Abstract
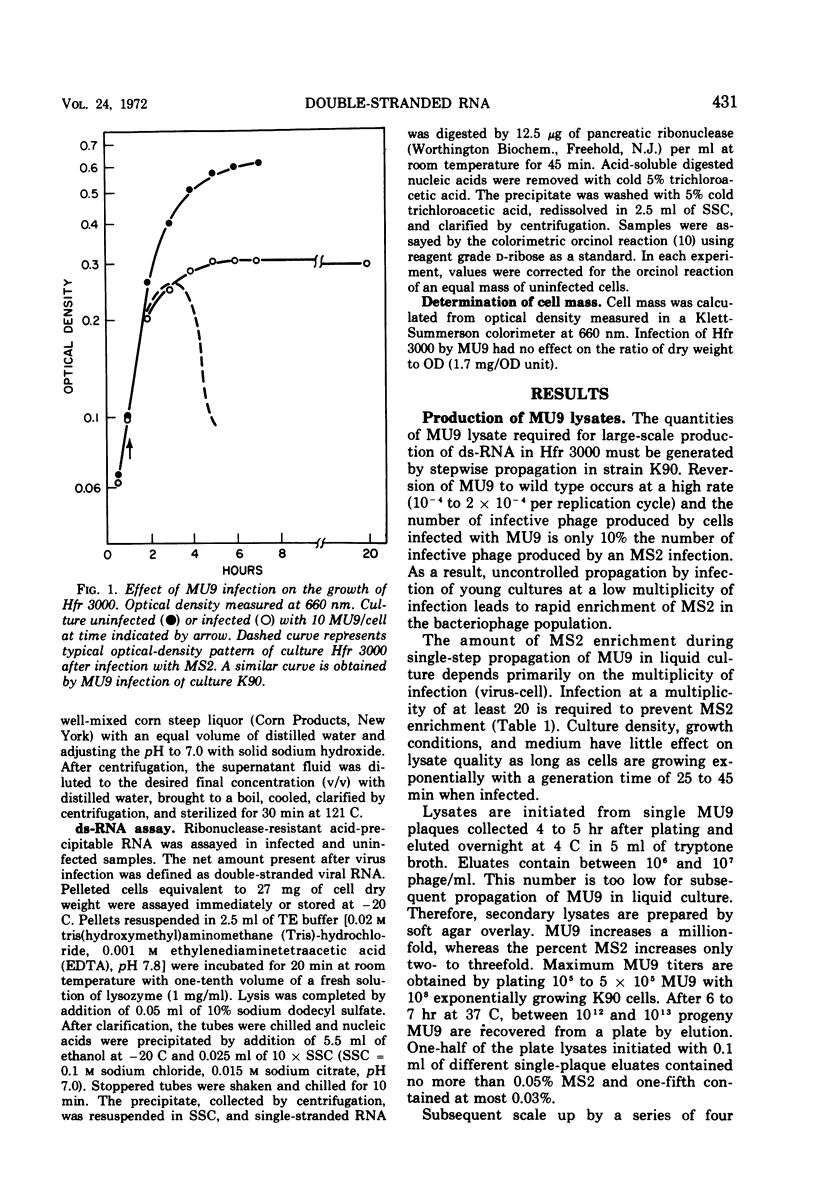
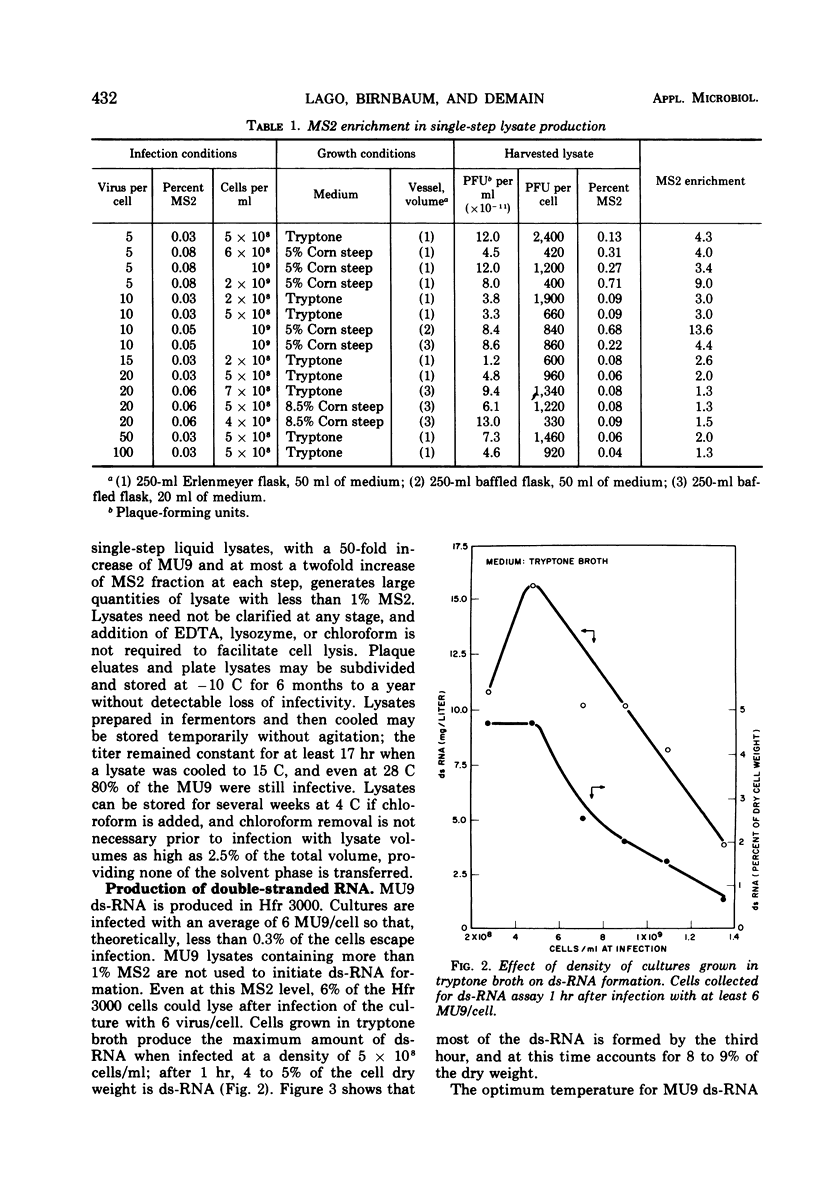
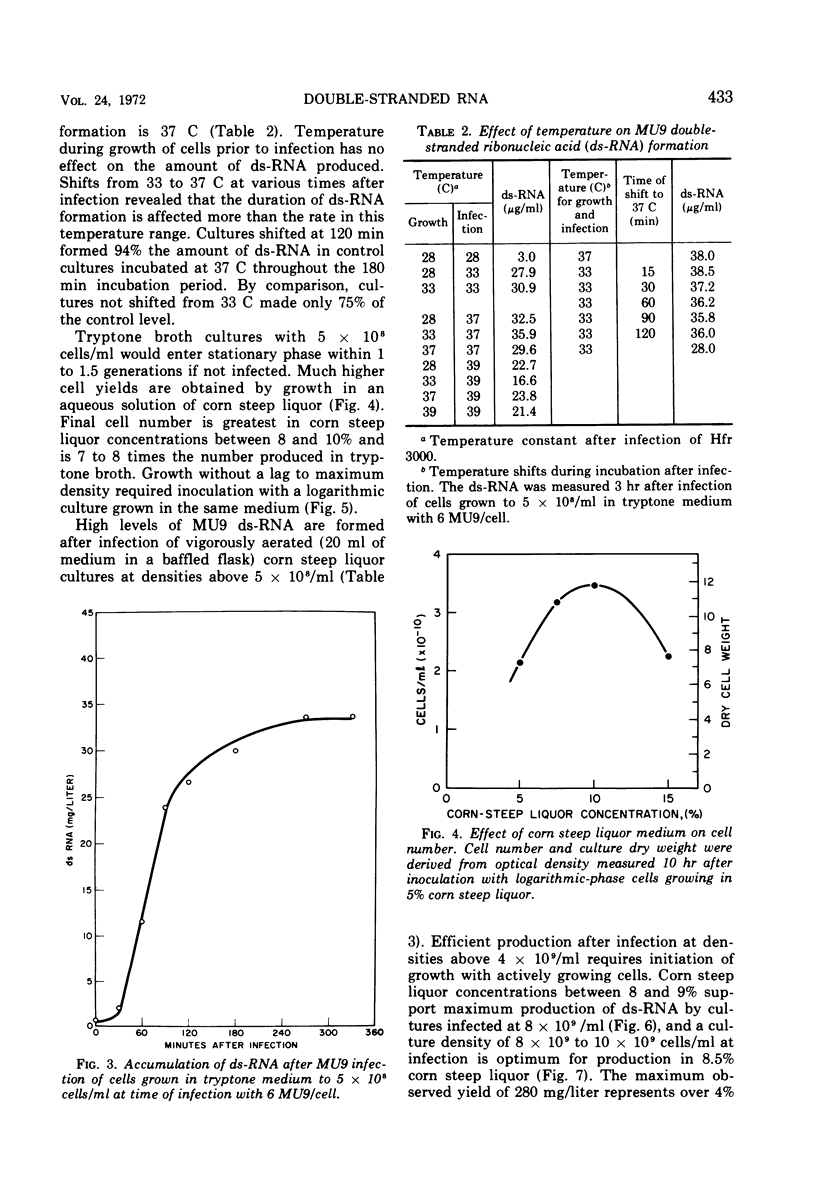
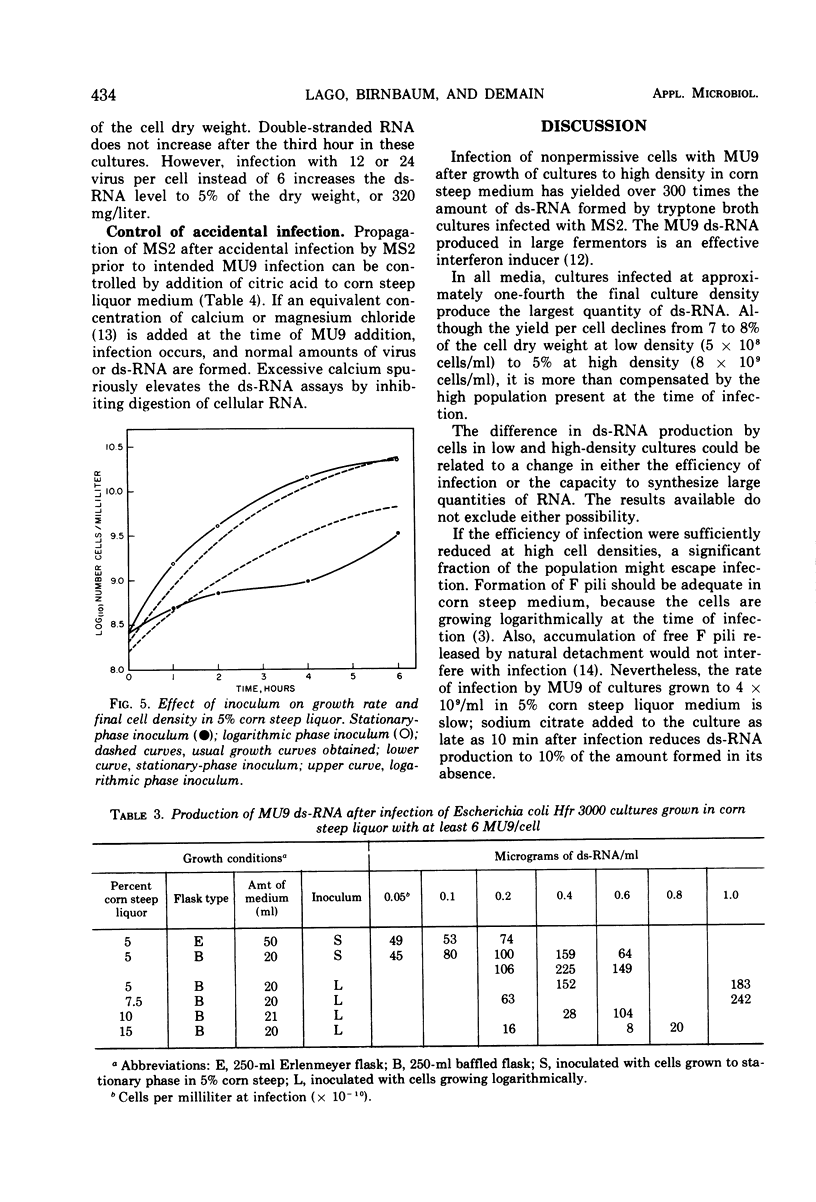
Double-stranded ribonucleic acid (ds-RNA) isolated from Escherichia coli infected with bacteriophage MS2 is a potent interferon inducer. High levels of ds-RNA are formed in nonpermissive cells infected with MU9, an amber coat protein mutant of MS2. This mutant has been used to develop a process for large-scale ds-RNA production. Preparation of quantities of MU9 lysate sufficient for ds-RNA production in fermentors is described. Over 300 μg of ds-RNA/ml can be accumulated after MU9 infection of cultures grown to high density in corn steep liquor medium. This is approximately 300 times the amount of ds-RNA made by MS2 infection of cells grown in tryptone medium. Maximum ds-RNA formation requires only 3 hr. The ds-RNA is stable and remains inside nonaerated cells for at least 17 hr.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Field A. K., Lampson G. P., Tytell A. A., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance, IV. Double-stranded replicative form RNA (MS2-Ff-RNA) from E. coli infected with MS2 coliphage. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2102–2108. doi: 10.1073/pnas.58.5.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Katze J., Konigsberg W. Position of the amber mutation in the MU9 coat protein. J Mol Biol. 1969 May 28;42(1):115–117. doi: 10.1016/0022-2836(69)90490-2. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Mutants of the bacteriophage f2. 8. Control mechanisms for phage-specific syntheses. J Mol Biol. 1966 Aug;19(2):333–348. doi: 10.1016/s0022-2836(66)80008-6. [DOI] [PubMed] [Google Scholar]
- Nathans D., Oeschger M. P., Polmar S. K., Eggen K. Regulation of protein synthesis directed by coliphage MS2 RNA. I. Phage protein and RNA synthesis in cells infected with suppressible mutants. J Mol Biol. 1969 Jan;39(2):279–292. doi: 10.1016/0022-2836(69)90317-9. [DOI] [PubMed] [Google Scholar]
- Nemes M. M., Tytell A. A., Lampson G. P., Field A. K., Hilleman M. R. Inducers of interferon and host resistance. VII. Antiviral efficacy of double-stranded RNA of natural origin. Proc Soc Exp Biol Med. 1969 Nov;132(2):784–789. doi: 10.3181/00379727-132-34309. [DOI] [PubMed] [Google Scholar]
- Novotny C., Raizen E., Knight W. S., Brinton C. C., Jr Functions of F pili in mating-pair formation and male bacteriophage infection studies by blending spectra and reappearance kinetics. J Bacteriol. 1969 Jun;98(3):1307–1319. doi: 10.1128/jb.98.3.1307-1319.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W. Stages in phage R17 infection: the role of divalent cations. Virology. 1966 Jan;28(1):90–99. doi: 10.1016/0042-6822(66)90309-6. [DOI] [PubMed] [Google Scholar]
- Roberts J., Burson G., Hill J. M. New procedures for purification of L-asparaginase with high yield from Escherichia coli. J Bacteriol. 1968 Jun;95(6):2117–2123. doi: 10.1128/jb.95.6.2117-2123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Stone H. O., Jr Protein synthesis directed by an amber coat-protein mutant of the RNA phage MS2. J Mol Biol. 1969 May 28;42(1):97–115. doi: 10.1016/0022-2836(69)90489-6. [DOI] [PubMed] [Google Scholar]
- Tooze J., Weber K. Isolation and characterization of amber mutants of bacteriophage R17. J Mol Biol. 1967 Sep 14;28(2):311–330. doi: 10.1016/s0022-2836(67)80012-3. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Feix G., Garwes D., Weissmann C., Ochoa S. Virus-specific proteins in Escherichia coli infected with some amber mutants of phage MS2. Biochim Biophys Acta. 1968 Feb 26;155(2):558–565. doi: 10.1016/0005-2787(68)90199-8. [DOI] [PubMed] [Google Scholar]
- WEIGERT M. G., GAREN A. AMINO ACID SUBSTITUTIONS RESULTING FROM SUPPRESSION OF NONSENSE MUTATIONS. I. SERINE INSERTION BY THE SU-1 SUPPRESSOR GENE. J Mol Biol. 1965 Jun;12:448–455. doi: 10.1016/s0022-2836(65)80267-4. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., COOPER S. HOST-DEPENDENT MUTANTS OF THE BACTERIOPHAGE F2. I. ISOLATION AND PRELIMINARY CLASSIFICATION. Virology. 1964 Jun;23:152–158. doi: 10.1016/0042-6822(64)90277-6. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Lyons L. B. Cell lysis: another function of the coat protein of the bacteriophage f2. Science. 1968 Jan 5;159(3810):84–86. [PubMed] [Google Scholar]



