Abstract
OBJECTIVE
Single-center studies have suggested that hypovitaminosis D is widespread. Our objective was to determine the serum levels of 25-hydroxyvitamin D (25[OH]D) in a nationally representative sample of US children aged 1 to 11 years.
METHODS
Data were obtained from the 2001–2006 National Health and Nutrition Examination Survey. Serum 25(OH)D levels were determined by radioimmunoassay and categorized as <25, <50, and <75 nmol/L. National estimates were obtained by using assigned patient visit weights and reported with 95% confidence intervals (CIs).
RESULTS
During the 2001–2006 time period, the mean serum 25(OH)D level for US children aged 1 to 11 years was 68 nmol/L (95% CI: 66 –70). Children aged 6 to 11 years had lower mean levels of 25(OH)D (66 nmol/L [95% CI: 64 –68]) compared with children aged 1 to 5 years (70 nmol/L [95% CI: 68 –73]). Overall, the prevalence of levels at <25 nmol/L was 1% (95% CI: 0.7–1.4), <50 nmol/L was 18% (95% CI: 16–21), and <75 nmol/L was 69% (95% CI: 65–73). The prevalence of serum 25(OH)D levels of <75 nmol/L was higher among children aged 6 to 11 years (73%) compared with children aged 1 to 5 years (63%); girls (71%) compared with boys (67%); and non-Hispanic black (92%) and Hispanic (80%) children compared with non-Hispanic white children (59%).
CONCLUSIONS
On the basis of a nationally representative sample of US children aged 1 to 11 years, millions of children may have suboptimal levels of 25(OH)D, especially non-Hispanic black and Hispanic children. More data in children are needed not only to understand better the health implications of specific serum levels of 25(OH)D but also to determine the appropriate vitamin D supplement requirements for children.
Keywords: vitamin D, deficiency, prevalence, supplementation
Vitamin D3 (cholecalciferol) comes from 2 sources: exposure to UV-B rays in natural sunlight and dietary intake (including supplements). Because few foods contain vitamin D precursors,1 sunlight exposure is the primary determinant of vitamin D status in humans. However, in northern latitudes between November and March there are insufficient UV-B rays to produce vitamin D.2,3 Moreover, sunscreen use in childhood, which protects against future melanoma,4–6 decreases vitamin D production in the skin.7 Together, these factors contribute to the recent reports of hypovitaminosis D in diverse populations.1,2
There is controversy, however, about what a healthy level of 25-hydroxyvitamin D (25[OH]D) is for children and even what level of 25(OH)D should be used to define vitamin D deficiency.8–11 In 1997, the Institute of Medicine defined vitamin D deficiency as a serum 25(OH)D level of <27.5 nmol/L (to convert to ng/mL divide by 2.496).8 In 2007, the Canadian Paediatric Society defined vitamin D deficiency as a serum 25(OH)D level of <25 nmol/L.12 Also, in a 2008 review written on behalf of the Lawson Wilkins Pediatric Endocrine Society, vitamin D deficiency was defined as a serum 25(OH)D level of <37.5 nmol/L.13 Although investigators’ definitions differ and different laboratory assays have been used to determine serum 25(OH)D levels, the preponderance of evidence from single-center studies suggests widespread hypovitaminosis D in children of all ages.11,14–18 A nationally representative study of children aged ≥12 years from 1988 –1994, using an even stricter criterion for deficiency (<17.5 nmol/L), found little evidence of deficiency, but found that lesser degrees of vitamin D deficiency are common.12 And there is a growing interest in these lesser degrees of vitamin D deficiency. Indeed, an examination of the 2000 –2004 National Health and Nutrition Examination Survey (NHANES) shows, among other data, the percentage of males and females 0 to 70 years of age with a 25(OH)D level of <50 nmol/L.19
Although controversial,20,21 higher levels of 25(OH)D have been shown in some studies to improve bone health22 and also may help prevent cancer,23–26 autoimmune diseases/type 1 diabetes,24,27–29 infectious diseases,30–32 myocardial infarction,33 and childhood wheezing.34,35 In adults, emerging data suggest that the optimal level of 25(OH)D may be as high as 75 or 100 nmol/L.36,37 In children, the optimal level of serum 25(OH)D for general health is unknown and even more controversial then in adults, because there are fewer outcome data.37,38
Our objective was to determine the prevalence of serum 25(OH)D levels of <25, <50, and <75 nmol/L in a nationally representative sample of US children aged 1 to 11 years.
METHODS
Study Design and Participants
Annually, the National Center for Health Statistics conducts the NHANES, a nationally representative probability sample of the noninstitutionalized US civilian population. Since 1999, NHANES data were released in 2-year cycles. We received a waiver from our institutional review board and analyzed the 3 most recent cycles of NHANES data (ie, January 2001 to December 2006).
Details of survey methodology are described elsewhere.39 Briefly, the sample was obtained by using a complex, stratified, multistage probability study design with unequal probabilities of selection. The NHANES oversamples certain subgroups of people including low-income persons, black persons, and Mexican Americans. The NHANES uses a 4-stage sampling strategy covering geographic primary sampling units, which are counties or small groups of contiguous counties, segments within primary sampling units (a block or group of blocks), households within segments, and 1 or more participants within households.
Serum 25(OH)D levels were obtained for participants aged 6 to 11 years from 2001–2006 and for those aged 1 to 5 years from 2003 to 2006. During 2001–2006, the NHANES collected household interview data for 3421 (87%) of 3951 invited participants aged 6 to 11. Subsequently, 3300 (96%) participants received physical and laboratory examination in a mobile examination center (MEC), of which 2759 had serum 25(OH)D testing (541 missing). During 2003–2006, the NHANES collected household interview data for 2677 (89%) of 3006 invited participants aged 1 to 5. Subsequently, 2346 (88%) participants had MEC evaluation, of which 1799 had serum 25(OH)D testing (547 missing).
Data Collection
Throughout the NHANES years, strategies for sampling and methodologies for data collection were very similar to maintain consistency and facilitate comparisons. To avoid weather issues and improve response, the NHANES MECs preferentially scheduled data collection in the lower latitudes (farther south) during winter months and higher latitudes (farther north) during the summer months. Demographic data (age, gender, race/ethnicity), based on self-report, were recorded. The poverty/income ratio is provided in the NHANES data set and was used to control for socioeconomic status; values of <1 were considered low socioeconomic status. For the vitamin supplement data, the participant showed the interviewer all of the vitamin/mineral supplements being used. The interviewer recorded the information and asked about frequency of use. Later, trained nutritionists matched each supplement to the National Center for Health Statistics database, which contained detailed information about ingredients. We recorded the vitamin D dosage for all supplements and the frequency of use. The results are stratified by those who were and were not taking vitamin D–containing supplements.
Blood samples for serum 25(OH)D testing collected during the examination were centrifuged, aliquoted, and frozen to −70°C on-site, and shipped on dry ice to central laboratories where they were stored at −70°C until analysis. Serum 25(OH)D levels were measured by a radioimmunoassay kit after extraction with acetonitrile (DiaSorin, Stillwater, MN) by the National Center for Environmental Health (Atlanta, GA).
Analysis
We performed statistical analyses by using Stata 10.1 (Stata Corp, College Station, TX). Using survey commands, we applied the recommended sub-sample weights for the interview plus examination data to account for unequal probabilities of selection. The NHANES provides weighting of the actual number of subjects to allow for the sample to represent the entire US population on the basis of census statistics. We used these weights to provide population estimates of 25(OH)D levels. Although all of results are presented as weighted values, we also provide the raw numbers to allow for insight about the sample size. We calculated variance on the basis of NHANES-provided masked variance units by using the Taylor series linearization method. We categorized serum 25(OH)D levels as <25, <50, and <75 nmol/L. Primary analysis is descriptive with 95% confidence intervals (CIs).We tested for interactions by using logistic regression using interaction terms for age and race/ethnicity while controlling for the primary effects of age, gender, and race/ethnicity.
RESULTS
During 2001–2006, the mean serum 25(OH)D for children aged 1 to 11 years was 68 nmol/L. Using the NHANES weighting of the actual number of subjects to provide population estimates, there were 90 participants with serum 25(OH)D levels of <25 nmol/L, representing 320 000 (95% CI: 220 000–430 000) children; 1268 participants with a serum 25(OH)D level of <50 nmol/L, representing 6.3 million (95% CI: 5.4 –7.2 million) children; and 3587 participants with a serum 25(OH)D level of <75 nmol/L, representing 24 million (95% CI: 21–26 million) children. Overall, the prevalence of serum 25(OH)D levels of <25 nmol/L was 1.0% (95% CI: 0.7–1.4), <50 nmol/L was 18% (95% CI: 16 –21), and <75 nmol/L was 95% (95% CI: 65–73).
Demographics
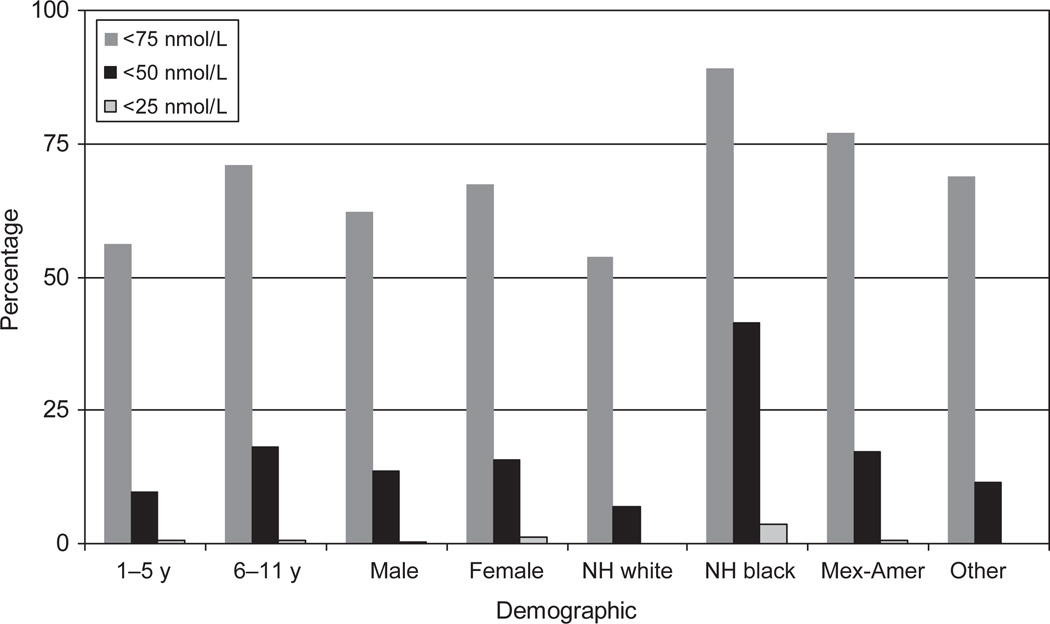
Fig 1 shows the prevalence of serum 25(OH)D levels for all of the children as a group (ie, everyone aged 1 to 11 years) at 3 thresholds: <25, <50, and <75 nmol/L. Although the prevalence of children aged 1 to 11 years with a serum 25(OH)D level of <25 nmol/L is not high (1.0%), non-Hispanic black children (4.8%) clearly have the highest percentage. When the Institute of Medicine’s definition of deficiency (<27.5 nmol/L) is used, the prevalence of vitamin D deficiency increases to 1.5% for all children aged 1 to 11 years.8 When the threshold of <50 nmol/L is used a substantial proportion of children have low levels of serum 25(OH)D. If a serum 25(OH)D level of <75 nmol/L is used, the prevalence of children aged 1 to 11 years in this category is higher among girls (71% [95% CI: 66 –76]) compared with boys (67% [95% CI: 62–72]), and non-Hispanic black (92% [95% CI: 89 –94]) and Hispanic children (80% [95%CI:77– 83]) compared with non-Hispanic white children (59% [95% CI: 53–65]). Adjustment for socioeconomic status, as measured by poverty/income ratio, did not materially change these results (data not shown).
FIGURE 1.
Demographics for children aged 1 to 11 years using 3 thresholds of serum 25(OH)D. NH indicates non-Hispanic.
Mean and categorical 25(OH)D levels stratified by age are presented in the Table 1, because the mean levels of 25(OH)D were lower in children aged 6 to 11 years (66 nmol/L [95% CI: 64 –68]) compared with children aged 1 to 5 years (70 nmol/L [95% CI: 68 –73]), whereas the prevalence of 25(OH)D levels at <75 nmol/L was higher among children aged 6 to 11 (73% [95% CI: 69 –78]) compared with children aged 1 to 5 (63% [95% CI: 58 –68]). More than 1 of every 5 children aged 6 to 11 years and over half of non-Hispanic black children aged 6 to 11 years has a 25(OH)D level of <50 nmol/L. If the threshold is changed to <75 nmol/L, almost all non-Hispanic black and Hispanic children aged 6 to 11 (96% and 86%, respectively) have low levels and most children aged 1 to 5 (85% and 73%, respectively) do as well. Although controlling for age, gender, and race/ethnicity, there was a statistically significant interaction between age and both non-Hispanic black (P < .001) and Hispanic (P = .03) ethnicity.
TABLE 1.
Demographic Characteristics of Children Aged 1 to 11 Years According to Serum 25(OH)D Level, 2001–2004
| Mean, nmol/L (95% CI) |
<25 nmol/L, % (95% CI) |
25–49.9 nmol/L, % (95% CI) |
50–74.9 nmol/L, % (95% CI) |
≥75 nmol/L, % (95% CI) |
|
|---|---|---|---|---|---|
| Ages 1–5 | |||||
| Total (n = 1799) | 70 (68–73) | <1 (NC) | 14 (11–18) | 48 (44–52) | 37 (32–42) |
| Gender | |||||
| Male (n = 904) | 71 (69–74) | <1 (NC) | 14 (11–18) | 46 (40–51) | 39 (33–45) |
| Female (n = 895) | 70 (67–72) | <1 (NC) | 14 (11–18) | 51 (45–56) | 35 (29–41) |
| Race/ethnicity | |||||
| NH white (n = 516) | 74 (72–78) | 0 | 8 (6–12) | 46 (39–52) | 46 (40–53) |
| NH black (n = 503) | 58 (55–61) | 3 (NC) | 31 (25–38) | 51 (45–56) | 15 (11–20) |
| Hispanic (n = 677) | 67 (65–69) | <1 (NC) | 17 (13–22) | 55 (51–59) | 27 (23–32) |
| Other (n = 103) | 69 (64–74) | <1 (NC) | 20 (NC) | 41 (32–51) | 39 (28–41) |
| Ages 6–11 | |||||
| Total (n = 2759) | 66 (64–68) | <1 (1–2) | 20 (17–23) | 52 (48–56) | 27 (22–31) |
| Sex | |||||
| Male (n = 1359) | 67 (65–70) | <1 (NC) | 18 (15–21) | 53 (49–58) | 28 (24–34) |
| Female (n = 1400) | 64 (62–66) | 2 (1–3) | 22 (19–26) | 51 (46–56) | 25 (20–30) |
| Race/ethnicity | |||||
| NH white (n = 751) | 72 (70–75) | <1 (NC) | 11 (8–14) | 52 (46–57) | 38 (31–44) |
| NH black (n = 895) | 49 (48–52) | 6 (4–8) | 48 (44–52) | 42 (38–46) | 4 (3–6) |
| Hispanic (n = 963) | 60 (58–62) | 1 (NC) | 26 (22–31) | 59 (55–63) | 14 (10–18) |
| Other (n = 105) | 62 (59–65) | 1 (NC) | 20 (12–30) | 61 (48–73) | 18 (11–28) |
NC indicates not calculable (n < 30 observations); NH, non-Hispanic
Vitamin D Supplementation
Any vitamin supplement was taken by 38% (95% CI: 35–41) of participants, of which 89% contained vitamin D. The vitamin D dosage was ≤100 IU for 9.5%, 100 –399 IU for 5.6%, 400 IU for 83%, and ≥400 IU for 2.3% of these supplements. Daily use was reported for 51% of participants, and 80% reported use of ≥15 of the past 30 days. The mean serum 25(OH)D level was higher among those reporting taking vitamin D–containing supplements (72.3 nmol/L) compared with those without supplementation (67.0 nmol/L). Among those children aged 1 to 11 years taking vitamin D–containing supplements, 10% had a 25(OH)D level of <50 nmol/L and 62% had a level of <75 nmol/L. Among those children aged 1 to 11 years not taking supplements, 21% had a 25(OH)D level of <50 nmol/L and 70% had a level of <75 nmol/L.
DISCUSSION
To our knowledge, this is the first nationally representative sample providing an in depth examination of the prevalence of 3 levels of serum 25(OH)D (<25, <50, and <75 nmol/L) in children aged 1 to 11 years. These data highlight that millions of children in the United States have levels of 25(OH)D some experts consider to be too low and that non-Hispanic black and Hispanic children have the lowest levels of 25(OH)D. Practitioners caring for children should become aware of the emerging data about the diverse health effects of vitamin D and understand that this group of children may require more vitamin D then they are currently making from sunlight or consuming in their diet (including supplements).
In children, there are sparse outcome data to help define a healthy or optimal level of 25(OH)D. Although vitamin D deficiency has been defined as a child having a serum 25(OH)D level of <25 nmol/L10 or <27.5 nmol/L,8 some have proposed that levels of <50 nmol/L should define vitamin D deficiency.9,13 The initial definitions and discussion about a healthy level of vitamin D concerned the prevention of rickets.8 Data however, demonstrate that vitamin D is a prohormone with receptors throughout the body.40 As a result, health outcomes beyond rickets are now being considered when experts and national panels try to define a healthy level of 25(OH)D for children.41
In terms of bone-related outcomes, Gordon et al22 recently demonstrated that one third of infants and toddlers with a serum 25(OH)D level of <50 nmol/L were noted to have some evidence of bone demineralization by standard radiograph. In addition, mild rickets has been found in North American infants with serum 25(OH)D levels close to 50 nmol/L.42 Considering nonbone health outcomes, a meta-analysis shows that the risk of type 1 diabetes is significantly reduced in children receiving vitamin D supplements compared with those not receiving supplements.29 Respiratory outcomes have also been investigated. Children aged 2 to 60 months hospitalized with acute lower respiratory tract infection had a 12.5-fold higher unadjusted odds of having a serum 25(OH)D level of <50 nmol/L compared with healthy controls.43 Two prospective birth cohorts, 1 from the United States34 and 1 from Scotland35 have both demonstrated that lower maternal intake of vitamin D during pregnancy is associated with increased risk of recurrent wheezing in these mothers’ children. Combining the infection and wheezing outcomes, Camargo et al44 found in a birth cohort of 922 New Zealand children an inverse relationship between the level of 25(OH)D in umbilical cord blood and parent-reported respiratory infection (ie, bronchiolitis, croup, pneumonia, throat infections, and ear infections) by 3 months of age and parent-reported wheezing illness at 15 months, 3 years, and 5 years of age. These results were independent of season, exclusive breastfeeding, and other potentially relevant confounders. The risk was lowest for children with a cord blood 25(OH)D level of ≥75 nmol/L.44 The mechanism underlying the association with vitamin D and risk of infections is being examined, but the innate immune system seems to play a role.45
In adults, there is more data describing the nonbone health effects of vitamin D.* Two recently published studies33,48 demonstrate that a healthy level of 25(OH)D may be at least 75 nmol/L. In 1 study, patients with colorectal cancer who had mean 25(OH)D levels of >100 nmol/L were almost 50% less likely to die (from all causes, including colon cancer) than those with a mean serum 25(OH)D level of <42 nmol/L.48 In another recent prospective study, the risk of myocardial infarction increased as a man’s serum 25(OH)D level decreased. Men with serum 25(OH)D levels of <37.5 nmol/L had a 2.4 relative risk increase in myocardial infarction compared with men with serum 25(OH)D levels of ≥75 nmol/L after controlling for multiple other risk factors.33 Adult data frequently do not translate to children, and the clinical outcomes examined in many of the adult vitamin D studies are not relevant for children. Nonetheless, these adult data should not be ignored by clinicians caring for children, because results from both adult and pediatric studies similarly suggest that healthier levels of 25(OH)D may prove to be 50, 75, or even >75 nmol/L.
The level of concern regarding the national prevalence data presented here will depend, in part, on what level of 25(OH)D is eventually found to be optimal for a child’s health. The Canadian Paediatric Society has termed children with levels of 25(OH)D of <75 nmol/L as insufficient.10 When this 75 nmol/L level is applied to US children aged 1 to 11 years, 2 of every 3 children in the United States are below this threshold and almost all non-Hispanic black and Hispanic children are below this threshold. If the threshold is changed to <50 nmol/L, then over half of non-Hispanic black children aged 6 to 11 years fall into this category. Considering these prevalence data, would there be risks associated with recommending that all children have vitamin D supplements until the short- and long-term health outcome data are elucidated? Toxicity, from excessive intake of vitamin D is rare but is considered for adults taking >10 000 IU per day.49 Case reports of toxicity describe a 42-year-old man who inadvertently may have taken between 156 000 and 2 640 000 IU of vitamin D3 per day for 2 years50 and 1 study in which children aged 3 to 36 months were given 300 000 IU of vitamin D.51 It should be noted, however, that appropriately designed studies may need to be conducted to examine if excessive vitamin D supplementation either causes less overt complications then those currently recognized or long-term negative effects on adult-onset diseases. Currently, only 1 of 3 children is taking a vitamin D–containing supplement. And although vitamin D supplementation raises 25(OH)D levels, 1 in 10 children taking vitamin D–containing supplements at current doses (100 –400 IU) had a 25(OH)D level of <50 nmol/L and over half of children had a level of <75 nmol/L. As suggested recently by the Canadian Paediatric Society and a recent state-of-the-art review on vitamin D,10,13 higher supplement doses may be required for children to reach the safe and potentially healthier levels of 25(OH)D of >50 or >75 nmol/L.
Sunlight, specifically UV-B rays, promotes the cutaneous generation of vitamin D3. This process, however, is reduced by increased melanin pigmentation of the skin.52 The effect of skin pigmentation on levels of 25(OH)D is evident in these data and may help explain the surprising finding that children aged 1 to 5 years have higher levels of 25(OH)D compared with older children aged 6 to 11 years. We hypothesize that as children age and begin playing outdoors more, the relative effects of skin pigmentation may become more evident and explain the lower levels of 25(OH)D in the older non-Hispanic black and Hispanic children. We plan to examine this idea in future work.
The present study has some potential limitations. We did not analyze latitude and season in this analysis, the most important determinants of the serum level of 25(OH)D. For these NHANES data, serum was collected at only 1 point in time and preferentially collected in northern states in the summer and southern states in the winter. Therefore, this geographical preference used for the sampling may slightly increase overall population 25(OH)D levels and, as a result, these data most likely represent the “best-case” scenario for 25(OH)D levels. If, the sampling methodology were changed to random sampling across all seasons, this methodology should yield an even higher prevalence of lower levels of 25(OH)D. Moreover, our goal in this manuscript was not to discuss or verify the causes of low levels of 25(OH)D, such as latitude and season, but rather to describe the prevalence of serum 25(OH)D levels in children aged 1 to 11 years. It should be assumed that at higher latitudes in the winter the prevalence of lower levels of 25(OH)D would be even greater. Another potential limitation is that demographic and vitamin supplementation information is self-report, and other factors associated with 25(OH)D levels were not analyzed (eg, estimated sunlight exposure and diet). However, the purpose of our analysis was to examine the prevalence of certain levels of 25(OH)D in children and not to predict or determine the cause of the observed levels.
CONCLUSIONS
There are millions of children with levels of 25(OH)D lower than some experts believe to be healthy, especially among non-Hispanic black and Hispanic children. These data are a call to action to determine in children the optimal level of serum 25(OH)D, the health effects of vitamin D in children, and the different approaches that one might use to reach a healthy level. In the meantime, however, on the basis of the limited risk of toxicity, we conclude that it is likely that many US children need more vitamin D.
WHAT’S KNOWN ON THIS SUBJECT
Single-center studies have suggested that hypovitaminosis D is widespread. Our objective was to determine the serum levels of 25(OH)D in a nationally representative sample of US children aged 1 to 11 years.
WHAT THIS STUDY ADDS
On the basis of a nationally representative sample of US children aged 1 to 11 years, millions of children may have suboptimal levels of 25(OH)D, especially non-Hispanic black and Mexican American children. It is likely that many US children need more vitamin D.
ACKNOWLEDGMENTS
Dr Camargo was supported by the Massachusetts General Hospital Center for D-receptor Activation Research (Boston, MA) and grants R01-HL84401 and K23 A1077801 (Bethesda, MD).
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- NHANES
National Health and Nutrition Examination Survey
- MEC
mobile examination center
- CI
confidence interval
Footnotes
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Marks R, Jolley D, Lectsas S, Foley P. The role of childhood exposure to sunlight in the development of solar keratoses and non-melanocytic skin cancer. Med J Aust. 1990;152(2):62–66. doi: 10.5694/j.1326-5377.1990.tb124456.x. [DOI] [PubMed] [Google Scholar]
- 5.Autier P, Dore JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EPIMEL and EORTC Melanoma Cooperative Group. European Organisation for Research and Treatment of Cancer. Int J Cancer. 1998;77(4):533–537. doi: 10.1002/(sici)1097-0215(19980812)77:4<533::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 7.Fuller KE, Casparian JM. Vitamin D: balancing cutaneous and systemic considerations. South Med J. 2001;94(1):58–64. [PubMed] [Google Scholar]
- 8.Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 9.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88(2):S529–S533. doi: 10.1093/ajcn/88.2.529S. [DOI] [PubMed] [Google Scholar]
- 10.Vitamin D. supplementation: recommendation for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583–589. [PMC free article] [PubMed] [Google Scholar]
- 11.Rovner AJ, O’Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 12.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 13.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118(2):603–610. doi: 10.1542/peds.2006-0108. [DOI] [PubMed] [Google Scholar]
- 16.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 17.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86(1):150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Bowden SA, Robinson RF, Carr R, Mahan JD. Prevalence of vitamin D deficiency and insufficiency in children with osteopenia or osteoporosis referred to a pediatric metabolic bone clinic. Pediatrics. 2008;121(6) doi: 10.1542/peds.2007-2111. Available at: www.pediatrics.org/cgi/content/full/121/6/e1585. [DOI] [PubMed] [Google Scholar]
- 19.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):S558–S564. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JA. Defining vitamin D deficiency in infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):583–584. doi: 10.1001/archpedi.162.6.583. [DOI] [PubMed] [Google Scholar]
- 21.Brannon PM, Yetley EA, Bailey RL, Picciano MF. Overview of the conference “vitamin D and health in the 21st century: an update.”. Am J Clin Nutr. 2008;88(2):S483–S490. doi: 10.1093/ajcn/88.2.483S. [DOI] [PubMed] [Google Scholar]
- 22.Gordon CM, Feldman HA, Sinclair L, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 25.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167(10):1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 27.Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 28.Zella JB, DeLuca HF. Vitamin D and autoimmune diabetes. J Cell Biochem. 2003;88(2):216–222. doi: 10.1002/jcb.10347. [DOI] [PubMed] [Google Scholar]
- 29.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 30.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 32.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 36.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 38.Greer FR. Vitamin D deficiency—it’s more than rickets. J Pediatr. 2003;143(4):422–423. doi: 10.1067/S0022-3476(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data, 1999–2004. Hyattsville, MD: US Department of Health and Human Services; [Accessed June 26, 2008]. National Center for Health Statistics. Available at: www.cdc.gov/nchs/about/major/nhanes/datalink.htm. [Google Scholar]
- 40.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(suppl 6):S1689–S1696. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 41.Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008;9(2):161–170. doi: 10.1007/s11154-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 42.Arnaud SB, Stickler GB, Haworth JC. Serum 25-hydroxyvitamin D in infantile rickets. Pediatrics. 1976;57(2):221–225. [PubMed] [Google Scholar]
- 43.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 44.Camargo CA, Ingham T, Wickens K, et al. Cord blood 25-hydroxyvitamin D levels and risk of childhood wheeze in New Zealand [abstract] Am J Respir Crit Care Med. 2008;177(suppl):A993. [Google Scholar]
- 45.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 46.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 47.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50(6):364–368. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 48.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 49.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 50.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 51.Cesur Y, Caksen H, Gundem A, Kirimi E, Odabas D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16(8):1105–1109. doi: 10.1515/jpem.2003.16.8.1105. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211(4482):590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]