Abstract
Background
We report the synthesis of benzimidazoles using lanthanum chloride as an efficient catalyst. One-pot synthesis of 2-substituted benzimidazole derivatives from o-phenylenediamine and a variety of aldehydes were developed under mild reaction conditions.
Results
We have examined the effect of different solvents using the same reaction conditions. The yield of the product varied with the nature of the solvents, and better conversion and easy isolation of products were found with acetonitrile. In a similar manner, the reaction with o-phenylenediamine and 3,4,5-trimethoxybenzaldehyde was carried out without any solvents. The observation shows that the reaction was not brought into completion, even after starting for a period of 9 h, and the reaction mixture showed a number of spots in thin-layer chromatography.
Conclusions
In conclusion, lanthanum chloride has been employed as a novel and efficient catalyst for the synthesis of benzimidazoles in good yields from o-phenylenediamine and a wide variety of aldehydes. All of the reactions were carried out in the presence of lanthanum chloride (10 mol%) in acetonitrile at room temperature.
Keywords: Benzimidazoles, Aldehydes, o-Phenylenediamine, Lanthanum chloride
Background
Benzimidazole nucleus is found in a variety of naturally occurring compounds such as vitamin B12 and its derivatives; it is structurally similar to purine bases. Benzimidazoles and its derivatives represent one of the most biologically active classes of compounds, possessing a wide spectrum of activities, and these are well documented in the literature. They show selective nonpeptide luteinizing hormone-releasing hormone antagonist, lymphocyte-specific kinase inhibitor, N-methyl-d-aspartate antagonist, 5-liopoxygenase inhibitor, NS5B polymerase inhibitor (Figure 1), neuropeptide YY1 receptor antagonist, nonpeptide thrombin inhibitor, γ-aminobutyric acid receptor, factor Xa inhibitor, and poly (ADP-ribose) polymerase inhibitor. DNA-minor groove-binding agents possess antitumor activity, topoisomerase I inhibitors, angiotensin II inhibitors, and proliferation inhibitors. Several benzimidazole derivatives find applications that include antimicrobial, antihypertensive, anticancer antiulcer, antifungal, antihistamine activity, herbicides, and other veterinary applications as promising drugs in different therapeutic categories. The benzimidazole moieties express a significant activity against several viruses such as HIV, herpes (HSV-1), RNA influenza, human cytomegalovirus, selective angiotensin II inhibitors, and 5-HT3 antagonists. In addition, benzimidazoles are very impotent intermediates in synthetic routes and serve as ligands for asymmetric catalysts [1-8]. The high profile of biological applications of the benzimidazole compounds has prompted the emergence of extensive studies of their syntheses. In this context, numerous efforts have been made to synthesize benzimidazole derivatives. One of the most common methods for the preparation of benzimidazole derivatives involves the condensation of an o-phenylenediamine and carbonyl compounds such as aldehydes and acid derivatives. The condensation of o-phenylenediamine with carboxylic acid often requires strong acidic conditions and high temperatures [9,10]. The other method involves the oxidative cyclodehydrogenation of Schiff bases, which is generated from o-phenylenediamine and aldehydes in the presence of various catalysts.
Figure 1.
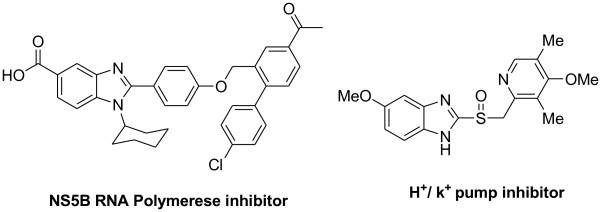
Scheme of NS5B polymerase and H+/K+ inhibitors.
This is the most popular approach in general for the synthesis of benzimidazole derivatives. The catalysts used are CAN, K3PO4, oxone, sulfamic acid, DDQ, PhI (OAc)2, iodine, and KHSO4[11-17]. In addition, several catalysts such as metal halides and metal oxychlorides, [18-22] metal oxides, PTSA, metal triflates, air, [23-30] ionic liquid, heteropoly acid, BDSB [31-33], proline, solid-supported catalysts, polymer-supported catalysts [34,35], and microwave-promoted [36-39] and clayzic [40] reactions have been reported in the literature. Unfortunately, many of these methods suffer from drawbacks such as drastic reaction conditions, low yields, tedious workup procedures, and co-occurrence of several side reactions. As a consequence, the introduction of an efficient and mild method is still needed to overcome these limitations.
As part of our research program in developing various synthetic methodologies [41-46], we report the synthesis of benzimidazoles using lanthanum chloride (LaCl3) as an efficient catalyst. The catalyst is known as an efficient catalyst in the literature for various organic transformations [47-52].
Methods
Melting points were recorded on a Buchi R-535 apparatus (BUCHI Labortechnik AG, Flawi, Switzerland) and were uncorrected. Infrared (IR) spectra were recorded on a PerkinElmer FT-IR 240-c spectrophotometer (PerkinElmer Instruments, Branford, CT, USA) using KBr discs. Hydrogen-1 nuclear magnetic resonance (1H NMR) spectra were recorded on a Gemini-200 spectrometer (Varian Medical Systems, Palo Alto, CA, USA) in CDCl3 using TMS as internal standard. Mass spectra were recorded on a Finnigan MAT 1020 (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer operating at 70 eV.
Results and discussion
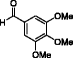
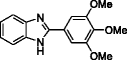
In a typical experiment, a reaction was made to occur between 1,2-phenylenediamine (OPD) (1) and 3,4,5-trimethoxybenzaldehyde (2) in the presence of LaCl3 in acetonitrile at room temperature to afford the corresponding product, 2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (3), in excellent yield. The reaction was completed within 2 h (Scheme 1).
Scheme 1.
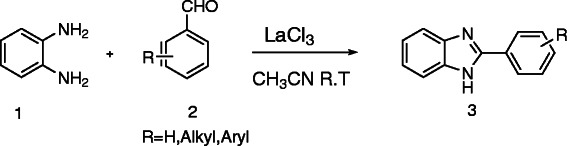
Reaction between 1,2-phenylenediamine (1) and 3,4,5-trimethoxybenzaldehyde (2) that yielded 2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (3).
We have examined the effect of different solvents using the same reaction conditions, as shown in Table 1. The yield of the product varied with the nature of the solvents; better conversion and easy isolation of products were found with acetonitrile. Acetonitrile dissolves a wide range of ionic and nonpolar compounds. In a similar manner, the reaction with o-phenylenediamine and 3,4,5-trimethoxybenzaldehyde was carried out without any solvents. The observation shows that the reaction was not brought into completion, even after starting for a period of 9 h, and the reaction mixture showed a number of spots in thin-layer chromatography (TLC).
Table 1.
Comparative study of the solvent system
| Number | Solvent | Time (h) | Yield (%) |
|---|---|---|---|
| 1 |
CH3CN |
2.0 |
95 |
| 2 |
CH3OH |
4.0 |
80 |
| 3 |
Dioxane |
5.0 |
75 |
| 4 |
THF |
6.0 |
70 |
| 5 |
Toluene |
7.0 |
65 |
| 6 |
DMF |
5.0 |
6 |
| 7 |
DCM |
8.0 |
50 |
| 8 | - | 9.0 | - |
In a similar manner, a comparative study on the role and requirement of the catalyst for condensation has been carried out, and the obtained results are clearly shown in Table 2. The reactants for this reaction are also o-phenylenediamine and 3,4,5-trimethoxybenzaldehyde in acetonitrile. From our observation, a catalytic amount (10 mol%) of LaCl3 was enough to complete the conversion of aldehyde and o-phenylenediamine into the required condensation product.
Table 2.
Comparative study of catalyst
| Number | Amount of catalyst (LaCl3) (eq) | Time (h) |
|---|---|---|
| 1 |
0.1 |
2.0 |
| 2 |
0.2 |
2.0 |
| 3 |
0.4 |
3.5 |
| 4 |
0.6 |
3.5 |
| 5 |
0.8 |
3.0 |
| 6 |
1.0 |
3.0 |
| 7 | No catalyst | 15 |
A blank experiment was carried out with o-phenylenediamine and 3,4,5-trimethoxybenzaldehyde in the absence of the catalyst LaCl3, and the required 3,4,5-trimethoxybenzimidazole product was not found even after stirring for 15 h. Finally, it was decided that the suitable conditions for condensation is in a solvent and in the presence of an activator or promoter. As shown in Table 3, aromatic, heteroaromatic, α-unsaturated and β-unsaturated aldehydes, and aliphatic aldehydes were reacted very well to afford the corresponding products of benzimidazole derivatives in very good to excellent yields. In general, the aromatic aldehydes having electron-donating groups and heteroaromatic compounds are reacting a little faster when compared with other aldehydes. In a similar manner, the aliphatic aldehydes and aromatic aldehydes containing electron-withdrawing groups are reacting comparatively a little slower in terms of conversion as well as yields, benzaldehyde and OPD, in the presence of the catalyst Lacl3. In general, all the reactions were completed within 2 to 4 h, and the obtained yields were 85% to 95%.
Table 3.
Lanthanum chloride-catalyzed synthesis of benzimidazoles
| Entry | Diamine | Aldehyde | Product (3a-3q) | Time (h) | Yields (%) | |||
|---|---|---|---|---|---|---|---|---|
| a |
|
|
|
2.0 |
95 |
|||
| b |
|
|
|
2.5 |
88 |
|||
| c |
|
|
|
2.0 |
91 |
|||
| d |
|
|
|
2.0 |
9.0 |
|||
| e |
|
|
|
3.0 |
87 |
|||
| f |
|
|
|
2.0 |
85 |
|||
| g |
|
|
|
2.5 |
92 |
|||
| h |
|
|
|
4.0 |
90 |
|||
| i |
|
|
|
3.0 |
88 |
|||
| j |
|
|
|
2.5 |
86 |
|||
| k |
|
|
|
3.0 |
90 |
|||
| l |
|
|
|
2.5 |
91 |
|||
| m |
|
|
|
3.0 |
87 |
|||
| n |
|
|
|
4.0 |
85 |
|||
| o |
|
|
|
3.0 |
88 |
|||
| p |
|
|
|
2.5 |
86 |
|||
| q | 2.5 | 89 |
Experimental
General procedure
A mixture of o-phenylenediamine (1.0 mmol) and aldehyde (1.2 mmol) in the presence of lanthanum chloride (10 mol%) was stirred in acetonitrile (5 ml) at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction as indicated by TLC, the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate and washed with water and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The crude products were purified by column chromatography. All the products were identified by their 1H NMR, IR, and mass spectroscopy data.
Spectral data for selected compounds
2-(3,4,5-Trimethoxyphenyl)-1H-benzo[d]imidazole (3a)
For this compound, the white solid’s melting point was 259°C. The IR (KBr) frequency (υ) values were as follows: 2,924, 2,851, 1,601, 1,495, 1,463, 1,416, 1,282, 1,096, 1,020, 899, 801, 749, and 693 cm−1. The 1H NMR (DMSO-d6) chemical shift (δ) values were as follows: 3.90 (s, 3H), 4.00 (s, 6H), 7.43 to 7.60 (m, 2H), 7.65 (s, 2H), and 7.85 to 7.95 (m, 2H). The electron ionized mass spectrometry (EIMS) mass-to-ratio (m/z) values and corresponding percentage were as follows: 285 (m+1 100%), 269 (10%), 255 (10%), and 224 (5%).
4-(1H-Benzo[d]imidazol-2-yl)-N,N-dimethyl benzenamine (3b)
For this compound, the white solid’s melting range was 288°C to 290°C. The IR (KBr) υ values were as follows: 2,853, 2,800, 1,740, 1,611, 1,561, 15,276, 1,446, 1,389, 1,362, 1,324, 1,278, 1,230, 1,200, 1,167, 1,106, 1,064, 948, 819, 800, 744, 769, and 583 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 2.90 (s, 6H), 6.70 (dd, 2H), 6.95 (d, 2H), 7.10 to 7.25 (m, 2H), and 7.60 (dd, 2H). The EIMS m/z values and percentage were as follows: 238 (m+1 100%), 157 (30%), 134 (80%), and 109 (10%).
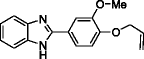
2-(4-(Allyoxy)-3-methoxyphenyl)-1H-benzo[d]imidazole (3c)
For this compound, the solid’s IR (KBr) υ values were as follows: 3,063, 2,923, 2,853, 1,886, 1,747, 1,649, 1,607, 1,580, 1,449, 1,460, 1,422, 1,387, 1,316, 1,250, 1,215, 1,180, 1,138, 1,027, 991, 924, 866, 805, 763, 743, 628, and 594 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 3.75 (s, 3H), 4.55 (d, 2H), 5.25 (d, 1H), 5.40 (t, 2H), 5.95 to 6.10 (m, 1H), 6.60 (d, 1H) 6.73 (t, 1H), 7.15 to 7.35 (m, 2H), 7.50 to 7.60 (m, 2H), and 7.80 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 280 (m+1 100%) and 242 (80%).
2-(Furan-2-yl)-1H-benzo[d]imidazole (3d)
For this compound, the solid’s melting point was 296°C. The IR (KBr) υ values were as follows: 2,927, 2,857, 1,741, 1,609, 1,545, 1,462, 1,379, 1,189, 1,069, 751, and 597 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 6.30 (d, 2H), 7.15 to 7.35 (m, 2H), 7.40 (d, 1H), and 7.65 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 184 (m+1 100%), 158 (20%), 137 (5%), and 133 (5%).
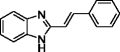
(E)-2-Styryl-1H-benzo[d]imidazole (3e)
For this compound, the solid’s melting range was 201°C to 203°C. The IR (KBr) υ values were as follows: 3,377, 3,027, 2,924, 2,853, 1,948, 1,805, 1,633, 1,598, 1,495, 1,449, 1,402, 1,355, 1,326, 1,284, 1,194, 1,153, 1,070, 1,018, 963, 918, 841, 737, 691, and 558 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 6.40 (dd, 1H), 6.55 (d, 1H), 7.15 to 7.55 (m, 7H), and 7.70 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 220 (m+1 15%), 195 (5%), 174 (5%), 155 (5%), 144 (5%), and 134 (5%).
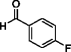
2-(4-Fluorophenyl)-1H-benzo[d]diazole (3f)
For this compound, the white solid’s melting point was 248°C. The IR (KBr) υ values were as follows: 3,053, 2,930, 1,663, 1,624, 1,545, 1,486, 1,440, 1,315, 1,277, 1,229, 1,094, 1,034, 1,004, 972, 833, 795, 746, 690, 618, and 568 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 7.15 to 7.20 (m, 2H), 7.20 to 7.40 (m, 2H), 7.45 to 7.52 (m, 2H), 7.60 to 7.70 (m, 2H), and 8.00 (brs, 1H). The EIMS m/z values and corresponding percentage were as follows: 212 (m+ 100%), 193 (5%), 215 (15%), 168 (5%), 155 (5%), 136 (5%), 129 (5%), and 95 (5%).
2-p-Tolyl-1H-benzo[d]imidazole (3g)
For this compound, the white solid’s melting point was 275°C. The IR (KBr) υ values were as follows: 3,397, 3,027, 2,922, 2,858, 1,813, 1,514, 1,481, 1,452, 1,412, 1,383, 1,348, 1,282, 1,250, 1,183, 1,157, 1,114, 1,021, 987, 823, 746, and 612.cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 2.35 (s, 3H), 4.42 (brs, 1 NH), 6.95 (d, 2H), 7.10 (d, 2H), 7.28 (d, 2H), and 7.55 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 208 (m+ 100%), 195 (15%), 179 (20%), 161 (10%), 153 (10%), 149 (5%), 140 (20%), 136 (5%), 126 (10%), and 122 (5%).
3-(1H-Benzo[d]imidazol-2-yl)-2-chloro-6-methylquinoline (3h)
For this compound, the solid’s IR (KBr) υ values were as follows: 3,073, 1,585, 1,493, 1,435, 1,392, 1,331, 1,280, 1,227, 1,147, 1,031, 929, 816, 748, 711, 646, 579, and 483 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 2.60 (s, 3H), 7.25 (d, 2H), 7.70 (d, 1H), 7.80 (d, 2H), 7.90 (d, 2H), and 8.80 (s 1H). The EIMS m/z values and corresponding percentage were as follows: 294 (m+ 70%), 290 (10%), 274 (40%), 258 (5%), 246 (5%), 230 (5%), 212 (5%), 191 (10%), and 169 (5%).
2-Phenyl-1H-benzo[d]imidazole (3i)
For this compound, the white powder’s melting point was 295°C. The IR (KBr) υ values were as follows: 3,406, 3,047, 1,589, 1,540, 1,443, 1,409, 1,483, 1,275, 1,118, 736, and 704 cm−1. The 1H NMR (DMSO-d6) δ values were 4.50 (brs, 1H), 7.20 to 7.40 (m, 2H), 7.50 to 7.75 (m, 5H), 7.70 (d, 2H), and 8.25 (d, 2H). EIMS m/z values and corresponding percentage were as follows: 195 (m+ 10%), 175 (5%), and 160 (5%).
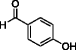
4-(1H-Benzo[d]imidazole-2yl) phenol (3j)
For this compound, the white powder’s melting range was 229°C to 230°C. The IR (KBr) υ values were as follows: 3,376, 3,290, 3,027, 2,807, 1,697, 1,611, 1,591, 1,515, 1,443, 1,394, 1,268, 1,246, 839, and 745 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 6.90 (d, 1H), 7.05 to 7.15 (m, 4H), and 7.75 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 210 (m+ 100%), 193 (5%), 191 (20%), 183 (10%), 181 (5%), 169 (40%), 154 (5%), 137 (5%).
2-(4-(Benzyloxy)-3-methoxyphenyl)-1H-benzo[d]imidazole (3k)
For this compound, the IR (KBr) υ values were as follows: 3,036, 2,924, 2,853, 1,738, 1,604, 1,497, 1,458, 1,384, 1,321, 1,240, 1,209, 1,175, 1,132, 1,028, 992, 905, 802, 740, 697, 641, and 572, and 465 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 3.73 (s, 3H), 5.15 (s, 2H), 6.55 (d, 1H), 6.55 (d, 1H), 6.75 (dd, 2H) 7.10 to 7.50 (m, 7H), and 7.80 (d, 2H). The EIMS m/z (first set) values and corresponding percentage were as follows: 330 (m+ 60%), 313 (10%), 305 (20%), 289 (5%), 261 (30%), 245 (20%), 227 (100%), 210 (20%), 201 (50%), 195 (20%), 157 (20%), 100 (30%), 91 (10%), and 89 (5%). The EIMS m/z (second set) values and corresponding percentage were as follows: 245 (m+ 100%), 243 (5%), and 141 (10%). The EIMS m/z (third set) values and corresponding percentage were as follows: 245 (m+ 100%), 243 (5%), and 141 (10%).
2-(3-Chlorophenyl)-1H-benzo[d]imidazole (31)
For this compound, the white powder’s melting range was 232°C to 234°C. The IR (KBr) υ values were as follows: 3,059, 1,619, 1,593, 1,440, 1,421, 1,269, 836, and 750 cm−1. The 1H NMR (DMSO-d6 MHz) δ values were as follows: 7.45 to 7.60 (m, 4H), 7.62 to 7.72 (m, 2H), and 8.30 to 8.45 (m, 2H). The EIMS m/z value with its corresponding percentage was 229 (m+ 100%).
2-(Naphthalene-2yl)-1H-benzo[d]imidazole (3m)
For this compound, the white powder’s melting range was 218°C to 219°C. The IR (KBr) υ values were as follows: 3,425, 3,047, 2,924, 2,853, 1,624, 1,605, 1,447, 1,385, and 748 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 6.70 to 6.90 (m, 2H), 7.20 to 7.35 (m, 2H), 7.55 to 7.80 (m 4H), and 7.90 to 8.10 (m, 2H). The EIMS m/z values and corresponding percentage were as follows: 245 (m+ 100%), 243 (5%), and 141 (10%).
(E)-2-(Pent-en-2-yl)-1H-benzo[d]imidazole (3n)
For this compound, the IR (KBr) υ values were as follows: 3,064, 2,963, 2,923, and 1,648 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 1.10 (t, 3H), 1.80 (m, 2H), 1.95 to 2.10 (m, 2H), 5.90 (dd, 1H), 7.30 (d, 2H), and 7.75 (d, 2H). The EIMS m/z value was 187.
2-(4-Nitrophenyl)-1H-benzo[d]imidazole (3o)
For this compound, the yellow powder’s melting point was 314°C. The IR (KBr) υ values were 3,042, 1,604, 1,515, 1,434, 1,353, 854, 745, and 710 cm−1.. The 1H NMR (DMSO-d6) δ values were as follows: 7.10 to 7.15 (m, 2H), 7.30 (d, 1H), 7.35 (d, 1H), 7.40 (t, 1H), 7.45 (t, 1H), 8.0 (dd, 2H), and 13.0 (brs, 1H). The EIMS m/z values and corresponding percentage were as follows: 240 (m+ 100%), 226 (5%), 211 (10%), 194 (20%), and 182 (5%).
2-(Pyridine-2-yl)-1H-benzo[d]imidazole (3p)
For this compound, the solid’s melting range was 245°C to 248°C. The IR (KBr) υ values were as follows: 3,068, 1,449, 1,402, 1,280, and 746 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 6.85 (m, 2H), 7.00 to 7.10 (m, 1H), 7.45 to 7.55 (m, 1H), 7.80 to 7.90 (m, 2H), 8.10 (t, 1H), and 8.65 (d, 1H). EIMS m/z value with its corresponding percentage was 196 (m+ 15%).
4-(1H-Benzo[d]imidazole-2yl) benzonitrile (3q)
For this compound, the white crystal solid’s melting point was 262°C. The IR (KBr) υ values were as follows: 3,417, 3,047, 2,912, 2,222, 1,605, 1,454, 1,408, and 748 cm−1. The 1H NMR (DMSO-d6) δ values were as follows: 5.50 (brs, 1H), 7.45 to 7.60 (m, 2H), 7.82 to 7.90 (m, 2H), 8.05 (d, 2H), and 8.50 (d, 2H). The EIMS m/z values and corresponding percentage were as follows: 220 (m+ 100%), 211(10%), 196 (5%), and 186 (5%).
Conclusions
In conclusion, lanthanum chloride has been employed as a novel and efficient catalyst for the synthesis of benzimidazoles in good yields from o-phenylenediamine and a wide variety of aldehydes. All the reactions were carried out at room temperature while using the catalyst lanthanum chloride in 10 mol%. The reaction conditions were very mild, and the isolation of products was also very easy.
Competing interests
The authors declare that they have no competing interests.
Authors’ information
YV and SRK are research scholars, and PL is a professor at the Department of Chemistry, University College for Women, Koti Osmania University, Hyderabad 500095, India.
Contributor Information
Yekkirala Venkateswarlu, Email: yekkiralavenkat@gmail.com.
Sudhagani Ramesh Kumar, Email: rameshteja_2001@yahoo.co.in.
Panuganti Leelavathi, Email: ameshteja_2001@yahoo.co.in.
Acknowledgments
The authors are thankful to the director of IICT for providing the working space and chemicals.
References
- Nakano H, Inoue T, Kawasaki N, Miyataka H, Matsumoto H, Taguchi T, Inagaki N, Nagai H, Satoh T. Synthesis of biologically activities of novel anti-allergic agents with 5-lipoxygenase inhibiting action. Bioorg Med Chem. 2000;3:373–380. doi: 10.1016/S0968-0896(99)00291-6. [DOI] [PubMed] [Google Scholar]
- Hauel HN, Nar H, Priepke H, Ries U, Stassen J, Wienen W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002;3:1757–1766. doi: 10.1021/jm0109513. [DOI] [PubMed] [Google Scholar]
- He Y, Wu B, Yang J, Robinson D, Risen L, Ranken R, Blyn L, Sheng S, Swayze EE. 2-Piperidin-4-yl-benzimidazoles with broad spectrum antibacterial activities. Bioorg Med Chem Lett. 2003;3:3253–3256. doi: 10.1016/S0960-894X(03)00661-9. [DOI] [PubMed] [Google Scholar]
- Porcari RA, Devivar RV, Kucera LS, Cdreach J, Townsend BL. Design, synthesis and antiviral evaluations of 1-(substituted benzyl)-2-substituted-5, 6-dichlorobenzimidazoles as non nucleoside analogues of 2,5,6-trichloro-1-(β-d-ribofuranosyl) benzimidazole. J Med Chem. 1998;3:1252–1262. doi: 10.1021/jm970559i. [DOI] [PubMed] [Google Scholar]
- Roth T, Morningstar LM, Boyer LP, Hughes MS, Buckheitjr RW, Michejda JC. Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem. 1997;3:4199–4207. doi: 10.1021/jm970096g. [DOI] [PubMed] [Google Scholar]
- Migawa TM, Girardet LJ, Walker AJ, Koszalka WG, Chamberjain DS, Drach C, Townsend BL. Design, synthesis, and antiviral activity of α-nucleosides: d-and l-isomers of lyxofuranosyl and (5-deoxylyxofuranosyl) benzimidazoles. J Med Chem. 1998;3:1242–1251. doi: 10.1021/jm970545c. [DOI] [PubMed] [Google Scholar]
- Mann J, Baron AY, Opoku-Boahen Y, Johansoon E, Parkmson G, Kelland RL, Neidle S. A new class of symmetric bisbenzimidazole based DNA minor groove-binding agents showing antitumor activity. J Med Chem. 2001;3:138–144. doi: 10.1021/jm000297b. [DOI] [PubMed] [Google Scholar]
- Figge A, Altenbach JH, Brauer DJ, Tielmann P. Synthesis and resolution of 2-(2-diphenylphosphinyl-naphthalen-1-yl)-1-isopropyl-1H-benzimidazole: a new atropisomeric P, N-chelating ligand for asymmetric catalysis. Tetrahedron-Asymmetry. 2002;3:137–144. doi: 10.1016/S0957-4166(02)00079-4. [DOI] [Google Scholar]
- Hisano T, Ichikawa M, Tsumoto K, Tasaki M. Synthesis of benzoxazoles, benzothiazoles and benzimidazoles and evaluation of their antifungal, insecticidal and herbicidal activities. Chem Pharm Bull. 1982;3:2996–3004. doi: 10.1248/cpb.30.2996. [DOI] [Google Scholar]
- Kumar BVS, Vaidya SD, Kumar RV, Bhirud SB, Mane RB. Synthesis and anti-bacterial activity of some novel 2-(6-fluorochroman-2-yl)-1-alkyl/acyl/aroyl-1H-benzimidazoles. Eur J Med Chem. 2006;3:599–604. doi: 10.1016/j.ejmech.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Bahrami K, Khodaei MM, Naali F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J Org Chem. 2008;3:6835–6837. doi: 10.1021/jo8010232. [DOI] [PubMed] [Google Scholar]
- Buchwald SL, Zheng N, Anderson KW, Huang X, Nguyen HN. A palladium-catalyzed regiospecific synthesis of N-arylbenzimidazoles. Angew Chem. 2007;3:7509–7512. doi: 10.1002/anie.200702542. [DOI] [PubMed] [Google Scholar]
- Beaulieu PL, Hache B, Moon VE. A practical oxone-mediated, high-through, solution-phase synthesis of benzimidazoles from 1,2-phenylenediamines and aldehydes and its application to preparative-scale synthesis. Synthesis. 2003;3:1683–1692. [Google Scholar]
- Eynde JJV, Delfosse F, Lor P, Van YH. 2,3-Dichloro-5, 6-dicyano-1,4-benzoquinone, a mild catalyst for the formation of C-N bonds. Tetrahedron. 1995;3:5813–5818. doi: 10.1016/0040-4020(95)00252-4. [DOI] [Google Scholar]
- Du LH, Wang YG. A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis. 2007;3:675–678. [Google Scholar]
- Du HL, Luo AP. Efficient and one-pot synthesis of and benzimidazoles under solvent free conditions. Synth Commun. 2010;3:2880–2886. doi: 10.1080/00397910903340629. [DOI] [Google Scholar]
- Gogoi P, Konwar D. An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of C-N bonds in water. Tetrahedron Lett. 2006;3:79–82. doi: 10.1016/j.tetlet.2005.10.134. [DOI] [Google Scholar]
- Singh PM, Sasmal S, Lu W, Chatterjee NM. Synthetic utility of catalytic Fe(III)/Fe(II) redox cycling towards fused heterocycles: a facile access to substituted benzimidazoles, bisbenzimidazoles and imidazopyridine derivatives. Synthesis. 2000;3:1380–1390. [Google Scholar]
- Wang XJ, Zhang L, Xu Y, Murthy DK, Senanayake CS. A practical synthesis of 2-(N-substituted)-amino-benzimidazoles utilizing CuCl-promoted intramolecular cyclization of N-(2-aminoaryl)thioureas. Tetrahedron Lett. 2004;3:7167–7170. doi: 10.1016/j.tetlet.2004.07.042. [DOI] [Google Scholar]
- Alloum AB, Bougrin K, Soufiaoui M. Synthese chimioselective des benzimidazoles sursilice traitee parlechlorure du thionyle. Tetrahedron Lett. 2003;3:5935–5937. doi: 10.1016/S0040-4039(03)01387-X. [DOI] [Google Scholar]
- Yang D, Fu H, Hu L, Jiang Y, Zhao Y. Copper-catalyzed synthesis of benzimidazoles via cascade reactions of o-halo acetanilide derivatives with amidine hydrochlorides. J Org Chem. 2008;3:7841–7844. doi: 10.1021/jo8014984. [DOI] [PubMed] [Google Scholar]
- Subramanyam SC, Narayanan S. Yttrium (III) chloride: a mild and efficient catalyst for the synthesis of benzimidazoles. Int J Apples Bio and Phar Tech. 2010;3:689–694. [Google Scholar]
- Tandon VK, Kumar M. BF3-Et2O-promoted one-pot expeditious and convenient synthesis of 2-substituted benzimidazoles and 3,1,5-benzoxadiazepines. Tetrahedron Lett. 2004;3:4185–4187. doi: 10.1016/j.tetlet.2004.03.117. [DOI] [Google Scholar]
- Shinde B, Nagawade RR. BF3-Et2O promoted solvent free synthesis of benzimidazole derivatives. Chin Chem Lett. 2006;3:453–456. [Google Scholar]
- Yulu W, Huiqiang M, Xiangming H. p-TsOH catalyzed synthesis of 2-arylsubstituted benzimidazoles. ARKIVOC. 2007;3(13):150–154. doi: 10.3998/ark.5550190.0008.d18. [DOI] [Google Scholar]
- Trivedi R, De SK, Gibbs RA. A convenient one-pot synthesis of 2-substituted benzimidazoles. J Mol Catal A. 2006;3:8–11. doi: 10.1016/j.molcata.2005.09.025. [DOI] [Google Scholar]
- Currini M, Epifano F, Motanari F, Rosati O, Taccone S. Yttrium triflate promoted synthesis of benzimidazole derivatives. Synlett. 2004;3:1832–1834. [Google Scholar]
- Chari MA, Sadanandam P, Shobha D, Mukkanti K. A Simple mild, and efficient procedure for benzimidazoles using copper triflate as catalyst. Heterocyclic Chem. 2010;3:153–155. [Google Scholar]
- Reddy AR, Narasaiah AV, Yadav JS. Mild and efficient protocol for the synthesis of benzimidazoles using samarium triflate. Synth Commun. 2011;3:262–267. [Google Scholar]
- Lin S, Yang L. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett. 2005;3:4315–4319. doi: 10.1016/j.tetlet.2005.04.101. [DOI] [Google Scholar]
- Nadaf RN, Siddiqui SA, Daniel T, Lahoti RJ, Srinivasan KV. Room-temperature ionic liquid-promoted region selective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions. J Mol Catal A. 2004;3:155–160. doi: 10.1016/j.molcata.2003.10.064. [DOI] [Google Scholar]
- Heravi MM, Sadjadi S, Oskooie HA, Shoar RH, Bamoharram FF. Heteropoly acids as heterogeneous and recyclable catalysts for the synthesis of benzimidazoles. Catal Commun. 2008;3:504–507. [Google Scholar]
- Das B, Holla H, Srinivas Y. Efficient (bromodimethysulfonium bromide mediated synthesis of benzimidazoles. Tetrahedron Lett. 2007;3:61–64. doi: 10.1016/j.tetlet.2006.11.018. [DOI] [Google Scholar]
- Zou B, Yuan Q, Ma D. Synthesis of 1,2-disubstituted benzimidazoles by a Cu-catalyzed cascade aryl amination/condensation process. Angew Chem. 2007;3:2598–2601. doi: 10.1002/anie.200700071. [DOI] [PubMed] [Google Scholar]
- Varala R, Nasreen A, Enugala R, Adapa SR. l-Proline catalyzed selective synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles. Tetrahedron Lett. 2007;3:69–72. doi: 10.1016/j.tetlet.2006.11.010. [DOI] [Google Scholar]
- Wu CH, Sun CM. Parallel synthesis of amino bis-benzimidazoles by multistep microwave irradiation. Tetrahedron Lett. 2006;3:2601–2604. doi: 10.1016/j.tetlet.2006.02.015. [DOI] [Google Scholar]
- Surpur MP, Singh PR, Patil SB, Samant SD. One-pot synthesis of benzimidazoles from o-nitro anilines under microwaves via a reductive cyclization. Synth Commun. 2007;3:1375–1379. doi: 10.1080/00397910701230170. [DOI] [Google Scholar]
- Wang Y, Sarris K, Sauer DR, Djuric SW. A simple and efficient one step synthesis of benzoxazoles and benzimidazoles from carboxs. Tetrahedron Lett. 2006;3:4823–4826. doi: 10.1016/j.tetlet.2006.05.052. [DOI] [Google Scholar]
- Mao Z, Wang Z, Li J, Song X, Luo Y. Rapid and cheap synthesis of benzimidazoles via intermittent microwave promotion: a simple and potential industrial application of air as oxidant. Synth Commun. 2010;3:1963–1977. doi: 10.1080/00397910903219328. [DOI] [Google Scholar]
- Pitchumani K, Kanagaraj K, Dhakshinamoorthy A. A Zn2+ K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011;3:69–73. doi: 10.1016/j.tetlet.2010.10.146. [DOI] [Google Scholar]
- Kumar SR, Venkateswarlu Y, Leelavathi P. Synthesis of 1,5-benzodiazepines catalysed by Zn(OTf)2 in solvent free medium. Asian J Chem. 2011;3:1611–1614. [Google Scholar]
- Venkateswarlu Y, Leelavathi P. NbCl5: an efficient catalyst for the synthesis of quinoxalines. Lett Org Chem. 2010;3:208–211. doi: 10.2174/157017810791112432. [DOI] [Google Scholar]
- Venkateswarlu Y, Kumar SR, Leelavathi P. CdCl2: a simple and efficient catalyst for the synthesis of 1,4-dihydropyridine. (Hantzsch pyridines) Int J Ind Chem. 2012;3:18. doi: 10.1186/2228-5547-3-18. [DOI] [Google Scholar]
- Venkateswarlu Y, Kumar SR, Leelavathi P. Eu(OTf)3: an efficient catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Organic Synthesis and Medicinal Chemistry ASAP. 2011. [DOI] [PMC free article] [PubMed]
- Venkateswarlu Y, Kumar SR, Leelavathi P. A simple and efficient protocol for the synthesis of quinolines catalyzed by chloramines-T. Org Commun. 2012;3(3):120. [Google Scholar]
- Kumar SR, Venkateswarlu Y, Leelavathi P. LaCl3: an efficient catalyst for the synthesis of substituted thiazoles. Organic synthesis and medicinal chemistry. 2012;3:11–14. doi: 10.1186/2191-2858-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsaiah AV. Lanthanum trichloride (LaCl3): accelerated conjugate addition of amines to electron-poor alkenes. Lett Org Chem. 2007;3(7):462–464. doi: 10.2174/157017807782006290. [DOI] [Google Scholar]
- Luche JL, Gemal AL. Efficient synthesis of acetals catalyzed by rare earth chlorides. Chem Commun. 1978;3:976–977. [Google Scholar]
- Narsaiah AV, Nagaiah K. LaCl3: mediated regeneration of carbonyl compounds from oximes in water. Ind J Chem. 2003;3(9):2045–2047. [Google Scholar]
- Narsaiah AV, Nagaiah K. An efficient Knoevenagel condensation catalyzed by LaCl3 in heterogeneous medium. Synth Commun. 2003;3:3825–3832. doi: 10.1081/SCC-120025194. [DOI] [Google Scholar]
- Narsaiah AV. LaCl3: an efficient catalyst for the silylation of hydroxyl groups by activating HMDS. J Orgnomet Chem. 2007;3:3614. doi: 10.1016/j.jorganchem.2007.05.002. [DOI] [Google Scholar]
- Lu J, Bai Y, Wang Z, Yang B, Ma H. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using LaCl3 as a catalyst. Tetrahedran Lett. 2000;3:9075–9078. doi: 10.1016/S0040-4039(00)01645-2. [DOI] [Google Scholar]