pH, which results in a concurrent increase in the intracellular Na+ concentration. By its turn, the increase in intracellular sodium concentration either slows or reverses the direction of the Na+/Ca2+ exchanger, which leads to an increase in the intracellular concentration of Ca2+. The mitochondria act as a buffer for intracellular calcium, and ultimately the rising cytosolic calcium levels causes calcium overload in the mitochondria [4]. This leads to an increase in ROS production from mitochondrial electron transfer complexes I and III and a consequent decrease in antioxidant defenses. ROS production rises steadily during ischemia and increases greatly at the onset of reperfusion as oxygen tension rises [5]. The generation of ROS during early reperfusion in association with mitochondrial Ca2+ overload leads to the opening of the mitochondrial permeability transition pore (MPTP) [4], which plays an important role in ischemia-reperfusion (IR) injury [6].
Cells acutely exposed to hypoxia have increased mitochondrial ROS generation and stabilization of Hypoxia-inducible factor 1 (HIF-1) [7]. HIF-1 induces expression of Bnip3 (Bcl-2 and adenovirus E1B 19 kDa-interacting protein 3), which functions as a redox sensor. Increased oxidative stress induces homodimerization and activation of Bnip3 [8, 9], resulting in mitochondrial matrix remodeling and large amplitude swelling of the inner membrane. The membrane rearrangements lead to disassembly of optic atrophy protein 1 (OPA1) complexes and release from the mitochondria, interfering with fission and fusion machinery [10].
Damaged mitochondria can release cytochrome c, triggering caspase activation and apoptosis. However, apoptosis is not the dominant mode of cell death in ischemia-reperfusion injury. Rather, it is necrosis, which is mediated in large part by the mitochondrial permeability transition (MPT). MPT is a common response to ischemia-reperfusion injury and is induced by stresses such as ROS and calcium overload. MPT makes the inner mitochondrial membrane permeable to solutes of up to 1,500 Da [11], resulting in depolarization of the membrane potential due to dissipation of the electrochemical gradient. Loss of the electrochemical gradient causes ATP synthase to operate in reverse, consuming ATP, preceded by a transient but massive release of ROS and calcium [12, 13] [14]. This ROS release signals neighboring mitochondria to do the same (ROS-induced ROS release), culminating in activation of calcium-dependent proteases (calpains) and lipases (cPLA2), as well as ROS-activated iPLA2, and ultimately necrotic cell death [2]. Damaged but still functional mitochondria can release up to ten-fold more H2O2 , representing 10-20% of the oxygen consumed [15].
A third mode of cell death has been described, termed autophagic cell death; however, it is not clear that the autophagosomes frequently observed in ischemic heart have been responsible for cell death. Indeed, Vatner’s group, in a study of chronic ischemia in pigs, noted that the cells showing upregulation of autophagy-related proteins were not the ones that were TUNEL-positive [16]. In this model, the peak of autophagic activity was concomitant with the decline in apoptosis, suggesting autophagy may be involved in the protection against apoptosis [16].
3. Mitochondrial-targeted cardioprotective mechanisms
3.1 Mitochondrial turnover
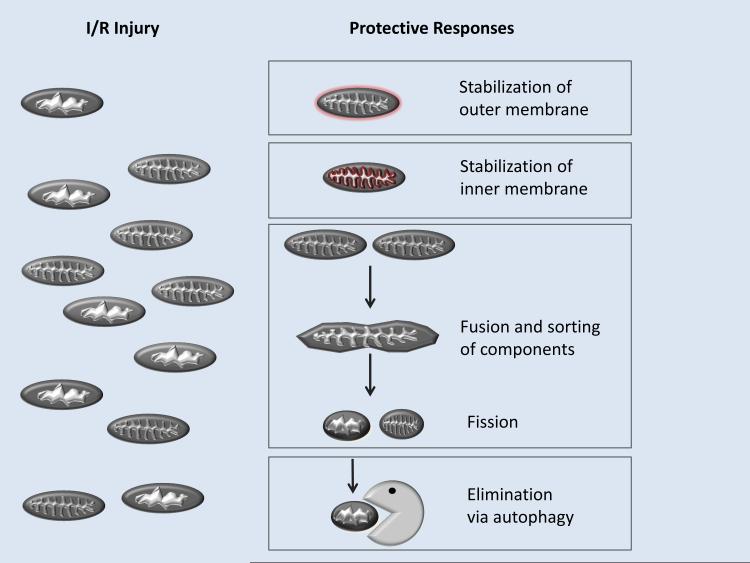
Cardiac mitochondrial turnover comprises mitochondrial biogenesis (growth and division of pre-existing mitochondria, including fission and fusion) in association with mitochondrial removal (activation of degrading pathways, namely, macroautophagy, microautophagy and chaperone-mediated autophagy [17]). Fusion and fission are important elements of mitochondrial quality control and turnover, as it allows for the redistribution of mtDNA and proteins (Figure 1).
Figure. Strategies for mitoprotection.
Reperfusion injury is largely due to mitochondrial dysfunction, which may be controlled by inhibiting the MPTP with cyclosporine A or by selective elimination of damaged mitochondria via autophagy.
3.1.1 Fission and fusion
Mitochondria constantly undergo cycles of fission and fusion. During fission, dynamin-related protein 1 (DRP1) translocates from the cytoplasm to the mitochondria, where it docks with fission protein 1 (FIS1) in the outer mitochondrial membrane. DRP1 forms a large homo-multimeric ring around the mitochondrion, which narrows until fission occurs [18, 19]. Fusion requires coordinated tethering of both the outer and inner mitochondrial membranes. Outer mitochondrial membrane fusion is directed by mitofusin 1 (MFN1) and mitofusin 2 (MFN2), large GTPases located in the outer membrane. Inner mitochondrial membrane fusion is directed by OPA1 (for review see [20]).
In pancreatic beta cells fission generates asymmetric daughter mitochondria: one subpopulation has increased membrane potential and high probability of fusion, while the other has decreased potential, decreased levels of the fusion protein OPA1 and reduced probability of fusion. The dysfunctional mitochondria with low membrane potential are segregated and selectively removed by autophagy, while the ones with high potential continue going through the fusion-fission cycles. Overexpressing OPA1, or inhibiting fission with dominant negative DRP1 or knocking down FIS1 decreases mitophagy, resulting in the accumulation of defective mitochondria [21].
Ong et al. [22] showed that stimulating fusion in cardiac cells by expressing MFN1, MFN2, dominant-negative Drp1 or treatment with mitochondrial division inhibitor-1, a pharmacological inhibitor of Drp1, raises the MPTP opening threshold and protects against ischemia-reperfusion injury. The cardioprotective effects were also observed after in vivo treatment with mitochondrial division inhibitor-1. However, Chen et al. [23] observed that although ischemia decreased OPA1 levels in H9c2 cells and that overexpression of OPA1 increased mitochondria tubularity, it did not protect against ischemia-induced apoptosis.
Hoppel’s group [24] reported that mitochondrial dysfunction in heart failure is associated with loss of respirasomes (assembly of electron transfer complexes into functional supercomplexes), although the total amount of mitochondrial electron transfer complexes remained constant. One mechanism by which removal and targeted replacement of components could occur would be through dynamic assembly and disassembly of respirasomes. We hypothesize that this dynamic process would result in exclusion of damaged and dysfunctional components. This sorting process could result in spatial segregation of supercomplexes from damaged isolated subunits or complexes and may underlie asymmetric fission. Regions enriched in supercomplexes might have more rigid membrane architecture and conceivably might be less likely to undergo fission, while individual electron transfer complexes not organized into supercomplexes, as well as any damaged/misfolded components, would be excluded and would end up in regions of increased membrane fluidity. Regions with damaged components or not organized into supercomplexes are more likely to have lower mitochondrial membrane potential, and therefore when fission ensues they would be targeted for autophagy [21, 24].
3.1.2 Autophagy
Autophagy is induced in response to nutrient deprivation, cellular stress, ROS, and accumulation of protein aggregates or damaged organelles. Autophagy is essential during nutrient limitation, degrading organelles and proteins to provide the cells with energy. The key sensor of the cell’s energetic status is the mammalian target of rapamycin (mTOR). mTOR senses growth factor signals, energy status, oxygen availability and amino acid concentrations [25]. For example, when nutrient availability is limited mTOR is inhibited and autophagy is activated. On the other hand, when mTOR is activated, autophagy is inhibited and protein synthesis is activated [25]. mTOR signaling can be override by other regulators of autophagy, such as AMP-activated protein kinase (AMPK) [26]. AMPK negatively regulates mTOR, thereby inducing autophagy [27]. The first step of autophagy is triggered by Beclin1 and vacuolar protein sorting 34 (Vps34), a class I PI3 kinase. Atg12 is activated by Atg7, an E1-like enzyme, which then transfers Atg12 to Atg10, an E2-like enzyme, which then conjugates Atg12 to Lys 130 of Atg5. A complex formed by the Atg5/Atg12 conjugate and a homodimer of Atg16 assembles on the forming membrane structure – the phagophore. Following the phagophore formation, Atg8 is cleaved by Atg4 (the cleaved Atg8 is also known as LC3-I), and then transferred to Atg3 by Atg7. Atg3, an E2-like enzyme, conjugates LC3-I onto phosphatidylethanolamine forming LC3-II. Intracellular targets may be labeled with ubiquitin and p62, which has binding motifs for ubiquitin and LC3, thereby recruiting the phagophore to elongate around the decorated aggregate or organelle. After elongation, the phagophore engulfs its content (e.g., protein aggregates or organelles) and closes, originating the autophagosome, a double membrane structure. The autophagosome then fuses with a lysosome and the contents are degraded via the action of multiple acidic hydrolases (proteases, lipases, amylases, and nucleases). The end-products (e.g. amino acids, fatty acids) are exported to the cytosol via efflux permeases; efflux of these nutrients will reactivate mTOR, permitting protein synthesis to resume [28, 29]. (For a review on autophagy basics see [30]).
Autophagy is critical for cardiomyocyte health and survival, and deficient autophagy has been associated with cardiac disorders. Mitochondrial DNA has a 10- to 20-fold higher mutation rate compared to nuclear DNA [31]. It is more prone to oxidative damage due to the abundance of ROS produced nearby as well as the absence of histones and relatively inefficient mitochondrial DNA repair enzymes [31, 32]. A defective version of mitochondrial DNA polymerase gamma (involved in synthesis and repair) introduces a high frequency of mutations in the mitochondrial genome. A mouse model expressing the mutant polymerase gives rise to a phenotype of accelerated aging [33]. Nekhaeva and collaborators [34] showed that in humans certain mutations in the mitochondrial DNA increase mitochondrial fission, resulting in the replacement of normal mitochondrial genomes with mutated copies. De Grey [35, 36] has suggested that the mutated mitochondria would be less susceptible to oxidative damage because of lower respiratory rates, and potentially less vulnerable to autophagy. As a result, these mutations amplify and accumulate with age. With increasing age, autophagy decreases and lysosomal activity becomes inefficient due to the accumulation of lipofuscin, a brown granular pigment that consists of cross-linked lipids and proteins produced during lysosomal digestion [17, 37]. In the aging heart, the intralysosomal accumulation of lipofuscin is partially responsible for inhibiting autophagy [38]. Senescent myocytes are characterized by the existence of giant mitochondria originating from oxidative damage followed by inefficient autophagy [39-42]. Terman and Brunk suggested that autophagic engulfment of large mitochondria requires more energy, and consequently, is less efficient [38]. Thus, the accumulation of defective mitochondria and lysosomes in aged myocytes is a reflection of inefficient autophagy [43]. The impairment or suppression of autophagy plays a critical role in the development of aging-related disorders in the heart.
Inhibition of autophagy in the mouse heart during pressure overload-induced heart failure causes mitochondrial aggregation, increased apoptosis and advances the progression of cardiac disease [44]. In adult mice, cardiac-specific, temporarily controlled deficiency of Atg5 leads to increased levels of ubiquitination, cardiac hypertrophy, left ventricular dilatation, and contractile dysfunction [44]. Silencing Atg7 in rat neonatal cardiomyocytes causes loss of cell viability, and morphological and biochemical features of cardiomyocyte hypertrophy [44]. Lysosome-associated membrane protein 2 (LAMP2, an important constituent of the lysosomal membrane)-deficient mice have excessive accumulation of autophagic vacuoles and impaired autophagic degradation of long-lived proteins, resulting in cardiomyopathy [45]. Collectively, these results indicate that in the heart, autophagy is required for protein quality control and normal cellular structure and function in physiological conditions.
Autophagy has been shown to be upregulated in pathological conditions, such as ischemia and reperfusion [46-49]. Decker et al. [48, 49] induced hypoxia-reoxygenation using a Langendorff perfused heart model and observed that 20 min of hypoxic perfusion was not sufficient to induce autophagy in rabbit hearts, while 40 min increased autophagy and the appearance of degenerating mitochondria. In both cases, the number of autophagosomes increased at reoxygenation and cardiomyocyte damage could be repaired. When the duration of hypoxia was extended to 60 min, large lysosomes were apparent at reoxygenation and the damage to cardiomyocytes was irreversible. The authors concluded that autophagy was important to repair hypoxia-reoxygenation induced injury. Autophagy has also been shown to be upregulated in ischemia-reperfusion in a swine model of stunning and in cardiac cells [16, 50]. Adult mice with cardiac-specific knockdown of Atg5 are autophagy-deficient and have contractile dysfunction, ventricular remodeling, and heart failure [44].
The most obvious trigger of autophagy during ischemia is nutrient deprivation and depletion of high energy phosphate reserves. ATP depletion leads to an increase in the AMP/ATP ratio, which, in combination with serine/threonine kinase 11 (STK11) [51], results in phosphorylation/activation of AMPK, inhibition of mTOR, and therefore activation of autophagy [47, 51, 52]. This is supported by studies showing that cardiac expression of dominant negative AMPK inhibits autophagy in mouse heart subjected to ischemia and increases myocardial infarct size [52]. While in ischemia autophagy seems to be AMPK-dependent, reperfusion studies have shown that there is upregulation of Beclin 1, but not activation of AMPK, and that induction of autophagy during reperfusion is significantly attenuated in Beclin 1(+/−) mice [47].
Autophagy is also triggered by Bnip3, a BH3 only apoptotic protein. Increased glycolysis and lactic acid production during ischemia lead to hypoxia and acidosis, which have been shown to activate Bnip3 [53, 54]. Bnip3 overexpression in cardiomyocytes activates autophagy, while expression of dominant negative Bnip3 attenuates autophagy [9]. Activation of autophagy may be a compensatory response to mitochondrial damage caused by Bnip3, as autophagy/mitophagy is triggered by a variety of interventions that cause mitochondrial depolarization [55].
Ischemia-reperfusion is also associated with increases in intracellular calcium concentration [56, 57], ROS production [12, 13], mitochondrial depolarization [58] and opening of the MPTP [4]. All these factors have been shown to induce autophagy [56, 57, 59-62]. Increases in free cytosolic calcium concentration induce autophagy; on the other hand, 2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), a calcium chelator, and thapsigargin, an inhibitor of the sarco / endoplasmic reticulum calcium ATPase inhibit nutrient deprivation-induced autophagy [56, 57]. The calcium effect is believed to be mediated by Bcl-2 [56, 57]. ROS directly activate autophagy via redox regulation of Atg4 [63]. Starvation, an inducer of autophagy, stimulates ROS formation from complex I, and antioxidants inhibit starvation-induced autophagy [61, 62]. Lemasters’ group has shown that induction of autophagy in rat hepatocytes by serum deprivation and glucagon causes an increase in the number of spontaneously depolarizing mitochondria, and these mitochondria are sequestered by autophagosomes [60, 64]. In the heart this depolarization is associated with the opening of MPTP and blocking the pore inhibits mitochondrial removal by autophagy [59].
The role of autophagy in cardiac disease is still controversial. An increase in the number of autophagosomes has been observed in cardiac tissues of patients with cardiovascular disorders such as left ventricular hypertrophy, aortic valve stenosis, hibernating myocardium, and heart failure [65-68]. It is unknown whether increased autophagy contributed to the disorder or if it was upregulated in an effort to limit it. Matsui et al. [47] suggested that autophagy increases ischemia-reperfusion injury because infarct size and apoptosis were diminished in Beclin1 (+/−) mice. However, Beclin1 not only induces autophagosome formation but also can regulate lysosomal fusion. Interaction between Beclin1 and a protein called Rubicon slows down autophagosome-lysosome fusion [69]. Therefore, the Beclin1 (+/−) mice may be more resistant to ischemia-reperfusion injury because they clear autophagosomes more efficiently through enhanced lysosomal fusion, i.e., they have increased autophagic flux. This scenario is consistent with the observation of fewer autophagosomes after ischemia-reperfusion. In chronically ischemic pig myocardium autophagy seems to be cardioprotective [16]. In this model, the peak of autophagic activity is concomitant with the decline in apoptosis, which suggests autophagy may be involved in the protection against apoptosis [16]. Autophagy is upregulated during pharmacological and ischemic preconditioning (IPC). Yan et al. [70] have shown that autophagy is increased in preconditioned hibernating myocardium. Using a cell culture model, we showed that activation of autophagy is protective against simulated ischemia-reperfusion in HL-1 cells [50]. We have also shown in neonatal rat cardiomyocytes and in vivo in αMHC-mCherry-LC3 transgenic mice that pharmacological preconditioning with the A1 adenosine receptor agonist 2-chloro- N(6)-cyclopentyladenosine (CCPA) causes an increase in the number of autophagosomes within 10 min of treatment [71]. The cardioprotective effect can be blocked by inhibiting autophagy with Tat-Atg5(K130R) [71]. Moreover, we have shown that ischemic preconditioning requires autophagy in the isolated perfused rat heart [72].
Our group and others [59, 60, 64] have shown that the depolarization associated with the opening of the mitochondrial permeability transition pore signals mitochondria to be removed by autophagy. We have shown that cardiomyocytes from mice deficient in cyclophilin D (a component of the mitochondrial permeability transition pore) do not upregulate autophagy in response to starvation [59]. Similar results were obtained in cardiomyocytes from wildtype mice treated with cyclosporin A (an inhibitor of the pore) and subjected to starvation. This led to the conclusion that cyclophilin D and the pore are necessary for mitochondrial removal by autophagy [59]. The exact mechanism by which the pore and mitochondrial depolarization signal the mitochondria to be removed by autophagy is unknown, but it might involve OPA1 or Parkin. OPA1 regulates mitochondria and cristae morphology and its function is affected by mitochondrial potential [73]. Parkin accumulates in depolarized mitochondria and promotes their removal by mitophagy [55, 74].
3.1.3 Mitophagy
The elimination of excess or damaged mitochondria by autophagy will preserve the integrity of the remaining population and maintain cellular homeostasis. Therefore, therapeutic approaches to increase autophagy and mitophagy may prove to be cardioprotective. If the energetic demand is low, excess mitochondria are unnecessary specially because they can generate excessive ROS and even consume ATP if they become uncoupled [14]. Furthermore, damaged or unstable mitochondria may release cytochrome c, apoptosis inducing factor (AIF), second mitochondria-derived activator of caspases/ direct IAP binding protein of low pI, (Smac/DIABLO), and other apoptosis-promoting factors which would promote damage to neighboring mitochondria and the entire cell [4]. Mitophagy is triggered by the same factors described above for autophagy, the most important triggers being Bnip3 and Nix (Bnip3-like protein X); PINK1, Parkin and p62/SQSTM1; mitochondrial depolarization and MPTP. The latter is described in more detail in the next section.
There is strong evidence that PINK1, Parkin and p62/SQSTM1 play a key role in mitophagy [55, 74, 75]. Parkin accumulates in depolarized mitochondria and promotes their removal by mitophagy [55]. The translocation of Parkin to the mitochondria is promoted by PINK1, a mitochondrial serine/threonine protein-kinase [75, 76]. The levels of PINK1 increase when mitochondrial membrane potential diminishes. PINK1 then triggers translocation of Parkin to the mitochondrial outer membrane [75]. Knockdown of PINK1 abolishes Parkin recruitment to mitochondria and mitophagy in response to CCCP treatment [77], whereas overexpression of PINK1 promotes translocation of Parkin to mitochondria with normal potential [76]. Parkin is an E3 ubiquitin ligase that modifies various mitochondrial outer membrane proteins; ubiquitination results in recruitment of p62/SQSTM1 to the mitochondrion [78]. P62 is an adapter protein that interacts with LC3 and thereby recruits a phagophore to engulf the ubiquitin-tagged mitochondrion. P62/SQSTM1 is not necessary for parkin translocation but is critical for clearance of damaged proteins; knockdown of p62/SQSTM1 completely blocks mitochondrial clearance [77, 79].
Bnip3 and Nix are proteins with homology to Bcl-2 in the BH3 domain, which induce mitophagy by triggering mitochondrial depolarization. The mitochondrial depolarization has been shown to be both sensitive and insensitive to MPTP inhibitors [10], and therefore there is a wide debate whether the induction of autophagy by Nix and Bnip3 is through the pore [7-9, 80, 81]NIX is required for mitophagy in erythroid cells. It functions as a selective autophagy receptor by binding to LC3/GABARAP proteins, which are ubiquitin-like modifiers necessary for the growth of autophagosomal membranes. Furthermore, ablation of the Nix:LC3/GABARAP interaction retards mitochondrial clearance in maturing murine reticulocytes. [82-84]. HIF-1-deficient cells when subjected to hypoxia fail to induce BNIP3 and mitophagy, and suffer ROS-induced cell death [80, 81]. Hypoxia-induced mitophagy requires HIF-1-dependent induction of BNIP3 and the constitutive expression of Beclin1 and Atg5 [80, 81] and it serves to remove mitochondria that would otherwise be producing excessive ROS.
In hypoxia, mitophagy is an adaptive response that prevents increased ROS production and cell death. It has been shown that the generation of ROS by mitochondria in cells acutely exposed to hypoxia is increased [7]. Therefore mitophagy serves to remove mitochondria that would otherwise be producing excessive ROS. There is a very delicate balance between mitophagy that is cardioprotective and mitophagy that can be deleterious to the cell. Damaged mitochondria can release apoptotic factors from the intermembrane space, including cytochrome c, apoptosis inducing factor, and Smac/Diablo [4]. It is possible that the proteins released may signal a particular mitochondrion to be removed by mitophagy. This connection between autophagy and apoptosis may explain why many known inducers of apoptosis have been shown to also activate autophagy, such as etoposide in mouse embryonic fibroblasts [85], ceramide in breast and colon carcinoma [86], activation of the TRAIL receptor-2 in cancer cells [87], tumor necrosis factor alpha [88-91], serum/growth factor deprivation [92, 93], staurosporin [94], lipopolysaccharide [88, 89], and Bnip3 [8, 9, 80, 81, 95].
3.1.4 Mitochondrial biogenesis
In an ideal situation of mitochondrial turnover, mitochondrial biogenesis should compensate for the removal of damaged mitochondria by providing the cell with sufficient functional mitochondrial mass, and it should be responsive to the heart’s energetic demand [8, 9]. Mitochondrial biogenesis is controlled by the PPARγ coactivator (PGC) family of transcriptional coactivators, most importantly PGC-1α, PGC-1β, and the PGC-related coactivator PRC. PGC-1α regulates nuclear respiratory factor 1 and 2 (NRF1, NRF2), which in turn control regulatory factors required for mitochondrial DNA transcription and translation, most importantly mitochondrial transcription factor A (Tfam) [96]. Although overexpression of PGC-1α is sufficient to induce mitochondrial biogenesis [97], acetylation of PGC-1α suppresses its transcriptional coactivator activity, limiting mitochondrial biogenesis. Deacetylation of PGC-1α by the histone deacetylase sirtuin 1 (Sirt1) restores its activity and stimulates mitochondrial biogenesis [98]. Sirtuins also regulate autophagy through forkhead box protein O1 and O3 (FoxO1 and FoxO3); and AMPK, one of the regulators of autophagy, also triggers Sirt1-dependent deacetylation of PGC-1α [98, 99]. Thus, autophagy and mitochondrial biogenesis are coordinately regulated. Overexpression of PGC-1α in the myocardium causes an increase in mitochondrial number and cardiomyopathy progressing to failure [100], demonstrating the need to maintain an adequate balance in mitochondrial abundance. Recently, the linkage between mitophagy and biogenesis was drawn even tighter by the identification of PARIS (a member of the family of KRAB zinc-finger protein transcriptional repressors) as a substrate of Parkin [101]. Parkin ubiquitinates PARIS, signaling its degradation. PARIS represses the expression of PGC-1α and the PGC-1α target gene, NRF-1, decreasing mitochondrial biogenesis [102]. Through this mechanism, mitochondrial biogenesis is coordinated with mitophagy.
Mitophagy and biogenesis are not always balanced. Mitochondrial biogenesis is increased in type I diabetes, with increased levels of mitochondrial proteins, mitochondrial area and number, mitochondrial protein content, and mitochondrial DNA [10]. Despite the higher number of mitochondria, their function is impaired. Similar results are observed in a murine model of metabolic syndrome [11]. It is possible that increased biogenesis is a compensatory mechanism for defective mitochondrial function. However, biogenesis in the absence of balanced mitophagy to remove defective mitochondria may be maladaptive. Further studies are warranted to clarify this.
3.2 Ischemic preconditioning
In 1986, Murry et al. [103] described a procedure by which brief sub-lethal ischemic episodes, each separated by periods of reperfusion, protect the heart against subsequent lethal ischemia. This phenomenon was termed IPC. IPC has two windows of protection. The first is called early IPC or first window of protection, and it protects the heart for an hour or two and then fades away; the second phase, termed delayed IPC or second window of protection, appears 24 h after the IPC protocol and can last for 3 days. In this section we will focus on early IPC. Murry and colleagues [103], while studying myocardial infarction caused by a prolonged period of coronary artery occlusion (index ischemia) in dogs, observed that when the index ischemia was preceded by 4 cycles of 5 min of ischemia alternating with 5 min of reperfusion, infarct size was greatly reduced to only 25% of that in the control group. Hausenloy and Yellon [104-106] demonstrated that IPC exerts its protection in the first minutes of reperfusion. The authors found that blocking PI3 kinase or ERK in the first minutes of reperfusion prevented IPC’s protection in rat hearts. Similar results were reported by Solenkova et al. in isolated rabbit hearts [107], who observed that adenosine receptors A1 and/or A2B initiate the protective signal transduction cascade at the onset of reperfusion. PI3K activity is necessary long into the reperfusion phase, but adenosine receptor occupancy is no longer essential after 30 min of reperfusion, and ERK activity is only required in the first few minutes of reperfusion. The exact mechanism through which IPC leads to cardioprotection is still under intense investigation. It is a very complex mechanism with a plethora of triggers and mediators. We will only cover the ones more related to mitochondria. We propose that mitochondrial cardioprotection involves the stabilization of mitochondria, through inhibition of the mitochondrial permeability transition pore or preservation of outer mitochondrial membrane integrity through activity of anti-apoptotic Bcl-2 family members; these have been covered in countless reviews. A second pathway involves elimination of damaged or unwanted mitochondria before they can cause irreversible injury. We propose that many cardioprotective interventions that trigger mitochondrial ROS production or mild depolarization serve to trigger mitophagy, and that the end-effector of protective signalling is elimination of unstable mitochondria (Figure 1).
3.3 Mitochondrial membrane potential
Although mitochondrial potential is a prerequisite for oxidative phosphorylation, an elevated mitochondrial transmembrane potential is associated with enhanced formation of mitochondrial ROS [108, 109]. On the other hand, small decreases in potential (mild uncoupling) have been shown to prevent ROS formation without seriously compromising cellular energetics [109-112]. Mild uncoupling reduces ROS formation by limiting the life span of reduced electron transport chain (ETC) intermediates capable of generating ROS, in addition to a decrease in local oxygen tensions [108, 109]. However, in isolated energized cardiac mitochondria it has been shown that hypoxia causes ETC inhibition and a mild decrease in membrane potential, which lead to ROS formation during reoxygenation [113]. The authors did not verify whether this decrease in mitochondrial potential was sufficient to impair the cell energetic status. Korge et al. [113] proposed that the increase in ROS formation can be prevented by NO•. The authors described that NO• can decrease ROS-induced damage to electron transport complexes, possibly by forming NO•-Fe2+ complexes in the presence of Fe2+ and matrix glutathione (GSH), which inhibits hydroxyl radical formation by Fenton reaction [113, 114]. An important endogenous mild uncoupling pathway that prevents ROS release is fatty acid (FA) cycling across the inner mitochondrial membrane [115, 116]. In the proton-rich intermembrane space, FA anions are protonated, become uncharged and flipflop across the inner membrane lipid bilayer. Once in the mitochondrial matrix, the proton is released and the FA anion transported back to the intermembrane space by anion carriers, which include mitochondrial uncoupling proteins [117, 118], and the adenine nucleotide translocator [116, 119, 120]. Uncoupling proteins (UCP) expression promotes tissue protection against ischemia-reperfusion [121, 122] and post-ischemic tissue survival in both heart and brain correlates closely with uncoupling proteins expression [122, 123]. UCP2 and UCP3 have been shown to mediate delayed ischemic protection in heart-derived cells by decreasing oxidative stress [122]. The non-deleterious formation of ROS during early IPC activates the adenine nucleotide translocator, increasing fatty acid transport and causing mild uncoupling, which can diminish ROS formation during ischemia-reperfusion [124]. Nadtochiy et al. [125] suggested that early IPC induces mitochondrial uncoupling by increasing uncoupling proteins activity, and that the greater uncoupling that occurs in ischemia-reperfusion is mediated by the adenine nucleotide translocator. However, in this study all experiments were conducted in the presence of BSA, which quenches FA necessary for uncoupling proteins and adenine nucleotide translocator activity. Given that both uncoupling proteins and the adenine nucleotide translocator are activated by ROS, and its activation decreases ROS, this illustrates an elegant negative feedback mechanism triggered by IPC that controls redox balance in mitochondria. In this setting as well, expression of UCPs may lead to mild depolarization and acceleration of autophagic removal of mitochondria with the lowest membrane potential.
3.4 The mitochondrial permeability transition pore
Interestingly, recent evidence sugests a physiological role for the MPTP in: i) calcium homeostasis [11, 126]; ii) cardioprotection against IR injury provided by ischemic and pharmacological preconditioning and postconditioning [127, 128]; and iii) mitochondrial removal by autophagy [21, 60, 64]. Mitochondria are known to be degraded by autophagy; however, the basis on which individual mitochondria are selected for autophagy and the signaling mechanisms are unknown. Lemasters’ group [60, 64] demonstrated that induction of autophagy in rat hepatocytes by serum deprivation and glucagon causes an increase of spontaneously depolarizing mitochondria and autophagosome number. The depolarized mitochondria are sequestered by autophagosomes. The authors concluded that MPT is the cause of mitophagy given that cyclosporin A, an inhibitor of mitochondrial permeability transition, prevents mitochondrial depolarization and blocks autophagosomal proliferation [60, 64]. In nicotinamide-treated human fibroblasts, nicotinamide activates autophagy leading to the selective removal of mitochondria with low membrane potential. In this model mitophagy is also attenuated by treatment with cyclosporin A [129]. Twig and colleagues [21] showed that in pancreatic beta cells, fission generates asymmetric daughter mitochondria: one subpopulation has increased membrane potential and high probability of fusion, while the other has decreased potential, decreased levels of the fusion protein OPA1, and reduced probability of fusion [21]. Dysfunctional mitochondria are excluded from subsequent rounds of fission and fusion, and eventually selectively removed by autophagy. The authors argued against the MPTP as the cause of the depolarization because the depolarization was still observed in the presence of 1 μM cyclosporin A. In cardiac cells, starvation-induced autophagy has been shown to cause mitochondrial depolarization which is prevented by cyclosporin A [130], indicating the MPT pore underlies starvation-induced mitochondrial depolarization. We have also shown that cyclophilin D, a component of the MPT pore, is required for starvation-induced autophagic removal of cardiac mitochondria [130]. Cardiomyocytes from cyclophilin D deficient mice do not upregulate autophagy when subjected to starvation, in contrast to cardiomyocytes from wild type mice [130]. These results implicate cyclophilin D and the MPT in the initiation of autophagy and removal of mitochondria in starvation-induced autophagy. In a hypothesis advanced by us and others [59, 60, 131, 132], we propose that the balance between apotosis and autophagy dependends on the severity of the injury to the heart and the extent of the MPTP opening. The MPT pore has two conductance states: low and high. The low-conductance state allows the diffusion of small ions like Ca2+, and has a physiological role in the maintenance of cellular calcium homeostasis, while the high-conductance state allows the nonselective diffusion of big molecules (up to 1500 Da) and is associated with cell death [126]. With mild injury, limited MPT onset may only increase mitophagy to rid cells of damaged mitochondria as a repair mechanism. With increasing stress, mitophagy may not be able to remove the majority of the dysfunctional mitochondria which release apoptotic factors, and apoptosis occurs. Finally, severe injury which triggers wholesale MPT onset will result in rapid ATP depletion. Because of bioenergetic failure, neither autophagy nor apoptosis can progress, and only necrotic cell death ensues.
The MPTP is involved in IPC both as a target and a mediator. IPC is cardioprotective by inhibiting the opening of the MPTP [133]. Pore inhibition in adult rat cardiomyocytes subjected to ischemia-reperfusion improves cell survival [134], blocking the pore at reperfusion preserves ATP levels, improves post-ischemic contractile recovery and decreases infarct size [135]. Interestingly, it has been shown that cyclophilin D, a component of the pore, is critical for IPC-induced cardioprotection. Mice deficient in cyclophilin D are resistant to cardioprotection conferred by ischemic and pharmacological preconditioning and postconditioning [128, 136]. Hausenloy et al. [136] proposed that cyclophilin D is required by IPC to generate mitochondrial ROS and phosphorylate Akt and Erk1/2, major steps in the IPC signaling pathway [136]. However, cyclophilin D is also necessary for mitochondrial removal by autophagy [59], and the inability to induce cardioprotection by IPC in mice deficient in cyclophilin D may be due to the lack of effective mitophagy. Studies in perfused rat heart show that the pore remains closed during myocardial ischemia and only opens in the first few minutes of myocardial reperfusion, thereby defining a critical time-window for cardioprotection [137].
IPC leads to phosphorylation and inactivation of glycogen synthase kinase-3beta (GSK-3beta), a downstream target of PI3-kinase and Akt [138]. GSK-3beta inhibition is an efficient strategy for limiting myocardial infarct size at the time of reperfusion in both pharmacological cardioprotection [139] and IPC [140]. Cardioprotection cannot be induced in cardiomyocytes containing a constitutively activated form of GSK-3beta, which is resistant to phosphorylation and inhibition, confirming the importance of this protein kinase [141]. Inhibition of GSK-3beta inhibits MPT pore opening, although the mechanism by which it occurs is not fully known. Das et al. [142] suggested that GSK-3beta inhibition leads to dephosphorylation of the voltage dependent anion channel (VDAC), which prevents the entry of adenine nucleotides into the mitochondria, causing mitochondrial depolarization. Lower mitochondrial potential leads to diminished mitochondrial calcium accumulation and generation of ROS during myocardial ischemia, thereby preventing MPTP opening at the time of myocardial reperfusion. However, it is hard to comprehend how this mechanism is cardioprotective given the need for adenine nucleotides to enter the mitochondria in order to enable mitochondrial re-energization and recovery of the cardiomyocyte. Furthermore, the existence of GSK-3beta in cardiac mitochondria isolated from preconditioned hearts has been challenged by Halestrap’s group who reported that using extracts following stringent mitochondrial purification failed to identify phosphorylated protein kinases [143]. Moreover, a GSK-3beta inhibitor has been shown to have no effect on MPTP opening in isolated mitochondria [144], indicating that the GSK-3beta isoform that is involved with cardioprotection must reside outside the mitochondria. The mitochondrial ATP-sensitive potassium channel (mitoKATP) blocker 5-hydroxydecanoate has been shown to block cardioprotection mediated by GSK-3beta inhibition, suggesting that a GSK-3beta-mitoKATP interaction is necessary for cardioprotection [145].
3.5 ROS derived from mitochondria
One mitochondrial function clearly involved in IPC is the generation of ROS at the level of the ETC. IPC is dependent on a moderate increase in ROS generation possibly due to mild inhibition of respiratory complexes [146, 147]. Increased ROS release during IPC activates a signaling pathway that protects against oxidative stress during ischemia-reperfusion [148-150]. The removal of ROS during IPC completely abrogates its beneficial effects [147, 150, 151], while transient exposure to an oxygen radical generating system mimics IPC [149, 150]. ROS-induced cardioprotection can be blocked by protein kinase C (PKC) inhibitors, indicating that the ROS signal occurs upstream of PKC, possibly through oxidation of PKC sulfhydryl groups [152, 153]. This is confirmed by the fact that cardioprotection induced by direct activators of PKC can not be blocked by ROS scavengers [154]. Liu et al. [155] described that activation of the Gi-coupled adenosine A1 receptors triggers IPC protection given that adenosine or the A1-selective agonist R(-)-N6-(2-phenylisopropyl) adenosine could mimic IPC, while an adenosine receptor antagonist blocked IPC-induced cardioprotection. The authors propose that during IPC’s ischemic episodes, adenosine is released activating A1 adenosine receptors and leading to preconditioning. Bradykinin [156] and opioids [156] are also released during IPC, and blockade of either adenosine, bradykinin or opioids receptors will prevent cardioprotection induced by 1 cycle of IPC, but not multiple cycles. Therefore it was proposed that the effects of the three receptors are additive [157]. With a single cycle of IPC, blockade of either receptor will diminish the total stimulus below threshold and prevent protection. A more intense preconditioning ischemic stimulus will overcome the blockade of one of the receptors, possibly by enhancing the release of ligands for the other two receptors that were not inhibited, reaching the threshold and being cardioprotective. The three triggers have a common target - PKC, and their cardioprotection can be prevented by PKC inhibitors. Adenosine receptors are believed to activate PKC through phospholipases that generate the second messenger diacylglycerol from membrane phospholipids [158, 159]. Opioid receptors are proposed to depend on metalloproteinase-mediated transactivation of the epidermal growth factor receptor (EGFR) which activates PI3 kinase. The EGRF receptor tyrosine kinase auto-phosphorylates its tyrosine residues when bound to its triggering growth factor [160]. Bradykinin also activates PI3 kinase but the activation seems to be independent of EGFR. PI3 kinase induces phosphorylation of Akt, via phospholipid-dependent kinases. Phosphorylated Akt directly phosphorylates endothelial nitric oxide synthase (eNOS) activating the enzyme and leading to NO production [161], which then stimulates guanylyl cyclase to produce cGMP which in turn stimulates PKG [162]. Active PKG activates the mitoKATP.
3.6 Mitochondrial ATP-sensitive potassium channel
Application of exogenous PKG and cGMP to isolated mitochondria opens the mitoKATP, effect that can be blocked by mitoKATP inhibitors 5-hydroxydecanoate, glibenclamide, and tetraphenylphosphonium [163]. It is not clear how PKG in the cytosol can target the mitoKATP in the inner mitochondrial membrane. MitoKATP mediates both pharmacological and ischemic preconditioning. MitoKATP agonists lead to cardioprotection in the absence of IPC; conversely, mitoKATP channel antagonists prevent the beneficial effects of IPC [148, 164-167]. Increases in mitochondrial ROS during preconditioning activate the mitoKATP [151, 168]. ROS scavengers like catalase reverse the beneficial effects of ischemic preconditioning but not of mitoKATP agonist diazoxide. On the other hand, 2-mercaptopropionylglycine (MPG) prevents cardioprotection induced by both IPC and diazoxide, suggesting that it may have effects other than scavenging ROS [168]. Indeed, MPG and another thiol-reducing agent, dithiothreitol, impair diazoxide-mediated activation of mitoKATP in isolated heart mitochondria. This suggests that mitoKATP activity is regulated by thiol redox status [168].
MitoKATP activation is cardioprotective by: i) decreasing ROS formation during reperfusion; ii) preventing mitochondrial calcium overload during ischemia; iii) preserving ATP levels and improving mitochondrial energy production after ischemia; iv) inhibiting apoptosis [168-174]. We would suggest that an important consequence of mitoKATP channel opening is mild uncoupling between mitochondrial respiration and oxidative phosphorylation, which may be sufficient to trigger autophagic removal of mitochondria having the lowest membrane potential. Indeed, we have shown that diazoxide triggers autophagy [175]. Mild depolarization also decreases secondary ROS during ischemia-reperfusion [151, 174]. As a result, the mitoKATP channel regulates mitochondrial redox state under physiological conditions and prevents oxidative stress under pathological conditions such as ischemia-reperfusion. MitoKATP activation also prevents mitochondrial Ca2+ overload when cytosolic Ca2+ homeostasis is perturbed [176, 177]. This is consistent with the finding that mitoKATP activity prevents post-ischemic MPT pore opening [178]. MitoKATP activation during ischemia prevents MPT by inhibiting Ca2+-stimulated mitochondrial oxidative stress [171]. MitoKATP activity protects against ATP loss during ischemia by limiting ATP transport and hydrolysis [169]. In the absence of oxygen and substrates, mitochondria can not form mitochondrial potential through electron transport. Lack of mitochondrial potential causes the mitochondrial ATP synthase to function in its reverse mode, hydrolyzing ATP to ADP and Pi, while pumping protons into the intermembrane space [169, 179]. Limitation of ATP hydrolysis and loss during ischemia is unquestionably one of the main protective roles for these channels. In addition, mitoKATP activity can also prevent apoptotic events such as Bax translocation and cytochrome c release [180]. Prolonged treatments with the mitoKATP agonist diazoxide lead to activation of transcription factors cAMP-response element binding protein (CREB) and NFkappaB, resulting in increased expression of the anti-apoptotic protein Bcl-xL [181].
3.7 Antioxidants
Mitochondria are one of the main sources of ROS in cardiomyocytes. Even in physiological conditions, 1-2% of the oxygen consumed by mitochondria is converted to superoxide (O2−•) and then to hydrogen peroxide (H2O2) and other ROS [182]. The heart is well equipped with antioxidant defenses, which include antioxidants such as alpha-tocopherol (vitamin E), ascorbic acid (vitamin C), glutathione and several antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSHPx). However, during IR the antioxidant defenses are overwhelmed. During ischemia there is an increase in ROS production from mitochondrial electron transfer complexes I and III, and a consequent decrease in antioxidant defenses. ROS production rises steadily during ischemia and increases greatly at the onset of reperfusion as oxygen tension rises [5]. IR-induced oxidative stress affects cardiomyocytes metabolism [183], signal transduction [184], and gene expression [185]. Interventions which lessen oxidative stress can decrease IR-induced myocardial damage.
One intervention that decreases oxidative stress involves the use of the cell’s natural antioxidant defenses or mimetics. Administration of SOD before thrombolysis in an acute coronary thrombosis model reduced reperfusion arrhythmias and preserved myocardial function [186]. In a canine model of in vivo ischemia-reperfusion, combined administration of SOD and catalase into the left atrium reduced infarct size when given before ischemia, but was not effective when given 40 minutes following reperfusion [187]. In a study with pigs subjected to LAD occlusion, retrograde delivery of SOD via the great cardiac vein decreased infarct size, whereas no effect was observed when the delivery was via the left atrium. Pigs lack coronary collaterals and, therefore, antegrade administration of SOD is ineffective [188]. In myocardial stunning, administration of MPG before ischemia or 1 min before reperfusion, but not 1 min after reperfusion, ameliorates stunning, suggesting that free radicals that lead to stunning are generated immediately after reperfusion [189]. These studies illustrate the importance of using the correct research models and that the timing of cardioprotective interventions is critical. In transgenic mice, overexpression of the mitochondrial SOD – MnSOD – decreases infarct size after in vivo left coronary artery ligation, suggesting that overexpression of MnSOD confers resistance to IR damage [190]. Overexpression of MnSOD also protects against IR injury in isolated rat hearts [191]. Jones et al. [192] showed that MnSOD overexpression in transgenic mice preserves cardiac function 7 days after in vivo myocardial IR. Decreases in the levels of MnSOD increase IR-induced oxidative stress, increase the susceptibility to IR damage and worsen post-ischemia myocardial function [193]. M40401 and M40403 are SOD mimetics which have been shown to decrease IR injury and graft coronary artery disease in rodent cardiac allografts, and myocardial damage in in vivo models of IR [194-198]. In myocardial IR, M40403 acts by decreasing lipid peroxidation and calcium overload, and in cardiac allografts subjected to IR, M40401 has been shown to lower myeloperoxidase activity and tumor necrosis factor-alpha concentrations [194-198].
Intracellular levels of GSH can be increased with N-acetylcysteine (NAC), a membrane permeable precursor of GSH synthesis. NAC has been show to increase myocardial glutathione content, limit oxidative stress and enhance contractile recovery in isolated rabbit hearts; and to decrease oxidative stress and improve cardiac function recovery in coronary artery disease patients undergoing coronary bypass grafting [199]. The ISLAND (Infarct Size Limitation: Acute Nacetylcysteine Defense) trial showed that in patients with successful reperfusion induced by a combination therapy of streptokinase and NAC, there was a reduction in infarct size associated with improved left ventricular function when compared to patients with reperfusion induced by streptokinase alone or compared to patients with failed reperfusion [200].
Two of the most widely described antioxidants are vitamin C and E. Administration of a combination of vitamin C and E to rabbits after coronary artery ligation improves calcium homeostasis, decreases oxidative stress, apoptosis and improves cardiac function in comparison to placebo [201]. Despite the results obtained in basic research, clinical studies have failed to show benefits from supplementing the diet with vitamin C and E in the prevention of cardiovascular diseases. The St. Francis Heart Study randomized clinical trial showed that vitamin C and E diet supplementation does not affect the progression of coronary calcification in asymptomatic adults with elevated coronary calcium scores [202]. Moreover, the HOPE (Heart Outcomes Prevention Evaluation) trial actually indicated an increase in the incidence of congestive heart failure in patients treated with vitamin E [203]. The GISSI-Prevenzione (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) trial showed that patients with left ventricular dysfunction who received vitamin E had a 50% increase in the risk of developing congestive heart failure [204]. Knekt et al. demonstrated that a high intake of vitamin C (greater than 700 mg of supplemental vitamin C per day) can lower coronary heart disease incidence while supplemental vitamin E intake does not significantly reduce coronary heart disease risk [205]. One concern regarding these studies is that antioxidant dietary supplementation can interact with statins therapy, which was used simultaneously with antioxidants in some of the studies, potentially causing some of the negative results. In fact, the ASAP (Antioxidant Supplementation in Atherosclerosis Prevention) study revealed no changes in statin-induced increases in HDL when the subjects diet was supplemented with vitamins E and C [206]. Nonetheless, this study also revealed some promising results in hypercholesterolemic patients who received vitamin C and E supplements for a long-term period – 6 years. The patients had a decreased progression rate of common carotid atherosclerosis [206].
The use of vitamin C and E derivatives, which have improved bioactivity might be a more efficient antioxidant therapy. As an example, vitamin E is insoluble in water and, thus, its antioxidant activity is limited to the cell’s lipid compartments. A phosphate ester derivative of vitamin C and vitamin E, EPC-K1, is soluble in both water and lipids, and has a better antioxidant activity than the vitamins from which it was derived [207]. EPC-K1 is a moderate scavenger of both hydroxyl (•OH) and alkyl radicals, a potent scavenger of lipid radicals, and inhibitor of lipid peroxidation [207]. In a canine model of cadaver heart transplantation, EPC-K1 reduced post-transplant reperfusion injury [208], and in canine coronary artery endothelium, in addition to scavenging free radicals that mediate endothelial cell injury, EPC-K1 improves vasodilation and stimulates the release of endothelium-derived nitric oxide [209]. These results indicate that EPC-K1 acts by two mechanisms – via modulation of nitric oxide signaling and inhibition of oxidative stress.
Polyphenolic compounds like resveratrol have been shown to be cardioprotective. Resveratrol is the major polyphenol found in grapes and red wine, and it has been shown to have antioxidant properties and to scavenge lipid hydroperoxyl free radicals as well as •OH and O2−• radicals [210]. In isolated perfused working rat hearts subjected to IR, resveratrol promotes myocardial protection [211]. Resveratrol decreases oxidative stress, improves recovery of post-ischemic ventricular function and decreases infarct size [211, 212]. The cardioprotective effects of resveratrol are also observed in isolated hearts of rats treated by IP injection during 7 days, where IR was performed 24hr after the last injection [213]. Resveratrol also improves mitochondrial turnover; the effects on mitochondrial turnover are described in detail in a separate section.
Free radical scavengers like 3-methyl-1-phenyl-1-phyrazolin-5-one (MCI-186, also known as edaravone), and 2,2,6,6-tetramethyl-N-[1-methyl-2-(2,6-dimethylphenoxy)ethyl]-1,2,3,6-tetrahydropyridin-4-carboxamide (HO-3073) prevent radical species-mediated cell damage and improve cell survival. In vivo studies in apolipoprotein E-deficient mice fed a high-fat diet show that MCI-186 suppresses atherosclerosis development associated with reduced expression of immune-activated cells and oxidative stress in fatty-streak plaques. In models of in vivo IR, MCI-186 decreases lipid peroxidation, MPTP opening, apoptosis, necrosis and infarct size, and improves myocardial ATP content and cardiac contractility [214-216]. In isolated perfused hearts, HO-3073 scavenges •OH and O2−• radicals, leading to decreased lipid and protein oxidation, decreased infarct size and improved myocardial function [217]. The cardioprotective effects of HO-3073 are also due to Akt activation. Wortmannin, a phosphatidylinositol-3-kinase antagonist, prevents HO-3073-induced Akt activation and inhibits protection against IR damage [218, 219].
NO• plays a crucial role in many aspects of the pathophysiology of heart failure, but it can also be cardioprotective. Many of the deleterious effects can be attributed to administration of NO• donors at high concentrations or to overproduction of NO• by NOS. Activation of any of the NOS isoforms [eNOS, inducible NOS (iNOS), and neuronal NOS (nNOS)], concomitant with oxidative stress results in NOS uncoupling, leading to further oxidative/nitrosative stress, which causes irreversible damage to proteins, lipids, and DNA [220]. In contrast, low doses of NO• are involved in IPC signaling, neovascularization after myocardial infarction, inhibition of I/R injury, inflammation and left ventricular remodeling [220, 221].
It is noteworthy that there are caveats in the use of antioxidants and radical scavengers as therapeutic agents. Although decreasing ROS and reactive nitrogen species (RNS) formation and oxidative/nitrosative stress is a potential therapeutic strategy against IR damage, antioxidants and ROS/RNS scavengers can also eliminate the cardioprotective effects of interventions like IPC [147, 150, 151] and exercise [222, 223]. An effective approach will need to remove ROS/RNS in a site-specific manner or inhibit the source of deleterious reactive species without affecting redox-sensitive survival signal transduction pathways.
3.8 Caloric restriction
Calorie restriction (CR) can prevent or delay several diseases including cancer, cardiovascular diseases, neurodegenerative disorders, diabetes and autoimmune diseases [224]. Regarding cardiovascular diseases, CR decreases blood pressure, alters the lipoprotein profile, improves glucoregulation, reduces sympathetic nervous system drive, and induces hormonal changes; as a consequence it delays the onset of age-related cardiac alterations and improves risk factors [224-227].
At the cellular level, cardioprotection afforded by CR is mediated by a decrease in oxidative stress, improvement of mitochondrial function, decrease in inflammation, and changes in autophagy. Oxidative stress is associated with ischemia-reperfusion injury [228], cardiac remodeling after myocardial infarction [229], left ventricular hypertrophy and heart failure [230]. Studies show that CR can decrease cardiac oxidative damage [231-233]. CR alters mitochondrial potential and respiratory activity, which results in lower ROS generation and oxidative damage. In addition, it also increases antioxidant defenses [233-236]. A period of CR as short as 35 days is enough to decrease cardiac oxidative stress. Diniz et al. [237] subjected rats to 50% CR for 35 days and observed an increase in glutathione peroxidase and catalase activity and a decrease in lipid peroxidation in comparison to rats fed ad libitum. Another study using rats subjected to 50% caloric restriction for one week followed by one week of re-feeding, showed that hepatic mitochondria become more efficient, as reflected by higher state 3 mitochondrial respiratory capacity and increased SOD activity [238]. Seymour et al. [239] using Dahl salt-sensitive rats fed a high-salt diet showed that 15% CR reduces lipid peroxidation, improving left ventricular remodeling, diastolic function and cardiac index, and delaying the onset of cardiac cachexia. Chandrasekar et al. [240] demonstrated that lifelong 40% CR attenuates cardiac oxidative damage in middle-aged rats following myocardial ischemia-reperfusion. Eight months-long CR improves recovery of cardiac function after 25 min of ischemia in working rat hearts [241]. The effect is mediated by an improvement in mitochondrial respiration and oxidative phosphorylation rate [242]. Short-term (4 weeks) and long-term (6 months) CR improves myocardial ischemic tolerance [243-245]. The cardioprotection afforded by short-term CR is associated with an increase in activated AMPK. The authors showed that short-term CR induces an increase in circulating adiponectin levels that activate myocardial AMPK, resulting in protection against ischemia [243, 244]. In long-term CR, there is also an increase in adiponectin, but AMPK is not activated [245]. The cardioprotection in long-term CR seems to occur via a nitric oxide-dependent increase in nuclear Sirt1 content [245]. A possible mechanism is that Sirt1 activates both autophagy, by deacetylating autophagy proteins [246], and mitochondrial biogenesis, via activation of PGC-1α [247-249]. The net result is that caloric restriction ensures good mitochondrial function by promoting mitochondrial turnover.
CR has also been shown to increase mitochondrial biogenesis and bioenergetic efficiency, in a process driven by Akt, eNOS and NO• signaling [249-254]. This increase in mitochondrial biogenesis has been proposed to be critical for the beneficial effects of CR [250, 251, 254, 255]. Nisoli et al. [251] showed that mice on CR (3 months) have higher levels of mitochondrial DNA, PGC-1α, NRF-1, Tfam, expression of cytochrome c oxidase, and cytochrome c when compared with mice fed ad libitum, indicating increased mitochondrial biogenesis. Cerqueira et al. [254] showed that mice fed with 60% of normal diet for 6 months had increased markers of mitochondrial biogenesis, namely PGC-1α, citrate synthase activity, and cytochrome c oxidase. Akt and eNOS expression and phosphorylation were activated and fasting plasma levels of NO• products were increased, which is believed to have led to increased mitochondrial biogenesis [254]. Similar results were obtained with the CR mimetic, 2,4-dinitrophenol [254]. The suggested mechanism is that CR activates Akt, which directly phosphorylates eNOS activating the enzyme and leading to NO production [161, 250-254]. Nitric oxide activates a NO/cGMP-dependent signaling pathway that induces PGC-1α, increasing mitochondrial biogenesis [255, 256]. Long term exposure to elevated ROS levels impairs eNOS activity [257, 258]. Given that mitochondria produce ROS and an excess of mitochondria will lead to increased ROS production, eNOS functions in a negative feedback loop preventing the generation of excessive ROS.
CR attenuates the age-related decline of autophagy [259, 260]. It has also been shown that caloric-restriction-induced autophagy is associated with increased longevity, and this may be due to the episodic removal of inefficient mitochondria followed by their replacement with new functional mitochondria [261, 262]. A lifelong 40% CR has been reported to increase the expression of autophagic markers (Atg7, Atg9 and LC3II) in the heart from adult and old rats compared to ad libitum-fed controls [260]. Shinmura et al. [263] showed that in aged rats, a 40% decrease in energy intake suppresses the mTOR pathway and activates autophagy, which translates into improved diastolic function in the senescent myocardium. Given that CR increases both autophagy and mitochondrial biogenesis, the net effect is an increase in mitochondrial turnover. Thus, the beneficial effects of CR might be the result of improved mitochondrial turnover.
3.9 Caloric restriction mimetics
Given the questionable feasibility of long-term dietary restriction, pharmacological alternatives to CR have been investigated. A strong candidate as an alternative is resveratrol.
Resveratrol is a naturally occurring polyphenol found in red wine with well-described cardioprotective properties. Resveratrol protects against cardiac dysfunction in ischemia-reperfusion, obesity, insulin resistance and type I diabetes [211, 264-268]. Resveratrol has been shown to inhibit cardiomyocyte apoptosis [269], induce autophagy in cardiac cells subjected to ischemia-reperfusion [265, 270], reduce ischemia-reperfusion-induced oxidative stress in isolated rat hearts [211], prevent left ventricular hypertrophy in aortic-banded rats [271], improve endothelial function in isolated rat aortas [272], inhibit platelet aggregation [273], and reduce inflammation [274].
Probably one of the most interesting effects of resveratrol at the mitochondrial level is its effect on mitochondrial turnover (i.e., on mitochondrial biogenesis and mitophagy). Resveratrol lowers Michaelis constant of Sirt1 for NAD+ [275] leading to activation of Sirt1. Deacetylation of PGC-1α by Sirt1 activates it. Deacetylated PGC-1α acts as a co-activator for NRF1, which transactivates genes involved in oxidative phosphorylation and mitochondrial biogenesis, such as Tfam [266]. Resveratrol was shown to activate PGC-1α, NRF-1 and TFAM in coronary arterial endothelial cells, and Sirt1 siRNA prevented resveratrol effects [276]. In aortas of type 2 diabetic mice, impaired mitochondrial biogenesis was normalized by chronic resveratrol treatment [276].
Resveratrol also increases FoxO1 activity by promoting FoxO1 nuclear translocation and expression of FoxO1 target genes, such as Atg proteins [277, 278]. Sirt1 inhibits mTOR and positively regulates autophagy [246, 279, 280]. It negatively regulates mTOR by interacting with TSC2, a component of mTOR inhibitory complex upstream of mTORC1 [246, 279, 280]. In addition, Sirt1 deacetylase activity stabilizes FoxO1, overriding phosphorylation-dependent mechanisms and preventing its proteasomal degradation [277]. In non-cardiac cells it has been described that resveratrol suppresses starvation-induced autophagy by inhibiting p70 S6 kinase [281]. However, studies in cardiac cells demonstrate that resveratrol activates autophagy [265, 270]. The contradictory results might be due to resveratrol hormetic behaviour (protective at low doses but deleterious at high concentrations) [282].
One concern regarding the use of resveratrol as a cardioprotective agent is that it was shown to induce cell death. Resveratrol can inhibit the activity of F1-ATPase, inhibiting ATP synthesis, cause loss of mitochondrial potential, ROS generation and mitochondrial outer membrane permeabilization in isolated mitochondria; factors which might contribute to cell death induction [283-285]. Despite these effects, resveratrol seems to be safe and reasonably well-tolerated in humans, which means it has the potential to be used as a cardioprotective drug in the future [286-288]. Currently, the NIH database for clinical trials (http://clinicaltrials.gov/) shows 30 clinical trials with resveratrol for different conditions, 10 of which are completed.
Another CR mimetic is 2,4-dinitrophenol, although its toxicity still precludes its direct clinical use. Low doses of 2,4-dinitrophenol (1 mg/L drinking water) have been shown to increase tissue respiratory rates, improve serum glucose, triglyceride and insulin levels, decrease ROS levels and DNA and protein oxidation, as well as to reduce body weight [289]. 2,4-dinitrophenol-treated animals also experienced enhanced longevity [289]. 2,4-dinitrophenol has also been shown to increase mitochondrial biogenesis, which is proposed to be critical for the beneficial effects of CR [250, 251, 254, 255]. In isolated rat hearts subjected to ischemia-reperfusion, 2,4-dinitrophenol pretreatment is cardioprotective, reducing infarct size to the same degree as ischemic preconditioning [290]. However, 2,4-dinitrophenol is far from being a perfect cardioprotective drug as shown by its negative side-effects when it was used as a diet pill in the 1930s.
Recently, statins have been recognized to trigger autophagy, although the significance of this observation is not yet apparent [291]. However, statins appear to be beneficial even in settings where cholesterol reduction is not thought to be clinically important. In one study, patients with average cholesterol levels (139 mg/dL) exhibited reductions in the number of recurring cardiovascular events after treatment with pravastatin, associated with a concurrent decrease in cholesterol level to 97 mg/dL [292]. Studies in non-hypercholesterolemic patients showed that statins decreased cardiac-related morbidity and mortality despite the presence of other risk factors such as diabetes [293] and hypertension [294]. Statins also have anti-inflammatory effects without affecting lipid levels, suggesting that their beneficial effects extend beyond simply lowering cholesterol [295]. Whether these pleiotropic beneficial effects are due to upregulation of autophagy remain to be explored.
3.10 Exercise
Exercise-induced cardioprotection against ischemia-reperfusion injury was first described in 1978 by McElroy and colleagues [296], who showed that chronic swim training induced a reduction in infarct size after coronary artery occlusion in rats. Regular exercise reduces the risk of death during myocardial ischemia-reperfusion insult [297]. In animals, regular sessions of aerobic exercise (i.e., running or swimming) protect the heart from ischemia-reperfusion-induced injury [298-309].
Endurance exercise training protects cardiac myocytes against ischemia-reperfusion-induced oxidative stress [301, 303, 306]. Endurance exercise promotes the expression of heat shock protein 72 (HSP72) in the heart, increasing myocardial HSP72 levels three-to five-fold [306, 310-312]. Overexpression of HSP72 provides myocardial protection against ischemia-reperfusion injury [313-315]. The mechanism by which HSP72 protects the myocardium during ischemia-reperfusion is still under debate. HSP72 is involved in synthesis, folding, transport, and degradation of proteins [316-318]. It has also been proposed that increased HSP72 can be cardioprotective by enhancing myocardial antioxidant capacity [315]. HSP72 improves the recovery from acute cellular injury and protects the cell from subsequent injury. This is achieved by preventing protein aggregation and denaturation along with the restoration of function of proteins damaged by stress [316, 317]. HSP72 has been shown to protect mitochondria against ischemia-reperfusion injury [314, 315] and to inhibit apoptosis [319]. It has also been proposed that endurance exercise increases the activity of antioxidant enzymes in the heart, such as manganese SOD (MnSOD), glutathione peroxidase and catalase [306, 320-324]. Yamashita et al. [309] demonstrated that an increase in MnSOD activity is required for exercise-induced cardioprotection against myocardial infarction. Hamilton et al. [325] showed that silencing the MnSOD gene results in the prevention of endurance exercise-induced protection against ischemia-reperfusion-mediated arrhythmias.
Exercise has also been shown to increase mitochondrial biogenesis and to improve mitochondrial function [326-330]. Skeletal muscle biopsies of humans performing high-intensity interval training showed an increase in Sirt1, nuclear PGC-1α and Tfam, which lead to an increase in skeletal muscle mitochondria and improved exercise performance [326, 328, 330]. Biopsies performed in older men showed that even with aging, exercise increases mitochondrial DNA and mitochondrial respiratory chain activity which is likely related to increases in mitochondria biogenesis [327, 329]. The exercise-induced increase in mitochondrial biogenesis is suggested to be mediated by ROS. Oral administration of the antioxidant vitamin C to rats lowers the exercise-induced increase in mitochondrial biogenesis [222]. It lowers mRNA and protein levels of PGC-1α, NRF-1 and Tfam. A decrease in the levels of cytochrome c is also observed [222]. Similar results were obtained by Ristow et al. [223] in humans. The authors observed that exercise induces an increase in PGC-1α and PGC-1β, ameliorates insulin resistance and causes an adaptive response promoting endogenous antioxidant defense capacity. However, when the subjects diet was supplemented with antioxidants these effects were not observed [223].
A study from 1984 shows that autophagic response is activated after strenuous exercise in mouse skeletal muscle fibers [331]. Another study from 1987 shows that liver protein degradation is increased during exercise as a result of autophagy and proteolysis of cell material inside the secondary lysosomes [332]. The rate of degradation of contractile proteins is decreased during exercise but is increased during the recovery period if the exercise is of high intensity and of long duration [332]. It has also been shown that mVps34 activity, a protein involved in autophagy induction, is increased after resistance exercise [333].
In sum, exercise has been shown to increase mitochondrial turnover, i.e., mitochondrial biogenesis and autophagy. Although this is better described for skeletal muscle, one can extrapolate that the same effects might be observed in cardiac muscle. Exercise-induced increase in cardiac mitochondrial turnover would lead to a functionally better mitochondrial population. This may explain the observation that exercise results in mitochondria with a higher threshold for permeability transition pore opening, and that it induces mitochondrial changes that lead to cardioprotection [334].
4. Conclusion
A steadily growing body of evidence points to mitochondrial turnover—balanced biogenesis and mitophagy—as essential to cellular homeostasis, optimal organ function, and longevity. Mitochondria are vulnerable to damage from ROS, pro-apoptotic signals originating in the cytosol, and from the multiple onslaughts of ischemia and reperfusion injury. When repair is not an option, then cellular survival depends upon elimination of irretrievably damaged mitochondria before they can activate programmed cell death. Mitochondrial numbers and metabolic activity are finely tuned to meet cellular demands; excess mitochondria may be eliminated by mitophagy, a process which is essential for cellular homeostasis. Conditions which interfere with this process, including diabetes and aging, lead to accumulation of dysfunctional mitochondria that can impose an added burden of ROS on the cell. Further work is needed to understand the degree to which mitophagy selectively removes the least functional mitochondria, and to establish the basis for such selective removal. Although evidence for the PINK1/Parkin pathway responsive to low membrane potential is solid, less is known about ROS-mediated triggers for mitophagy. A number of agents and interventions are now recognized to trigger mitophagy and biogenesis, including caloric restriction, exercise, and resveratrol.
It is clear that mitochondria are the central target of cardioprotection. However, it is not clear how cardioprotective signaling pathways impact mitochondria to make them more resilient to ischemic injury. Many cardioprotective interventions involve mitochondrial depolarization. It has been suggested that depolarization limits Ca++ uptake and prevents MPTP opening. However, it is equally plausible to suggest that depolarization induces mitophagy, the remaining mitochondria may be more robust. More work is needed to understand whether mitophagy and subsequent biogenesis are essential to the mechanism of cardioprotection. It may be possible to identify new drugs on the basis of their effects on mitophagy and biogenesis. As the field progresses, it is expected that pharmacologic agents and interventions that enhance mitophagy and balanced mitochondrial biogenesis will become a focus of therapeutic intervention for a variety of disease processes as well as natural aging.
Abbreviations
- AIF
apoptosis inducing factor
- AMPK
AMP-activated protein kinase
- ASAP
Antioxidant Supplementation in Atherosclerosis Prevention
- BAPTA-AM
1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis(acetoxymethyl ester)
- Bnip3
Bcl-2 and adenovirus E1B 19 kDa-interacting protein 3
- CCPA
2-chloro-N(6)-cyclopentyladenosine
- CR
caloric restriction
- CREB
cAMP-response element binding protein
- DRP1
dynamin-related protein 1
- EGFR
epidermal growth factor receptor
- eNOS
endothelial nitric oxide synthase (eNOS)
- ETC
electron transport chain
- FA
fatty acid
- FIS1
fission protein 1
- FoxO1
forkhead box protein O1
- FoxO3
forkhead box protein O3
- GABARAP
Gamma-aminobutyric acid receptor-associated protein
- GISSI
Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico
- GSH
glutathione
- GSHPx
glutathione peroxidase
- GSK-3beta
glycogen synthase kinase-3beta
- HIF-1
hypoxia-inducible factor 1
- HO-3073
2,2,6,6-tetramethyl-N-[1-methyl-2-(2,6-dimethylphenoxy)ethyl]-1,2,3,6-tetrahydropyridin-4-carboxamide
- H2O2
hydrogen peroxide
- HOPE
Heart Outcomes Prevention Evaluation
- HSP72
heat shock protein 72
- iNOS
inducible NOS
- IPC
ischemic preconditioning
- IR
ischemia-reperfusion
- ISLAND
Infarct Size Limitation: Acute Nacetylcysteine Defense
- LAMP2
lysosome-associated membrane protein 2
- MCI-186
3-methyl-1-phenyl-1-phyrazolin-5-one
- MFN1
mitofusin 1
- MFN2
mitofusin 2
- mitoKATP
mitochondrial ATP-sensitive potassium channel
- MnSOD
manganese superoxide dismutase
- MPG
2-mercaptopropionylglycine
- MPT
mitochondrial permeability transition
- MPTP
mitochondrial permeability transition pore
- mTOR
mammalian target of rapamycin
- NAC
N-acetylcysteine
- NIX
Bnip3-like protein X
- nNOS
neuronal NOS
- NRF1
nuclear respiratory factor 1
- NRF2
nuclear respiratory factor 2
- OPA1
optic atrophy protein 1
- p62/SQSTM1
p62/Sequestosome 1
- PGC-1α
peroxisome proliferator-activated receptor - γ coactivator-1α
- PINK1
PTEN-induced putative kinase 1
- PKC
protein kinase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Sirt
sirtuin
- Smac/DIABLO
second mitochondria-derived activator of caspases/ direct IAP binding protein of low pI
- SOD
superoxide dismutase
- O2−•
superoxide
- STK11
serine/threonine kinase 11
- Tfam
mitochondrial transcription factor A
- UCP
uncoupling proteins
- VDAC
voltage dependent anion channel
- Vps34
Vacuolar protein sorting 34
Footnotes
DISCLOSURES: None of the authors have any conflicts of interest relevant to this manuscript. RAG is CEO of Radical Therapeutix, Inc.
References
- [1].Sommer JR, Johnson EA. Handbook of Physiology. The Cardiovascular System. The Heart. Vol. 1. Am. Physiol. Soc.; Bethesda, MD: 1979. pp. 113–86. [Google Scholar]
- [2].Mancuso DJ, Abendschein DR, Jenkins CM, et al. Cardiac ischemia activates calcium-independent phospholipase A2beta, precipitating ventricular tachyarrhythmias in transgenic mice: rescue of the lethal electrophysiologic phenotype by mechanism-based inhibition. J Biol Chem. 2003;278:22231–6. doi: 10.1074/jbc.C300033200. [DOI] [PubMed] [Google Scholar]
- [3].Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- [4].Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- [5].Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- [6].Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- [7].Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- [8].Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2025–31. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- [10].Quinsay MN, Lee Y, Rikka S, et al. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–55. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- [12].Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–65. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- [13].Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- [15].Grivennikova VG, Kareyeva AV, Vinogradov AD. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim Biophys Acta. doi: 10.1016/j.bbabio.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–12. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Terman A, Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- [18].Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–8. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–84. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- [21].Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ong SB, Subrayan S, Lim SY, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- [23].Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–9. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosca MG, Hoppel CL. New aspects of impaired mitochondrial function in heart failure. J Bioenerg Biomembr. 2009;41:107–12. doi: 10.1007/s10863-009-9215-9. [DOI] [PubMed] [Google Scholar]
- [25].Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [27].Deshmukh AS, Treebak JT, Long YC, et al. Role of adenosine 5′-monophosphate-activated protein kinase subunits in skeletal muscle mammalian target of rapamycin signaling. Mol Endocrinol. 2008;22:1105–12. doi: 10.1210/me.2007-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–6. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rong Y, McPhee C, Deng S, et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tanida I. Autophagy basics. Microbiol Immunol. 2011;55:1–11. doi: 10.1111/j.1348-0421.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- [31].Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–9. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vermulst M, Wanagat J, Kujoth GC, et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–4. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- [34].Nekhaeva E, Bodyak ND, Kraytsberg Y, et al. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proc Natl Acad Sci U S A. 2002;99:5521–6. doi: 10.1073/pnas.072670199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Grey AD. A proposed refinement of the mitochondrial free radical theory of aging. Bioessays. 1997;19:161–6. doi: 10.1002/bies.950190211. [DOI] [PubMed] [Google Scholar]
- [36].de Grey AD. The reductive hotspot hypothesis of mammalian aging: membrane metabolism magnifies mutant mitochondrial mischief. Eur J Biochem. 2002;269:2003–9. doi: 10.1046/j.1432-1033.2002.02868.x. [DOI] [PubMed] [Google Scholar]
- [37].Keller JN, Dimayuga E, Chen Q, et al. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–91. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [38].Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68:355–65. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [39].Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [40].Coleman R, Silbermann M, Gershon D, Reznick AZ. Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology. 1987;33:34–9. doi: 10.1159/000212851. [DOI] [PubMed] [Google Scholar]
- [41].Terman A. Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep. 2001;6:15–26. doi: 10.1179/135100001101535996. [DOI] [PubMed] [Google Scholar]
- [42].Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863–76. doi: 10.1016/s0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- [43].Terman A, Gustafsson B, Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006;163:29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- [44].Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- [45].Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–6. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- [46].Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–81. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- [47].Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- [48].Decker RS, Poole AR, Crie JS, Dingle JT, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. II. Immunohistochemical and biochemical changes in cathepsin D. Am J Pathol. 1980;98:445–56. [PMC free article] [PubMed] [Google Scholar]
- [49].Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–44. [PMC free article] [PubMed] [Google Scholar]
- [50].Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- [51].Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- [52].Takagi H, Matsui Y, Hirotani S, et al. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–7. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- [53].Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–30. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–31. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- [55].Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J. 2007;274:3184–97. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- [57].Hoyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- [58].Levraut J, Iwase H, Shao ZH, Vanden Hoek TL, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am J Physiol Heart Circ Physiol. 2003;284:H549–58. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- [59].Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–72. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–7. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- [61].Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–52. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- [62].Scherz-Shouval R, Shvets E, Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3:371–3. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- [63].Scherz-Shouval R, Shvets E, Fass E, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006;2:39–46. doi: 10.4161/auto.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Elsasser A, Vogt AM, Nef H, et al. Human hibernating myocardium is jeopardized by apoptotic and autophagic cell death. J Am Coll Cardiol. 2004;43:2191–9. doi: 10.1016/j.jacc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- [66].Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- [67].Kostin S, Pool L, Elsasser A, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–24. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- [68].Yamamoto S, Sawada K, Shimomura H, Kawamura K, James TN. On the nature of cell death during remodeling of hypertrophied human myocardium. J Mol Cell Cardiol. 2000;32:161–75. doi: 10.1006/jmcc.1999.1064. [DOI] [PubMed] [Google Scholar]
- [69].Zhong Y, Wang QJ, Li X, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–76. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy. 2009;5:709–12. doi: 10.4161/auto.5.5.8510. [DOI] [PubMed] [Google Scholar]
- [71].Yitzhaki S, Huang C, Liu W, et al. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104:157–67. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Huang C, Yitzhaki S, Perry CN, et al. Autophagy Induced by Ischemic Preconditioning is Essential for Cardioprotection. J Cardiovasc Transl Res. 2010 doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–77. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–8. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- [75].Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–83. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Geisler S, Holmstrom KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- [78].Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–9. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang H, Bosch-Marce M, Shimoda LA, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [81].Semenza GL. Mitochondrial autophagy: life and breath of the cell. Autophagy. 2008;4:534–6. doi: 10.4161/auto.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Novak I, Kirkin V, McEwan DG, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schweers RL, Zhang J, Randall MS, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–5. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–46. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pattingre S, Bauvy C, Carpentier S, et al. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–28. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Park KJ, Lee SH, Kim TI, et al. A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res. 2007;67:7327–34. doi: 10.1158/0008-5472.CAN-06-4766. [DOI] [PubMed] [Google Scholar]
- [88].Yuan H, Perry CN, Huang C, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–9. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Comstock KL, Krown KA, Page MT, et al. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–75. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- [90].Jia L, Dourmashkin RR, Allen PD, et al. Inhibition of autophagy abrogates tumour necrosis factor alpha induced apoptosis in human T-lymphoblastic leukaemic cells. Br J Haematol. 1997;98:673–85. doi: 10.1046/j.1365-2141.1997.2623081.x. [DOI] [PubMed] [Google Scholar]
- [91].Prins JB, Ledgerwood EC, Ameloot P, et al. Tumor necrosis factor-induced cytotoxicity is not related to rates of mitochondrial morphological abnormalities or autophagy-changes that can be mediated by TNFR-I or TNFR-II. Biosci Rep. 1998;18:329–40. doi: 10.1023/a:1020261316486. [DOI] [PubMed] [Google Scholar]
- [92].Ohsawa Y, Isahara K, Kanamori S, et al. An ultrastructural and immunohistochemical study of PC12 cells during apoptosis induced by serum deprivation with special reference to autophagy and lysosomal cathepsins. Arch Histol Cytol. 1998;61:395–403. doi: 10.1679/aohc.61.395. [DOI] [PubMed] [Google Scholar]
- [93].Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–98. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- [94].Christensen ST, Chemnitz J, Straarup EM, et al. Staurosporine-induced cell death in Tetrahymena thermophila has mixed characteristics of both apoptotic and autophagic degeneration. Cell Biol Int. 1998;22:591–8. doi: 10.1006/cbir.1998.0320. [DOI] [PubMed] [Google Scholar]
- [95].Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006;2:307–9. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- [96].Scarpulla RC. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008;88:611–38. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- [97].Wende AR, Schaeffer PJ, Parker GJ, et al. A Role for the Transcriptional Coactivator PGC-1α in Muscle Refueling. Journal of Biological Chemistry. 2007;282:36642–51. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- [98].Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- [99].Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–9. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Russell LK, Mansfield CM, Lehman JJ, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–33. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- [101].Looman C, Abrink M, Mark C, Hellman L. KRAB zinc finger proteins: an analysis of the molecular mechanisms governing their increase in numbers and complexity during evolution. Mol Biol Evol. 2002;19:2118–30. doi: 10.1093/oxfordjournals.molbev.a004037. [DOI] [PubMed] [Google Scholar]
- [102].Shin JH, Ko HS, Kang H, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [104].Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- [105].Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–12. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- [106].Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- [107].Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–9. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- [108].Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–24. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- [109].Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–8. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- [110].Brand MD, Buckingham JA, Esteves TC, et al. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004:203–13. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- [111].Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- [112].Starkov AA. “Mild” uncoupling of mitochondria. Biosci Rep. 1997;17:273–9. doi: 10.1023/a:1027380527769. [DOI] [PubMed] [Google Scholar]
- [113].Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008;103:873–80. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Richardson DR, Lok HC. The nitric oxide-iron interplay in mammalian cells: transport and storage of dinitrosyl iron complexes. Biochim Biophys Acta. 2008;1780:638–51. doi: 10.1016/j.bbagen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- [115].Jezek P, Engstova H, Zackova M, et al. Fatty acid cycling mechanism and mitochondrial uncoupling proteins. Biochim Biophys Acta. 1998;1365:319–27. doi: 10.1016/s0005-2728(98)00084-x. [DOI] [PubMed] [Google Scholar]
- [116].Skulachev VP. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–62. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- [117].Pecqueur C, Alves-Guerra MC, Gelly C, et al. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–12. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- [118].Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345(Pt 2):161–79. [PMC free article] [PubMed] [Google Scholar]
- [119].Andreyev A, Bondareva TO, Dedukhova VI, et al. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem. 1989;182:585–92. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
- [120].Andreyev A, Bondareva TO, Dedukhova VI, et al. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988;226:265–9. doi: 10.1016/0014-5793(88)81436-4. [DOI] [PubMed] [Google Scholar]
- [121].Hoerter J, Gonzalez-Barroso MD, Couplan E, et al. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528–33. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- [122].McLeod CJ, Aziz A, Hoyt RF, Jr., McCoy JP, Jr., Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–6. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- [123].Mattiasson G, Shamloo M, Gido G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–8. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- [124].Carreira RS, Miyamoto S, Di Mascio P, et al. Ischemic preconditioning enhances fatty acid-dependent mitochondrial uncoupling. J Bioenerg Biomembr. 2007;39:313–20. doi: 10.1007/s10863-007-9093-y. [DOI] [PubMed] [Google Scholar]
- [125].Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–8. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- [127].Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–7. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- [128].Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–5. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kang HT, Hwang ES. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell. 2009;8:426–38. doi: 10.1111/j.1474-9726.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- [130].Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010 doi: 10.4161/auto.6.4.11553. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lemasters JJ, Nieminen AL, Qian T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–96. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- [132].Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–53. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–43. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- [134].Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–4. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- [135].Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–9. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- [136].Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning. Cardiovasc Res. 2010;88:67–74. doi: 10.1093/cvr/cvq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–9. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- [139].Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–6. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- [140].Nishihara M, Miura T, Miki T, et al. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–70. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [141].Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–91. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Clarke SJ, Khaliulin I, Das M, et al. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–90. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–66. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gross ER, Hsu AK, Gross GJ. GSK3beta inhibition and K(ATP) channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102:341–9. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- [146].da Silva MM, Sartori A, Belisle E, Kowaltowski AJ. Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am J Physiol Heart Circ Physiol. 2003;285:H154–62. doi: 10.1152/ajpheart.00955.2002. [DOI] [PubMed] [Google Scholar]
- [147].Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- [148].Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–8. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- [149].Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- [150].Tritto I, D’Andrea D, Eramo N, et al. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–8. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- [151].Facundo HT, Carreira RS, de Paula JG, et al. Ischemic preconditioning requires increases in reactive oxygen release independent of mitochondrial K+ channel activity. Free Radic Biol Med. 2006;40:469–79. doi: 10.1016/j.freeradbiomed.2005.08.041. [DOI] [PubMed] [Google Scholar]
- [152].Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–69. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- [153].Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–31. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- [154].Liu Y, Yang XM, Iliodromitis EK, et al. Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol. 2008;103:54–9. doi: 10.1007/s00395-007-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Liu GS, Thornton J, Van Winkle DM, et al. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–6. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- [156].Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681–9. [PubMed] [Google Scholar]
- [157].Goto M, Liu Y, Yang XM, et al. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611–21. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- [158].Cohen MV, Downey JM. Myocardial preconditioning promises to be a novel approach to the treatment of ischemic heart disease. Annu Rev Med. 1996;47:21–9. doi: 10.1146/annurev.med.47.1.21. [DOI] [PubMed] [Google Scholar]
- [159].Tosaki A, Maulik N, Cordis G, et al. Ischemic preconditioning triggers phospholipase D signaling in rat heart. Am J Physiol. 1997;273:H1860–6. doi: 10.1152/ajpheart.1997.273.4.H1860. [DOI] [PubMed] [Google Scholar]
- [160].Cohen MV, Philipp S, Krieg T, et al. Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. J Mol Cell Cardiol. 2007;42:842–51. doi: 10.1016/j.yjmcc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Oldenburg O, Qin Q, Krieg T, et al. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286:H468–76. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- [163].Costa AD, Garlid KD, West IC, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- [164].Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000;278:H305–12. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- [165].Garlid KD, Paucek P, Yarov-Yarovoy V, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–82. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- [166].Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–82. [PubMed] [Google Scholar]
- [167].Wang Y, Hirai K, Ashraf M. Activation of mitochondrial ATP-sensitive K(+) channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circ Res. 1999;85:731–41. doi: 10.1161/01.res.85.8.731. [DOI] [PubMed] [Google Scholar]
- [168].Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039–48. doi: 10.1016/j.freeradbiomed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- [169].Belisle E, Kowaltowski AJ. Opening of mitochondrial K+ channels increases ischemic ATP levels by preventing hydrolysis. J Bioenerg Biomembr. 2002;34:285–98. doi: 10.1023/a:1020256502583. [DOI] [PubMed] [Google Scholar]
- [170].Costa AD, Quinlan CL, Andrukhiv A, et al. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–15. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- [171].Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels prevent oxidative stress, permeability transition and cell death. J Bioenerg Biomembr. 2005;37:75–82. doi: 10.1007/s10863-005-4130-1. [DOI] [PubMed] [Google Scholar]
- [172].Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–30. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- [173].O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–32. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ferranti R, da Silva MM, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003;536:51–5. doi: 10.1016/s0014-5793(03)00007-3. [DOI] [PubMed] [Google Scholar]
- [175].Huang C, Yitzhaki S, Perry CN, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 2010;3:365–73. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ishida H, Hirota Y, Genka C, et al. Opening of mitochondrial K(ATP) channels attenuates the ouabain-induced calcium overload in mitochondria. Circ Res. 2001;89:856–8. doi: 10.1161/hh2201.100341. [DOI] [PubMed] [Google Scholar]
- [177].Murata M, Akao M, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–8. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- [178].Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287:H841–9. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- [179].Dos Santos P, Kowaltowski AJ, Laclau MN, et al. Mechanisms by which opening the mitochondrial ATP-sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–95. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- [180].Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–43. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- [181].Eliseev RA, Vanwinkle B, Rosier RN, Gunter TE. Diazoxide-mediated preconditioning against apoptosis involves activation of cAMP-response element-binding protein (CREB) and NFkappaB. J Biol Chem. 2004;279:46748–54. doi: 10.1074/jbc.M406217200. [DOI] [PubMed] [Google Scholar]
- [182].Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Marsin AS, Bertrand L, Rider MH, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- [184].Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [185].Nordlie MA, Wold LE, Simkhovich BZ, Sesti C, Kloner RA. Molecular aspects of ischemic heart disease: ischemia/reperfusion-induced genetic changes and potential applications of gene and RNA interference therapy. J Cardiovasc Pharmacol Ther. 2006;11:17–30. doi: 10.1177/107424840601100102. [DOI] [PubMed] [Google Scholar]
- [186].Mehta JL, Nichols WW, Saldeen TG, et al. Superoxide dismutase decreases reperfusion arrhythmias and preserves myocardial function during thrombolysis with tissue plasminogen activator. J Cardiovasc Pharmacol. 1990;16:112–20. doi: 10.1097/00005344-199007000-00016. [DOI] [PubMed] [Google Scholar]
- [187].Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984;54:277–85. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- [188].Hatori N, Tadokoro H, Satomura K, et al. Beneficial effects of coronary venous retroinfusion but not left atrial administration of superoxide dismutase on myocardial necrosis in pigs. Eur Heart J. 1991;12:442–50. doi: 10.1093/oxfordjournals.eurheartj.a059915. [DOI] [PubMed] [Google Scholar]
- [189].Bolli R, Jeroudi MO, Patel BS, et al. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res. 1989;65:607–22. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- [190].Chen Z, Siu B, Ho YS, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–9. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- [191].Suzuki K, Sawa Y, Ichikawa H, Kaneda Y, Matsuda H. Myocardial protection with endogenous overexpression of manganese superoxide dismutase. Ann Thorac Surg. 1999;68:1266–71. doi: 10.1016/s0003-4975(99)00726-2. [DOI] [PubMed] [Google Scholar]
- [192].Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H277–82. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- [193].Asimakis GK, Lick S, Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/−) but not SOD1(+/−) mouse hearts. Circulation. 2002;105:981–6. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- [194].Di Filippo C, Cuzzocrea S, Marfella R, et al. M40403 prevents myocardial injury induced by acute hyperglycaemia in perfused rat heart. Eur J Pharmacol. 2004;497:65–74. doi: 10.1016/j.ejphar.2004.06.037. [DOI] [PubMed] [Google Scholar]
- [195].Marzocca C, Vannacci A, Cuzzocrea S, et al. Effects of the SOD mimetic, M40403, on prostaglandin production in an in vivo model of ischemia and reperfusion in rat heart. Inflamm Res. 2003;52(Suppl 1):S23–4. doi: 10.1007/s000110300037. [DOI] [PubMed] [Google Scholar]
- [196].Masini E, Cuzzocrea S, Mazzon E, et al. Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Br J Pharmacol. 2002;136:905–17. doi: 10.1038/sj.bjp.0704774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Salvemini D, Wang ZQ, Zweier JL, et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–6. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- [198].Murata S, Miniati DN, Kown MH, et al. Superoxide dismutase mimetic m40401 reduces ischemia-reperfusion injury and graft coronary artery disease in rodent cardiac allografts. Transplantation. 2004;78:1166–71. doi: 10.1097/01.tp.0000137321.34200.fa. [DOI] [PubMed] [Google Scholar]
- [199].Ferrari R, Ceconi C, Curello S, et al. Oxygen free radicals and myocardial damage: protective role of thiol-containing agents. Am J Med. 1991;91:95S–105S. doi: 10.1016/0002-9343(91)90291-5. [DOI] [PubMed] [Google Scholar]
- [200].Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know? J Am Coll Cardiol. 2002;39:1422–8. doi: 10.1016/s0735-1097(02)01797-7. [DOI] [PubMed] [Google Scholar]
- [201].Qin F, Yan C, Patel R, Liu W, Dong E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic Biol Med. 2006;40:1827–42. doi: 10.1016/j.freeradbiomed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- [202].Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–72. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- [203].McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scand J Clin Lab Invest Suppl. 2005;240:143–56. doi: 10.1080/00365510500236366. [DOI] [PubMed] [Google Scholar]
- [204].Marchioli R, Levantesi G, Macchia A, et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 2006;7:347–50. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- [205].Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–20. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- [206].Salonen RM, Nyyssonen K, Kaikkonen J, et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003;107:947–53. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- [207].Wei T, Chen C, Li F, et al. Antioxidant properties of EPC-K1: a study on mechanisms. Biophys Chem. 1999;77:153–60. doi: 10.1016/s0301-4622(99)00017-4. [DOI] [PubMed] [Google Scholar]
- [208].Hisamochi K, Morimoto T, Bando K, Senoo Y, Teramoto S. A new hydroxyl radical scavenger “EPC” on cadaver heart transplantation in a canine model. Surg Today. 1997;27:930–5. doi: 10.1007/BF02388141. [DOI] [PubMed] [Google Scholar]
- [209].Takayama H, Hamner CE, Caccitolo JA, et al. A novel antioxidant, EPC-K1, stimulates endothelial nitric oxide production and scavenges hydroxyl radicals. Circ J. 2003;67:1046–52. doi: 10.1253/circj.67.1046. [DOI] [PubMed] [Google Scholar]
- [210].Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- [211].Ray PS, Maulik G, Cordis GA, et al. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999;27:160–9. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- [212].Dernek S, Ikizler M, Erkasap N, et al. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand Cardiovasc J. 2004;38:245–54. doi: 10.1080/14017430410035476. [DOI] [PubMed] [Google Scholar]
- [213].Mokni M, Limam F, Elkahoui S, Amri M, Aouani E. Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Arch Biochem Biophys. 2007;457:1–6. doi: 10.1016/j.abb.2006.10.015. [DOI] [PubMed] [Google Scholar]
- [214].Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am J Physiol Heart Circ Physiol. 2003;285:H2171–8. doi: 10.1152/ajpheart.00143.2003. [DOI] [PubMed] [Google Scholar]
- [215].Wu TW, Zeng LH, Wu J, Fung KP. Myocardial protection of MCI-186 in rabbit ischemia-reperfusion. Life Sci. 2002;71:2249–55. doi: 10.1016/s0024-3205(02)01965-3. [DOI] [PubMed] [Google Scholar]
- [216].Yagi H, Horinaka S, Matsuoka H. Edaravone prevented deteriorated cardiac function after myocardial ischemia-reperfusion via inhibiting lipid peroxidation in rat. J Cardiovasc Pharmacol. 2005;46:46–51. doi: 10.1097/01.fjc.0000162772.16797.7f. [DOI] [PubMed] [Google Scholar]
- [217].Halmosi R, Deres P, Toth A, et al. 2,2,5,5-Tetramethylpyrroline-based compounds in prevention of oxyradical-induced myocardial damage. J Cardiovasc Pharmacol. 2002;40:854–67. doi: 10.1097/00005344-200212000-00006. [DOI] [PubMed] [Google Scholar]
- [218].Toth A, Halmosi R, Kovacs K, et al. Akt activation induced by an antioxidant compound during ischemia-reperfusion. Free Radic Biol Med. 2003;35:1051–63. doi: 10.1016/s0891-5849(03)00467-2. [DOI] [PubMed] [Google Scholar]
- [219].Toth A, Kovacs K, Deres P, et al. Impact of a novel cardioprotective agent on the ischaemia-reperfusion-induced Akt kinase activation. Biochem Pharmacol. 2003;66:2263–72. doi: 10.1016/j.bcp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- [220].Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–99. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- [221].Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11:1913–28. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- [222].Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–9. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- [223].Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [225].Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Kemi M, Keenan KP, McCoy C, et al. The relative protective effects of moderate dietary restriction versus dietary modification on spontaneous cardiomyopathy in male Sprague-Dawley rats. Toxicol Pathol. 2000;28:285–96. doi: 10.1177/019262330002800208. [DOI] [PubMed] [Google Scholar]
- [227].Meyer TE, Kovacs SJ, Ehsani AA, et al. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- [228].Dhalla NS, Golfman L, Takeda S, Takeda N, Nagano M. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can J Cardiol. 1999;15:587–93. [PubMed] [Google Scholar]
- [229].Grieve DJ, Byrne JA, Cave AC, Shah AM. Role of oxidative stress in cardiac remodelling after myocardial infarction. Heart Lung Circ. 2004;13:132–8. doi: 10.1016/j.hlc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- [230].Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–7. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Kaneko T, Tahara S, Matsuo M. Retarding effect of dietary restriction on the accumulation of 8-hydroxy-2′-deoxyguanosine in organs of Fischer 344 rats during aging. Free Radic Biol Med. 1997;23:76–81. doi: 10.1016/s0891-5849(96)00622-3. [DOI] [PubMed] [Google Scholar]
- [232].Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–24. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- [233].Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–33. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- [234].Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–91. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- [235].Lambert AJ, Merry BJ. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am J Physiol Regul Integr Comp Physiol. 2004;286:R71–9. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- [236].Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Diniz YS, Cicogna AC, Padovani CR, et al. Dietary restriction and fibre supplementation: oxidative stress and metabolic shifting for cardiac health. Can J Physiol Pharmacol. 2003;81:1042–8. doi: 10.1139/y03-097. [DOI] [PubMed] [Google Scholar]
- [238].Crescenzo R, Lionetti L, Mollica MP, et al. Altered skeletal muscle subsarcolemmal mitochondrial compartment during catch-up fat after caloric restriction. Diabetes. 2006;55:2286–93. doi: 10.2337/db06-0312. [DOI] [PubMed] [Google Scholar]
- [239].Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–8. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [240].Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;280:H2094–102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- [241].Broderick TL, Driedzic WR, Gillis M, Jacob J, Belke T. Effects of chronic food restriction and exercise training on the recovery of cardiac function following ischemia. J Gerontol A Biol Sci Med Sci. 2001;56:B33–7. doi: 10.1093/gerona/56.1.b33. [DOI] [PubMed] [Google Scholar]
- [242].Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–25. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- [243].Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–96. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [244].Shinmura K, Tamaki K, Saito K, et al. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–17. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- [245].Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–55. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–9. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- [249].Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–73. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- [251].Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- [252].Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates the endothelial nitric oxide synthase through protein kinase A and C pathways. Nephrol Dial Transplant. 2007;22:2831–7. doi: 10.1093/ndt/gfm269. [DOI] [PubMed] [Google Scholar]
- [253].Ritchie SA, Kohlhaas CF, Boyd AR, et al. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J. 2010;426:85–90. doi: 10.1042/BJ20091580. [DOI] [PubMed] [Google Scholar]
- [254].Cerqueira FM, Laurindo FR, Kowaltowski AJ. Mild mitochondrial uncoupling and calorie restriction increase fasting eNOS, akt and mitochondrial biogenesis. PLoS One. 2011;6:e18433. doi: 10.1371/journal.pone.0018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- [256].Nisoli E, Falcone S, Tonello C, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101:16507–12. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem. 2008;283:25256–63. doi: 10.1074/jbc.M802455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol. 2009;297:H2227–33. doi: 10.1152/ajpheart.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Donati A, Cavallini G, Paradiso C, et al. Age-related changes in the autophagic proteolysis of rat isolated liver cells: effects of antiaging dietary restrictions. J Gerontol A Biol Sci Med Sci. 2001;56:B375–83. doi: 10.1093/gerona/56.9.b375. [DOI] [PubMed] [Google Scholar]
- [260].Wohlgemuth SE, Julian D, Akin DE, et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–92. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- [261].Bergamini E, Cavallini G, Donati A, Gori Z. The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction. Ann N Y Acad Sci. 2007;1114:69–78. doi: 10.1196/annals.1396.020. [DOI] [PubMed] [Google Scholar]
- [262].Simonsen A, Cumming RC, Brech A, et al. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–84. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- [263].Shinmura K, Tamaki K, Sano M, et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–27. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- [264].Lekli I, Szabo G, Juhasz B, et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol. 2008;294:H859–66. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- [265].Lekli I, Ray D, Mukherjee S, et al. Co-ordinated autophagy with resveratrol and gamma-tocotrienol confers synergetic cardioprotection. J Cell Mol Med. 2010;14:2506–18. doi: 10.1111/j.1582-4934.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [267].Sulaiman M, Matta MJ, Sunderesan NR, et al. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:H833–43. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Thirunavukkarasu M, Penumathsa SV, Koneru S, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Seya K, Kanemaru K, Sugimoto C, et al. Opposite effects of two resveratrol (trans-3,5,4′-trihydroxystilbene) tetramers, vitisin A and hopeaphenol, on apoptosis of myocytes isolated from adult rat heart. J Pharmacol Exp Ther. 2009;328:90–8. doi: 10.1124/jpet.108.143172. [DOI] [PubMed] [Google Scholar]
- [270].Gurusamy N, Lekli I, Mukherjee S, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–12. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Juric D, Wojciechowski P, Das DK, Netticadan T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am J Physiol Heart Circ Physiol. 2007;292:H2138–43. doi: 10.1152/ajpheart.00852.2006. [DOI] [PubMed] [Google Scholar]
- [272].Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–6. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- [273].Bertelli AA, Giovannini L, Giannessi D, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- [274].Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–35. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- [276].Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Ni YG, Wang N, Cao DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–22. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–31. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [281].Armour SM, Baur JA, Hsieh SN, et al. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1:515–28. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol. 2010;15:e134–8. [PMC free article] [PubMed] [Google Scholar]
- [283].Chen C, Ko Y, Delannoy M, et al. Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem. 2004;279:31761–8. doi: 10.1074/jbc.M401353200. [DOI] [PubMed] [Google Scholar]
- [284].Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104:13632–7. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Tinhofer I, Bernhard D, Senfter M, et al. Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl-2. FASEB J. 2001;15:1613–5. doi: 10.1096/fj.00-0675fje. [DOI] [PubMed] [Google Scholar]
- [286].Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–75. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Patel KR, Scott E, Brown VA, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–9. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- [289].Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–60. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- [290].Minners J, van den Bos EJ, Yellon DM, et al. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- [291].Araki M, Motojima K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2008;367:462–7. doi: 10.1016/j.bbrc.2007.12.166. [DOI] [PubMed] [Google Scholar]
- [292].Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- [293].Haffner SM, Alexander CM, Cook TJ, et al. Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1999;159:2661–7. doi: 10.1001/archinte.159.22.2661. [DOI] [PubMed] [Google Scholar]
- [294].Strazzullo P, Kerry SM, Barbato A, et al. Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Hypertension. 2007;49:792–8. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- [295].Sukhova G, Williams J, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–8. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- [296].McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation. 1978;57:958–62. doi: 10.1161/01.cir.57.5.958. [DOI] [PubMed] [Google Scholar]
- [297].Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73:326–40. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- [298].Ascensao A, Magalhaes J, Soares JM, et al. Endurance training limits the functional alterations of rat heart mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol. 2006;109:169–78. doi: 10.1016/j.ijcard.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [299].Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol. 1994;76:1608–14. doi: 10.1152/jappl.1994.76.4.1608. [DOI] [PubMed] [Google Scholar]
- [300].Brown DA, Chicco AJ, Jew KN, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569:913–24. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Demirel HA, Powers SK, Caillaud C, et al. Exercise training reduces myocardial lipid peroxidation following short-term ischemia-reperfusion. Med Sci Sports Exerc. 1998;30:1211–6. doi: 10.1097/00005768-199808000-00005. [DOI] [PubMed] [Google Scholar]
- [302].Demirel HA, Powers SK, Zergeroglu MA, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–12. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- [303].Hamilton KL, Powers SK, Sugiura T, et al. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–52. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- [304].Lennon SL, Quindry JC, French JP, et al. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand. 2004;182:161–9. doi: 10.1111/j.1365-201X.2004.01346.x. [DOI] [PubMed] [Google Scholar]
- [305].Libonati JR, Gaughan JP, Hefner CA, et al. Reduced ischemia and reperfusion injury following exercise training. Med Sci Sports Exerc. 1997;29:509–16. doi: 10.1097/00005768-199704000-00013. [DOI] [PubMed] [Google Scholar]
- [306].Powers SK, Demirel HA, Vincent HK, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998;275:R1468–77. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- [307].Quindry J, French J, Hamilton K, et al. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40:416–25. doi: 10.1016/j.exger.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [308].Yamashita N, Baxter GF, Yellon DM. Exercise directly enhances myocardial tolerance to ischaemia-reperfusion injury in the rat through a protein kinase C mediated mechanism. Heart. 2001;85:331–6. doi: 10.1136/heart.85.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Yamashita N, Hoshida S, Otsu K, et al. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Hamilton KL, Staib JL, Phillips T, et al. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med. 2003;34:800–9. doi: 10.1016/s0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- [311].Taylor RP, Harris MB, Starnes JW. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol. 1999;276:H1098–102. doi: 10.1152/ajpheart.1999.276.3.H1098. [DOI] [PubMed] [Google Scholar]
- [312].Demirel HA, Hamilton KL, Shanely RA, et al. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am J Physiol Heart Circ Physiol. 2003;285:H1609–15. doi: 10.1152/ajpheart.00982.2002. [DOI] [PubMed] [Google Scholar]
- [313].Hutter JJ, Mestril R, Tam EK, et al. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–11. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- [314].Jayakumar J, Suzuki K, Sammut IA, et al. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104:I303–7. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- [315].Suzuki K, Murtuza B, Sammut IA, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–6. [PubMed] [Google Scholar]
- [316].Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–32. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- [317].Knowlton AA. Heat-shock proteins, stress, and the heart. Ann N Y Acad Sci. 1994;723:128–37. [PubMed] [Google Scholar]
- [318].Powers SK, Locke, Demirel HA. Exercise, heat shock proteins, and myocardial protection from I-R injury. Med Sci Sports Exerc. 2001;33:386–92. doi: 10.1097/00005768-200103000-00009. [DOI] [PubMed] [Google Scholar]
- [319].Steel R, Doherty JP, Buzzard K, et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–9. doi: 10.1074/jbc.M401314200. [DOI] [PubMed] [Google Scholar]
- [320].Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol. 1985;59:1298–303. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- [321].Lew H, Quintanilha A. Effects of endurance training and exercise on tissue antioxidative capacity and acetaminophen detoxification. Eur J Drug Metab Pharmacokinet. 1991;16:59–68. doi: 10.1007/BF03189876. [DOI] [PubMed] [Google Scholar]
- [322].Powers SK, Criswell D, Lawler J, et al. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993;265:H2094–8. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- [323].Somani SM, Frank S, Rybak LP. Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacol Biochem Behav. 1995;51:627–34. doi: 10.1016/0091-3057(94)00427-k. [DOI] [PubMed] [Google Scholar]
- [324].Venditti P, Di Meo S. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch Biochem Biophys. 1996;331:63–8. doi: 10.1006/abbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- [325].Hamilton KL, Quindry JC, French JP, et al. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med. 2004;37:1360–8. doi: 10.1016/j.freeradbiomed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- [326].Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–22. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Menshikova EV, Ritov VB, Fairfull L, et al. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Safdar A, Little JP, Stokl AJ, et al. Exercise Increases Mitochondrial PGC-1{alpha} Content and Promotes Nuclear-Mitochondrial Cross-talk to Coordinate Mitochondrial Biogenesis. J Biol Chem. 2011;286:10605–17. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [329].Vina J, Gomez-Cabrera MC, Borras C, et al. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61:1369–74. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [330].Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R912–7. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- [331].Salminen A, Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:97–106. doi: 10.1007/BF02889856. [DOI] [PubMed] [Google Scholar]
- [332].Dohm GL, Tapscott EB, Kasperek GJ. Protein degradation during endurance exercise and recovery. Med Sci Sports Exerc. 1987;19:S166–71. [PubMed] [Google Scholar]
- [333].Mackenzie MG, Hamilton DL, Murray JT, Baar K. mVps34 is activated by an acute bout of resistance exercise. Biochem Soc Trans. 2007;35:1314–6. doi: 10.1042/BST0351314. [DOI] [PubMed] [Google Scholar]
- [334].Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol. 2008;294:H928–35. doi: 10.1152/ajpheart.01231.2007. [DOI] [PubMed] [Google Scholar]